Hashimoto’s Thyroiditis and Female Fertility: Endocrine, Immune, and Microbiota Perspectives in Assisted Reproduction—A Narrative Review
Abstract
1. Introduction
2. Pathophysiological Mechanisms
3. Ovarian Reserve and Thyroid Dysfunction
4. Clinical Implications in Assisted Reproduction
5. International Guidelines and Recommendations
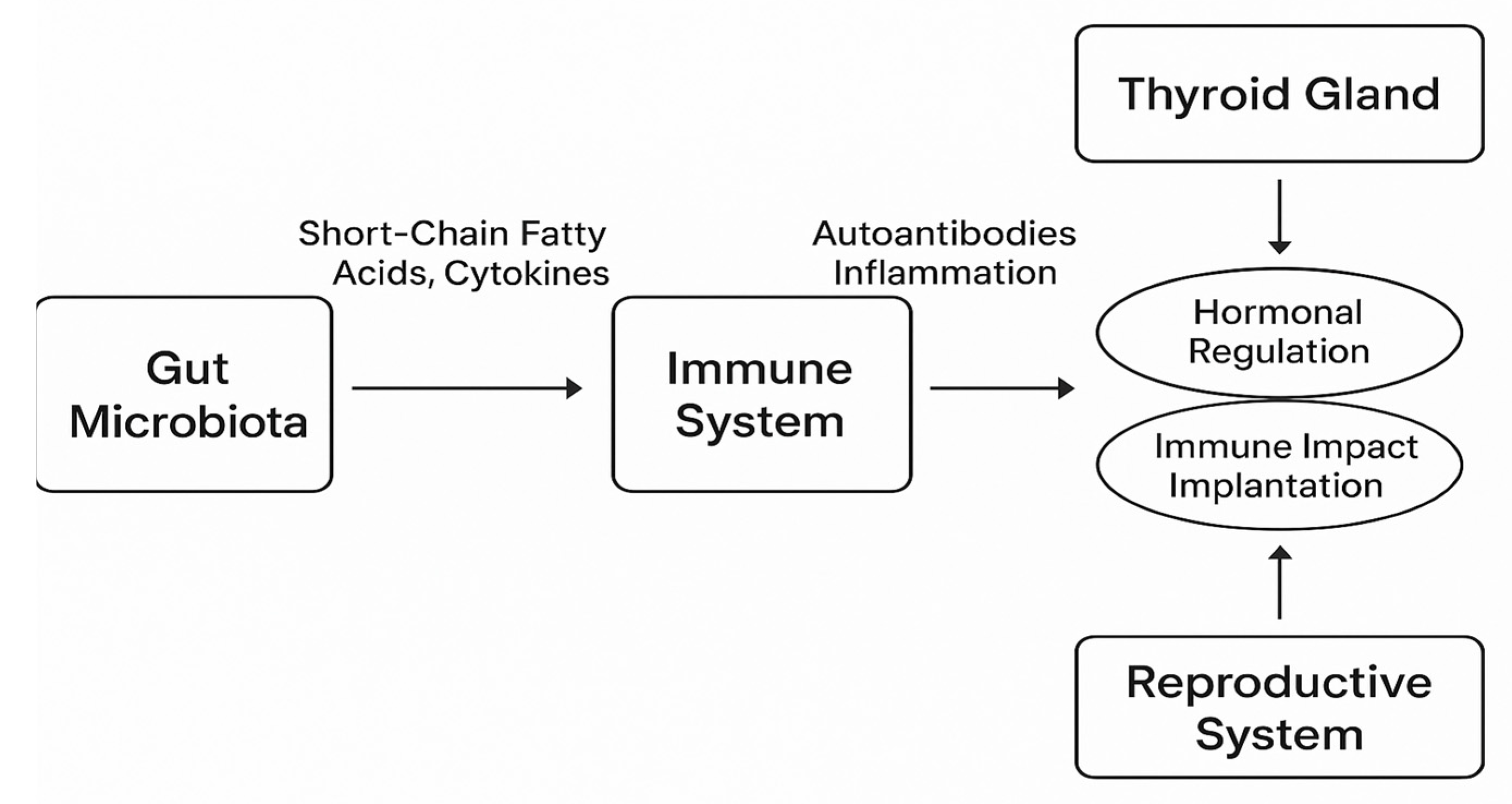
6. Gut Microbiota and Research Directions
7. Limitations and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Krysiak, R.; Kowalcze, K.; Okopien, B. Selenium Supplementation in Patients with Hashimoto Thyroiditis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Thyroid 2024, 34, 151–162. [Google Scholar] [CrossRef]
- Pan, M.; Qi, Q.; Li, C.; Wang, J.; Pan, X.; Zhou, J.; Sun, H.; Li, L.; Wang, L. Effect and Mechanism of Hashimoto Thyroiditis on Female Infertility: A Clinical Trial, Bioinformatics Analysis, and Experiments-Based Study. Biol. Pharm. Bull. 2024, 18, 201–212. [Google Scholar] [CrossRef]
- Ximenes de Souza, R.S.; Quintino-Moro, A.; Zantut-Wittmann, D.E.; Fernandes, A. Antithyroid Antibodies and Reproductive Parameters of Women with Hashimoto’s Thyroiditis. Endocr. Res. 2025, 50, 57–64. [Google Scholar] [CrossRef]
- Sun, J.; Xu, H.; Liu, Y. Global Prevalence and Epidemiological Trends of Hashimoto’s Thyroiditis in Adults: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto Thyroiditis: An Evidence-Based Guide to Etiology, Diagnosis and Treatment. Pol. Arch. Intern. Med. 2022, 132, 34–41. [Google Scholar]
- Poppe, K.; Velkeniers, B. Thyroid Autoimmunity in Female Infertility and Assisted Reproductive Technology Outcome. Front. Endocrinol. 2022, 13, 768363. [Google Scholar]
- Ding, X.; Wang, L.; Zhao, Y. Association Between Hashimoto’s Thyroiditis and Ovarian Reserve: A Systematic Review and Meta-Analysis. J. Endocrinol. Invest. 2021, 44, 519–529. [Google Scholar]
- Hong, Y.; Zhang, Y.; Yang, X. Levothyroxine Supplementation Improves Serum Anti-Müllerian Hormone Levels in Infertile Patients with Hashimoto’s Thyroiditis. J. Obstet. Gynaecol. Res. 2018, 44, 59–65. [Google Scholar]
- Lazzarin, N.; Chiandetti, L.; Mangano, E. Does Subclinical Hypothyroidism and/or Thyroid Autoimmunity Influence IVF/ICSI Outcome? Gynecol. Endocrinol. 2021, 37, 99–106. [Google Scholar]
- Shi, C.-J.; Shao, T.-R.; Zhao, X.; Wang, B. Evaluation of the Ovarian Reserve in Women and Adolescent Girls with Hashimoto’s Thyroiditis by Serum Anti-Müllerian Hormone Level: A Systematic Review and Meta-Analysis. Heliyon 2023, 9, e19204. [Google Scholar] [CrossRef]
- Song, Y.; Qiu, C.; Liu, L. The Impact of Gut Microbiota on Autoimmune Thyroiditis and Relationship with Pregnancy Outcomes: A Review. J. Autoimmun. 2023, 138, 102986. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yu, X.; Qiu, Y. Killer Cell Immunoglobulin-Like Receptor Genes and Their HLA-C Ligands in Hashimoto Thyroiditis in a Chinese Population. Endocr. Pract. 2016, 22, 418–426. [Google Scholar]
- Moon, K.Y.; Paik, H.; Jee, B.C.; Kim, S.H. Impact of Thyroid Autoantibodies and Serum TSH Level on Clinical IVF Outcomes: A Retrospective Study. Gynecol. Endocrinol. 2023, 39, 890–896. [Google Scholar]
- de Vet, A.; Laven, J.S.; de Jong, F.H.; Themmen, A.P.; Fauser, B.C. Antimüllerian Hormone Serum Levels: A Putative Marker for Ovarian Aging. Fertil. Steril. 2002, 77, 357–362. [Google Scholar] [CrossRef] [PubMed]
- De Leo, S.; Pearce, E.N. Hashimoto’s Thyroiditis Negatively Influences Intracytoplasmic Sperm Injection Outcome in Euthyroid Women on T4 Substitution Therapy: A Retrospective Study. Gynecol. Endocrinol. 2024, 40, 1–8. [Google Scholar]
- Wu, H.; Wang, R.; Zhang, Y. Intracytoplasmic Sperm Injection Versus In Vitro Fertilization in Infertile Women with Thyroid Autoimmunity. Thyroid 2024, 34, 232–240. [Google Scholar]
- Wang, H.; Zhang, C.; Liu, M. Effect of Levothyroxine Supplementation on Pregnancy Outcomes in Women with Subclinical Hypothyroidism and Thyroid Autoimmunity Undergoing IVF/ICSI: A Meta-Analysis. Reprod. Biol. Endocrinol. 2018, 16, 92. [Google Scholar]
- Li, Y.; Zhao, H.; Chen, C. Thyroid Autoimmunity and Its Negative Impact on Female Fertility and Maternal Pregnancy Outcomes. Front. Endocrinol. 2023, 13, 1049665. [Google Scholar]
- Bogović Crnčić, T.; Ćurko-Cofek, B.; Batičić, L.; Girotto, N.; Ilić Tomaš, M.; Kršek, A.; Krištofić, I.; Štimac, T.; Perić, I.; Sotošek, V.; et al. Autoimmune Thyroid Disease and Pregnancy: The Interaction Between Genetics, Epigenetics and Environmental Factors. J. Clin. Med. 2025, 14, 190. [Google Scholar] [CrossRef]
- Wiles, K.; Kumar, S.; Osei-Kuffour, D. Thyroxine Replacement for Subfertile Females with Subclinical Hypothyroidism and Autoimmune Thyroiditis: A Systematic Review. Cureus 2021, 13, e16872. [Google Scholar]
- De Groot, L.; Abalovich, M.; Alexander, E. A Review of Autoimmune Thyroid Diseases and Their Complex Interplay with Female Fertility. Semin. Reprod. Med. 2024, 42, 21–34. [Google Scholar]
- Chen, L.; Hu, R. Thyroid Autoimmunity and Miscarriage: A Meta-Analysis. Clin. Endocrinol. 2011, 74, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Chen, L.; Yan, Z.; Chi, H.; Qiao, J. Impact of Thyroid Autoimmunity on the Cumulative Live Birth Rates after IVF/ICSI Treatment Cycles: A Retrospective Cohort Study. BMC Pregnancy Childbirth 2024, 24, 230. [Google Scholar] [CrossRef]
- Virili, C.; Stramazzo, I.; Centanni, M. Gut Microbiome and Thyroid Autoimmunity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101506. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhu, X. Impact of Thyroid Autoimmunity on IVF/ICSI Outcomes and Fetal Weight. Fertil. Steril. 2022, 117, 984–992. [Google Scholar]
- Weetman, A.P. An Update on the Pathogenesis of Hashimoto’s Thyroiditis. J. Endocrinol. Investig. 2021, 44, 883–890. [Google Scholar] [CrossRef]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.C.D.S.; Chiamolera, M.I.; Silva, R.E.; Souza, M.D.C.B.D.; Antunes, R.A.; Souza, M.M.; Mancebo, A.C.A.; Arêas, P.C.F.; Reis, F.M.; Lo Turco, E.G.; et al. Metabolomic Profiling of Follicular Fluid in Women with Hashimoto’s Thyroiditis Undergoing IVF. Sci. Rep. 2023, 13, 6789. [Google Scholar] [CrossRef]
- Wu, J.; Qiu, Y.; Zhao, W.; Liu, J.; Gao, X. Recent Advances in Gut Microbiota and Thyroid Autoimmune Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2024, 14, 1361660. [Google Scholar] [CrossRef]
- Monteleone, P.; Parrini, D.; Faviana, P.; Artini, P.G. Female Infertility Related to Thyroid Autoimmunity: The Ovarian Follicle Hypothesis. Am. J. Reprod. Immunol. 2011, 66, 108–114. [Google Scholar] [CrossRef]
- Medenica, S.; Garalejić, E.; Abazović, D.; Žarković, M. Pregnancy Outcomes and Newborn Characteristics in Women with Follicular Fluid Thyroid Autoantibodies Undergoing Assisted Reproduction. Reprod. Biomed. Online 2022, 44, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimoto’s Thyroiditis: Epidemiology, Pathogenesis, Clinic and Therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef]
- Pellatt, L.; Rice, S.; Dilaver, N.; Heshri, A.; Galea, R.; Brincat, M.; Brown, K.; Simpson, E.R.; Mason, H.D. Anti-Müllerian Hormone Reduces Follicle Sensitivity to Follicle-Stimulating Hormone in Human Granulosa Cells. Fertil. Steril. 2011, 96, 1246–1251.e1. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Testing and Interpreting Measures of Ovarian Reserve: A Committee Opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef]
- Misirgis, M.; Gkouvi, A.; Mintziori, G.; Grammatikopoulou, M.; Goulis, D. The Impact of Thyroid Autoimmunity on Clinical Outcomes in Euthyroid Women Undergoing ART: A Systematic Review. Endocr. Abstr. 2025, 110, EP1305. [Google Scholar] [CrossRef]
- Lewiński, A.; Płazińska, M.; Ruchała, M. Thyroid Diseases and Fertility Disorders—Guidelines of the Polish Society of Endocrinology. Endokrynol. Pol. 2020, 71, 295–314. [Google Scholar]
- Gong, B.; Meng, F.; Wang, X.; Han, Y.; Yang, W.; Wang, C.; Shan, Z. Effects of Iodine Intake on Gut Microbiota and Gut Metabolites in Hashimoto Thyroiditis-Diseased Humans and Mice. Commun. Biol. 2024, 7, 136. [Google Scholar] [CrossRef]
- Zhang, C.; Teng, W.; Wang, C.; Shan, Z. The Gut Microbiota and Its Metabolites and Their Association with the Risk of Autoimmune Thyroid Disease: A Mendelian Randomization Study. Nutrients 2024, 16, 3898. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid–Gut Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Virili, C.; Stramazzo, I.; Centanni, M. Exploring Oral and Gut Microbiomes in Autoimmune Thyroid Disorders. J. Endocrinol. Investig. 2025, 48, 245–259. [Google Scholar]
- Wei, S.-X.; Wang, L.; Liu, Y.-B.; Fan, Q.-L.; Fan, Y.; Qiao, K. Positive Anti-TPO Antibodies Are Associated with Lower Ovarian Reserve and Embryo Quality in Euthyroid Women. Gynecol. Endocrinol. 2023, 39, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Thompson, G.A. Thyroid Autoimmunity in Euthyroid Women Undergoing IUI: Clinical Outcomes and Pathophysiologic Insights. Front. Endocrinol. 2024, 15, 1359210. [Google Scholar]
- Sankoda, A.; Suzuki, H.; Imaizumi, M.; Yoshihara, A.; Kobayashi, S.; Katai, M.; Hamada, K.; Hidaka, Y.; Yoshihara, A.; Nakamura, H.; et al. Effects of Levothyroxine Treatment on Fertility and Pregnancy Outcomes in Subclinical Hypothyroidism: A Systematic Review and Meta Analysis of Randomized Controlled Trials. Thyroid 2024, 34, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Unuane, D.; Velkeniers, B.; Bravenboer, B.; Drakopoulos, P.; Tournaye, H.; Parra, J.; De Brucker, M. Impact of Thyroid Autoimmunity in Euthyroid Women on Live Birth Rate after IUI. Hum. Reprod. 2017, 32, 915–922. [Google Scholar] [CrossRef][Green Version]
- Poppe, K.; Autin, C.; Veltri, F.; Kleynen, P.; Grabczan, L.; Rozenberg, S.; Ameye, L. Thyroid Autoimmunity and Intracytoplasmic Sperm Injection Outcome: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2018, 103, 1755–1766. [Google Scholar] [CrossRef]
- Xie, J.; Gu, A.; He, H.; Zhao, Q.; Yu, Y.; Chen, J.; Cheng, Z.; Zhou, P.; Zhou, Q.; Jin, M. Autoimmune Thyroid Disease Disrupts Immune Homeostasis in the Endometrium of Unexplained Infertility Women—A Single-Cell RNA Transcriptome Study during the Implantation Window. Front. Endocrinol. 2023, 14, 1185147. [Google Scholar] [CrossRef]
- Singh, P.; Boelaert, K. Controversies in Thyroid Disease Management in Pregnancy. Clin. Med. 2025, 25, 100287. [Google Scholar] [CrossRef]
- Concepción-Zavaleta, M.J.; Coronado-Arroyo, J.C.; Quiroz-Aldave, J.E.; Concepción-Urteaga, L.A.; Paz-Ibarra, J. Thyroid Dysfunction and Female Infertility: A Comprehensive Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102876. [Google Scholar] [CrossRef]
- Urgatz, B.; Razvi, S. Subclinical Hypothyroidism, Outcomes and Management Guidelines: A Narrative Review and Update of Recent Literature. Endocrinology 2023, 39, 351–365. [Google Scholar] [CrossRef]
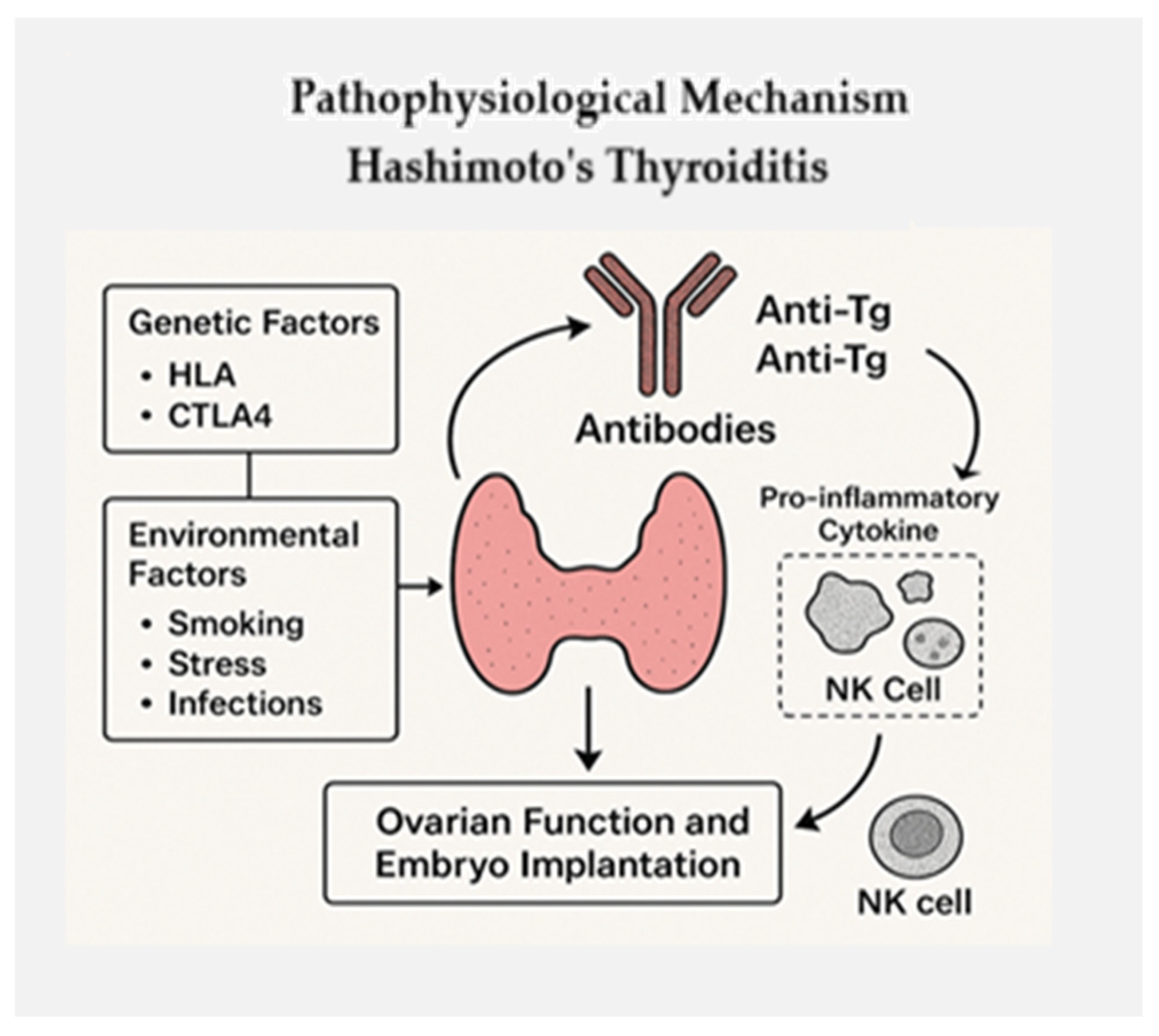
| Immune Factor | Effect on Fertility | Clinical Evidence |
|---|---|---|
| Anti-TPO antibodies | ↑ Risk of miscarriage, ↓ implantation rate | Meta-analyses; associated with 2–4× miscarriage risk [3] |
| Anti-Tg antibodies | ↑ Risk of immune ovarian damage | Observed in ovarian dysfunction in autoimmune settings [3,19,20,21] |
| IL-17α | ↓ Oocyte quality and implantation potential | Elevated in Hashimoto patients; correlates with lower pregnancy rates [13,22] |
| NK cells | ↓ Endometrial receptivity, ↑ inflammation | Increased activity in infertile women with TAI [13] |
| KIR-HLA-C mismatch | ↑ Implantation failure risk | Linked with poor ART outcomes in observational studies [13,19,20,21,22,23] |
| Parameter | Normal Role in Fertility | Impact in Hashimoto’s Thyroiditis | Clinical Implication |
|---|---|---|---|
| AMH (Anti-Müllerian Hormone) | Reflects ovarian reserve; lower levels indicate reduced fertility potential | ↓ AMH levels observed in subclinical hypothyroidism | May indicate premature ovarian insufficiency in euthyroid women with Hashimoto’s [9,10,11,25] |
| FSH (Follicle-Stimulating Hormone) | Stimulates follicle development; high levels may indicate ovarian aging | ↑ FSH levels are reported due to impaired ovarian feedback | Requires monitoring to guide ART stimulation protocols [9,10,11,25,26] |
| TSH (Thyroid-Stimulating Hormone) | Regulates thyroid hormone production; optimal range critical for ovulatory function | ↑ TSH is standard, even in subclinical cases, and may impair folliculogenesis | TSH normalization is critical before ART initiation [27] |
| FT4 (Free Thyroxine) | Supports metabolic and reproductive function; needed for endometrial receptivity | FT4 may remain normal, but it fluctuates in some patients with TAI | Needs regular assessment to ensure euthyroidism [28] |
| AFC (Antral Follicle Count) | Assesses follicle pool via ultrasound; fewer follicles reflect diminished reserve | ↓ AFC reported in women with autoimmune thyroid disease | Can help determine candidacy and response to ART [25,28] |
| ART Procedure | Outcome Without Thyroid Autoimmunity | Outcome with Thyroid Autoimmunity | Observed Effect |
|---|---|---|---|
| IVF [In Vitro Fertilization] | 38% clinical pregnancy rate | 34% clinical pregnancy rate | ↓ implantation and ↑ miscarriage [30] |
| ICSI [Intracytoplasmic Sperm Injection] | 42% clinical pregnancy rate | 28% clinical pregnancy rate | ↓ fertilization, ↓ implantation, ↑ miscarriage [30] |
| Intervention Type | Evidence Status | Clinical Recommendation |
|---|---|---|
| Probiotics | Limited pilot studies, no RCTs | Investigational only [22,23,34] |
| Prebiotics | Experimental models | Not recommended for clinical use [23,34] |
| Dietary modifications | Observational evidence | Potentially beneficial, not proven [22,23] |
| Fecal microbiota transplant (FMT) | Theoretical only | Not applicable in current practice [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, E.C.; Maghiar, L.; Maghiar, T.A.; Brihan, I.; Georgescu, L.M.; Toderaș, B.A.; Sachelarie, L.; Huniadi, A. Hashimoto’s Thyroiditis and Female Fertility: Endocrine, Immune, and Microbiota Perspectives in Assisted Reproduction—A Narrative Review. Biomedicines 2025, 13, 1495. https://doi.org/10.3390/biomedicines13061495
Popa EC, Maghiar L, Maghiar TA, Brihan I, Georgescu LM, Toderaș BA, Sachelarie L, Huniadi A. Hashimoto’s Thyroiditis and Female Fertility: Endocrine, Immune, and Microbiota Perspectives in Assisted Reproduction—A Narrative Review. Biomedicines. 2025; 13(6):1495. https://doi.org/10.3390/biomedicines13061495
Chicago/Turabian StylePopa, Emilia Cristina, Laura Maghiar, Teodor Andrei Maghiar, Ilarie Brihan, Laura Monica Georgescu, Bianca Anamaria Toderaș, Liliana Sachelarie, and Anca Huniadi. 2025. "Hashimoto’s Thyroiditis and Female Fertility: Endocrine, Immune, and Microbiota Perspectives in Assisted Reproduction—A Narrative Review" Biomedicines 13, no. 6: 1495. https://doi.org/10.3390/biomedicines13061495
APA StylePopa, E. C., Maghiar, L., Maghiar, T. A., Brihan, I., Georgescu, L. M., Toderaș, B. A., Sachelarie, L., & Huniadi, A. (2025). Hashimoto’s Thyroiditis and Female Fertility: Endocrine, Immune, and Microbiota Perspectives in Assisted Reproduction—A Narrative Review. Biomedicines, 13(6), 1495. https://doi.org/10.3390/biomedicines13061495







