Post-COVID Metabolic Fallout: A Growing Threat of New-Onset and Exacerbated Diabetes
Abstract
1. Introduction
2. Incidence of Post-COVID-19 Diabetes
2.1. Epidemiological Evidence
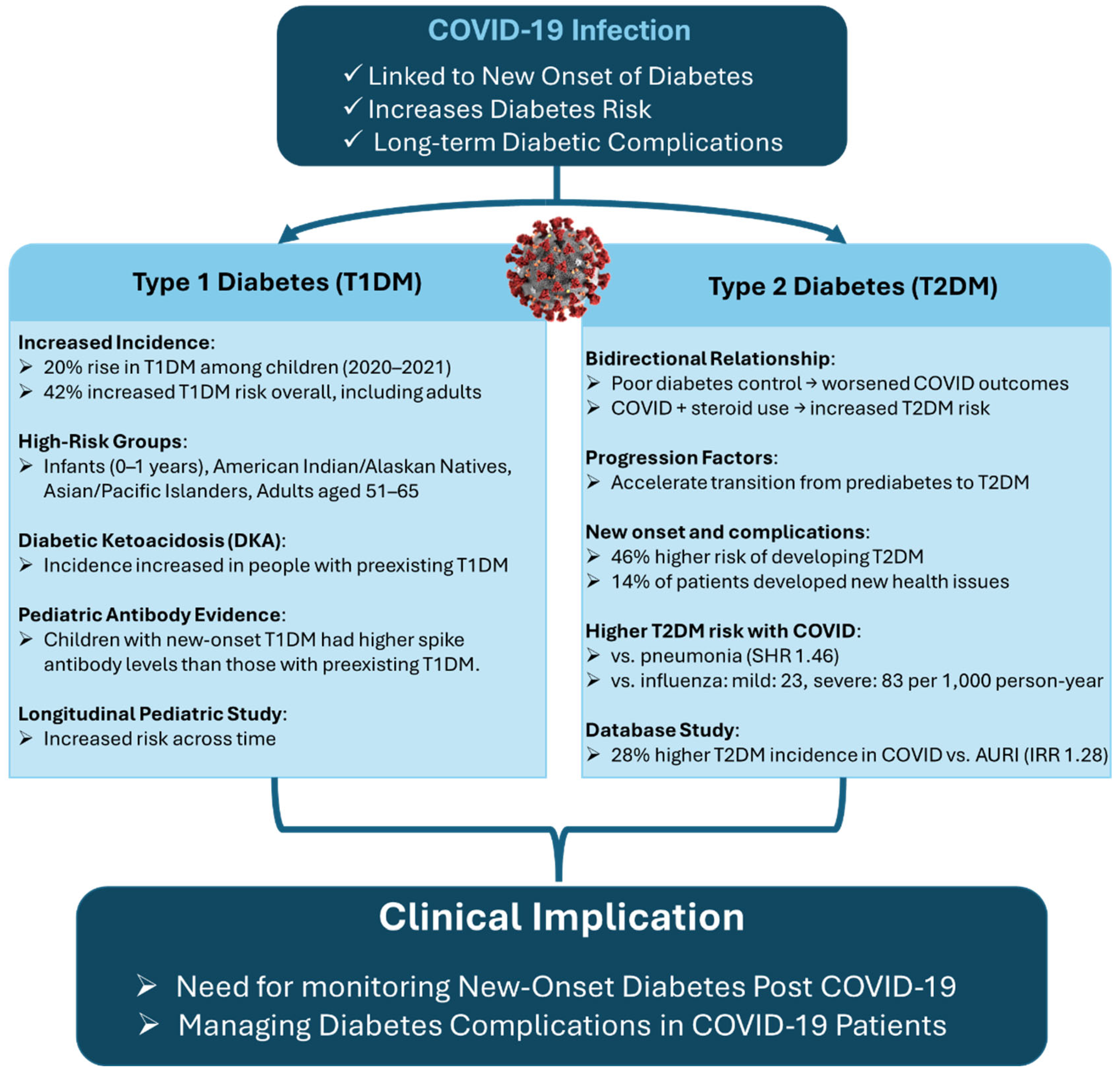
2.2. Divergent Diabetes Risks After COVID-19: Evidence for Type 1 and Type 2 Onset
3. Iological Underpinnings of COVID-19–Related Diabetes
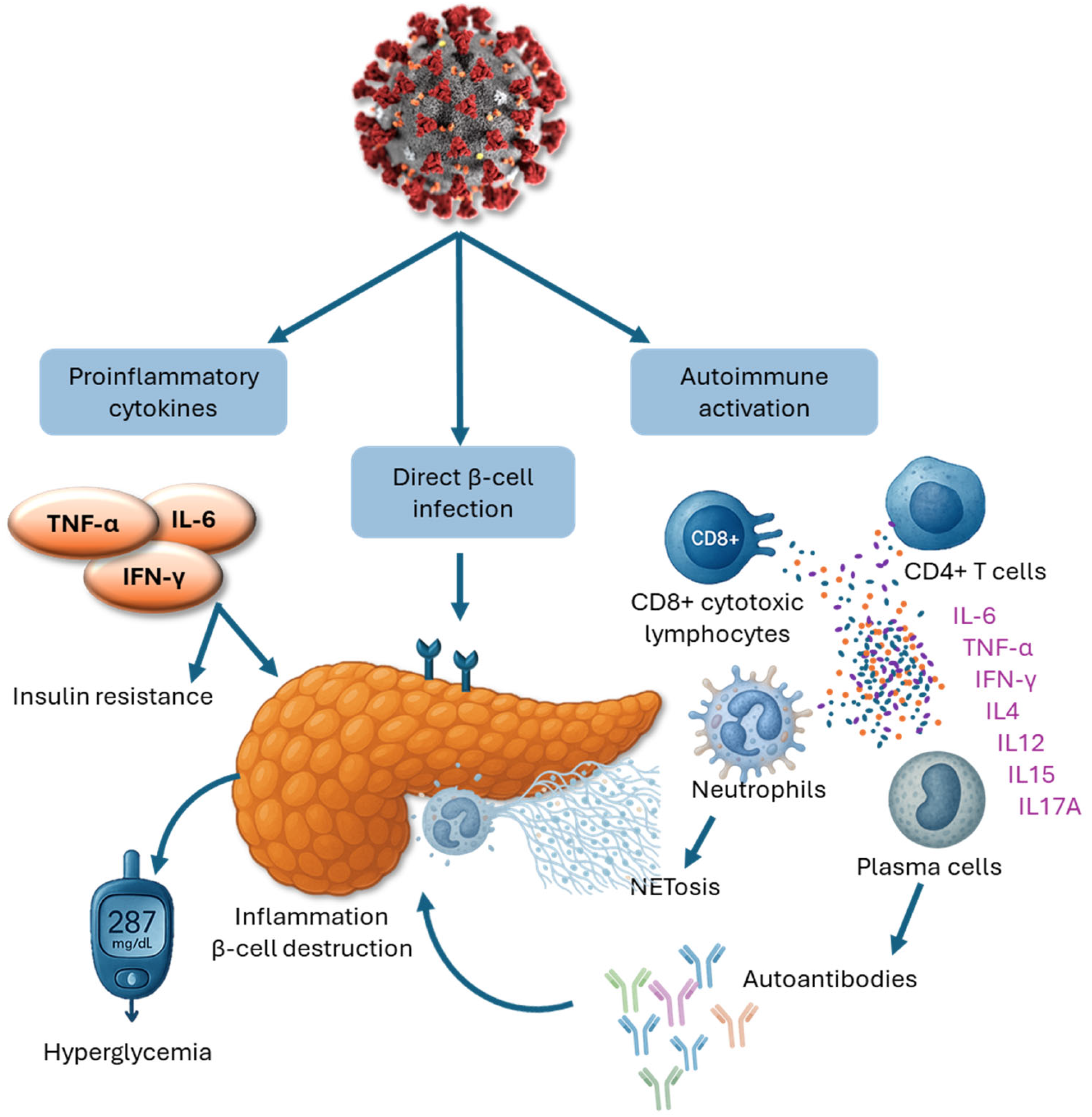
3.1. Direct Viral Impact on Pancreatic Beta Cells
3.2. Immune-Mediated Mechanisms
3.3. Impact of COVID-19 Therapies on Glycemic Control and Diabetes Risk
3.4. Post-COVID Insulin Resistance: A Persistent Metabolic Consequence
3.5. SARS-CoV-2 and Systemic Metabolic Injury
4. Demographic Disparities in Post-COVID Diabetes Risk
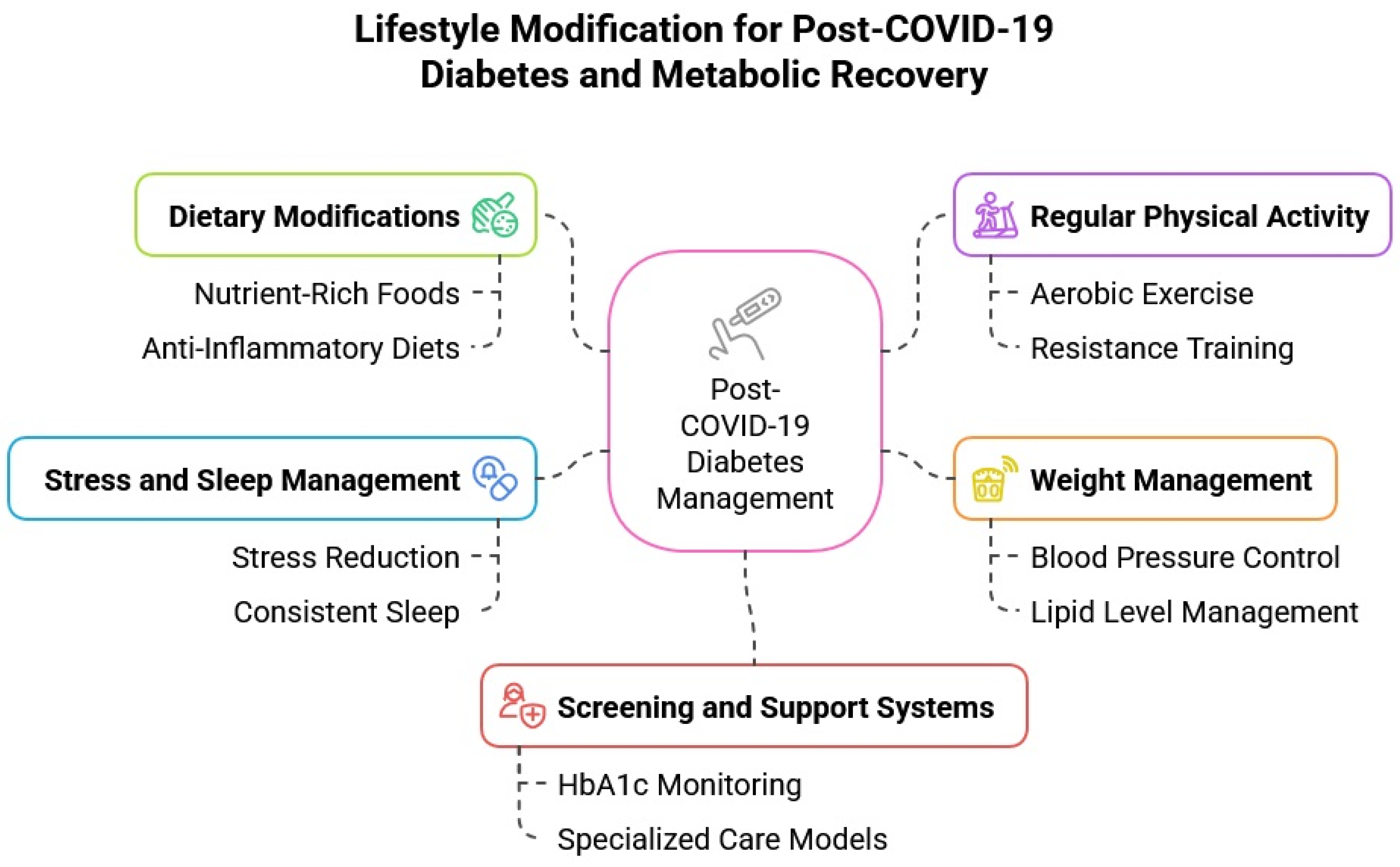
5. Targeting Post-COVID-19 Diabetes Through Lifestyle Modification
5.1. Dietary Recommendations in Post-COVID Recovery
5.2. Physical Activity and Exercise
5.3. Weight Management and Metabolic Health
5.4. The Role of Stress, Steroids, and Sleep Disruption in Post-COVID Diabetes Risk
5.5. Screening, Surveillance, and Support for At-Risk Populations
5.6. Recommended Clinical Approach for Managing Post-COVID Diabetes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Metzendorf, M.-I.; Fischer, A.-L.; Stegemann, M.; Spagl, M.; Nair, A.A.; Daniel, J.; Fichtner, F. Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence. Cochrane Database Syst. Rev. 2022, 2022, CD014963. [Google Scholar]
- Chavda, V.P.; Vuppu, S.; Mishra, T.; Kamaraj, S.; Patel, A.B.; Sharma, N.; Chen, Z.-S. Recent review of COVID-19 management: Diagnosis, treatment and vaccination. Pharmacol. Rep. 2022, 74, 1120–1148. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Oviedo, S.A.; Ali, M.K.; Ofotokun, I.; Gander, J.C.; Patel, S.A.; Magliano, D.J.; Patzer, R.E. The bidirectional association between diabetes and long-COVID-19–A systematic review. Diabetes Res. Clin. Prac. 2023, 195, 110202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Mei, Q.; Zhang, Z.; Walline, J.H.; Liu, Y.; Zhu, H.; Zhang, S. Risk for newly diagnosed diabetes after COVID-19: A systematic review and meta-analysis. BMC Med. 2022, 20, 444. [Google Scholar] [CrossRef]
- Paschou, S.A.; Papadopoulou-Marketou, N.; Chrousos, G.P.; Kanaka-Gantenbein, C. On type 1 diabetes mellitus pathogenesis. Endocr. Connect. 2018, 7, R38–R46. [Google Scholar] [CrossRef]
- Herczeg, V.; Luczay, A.; Ténai, N.; Czine, G.; Tóth-Heyn, P. Anti-SARS-CoV-2 Seropositivity Among Children With Newly Diagnosed Type 1 Diabetes Mellitus: A Case-Control Study. Indian Pediatr. 2022, 59, 809–810. [Google Scholar] [CrossRef]
- Kendall, E.K.; Olaker, V.R.; Kaelber, D.C.; Xu, R.; Davis, P.B. Association of SARS-CoV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Netw. Open 2022, 5, e2233014. [Google Scholar] [CrossRef]
- McKeigue, P.M.; McGurnaghan, S.; Blackbourn, L.; Bath, L.E.; McAllister, D.A.; Caparrotta, T.M.; Wild, S.H.; Wood, S.N.; Stockton, D.; Colhoun, H.M. Relation of incident type 1 diabetes to recent COVID-19 infection: Cohort study using e-health record linkage in Scotland. Diabetes Care 2023, 46, 921–928. [Google Scholar] [CrossRef]
- Qeadan, F.; Tingey, B.; Egbert, J.; Pezzolesi, M.G.; Burge, M.R.; Peterson, K.A.; Honda, T. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: A nationwide cohort from the US using the Cerner Real-World Data. PLoS ONE 2022, 17, e0266809. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Birabaharan, M.; Kaelber, D.C.; Pettus, J.H.; Smith, D.M. Risk of new-onset type 2 diabetes Mellitus in 600,055 persons after COVID-19: A cohort study. Diabetes Obes. Metab. 2022, 24, 1176. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Ren, S.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Guo, Y.; Lipsitch, M.; Daugherty, S.E. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2022, 376, e068414. [Google Scholar] [CrossRef] [PubMed]
- Collaborative, T.O.; Tazare, J.; Walker, A.J.; Tomlinson, L.A.; Hickman, G.; Rentsch, C.T.; Williamson, E.J.; Bhaskaran, K.; Evans, D.; Wing, K. Rates of serious clinical outcomes in survivors of hospitalisation with COVID-19 in England: A descriptive cohort study within the OpenSAFELY platform. Wellcome Open Res. 2022, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, n1098. [Google Scholar] [CrossRef]
- Rathmann, W.; Kuss, O.; Kostev, K. Incidence of newly diagnosed diabetes after COVID-19. Diabetologia 2022, 65, 949–954. [Google Scholar] [CrossRef]
- Bellia, C.; Andreadi, A.; D’Ippolito, I.; Scola, L.; Barraco, S.; Meloni, M.; Lauro, D.; Bellia, A. Prevalence and risk of new-onset diabetes mellitus after COVID-19: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1215879. [Google Scholar] [CrossRef]
- Mohamed-Jawad, N.K.; Abdul-Nabi, Z.N. COVID-19 and the Risk of Diabetes: A Systematic Review Article. Asia Pac. J. Med. Toxicol. 2023, 12, 66. [Google Scholar]
- Chen, M.; Zhu, B.; Chen, D.; Hu, X.; Xu, X.; Shen, W.-J.; Hu, C.; Li, J.; Qu, S. COVID-19 may increase the risk of insulin resistance in adult patients without diabetes: A 6-month prospective study. Endocr. Pract. 2021, 27, 834–841. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Reges, O.; Test, T.; Hoshen, M.; Cicurel, A.; Saliba, W.; Greenland, P.; Dicker, D.; Lavie, G. Time-varying association of acute and post-acute COVID-19 with new-onset diabetes mellitus among hospitalized and non-hospitalized patients. BMJ Open Diabetes Res. Care 2023, 11, e003052. [Google Scholar] [CrossRef]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Wander, P.L.; Lowy, E.; Beste, L.A.; Tulloch-Palomino, L.; Korpak, A.; Peterson, A.C.; Kahn, S.E.; Boyko, E.J. The incidence of diabetes among 2,808,106 veterans with and without recent SARS-CoV-2 infection. Diabetes Care 2022, 45, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Pal, R.; Dutta, S. Risk of incident diabetes post-COVID-19: A systematic review and meta-analysis. Prim. Care Diabetes 2022, 16, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, V.; Kar, S.S.; Duraiswamy, M.; Rajaram, M.; Menon, V.; Wyawahare, M.; Priya, D.V.; Lakshmanasamy, R.; Ravel, V.; Sivaji, R. New-onset Diabetes Mellitus among adults as sequelae of COVID-19 in selected tertiary care hospital, Puducherry—A cohort study. Clin. Epidemiol. Glob. Health 2025, 31, 101897. [Google Scholar] [CrossRef]
- Omotosho, Y.B.; Ying, G.W.; Stolar, M.; Mallari, A.J.P. COVID-19-induced diabetic ketoacidosis in an adult with latent autoimmune diabetes. Cureus 2021, 13, e12690. [Google Scholar] [CrossRef]
- Marchand, L.; Pecquet, M.; Luyton, C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020, 57, 1265–1266. [Google Scholar] [CrossRef]
- Najafi, M.B.; Javanmard, S.H. Post-COVID-19 syndrome mechanisms, prevention and management. Int. J. Prev. Med. 2023, 14, 59. [Google Scholar] [CrossRef]
- Li, G.; Chen, Z.; Lv, Z.; Li, H.; Chang, D.; Lu, J. Diabetes Mellitus and COVID-19: Associations and Possible Mechanisms. Int. J. Endocrinol. 2021, 2021, 7394378. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Yang, J.-K.; Lin, S.-S.; Ji, X.-J.; Guo, L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010, 47, 193–199. [Google Scholar] [CrossRef]
- Kai, H.; Kai, M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—Lessons from available evidence and insights into COVID-19. Hypertens. Res. 2020, 43, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Steenblock, C.; Richter, S.; Berger, I.; Barovic, M.; Schmid, J.; Schubert, U.; Jarzebska, N.; von Mässenhausen, A.; Linkermann, A.; Schürmann, A. Viral infiltration of pancreatic islets in patients with COVID-19. Nat. Commun. 2021, 12, 3534. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alblihed, M.; Guerreiro, S.G.; Cruz-Martins, N.; Batiha, G.E.-S. COVID-19 in relation to hyperglycemia and diabetes mellitus. Front. Cardiovasc. Med. 2021, 8, 644095. [Google Scholar] [CrossRef] [PubMed]
- Debuysschere, C.; Nekoua, M.P.; Alidjinou, E.K.; Hober, D. The relationship between SARS-CoV-2 infection and type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2024, 20, 588–599. [Google Scholar] [CrossRef]
- Tang, X.; Uhl, S.; Zhang, T.; Xue, D.; Li, B.; Vandana, J.J.; Acklin, J.A.; Bonnycastle, L.L.; Narisu, N.; Erdos, M.R. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2021, 33, 1577–1591.e1577. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Koganti, R.; Agelidis, A.; Patil, C.D.; Shukla, D. Dysregulation of cell signaling by SARS-CoV-2. Trends Microbiol. 2021, 29, 224–237. [Google Scholar] [CrossRef]
- Shin, J.; Toyoda, S.; Nishitani, S.; Onodera, T.; Fukuda, S.; Kita, S.; Fukuhara, A.; Shimomura, I. SARS-CoV-2 infection impairs the insulin/IGF signaling pathway in the lung, liver, adipose tissue, and pancreatic cells via IRF1. Metabolism 2022, 133, 155236. [Google Scholar] [CrossRef]
- Kelesidis, T.; Mantzoros, C.S. Cross-talk between SARS-CoV-2 infection and the insulin/IGF signaling pathway: Implications for metabolic diseases in COVID-19 and for post-acute sequelae of SARS-CoV-2 infection. Metab.-Clin. Exp. 2022, 134, 155267. [Google Scholar] [CrossRef]
- Wu, C.-T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576.e1565. [Google Scholar] [CrossRef]
- Deng, W.; Bao, L.; Song, Z.; Zhang, L.; Yu, P.; Xu, Y.; Wang, J.; Zhao, W.; Zhang, X.; Han, Y. Infection with SARS-CoV-2 can cause pancreatic impairment. Signal Transduct. Target. Ther. 2024, 9, 98. [Google Scholar] [CrossRef]
- Cromer, S.J.; Colling, C.; Schatoff, D.; Leary, M.; Stamou, M.I.; Selen, D.J.; Putman, M.S.; Wexler, D.J. Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: Associated factors, short-term outcomes, and long-term glycemic phenotypes. J. Diabetes Its Complicat. 2022, 36, 108145. [Google Scholar] [CrossRef] [PubMed]
- Steenblock, C.; Schwarz, P.E.; Ludwig, B.; Linkermann, A.; Zimmet, P.; Kulebyakin, K.; Tkachuk, V.A.; Markov, A.G.; Lehnert, H.; De Angelis, M.H. COVID-19 and metabolic disease: Mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021, 9, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Christen, U.; Edelmann, K.H.; McGavern, D.B.; Wolfe, T.; Coon, B.; Teague, M.K.; Miller, S.D.; Oldstone, M.B.; Von Herrath, M.G. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J. Clin. Investig. 2004, 114, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Boddu, S.K.; Aurangabadkar, G.; Kuchay, M.S. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2211–2217. [Google Scholar] [CrossRef]
- Taplin, C.E.; Barker, J.M. Autoantibodies in type 1 diabetes. Autoimmunity 2008, 41, 11–18. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Santos, A.; Magro, D.O.; Evangelista-Poderoso, R.; Saad, M.J.A. Diabetes, obesity, and insulin resistance in COVID-19: Molecular interrelationship and therapeutic implications. Diabetol. Metab. Syndr. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Iwasaki, M.; Saito, J.; Zhao, H.; Sakamoto, A.; Hirota, K.; Ma, D. Inflammation triggered by SARS-CoV-2 and ACE2 augment drives multiple organ failure of severe COVID-19: Molecular mechanisms and implications. Inflammation 2021, 44, 13–34. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sinha, A.; Banach, M.; Mittoo, S.; Weissert, R.; Kass, J.S.; Rajagopal, S.; Pai, A.R.; Kutty, S. Cytokine storm in COVID-19—Immunopathological mechanisms, clinical considerations, and therapeutic approaches: The REPROGRAM consortium position paper. Front. Immunol. 2020, 11, 1648. [Google Scholar] [CrossRef]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Chee, Y.J.; Ng, S.J.H.; Yeoh, E. Diabetic ketoacidosis precipitated by COVID-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 164, 108166. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.A.F.; Neves, P.F.M.d.; Lima, S.S.; Lopes, J.d.C.; Torres, M.K.d.S.; Vallinoto, I.M.V.C.; Bichara, C.D.A.; Santos, E.F.d.; de Brito, M.T.F.M.; da Silva, A.L.S. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front. Cell. Infect. Microbiol. 2022, 12, 922422. [Google Scholar] [CrossRef] [PubMed]
- Nhau, P.T.; Gamede, M.; Sibiya, N. COVID-19-induced diabetes mellitus: Comprehensive cellular and molecular mechanistic insights. Pathophysiology 2024, 31, 197–209. [Google Scholar] [CrossRef]
- Liu, F.; Long, X.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2128. [Google Scholar] [CrossRef]
- Pickup, J.; Mattock, M.; Chusney, G.; Burt, D. NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997, 40, 1286–1292. [Google Scholar] [CrossRef]
- Plomgaard, P.; Bouzakri, K.; Krogh-Madsen, R.; Mittendorfer, B.; Zierath, J.R.; Pedersen, B.K. Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005, 54, 2939–2945. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Michell, B.J.; van Denderen, B.J.; Watt, M.J.; Carey, A.L.; Fam, B.C.; Andrikopoulos, S.; Proietto, J.; Görgün, C.Z.; Carling, D. Tumor necrosis factor α-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006, 4, 465–474. [Google Scholar] [CrossRef]
- Govender, N.; Khaliq, O.P.; Moodley, J.; Naicker, T. Insulin resistance in COVID-19 and diabetes. Prim. Care Diabetes 2021, 15, 629–634. [Google Scholar] [CrossRef]
- Montefusco, L.; Ben Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef]
- Korytkowski, M.; Antinori-Lent, K.; Drincic, A.; Hirsch, I.B.; McDonnell, M.E.; Rushakoff, R.; Muniyappa, R. A pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. J. Clin. Endocrinol. Metab. 2020, 105, 3076–3087. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, M.; Athavale, A.M.; Abraham, M.; Vernik, J.; Amarah, A.R.; Ruiz, J.P.; Joshi, A.J.; Itteera, M.; Zhukovski, S.D.; Madaiah, R.P. Association of hyperglycaemia with hospital mortality in nondiabetic COVID-19 patients: A cohort study. Diabetes Metab. 2021, 47, 101254. [Google Scholar] [CrossRef] [PubMed]
- Knapp, S. Diabetes and infection: Is there a link?-A mini-review. Gerontology 2013, 59, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes/Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Parise, R.; Deruiter, J.; Ren, J.; Govindarajulu, M.; Ramesh, S.; Nadar, R.M.; Moore, T.; Dhanasekaran, M. Impact of COVID-19 therapy on hyperglycemia. Diabetes Vasc. Dis. Res. 2022, 19, 14791641221095091. [Google Scholar] [CrossRef]
- He, X.; Liu, C.; Peng, J.; Li, Z.; Li, F.; Wang, J.; Hu, A.; Peng, M.; Huang, K.; Fan, D. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal Transduct. Target. Ther. 2021, 6, 427. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Cron, R.Q.; Caricchio, R.; Chatham, W.W. Calming the cytokine storm in COVID-19. Nat. Med. 2021, 27, 1674–1675. [Google Scholar] [CrossRef]
- Hirano, T.; Murakami, M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020, 52, 731–733. [Google Scholar] [CrossRef]
- Šestan, M.; Marinović, S.; Kavazović, I.; Cekinović, Đ.; Wueest, S.; Wensveen, T.T.; Brizić, I.; Jonjić, S.; Konrad, D.; Wensveen, F.M. Virus-induced interferon-γ causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity 2018, 49, 164–177.e166. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Bosoni, P.; Dilillo, D.; Mannarino, S.; Fiori, L.; Fabiano, V.; Carlucci, P.; Di Profio, E.; Verduci, E.; Mameli, C. Impaired glucose-insulin metabolism in multisystem inflammatory syndrome related to SARS-CoV-2 in children. Children 2021, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Ilias, I.; Diamantopoulos, A.; Pratikaki, M.; Botoula, E.; Jahaj, E.; Athanasiou, N.; Tsipilis, S.; Zacharis, A.; Vassiliou, A.G.; Vassiliadi, D.A. Glycemia, beta-cell function and sensitivity to insulin in mildly to critically ill COVID-19 patients. Medicina 2021, 57, 68. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The major cell entry receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Barreto, E.A.; Cruz, A.S.; Veras, F.P.; Martins, R.; Bernardelli, R.S.; Paiva, I.M.; Lima, T.M.; Singh, Y.; Guimarães, R.C.; Damasceno, S. COVID-19-related hyperglycemia is associated with infection of hepatocytes and stimulation of gluconeogenesis. Proc. Natl. Acad. Sci. 2023, 120, e2217119120. [Google Scholar] [CrossRef]
- Mercado-Gómez, M.; Prieto-Fernández, E.; Goikoetxea-Usandizaga, N.; Vila-Vecilla, L.; Azkargorta, M.; Bravo, M.; Serrano-Maciá, M.; Egia-Mendikute, L.; Rodríguez-Agudo, R.; Lachiondo-Ortega, S. The spike of SARS-CoV-2 promotes metabolic rewiring in hepatocytes. Commun. Biol. 2022, 5, 827. [Google Scholar] [CrossRef]
- Choi, C.S.; Kim, Y.-B.; Lee, F.N.; Zabolotny, J.M.; Kahn, B.B.; Youn, J.H. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am. J. Physiol. -Endocrinol. Metab. 2002, 283, E233–E240. [Google Scholar] [CrossRef]
- Wang, X.; Lei, J.; Li, Z.; Yan, L. Potential effects of coronaviruses on the liver: An update. Front. Med. 2021, 8, 651658. [Google Scholar] [CrossRef]
- Reiterer, M.; Rajan, M.; Gómez-Banoy, N.; Lau, J.D.; Gomez-Escobar, L.G.; Gilani, A.; Alvarez-Mulett, S.; Sholle, E.T.; Chandar, V.; Bram, Y. Hyperglycemia in acute COVID-19 is characterized by adipose tissue dysfunction and insulin resistance. medRxiv 2021. [Google Scholar] [CrossRef]
- Zaletel, J.; Pongrac Barlovic, D.; Prezelj, J. Adiponectin-leptin ratio: A useful estimate of insulin resistance in patients with Type 2 diabetes. J. Endocrinol. Investig. 2010, 33, 514–518. [Google Scholar] [CrossRef]
- Finucane, F.; Luan, J.; Wareham, N.; Sharp, S.; O’Rahilly, S.; Balkau, B.; Flyvbjerg, A.; Walker, M.; Hojlund, K.; Nolan, J. Relationship between Insulin Sensitivity and Cardiovascular Disease Risk Study Group, Savage DB. Correlation of the leptin: Adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia 2009, 52, 2345–2349. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Colón, G.J.; Ratnasiri, K.; Chen, H.; Jiang, S.; Zanley, E.; Rustagi, A.; Verma, R.; Chen, H.; Andrews, J.R.; Mertz, K.D. SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. Sci. Transl. Med. 2022, 14, eabm9151. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.T.; Navis, G.v.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Kousathana, F.; Raptis, A.; Katogiannis, K.; Kokkinos, A.; Ikonomidis, I. Pre-existing cytokine and NLRP3 inflammasome activation and increased vascular permeability in diabetes: A possible fatal link with worst COVID-19 infection outcomes? Front. Immunol. 2020, 11, 557235. [Google Scholar] [CrossRef]
- Luther, T.; Eckerbom, P.; Cox, E.; Lipcsey, M.; Bülow, S.; Hultström, M.; Torrente, F.M.; Weis, J.; Palm, F.; Francis, S. Decreased renal perfusion during acute kidney injury in critical COVID-19 assessed by magnetic resonance imaging: A prospective case control study. Crit. Care 2022, 26, 262. [Google Scholar] [CrossRef]
- Sylvester, S.V.; Rusu, R.; Chan, B.; Bellows, M.; O’Keefe, C.; Nicholson, S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: A review. Curr. Med. Res. Opin. 2022, 38, 1391–1399. [Google Scholar] [CrossRef]
- Khunti, K.; Feldman, E.L.; Laiteerapong, N.; Parker, W.; Routen, A.; Peek, M. The impact of the COVID-19 pandemic on ethnic minority groups with diabetes. Diabetes Care 2023, 46, 228–236. [Google Scholar] [CrossRef]
- Barrett, C.E. Risk for newly diagnosed diabetes 30 days after SARS-CoV-2 infection among persons aged 18 years—United States, March 1, 2020–June 28, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 59–65. [Google Scholar] [CrossRef]
- Wang, Y.; Su, B.; Alcalde-Herraiz, M.; Barclay, N.L.; Tian, Y.; Li, C.; Wareham, N.J.; Paredes, R.; Xie, J.; Prieto-Alhambra, D. Healthy lifestyle for the prevention of post-COVID-19 multisystem sequelae, hospitalization, and death: A prospective cohort study. medRxiv 2024. [Google Scholar]
- Ayenigbara, I.O. Diabetes prevention and measures to ensuring a healthy lifestyle during COVID-19 pandemic and after. Korean J. Fam. Med. 2023, 44, 11. [Google Scholar] [CrossRef]
- Dartora, W.J.; Schmidt, M.I.; Griep, R.H.; Duncan, B.B. Changes in Lifestyle Habits in Individuals with Diabetes during the COVID-19 Pandemic: The ELSA-Brasil Cohort Study. COVID 2023, 3, 1601–1611. [Google Scholar] [CrossRef]
- Alah, M.A.; Abdeen, S.; Kehyayan, V.; Bougmiza, I. Impact of COVID-19 related home confinement measures on the lifestyle, body weight, and perceived glycemic control of diabetics. Metab. Open 2021, 12, 100144. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Kivimäki, M.; Gale, C.R.; Batty, G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387,109 adults in UK. Brain Behav. Immun. 2020, 87, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Arora, I.; Hsia, D.S.; Knowler, W.C.; LeBlanc, E.; Mylonakis, E.; Pratley, R.; Pittas, A.G. New-onset diabetes after COVID-19. J. Clin. Endocrinol. Metab. 2023, 108, e1164–e1174. [Google Scholar] [CrossRef]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E. Mitochondrial dysfunction in long COVID: Mechanisms, consequences, and potential therapeutic approaches. Geroscience 2024, 46, 5267–5286. [Google Scholar] [CrossRef]
- Perez-Araluce, R.; Martinez-Gonzalez, M.; Fernández-Lázaro, C.; Bes-Rastrollo, M.; Gea, A.; Carlos, S. Mediterranean diet and the risk of COVID-19 in the ‘Seguimiento Universidad de Navarra’cohort. Clin. Nutr. 2022, 41, 3061–3068. [Google Scholar] [CrossRef]
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020, 87, 53–54. [Google Scholar] [CrossRef]
- Marçal, I.R.; Fernandes, B.; Viana, A.A.; Ciolac, E.G. The urgent need for recommending physical activity for the management of diabetes during and beyond COVID-19 outbreak. Front. Endocrinol. 2020, 11, 584642. [Google Scholar] [CrossRef]
- Lee, I.; Shiroma, E.; Lobelo, F.; Puska, P.; Blair, S.; Katzmarzyk, P. PhD for the Lancet Physical Activity Series Working Group. Impact of Physical Inactivity on the World’s Major Non-Communicable Diseases. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Chastin, S.F.; Palarea-Albaladejo, J.; Dontje, M.L.; Skelton, D.A. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: A novel compositional data analysis approach. PLoS ONE 2015, 10, e0139984. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Clevel. Clin. J. Med. 2017, 84, S15. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, M.P.; da Silva Fagundes, K.K.; Bizuti, M.R.; Starck, É.; Rossi, R.C.; de Resende e Silva, D.T. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin. Exp. Med. 2021, 21, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Furtado, G.E.; Letieri, R.V.; Caldo-Silva, A.; Sardão, V.A.; Teixeira, A.M.; de Barros, M.P.; Vieira, R.P.; Bachi, A.L.L. Sustaining efficient immune functions with regular physical exercise in the COVID-19 era and beyond. Eur. J. Clin. Investig. 2021, 51, e13485. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar] [CrossRef]
- Casciano, F.; Caruso, L.; Zauli, E.; Gonelli, A.; Zauli, G.; Vaccarezza, M. Emerging Mechanisms of Physical Exercise Benefits in Adjuvant and Neoadjuvant Cancer Immunotherapy. Biomedicines 2024, 12, 2528. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport. Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Chastin, S.F.M.; Abaraogu, U.; Bourgois, J.G.; Dall, P.M.; Darnborough, J.; Duncan, E.; Dumortier, J.; Pavon, D.J.; McParland, J.; Roberts, N.J.; et al. Effects of Regular Physical Activity on the Immune System, Vaccination and Risk of Community-Acquired Infectious Disease in the General Population: Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1673–1686. [Google Scholar] [CrossRef]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.N.; Cohen, D.A. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48 440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef]
- Jimenez-Pavon, D.; Carbonell-Baeza, A.; Lavie, C.J. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: Special focus in older people. Prog. Cardiovasc. Dis. 2020, 63, 386–388. [Google Scholar] [CrossRef]
- Souza, E.d.; Meneses-Santos, D.; Santos, J.C.; Aidar, F.J.; Carvalho, C.R.d.O.; Santos, J.L.d.; Marçal, A.C. “Does Physical Exercise Promote Health Benefits for Diabetic Patients during the COVID-19 Pandemic?”: A Systematic Review. Sports 2023, 11, 192. [Google Scholar] [CrossRef]
- Antonarelli, M.; Fogante, M. Chest CT-derived muscle analysis in COVID-19 patients. Tomography 2022, 8, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lim, Y.; Jeong, S.; Han, H.W. COVID-19-related cardiovascular disease risk due to weight gain: A nationwide cohort study. Eur. J. Med. Res. 2024, 29, 2. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.-C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Jivanji, C.J.; Asrani, V.M.; Windsor, J.A.; Petrov, M.S. New-onset diabetes after acute and critical illness: A systematic review. Mayo Clin. Proc. 2017, 92, 762–773. [Google Scholar] [CrossRef]
- Forde, R.; Arente, L.; Ausili, D.; De Backer, K.; Due-Christensen, M.; Epps, A.; Fitzpatrick, A.; Grixti, M.; Groen, S.; Halkoaho, A. The impact of the COVID-19 pandemic on people with diabetes and diabetes services: A pan-European survey of diabetes specialist nurses undertaken by the Foundation of European Nurses in Diabetes survey consortium. Diabet. Med. 2021, 38, e14498. [Google Scholar] [CrossRef]
- Chourasia, P.; Goyal, L.; Kansal, D.; Roy, S.; Singh, R.; Mahata, I.; Sheikh, A.B.; Shekhar, R. Risk of new-onset diabetes mellitus as a post-COVID-19 condition and possible mechanisms: A scoping review. J. Clin. Med. 2023, 12, 1159. [Google Scholar] [CrossRef]
- Abd Elmageed, A.M.; Sherif, W.; Abass, R. The Impact of Implementing Sleep Hygiene Program for Patients with Type Two Diabetes Mellitus and Uncontrolled Glucose Level. Mansoura Nurs. J. 2022, 9, 405–418. [Google Scholar] [CrossRef]
- Klingenberg, L.; Chaput, J.-P.; Holmbäck, U.; Visby, T.; Jennum, P.; Nikolic, M.; Astrup, A.; Sjödin, A. Acute sleep restriction reduces insulin sensitivity in adolescent boys. Sleep 2013, 36, 1085–1090. [Google Scholar] [CrossRef]
- Shan, Z.; Ma, H.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef]
- Ramalho, S.; Martins-Mendes, D.; Macedo, J.M.; Barros, C.; Luis, C.; Sá, S.; Gestoso, Á.; Pereira, A.C.; Baylina, P.; Fernandes, R. Unveiling the path to resilience: Prioritizing mental health, sleep, and nutrition in the post-COVID era. Healthcare 2023, 11, 2463. [Google Scholar] [CrossRef]
- Tiwari, R.; Tam, D.N.H.; Shah, J.; Moriyama, M.; Varney, J.; Huy, N.T. Effects of sleep intervention on glucose control: A narrative review of clinical evidence. Prim. Care Diabetes 2021, 15, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Cangelosi, G.; Grappasonni, I.; Pantanetti, P.; Scuri, S.; Garda, G.; Cuc Thi Thu, N.; Petrelli, F. Nurse Case Manager Lifestyle Medicine (NCMLM) in the Type Two Diabetes patient concerning post COVID-19 Pandemic management: Integrated-Scoping literature review. Ann. Di Ig. Med. Prev. E Di Comunità 2022, 34, 585–602. [Google Scholar]
- Wrona, M.; Skrypnik, D. New-onset diabetes mellitus, hypertension, dyslipidaemia as sequelae of COVID-19 infection—Systematic review. Int. J. Environ. Res. Public Health 2022, 19, 13280. [Google Scholar] [CrossRef] [PubMed]
- Keerthi, B.; Sushmita, G.; Khan, E.A.; Thomas, V.; Cheryala, V.; Shah, C.; Kumar, G.R.; Haritha, V. New onset diabetes mellitus in post-COVID-19 patients. J. Fam. Med. Prim. Care 2022, 11, 5961–5968. [Google Scholar] [CrossRef]
- van der Feltz-Cornelis, C.M.; Sweetman, J.; Turk, F.; Allsopp, G.; Gabbay, M.; Khunti, K.; Williams, N.; Montgomery, H.; Heightman, M.; Lip, G.Y.H.; et al. Integrated care policy recommendations for complex multisystem long term conditions and long COVID. Sci. Rep. 2024, 14, 13634. [Google Scholar] [CrossRef]
- Talanki, A.S.; Bajaj, N.; Trehan, T.; Thirunavukkarasu, S. Incidence, Risk, and Clinical Course of New-Onset Diabetes in Long COVID: Protocol for a Systematic Review and Meta-Analysis of Cohort Studies. JMIR Res. Protoc. 2024, 13, e54853. [Google Scholar] [CrossRef]
- Kawasaki, E. Anti-Islet Autoantibodies in Type 1 Diabetes. Int. J. Mol. Sci. 2023, 24, 10012. [Google Scholar] [CrossRef]
- Chen, X.; Shi, S.; Sun, H.; Zhou, L.; Wang, H.; Li, Y.; Gilson, E.; Lu, Y.; Hu, L.; Ye, J. Metformin alleviates inflammatory response and severity rate of COVID-19 infection in elderly individuals. Sci. Rep. 2025, 15, 11340. [Google Scholar] [CrossRef]
- Suresh, V.; Shamim, M.A.; Ghosh, V.; Dave, T.; Jayan, M.; Verma, A.; Sanker, V.; Roy, P.; Bardhan, M. SGLT2 Inhibitors in COVID-19: Umbrella Review, Meta-Analysis, and Bayesian Sensitivity Assessment. Diseases 2025, 13, 67. [Google Scholar] [CrossRef]
- Huang, T.S.; Chao, J.Y.; Chang, H.H.; Lin, W.R.; Lin, W.H. COVID-19 and Diabetes: Persistent Cardiovascular and Renal Risks in the Post-Pandemic Landscape. Life 2025, 15, 726. [Google Scholar] [CrossRef]
- Goel, V.; Raizada, A.; Aggarwal, A.; Madhu, S.V.; Kar, R.; Agrawal, A.; Mahla, V.; Goel, A. Long-Term Persistence of COVID-Induced Hyperglycemia: A Cohort Study. Am. J. Trop. Med. Hyg. 2024, 110, 512–517. [Google Scholar] [CrossRef] [PubMed]


| Reference | Study Type | Sample Size | Follow-Up Duration | Key Findings | Risk Metrics |
|---|---|---|---|---|---|
| Chen, M. (2021) [18] | Longitudinal | 64 | 6 months | Insulin resistance increased in patients without prior DM; elevated FBG at 6 months | N/A |
| Ayoubkhani, D. (2021) [19] | Cohort Study | 47,780 | 140 days | Higher rates of new-onset DM in hospitalized patients compared to controls. | HR 2.47 (acute phase), HR 1.60 (4 months post) |
| Reges, O. (2023) [20] | Retrospective Cohort Study | 157,936 | 18 months | Acute and post-acute COVID-19 were associated with new DM in severe hospitalized patients | HR 2.47 (acute phase) HR 1.60 (post-infection) |
| Xie, Y. (2022) [21] | Cohort Study | 181,280 | 352 days | Increased risk of new-onset DM and anti-hyperglycemic therapy initiation post-COVID. | HR 1.40 (new-onset DM), HR 1.85 (therapy initiation) |
| Wander, P.L. (2022) [22] | Retrospective Cohort study | 128,255 | 120 days | Significant association between COVID-19 and increased DM risk, particularly in men | OR 1.75 (men at 120 days) |
| Banerjee, M. (2022) [23] | Meta-Analysis | 5.7 million | 28 days | 59% increased risk of DM in the post-acute phase of COVID-19 | HR 1.59 (overall), HR 1.52 (hospitalized), HR 1.22 (mild) |
| Jayaseelan, V. (2022) [24] | Prospective Cohort | 724 | 3 months | Increased risk of DM in patients with moderate to severe COVID-19 | RR 2.83 (moderate/severe cases) |
| Kendall, E.K. (2021) [7] | Retrospective Cohort | 1 million | 6 months | Significant increase in new-onset T1DM in children post-COVID-19 | HR 1.96 (1 month), HR 2.10 (3 months), HR 1.83 (6 months) |
| Birabaharan, M. (2022) [11] | Retrospective Analysis | 600,055 | 180 days | Higher incidence of T2DM in COVID-19 patients compared to influenza | RR 1.54 (mild), RR 1.46 (moderate/severe) |
| Herczeg, V. (2022) [6] | Retrospective Analysis | 26 | 3 months | The newly diagnosed T1DM patients had a higher rate of anti-SARS-CoV-2 | OR 3.74 |
| Tazare, J. (2022) [13] | Cohort Study | 77,347 | 4 months | COVID-19 patients had higher risk of T2DM | HR 1.46 |
| Rathmann, W. (2022) [15] | Retrospective Cohort Study | 35,865 | 30 days | COVID-19 confers an increased risk for type 2 diabetes | OR 1.28 |
| Stage | Recommended Actions in Primary Care | Referral Guidelines for Endocrinology |
|---|---|---|
| Risk stratification (during or at discharge) | Identify adults or children with any of the following: -COVID-19-related hospitalization/ICU stay -Pre-existing obesity, metabolic syndrome, gestational diabetes, PCOS, or pre-diabetes -Prolonged systemic steroid use for COVID-19 -Symptoms of hyperglycemia (polyuria, polydipsia, weight loss) | -Immediate referral if ketosis or marked hyperglycemia (random glucose ≥ 300 mg/dL) is detected |
| Baseline screening (4–12 weeks post-infection) | -Order HbA1c + fasting plasma glucose (FPG). -In high-risk or symptomatic patients, add a 2-h OGTT. -Diagnose using ADA cut-offs (A1c ≥ 6.5%, FPG ≥ 126 mg/dL, 2-h PG ≥ 200 mg/dL, or random PG ≥ 200 mg/dL + symptoms). | -Clarify diabetes type (autoantibodies/C-peptide if T1DM suspected). -Assess for acute complications (DKA, HHS). |
| Follow-up surveillance | -Normal results: repeat A1c or FPG at 6 months and 12 months. -Prediabetes: lifestyle program ± metformin (if BMI ≥ 35 kg/m2 or age < 60 y), retest every 3–6 months. | -Consider CGM or flash monitoring if glucose variability is suspected, CKD stage ≥ 3, or steroid taper is ongoing. |
| Management of confirmed post-COVID diabetes | -Initiate lifestyle therapy (medical nutrition, weight-loss targets ≥ 5–7%). -Start metformin unless contraindicated; if A1c > 8%, add GLP-1 RA or SGLT2-i early for cardio-renal benefit. -Update vaccinations (COVID-19 boosters, pneumococcal, influenza) | -Optimize regimen for cardio-renal risk; screen for pancreatic autoimmunity if insulin requirements rise rapidly. -Arrange complication screening (retina, kidneys, feet) at diagnosis, then annually. |
| Long-term monitoring | -A1c every 3 months until goal (<7% for most), then every 6 months. -Blood pressure, lipids, and BMI at each visit. | -Evaluate persistent or atypical hyperglycemia, insulin dependence, or severe hypoglycemia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemat Jouy, S.; Tonchev, H.; Mostafa, S.M.; Mahmoud, A.M. Post-COVID Metabolic Fallout: A Growing Threat of New-Onset and Exacerbated Diabetes. Biomedicines 2025, 13, 1482. https://doi.org/10.3390/biomedicines13061482
Hemat Jouy S, Tonchev H, Mostafa SM, Mahmoud AM. Post-COVID Metabolic Fallout: A Growing Threat of New-Onset and Exacerbated Diabetes. Biomedicines. 2025; 13(6):1482. https://doi.org/10.3390/biomedicines13061482
Chicago/Turabian StyleHemat Jouy, Shaghayegh, Harry Tonchev, Sarah M. Mostafa, and Abeer M. Mahmoud. 2025. "Post-COVID Metabolic Fallout: A Growing Threat of New-Onset and Exacerbated Diabetes" Biomedicines 13, no. 6: 1482. https://doi.org/10.3390/biomedicines13061482
APA StyleHemat Jouy, S., Tonchev, H., Mostafa, S. M., & Mahmoud, A. M. (2025). Post-COVID Metabolic Fallout: A Growing Threat of New-Onset and Exacerbated Diabetes. Biomedicines, 13(6), 1482. https://doi.org/10.3390/biomedicines13061482







