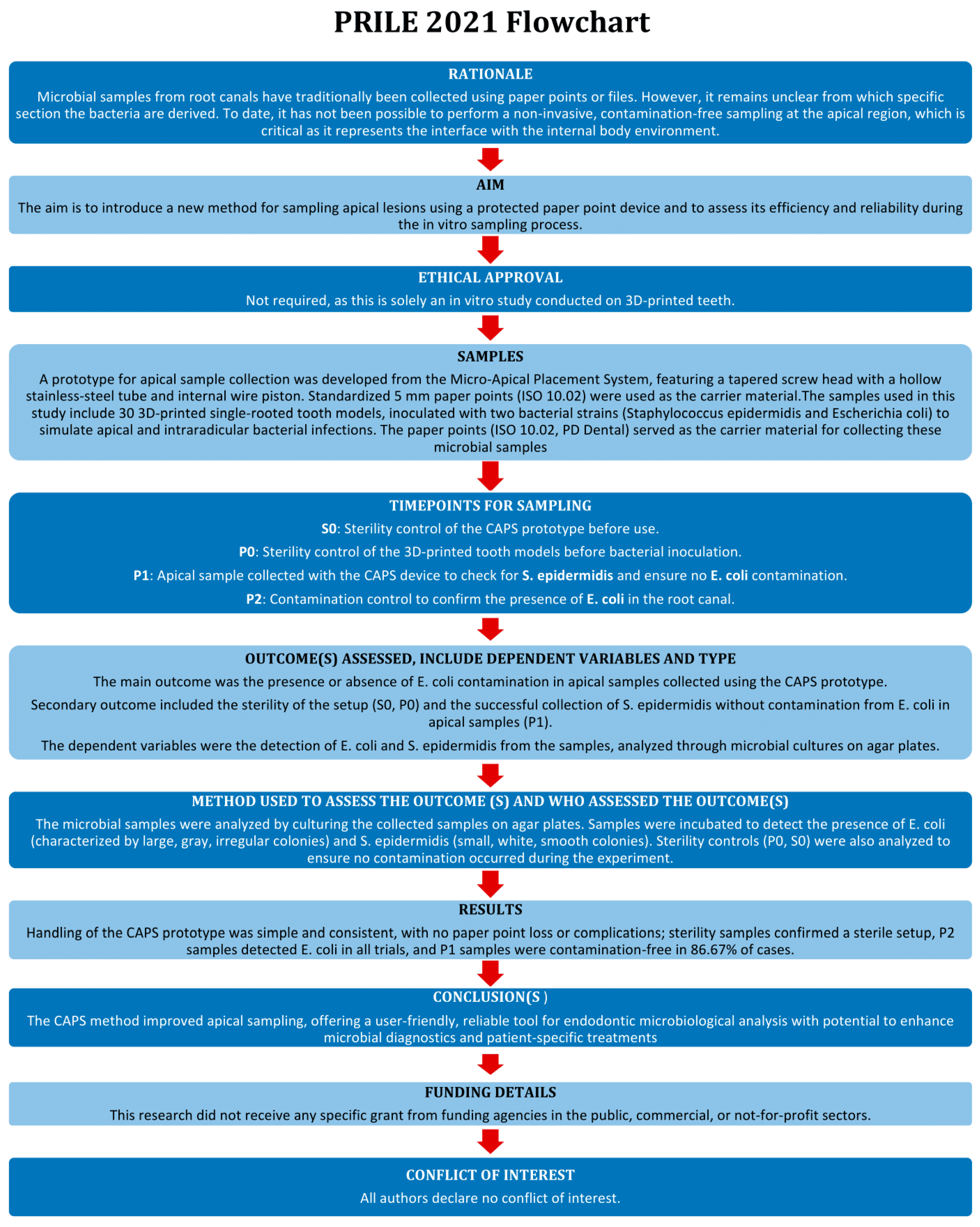
Introducing a Novel Paper Point Method for Isolated Apical Sampling—The Controlled Apical Sampling Device: A Methodological Study
Abstract
1. Introduction
2. Materials and Methods
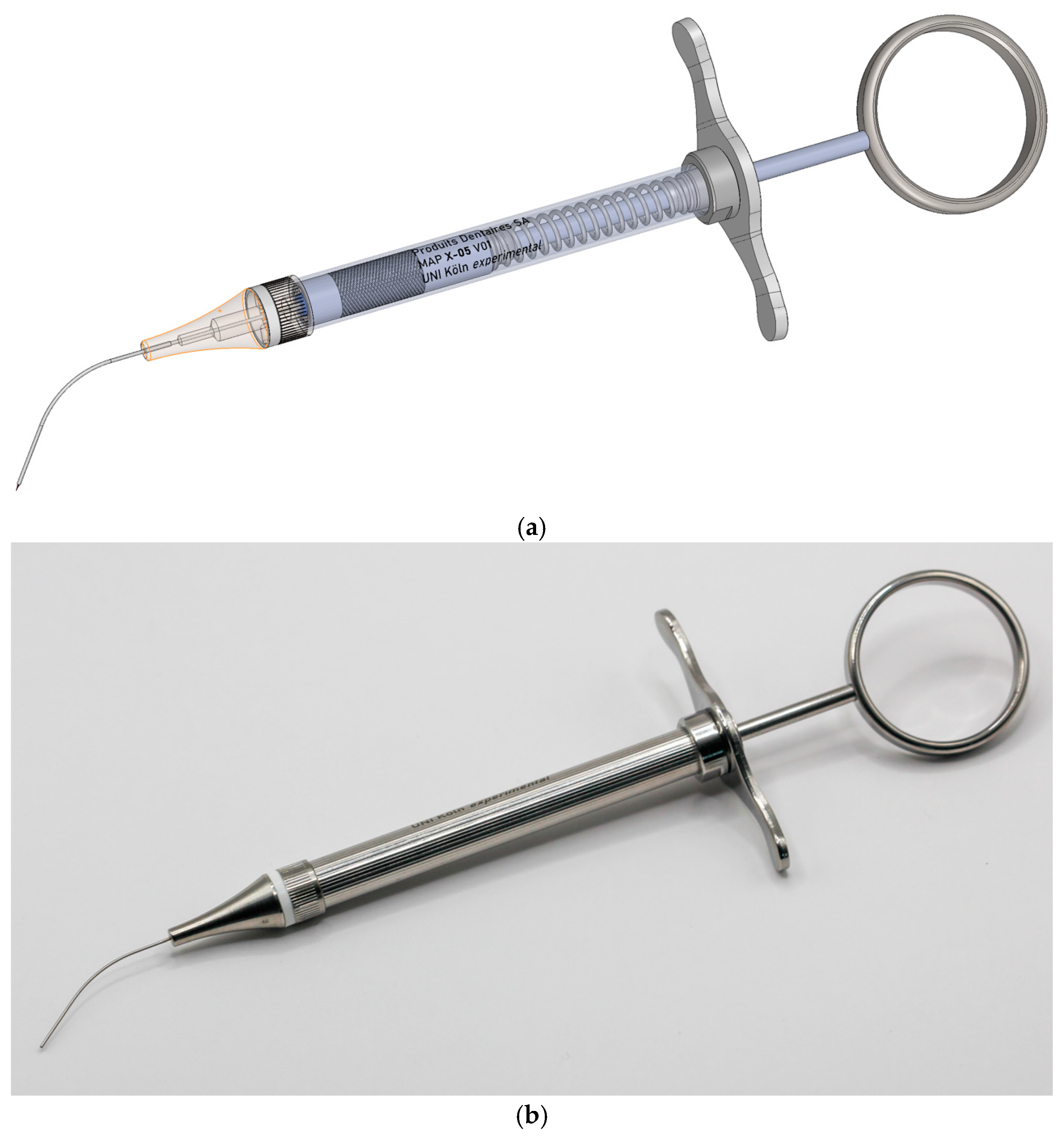
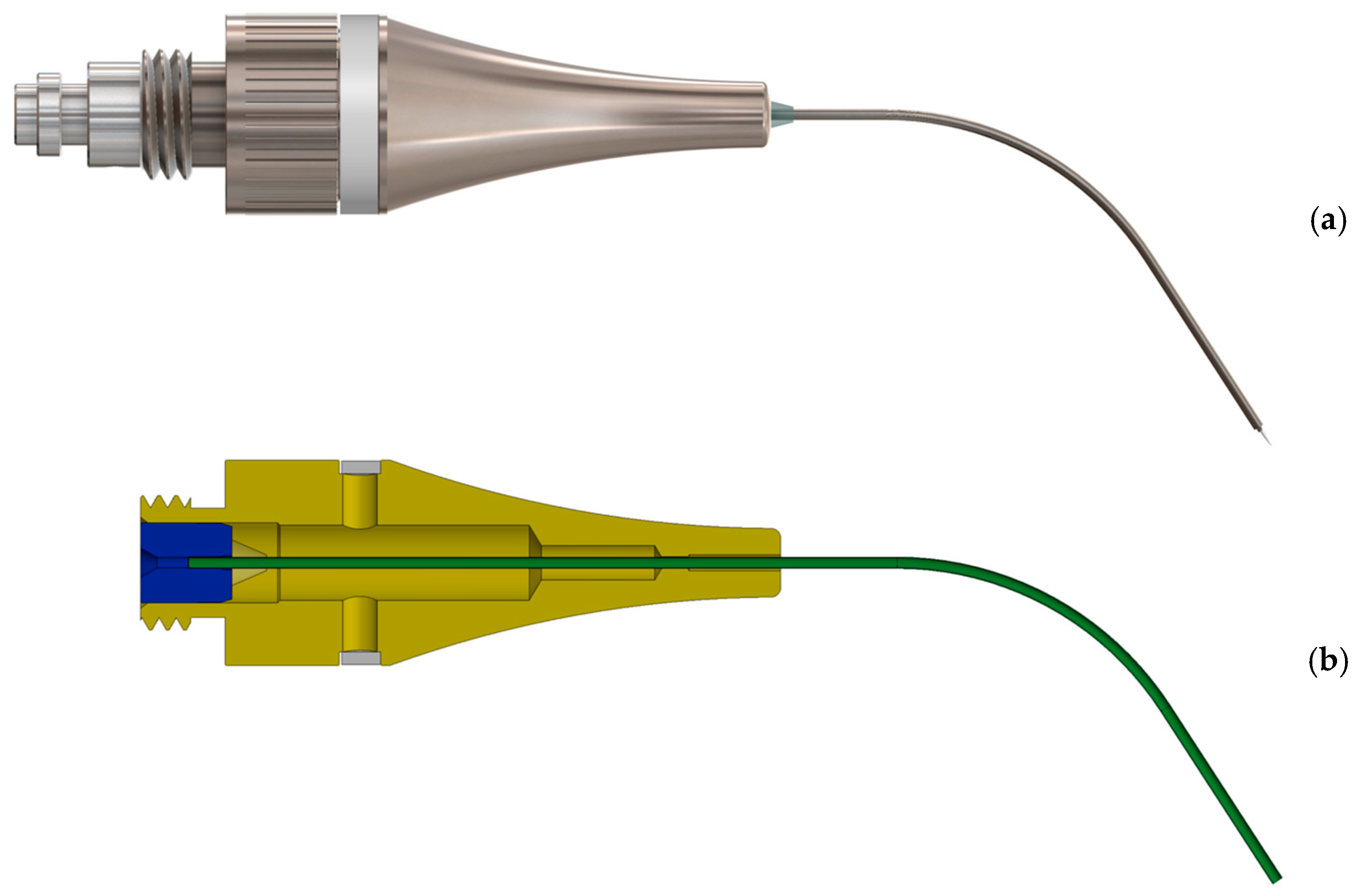
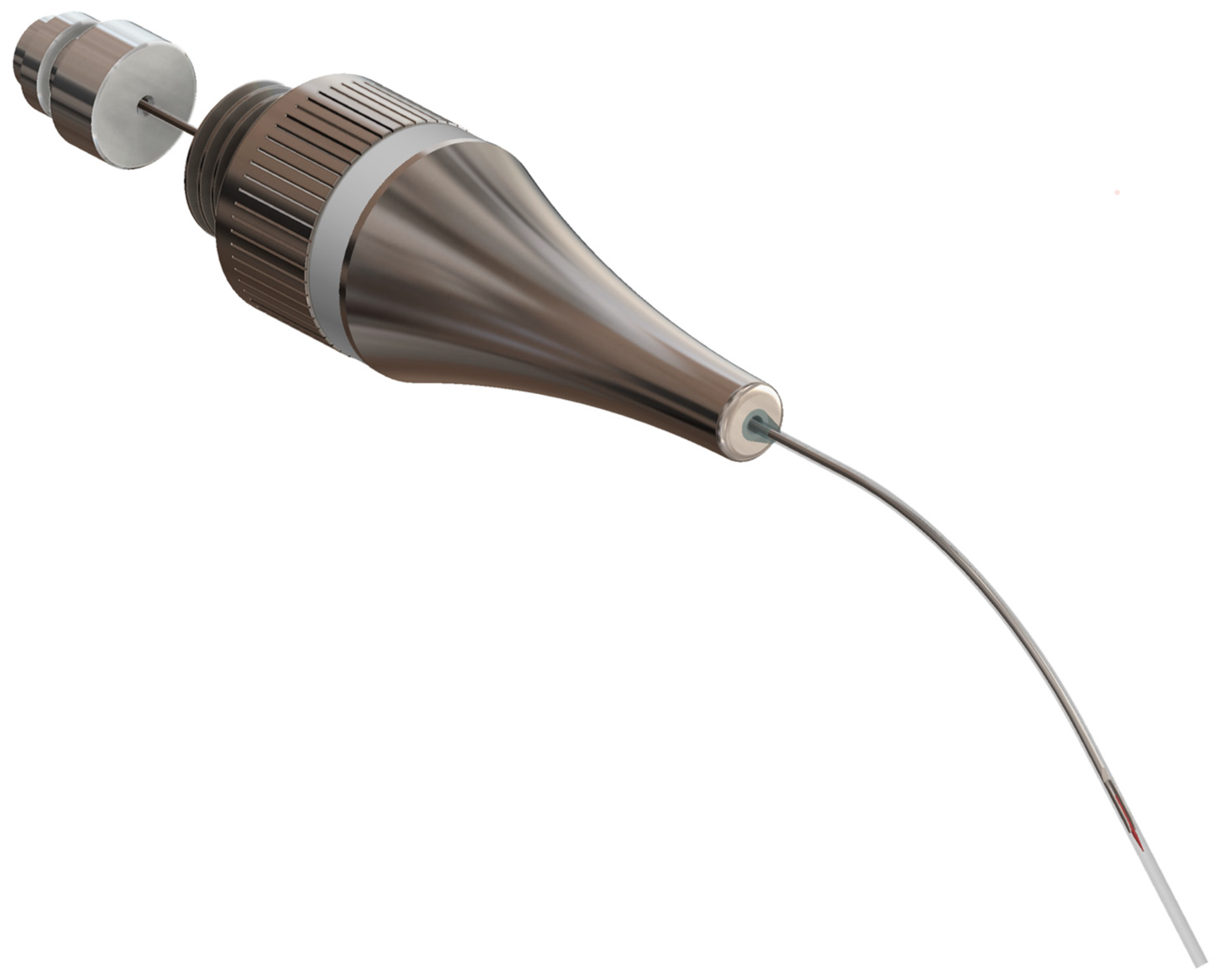
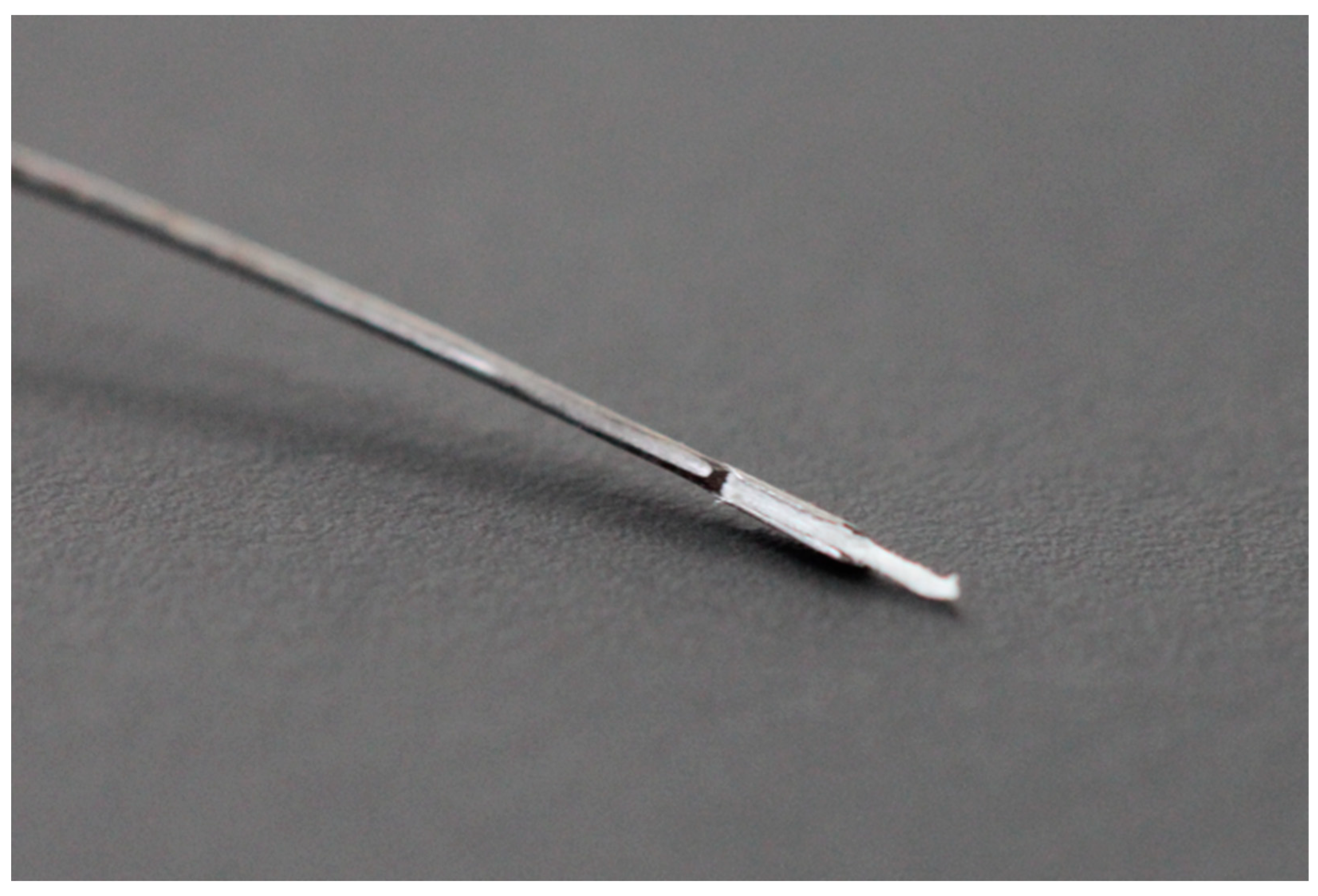
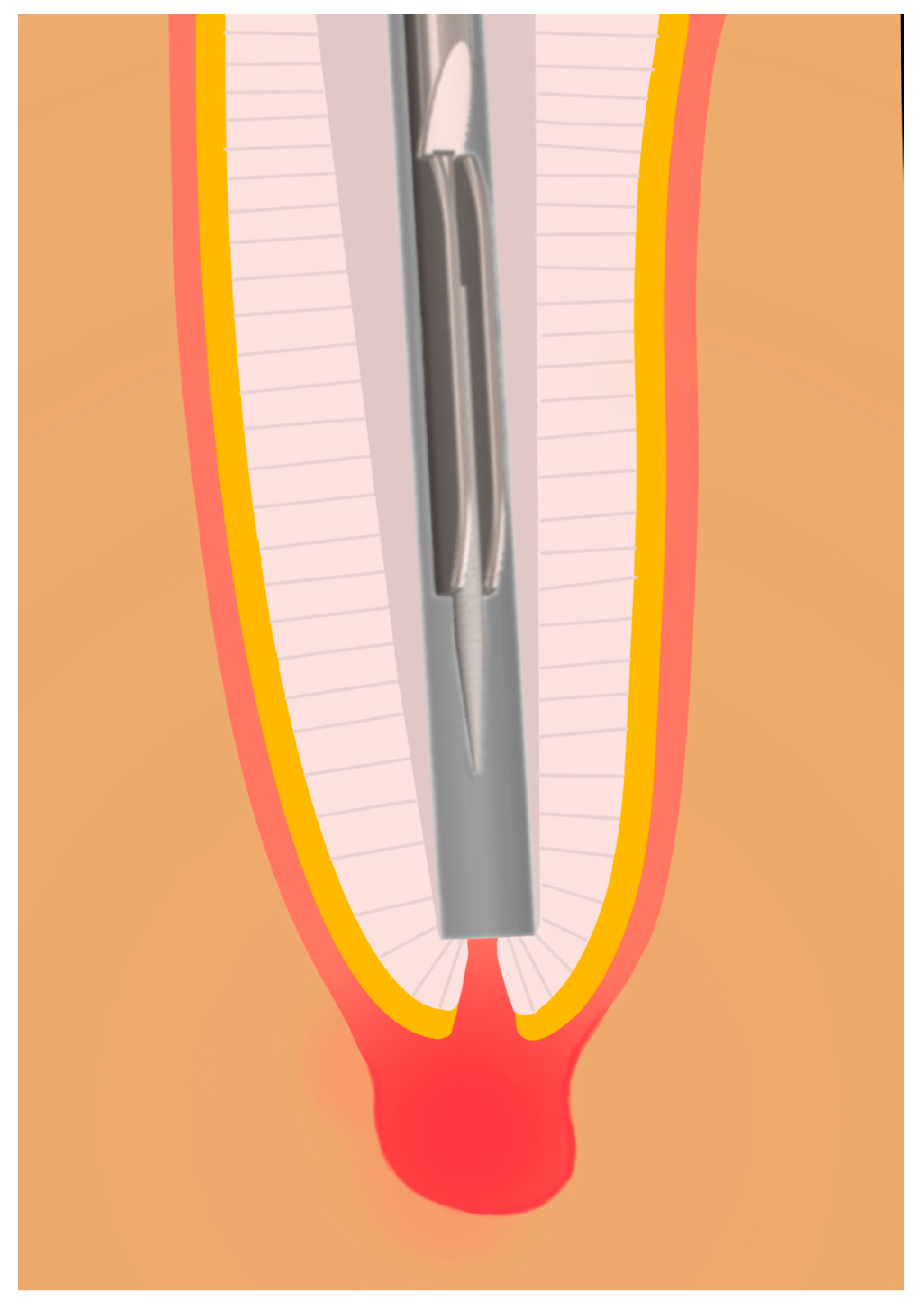
2.1. Development and Functionality of the Prototype
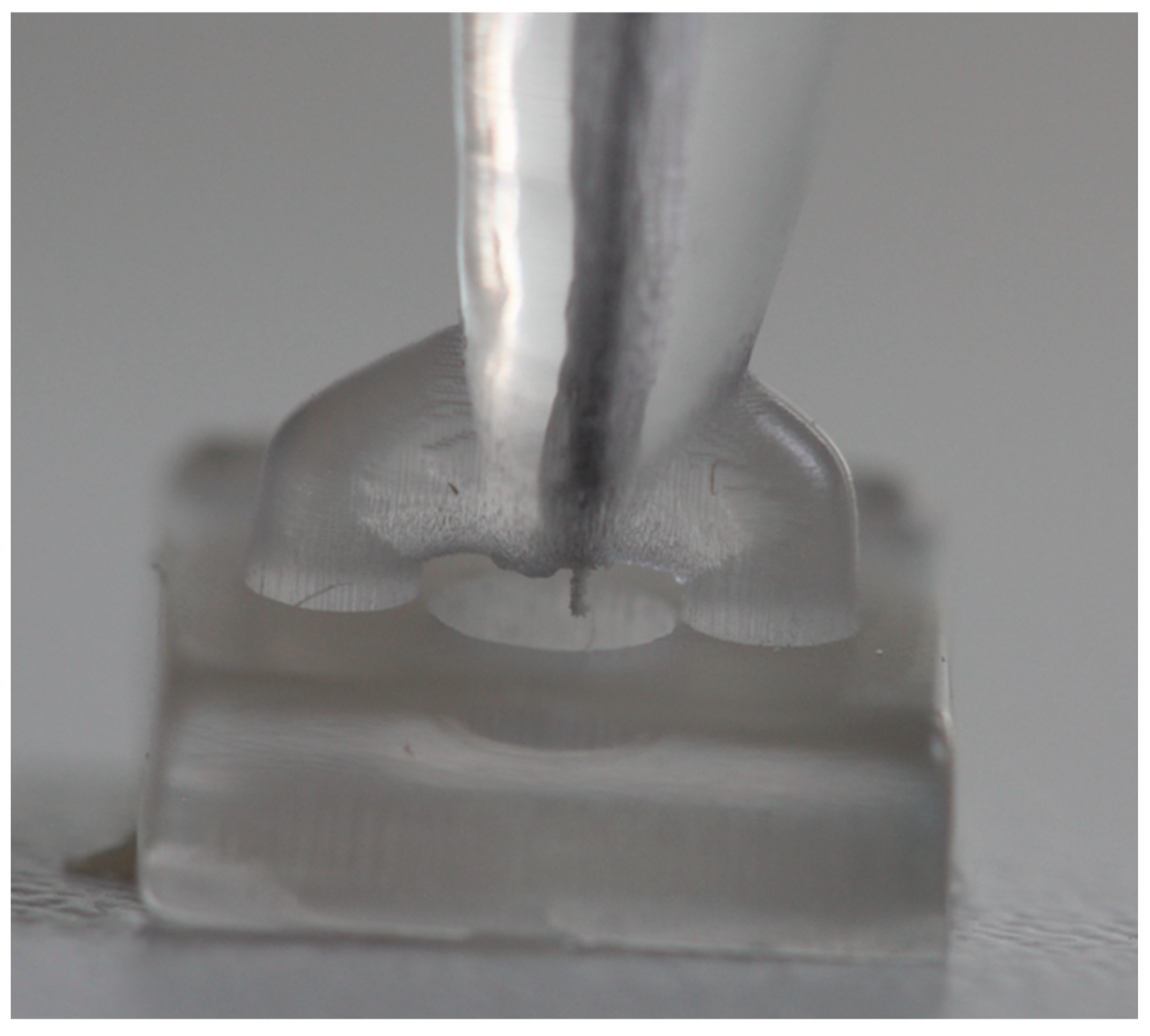
2.2. Creation of the 3D Model
2.3. Bacteria
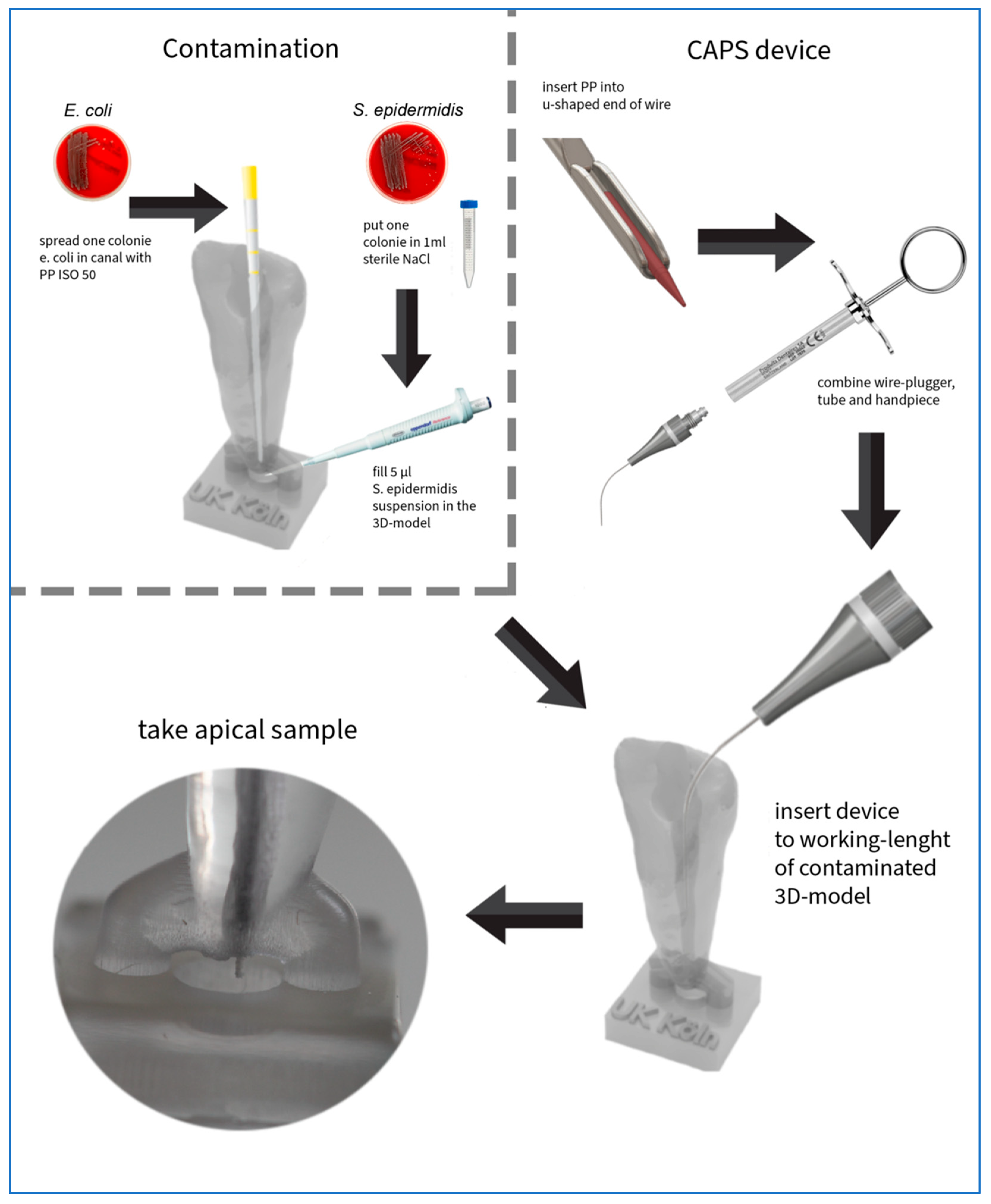
2.4. Test Procedure
2.5. Inoculation
2.6. Sampling
3. Results
4. Discussion
4.1. CAPS Prototype
4.2. Sampling Process
4.3. Conventional Sampling Methods
4.4. Bacterial Colonization
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAPS | Controlled apical sampling |
| MAP | Micro-Apical Placement |
| ISO | International Organization for Standardization |
| NaCl | Sodium chloride |
| PC | Positive control (NaCl control) |
| P0 | Sterility control of the 3D tooth model |
| P1A | Apical sample applied directly to agar plate |
| P1B | Apical sample after vortexing in saline solution and plating |
| P2 | Contamination control sample (E. coli detection from root canal) |
| S0 | Sterility control of the prototype device |
| VDW | Variable Drehmoment Werkstofftechnik (Manufacturer of endodontic equipment) |
| 3D | Three-dimensional |
| PD Dental | Produits Dentaires Dental Company |
| E. coli | Escherichia coli |
| S. epidermidis | Staphylococcus epidermidis |
| PRILE | Preferred Reporting Items for Laboratory studies in Endodontology |
References
- Kakehashi, S.; Stanley, H.R.; Fitzgerald, R.J. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Siqueira, J.F. Biofilms and Apical Periodontitis: Study of Prevalence and Association with Clinical and Histopathologic Findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, G. Bacteriological Studies of Necrotic Dental Pulps. Ph.D. Thesis, University of Umea, Umea, Sweden, 1976. [Google Scholar]
- Siqueira, J.F., Jr.; Rôças, I.N. Diversity of endodontic microbiota revisited. J. Dent. Res. 2009, 88, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N. Treatment of Endodontic Infections, 2nd ed.; Quintessence Publishing Company, Ltd.: New Malden, UK, 2022; p. 576. [Google Scholar]
- Siqueira, J.F., Jr.; Rôças, I.N. Microbiology and treatment of acute apical abscesses. Clin. Microbiol. Rev. 2013, 26, 255–273. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Community as the unit of pathogenicity: An emerging concept as to the microbial pathogenesis of apical periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radioliol. Endod. 2009, 107, 870–878. [Google Scholar] [CrossRef]
- Tak, E.J.; Park, O.-J.; Lee, J.-S.; Yoo, Y.-J.; Perinpanayagam, H.; Jeong, Y.-S.; Lee, J.-Y.; Bae, J.-W.; Kum, K.-Y.; Han, S.H. Microbiota associated with caries and apical periodontitis: A next-generation sequencing study. Int. Endod. J. 2025, 58, 890–901. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 2022, 55 (Suppl. S3), 512–530. [Google Scholar] [CrossRef]
- Manoil, D.; Al-Manei, K.; Belibasakis, G.N. A Systematic Review of the Root Canal Microbiota Associated with Apical Periodontitis: Lessons from Next-Generation Sequencing. Proteom. Clin. Appl. 2020, 14, e1900060. [Google Scholar] [CrossRef]
- de Paz, L.C. Gram-positive organisms in endodontic infections. Endod. Top. 2004, 9, 79–96. [Google Scholar] [CrossRef]
- Fries, J.F. The compression of morbidity. Ann. Acad. Med. Singap. 1983, 12, 358–367. [Google Scholar] [CrossRef]
- Pinheiro, E.T.; Gomes, B.P.; Ferraz, C.C.; Sousa, E.L.; Teixeira, F.B.; Souza-Filho, F.J. Microorganisms from canals of root-filled teeth with periapical lesions. Int. Endod. J. 2003, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Donnermeyer, D.; Matern, J.; Prior, K.; Ibing, M.; Hagenfeld, D.; Schäfer, E.; Bürklein, S.; Harmsen, D.; Ehmke, B. A Methodological Study on Microbial In Vivo Sampling Methods of Root Canal Microbiota for Next-Generation Gene Sequencing Analysis. J. Endod. 2025, 51, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Alquria, T.A.; Alfirdous, R.A.; Gupta, S.; Santamaria, M.P.; Santamaria, I.F.; Gomes, A.P.M.; Tiradentes, N.; Silva, E.G.; Martinho, F.C. Comparison of conventional and contemporary root canal disinfection protocols against bacteria, lipoteichoic acid (LTA), and lipopolysaccharide (LPS). Sci. Rep. 2023, 13, 1206. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. A critical analysis of research methods and experimental models to study the root canal microbiome. Int. Endod. J. 2022, 55 (Suppl. S1), 46–71. [Google Scholar] [CrossRef]
- Lijnen, I.; Willems, G. DNA research in forensic dentistry. Methods Find. Exp. Clin. Pharmacol. 2001, 23, 511–517. [Google Scholar] [CrossRef]
- Sweet, D.; Hildebrand, D. Recovery of DNA from human teeth by cryogenic grinding. J. Forensic Sci. 1998, 43, 1199–1202. [Google Scholar] [CrossRef]
- Sweet, D.; Hildebrand, D.; Phillips, D. Identification of a skeleton using DNA from teeth and a PAP smear. J. Forensic Sci. 1999, 44, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Hajihassani, N.; Ramezani, M.; Tofangchiha, M.; Bayereh, F.; Ranjbaran, M.; Zanza, A.; Reda, R.; Testarelli, L. Pattern of Endodontic Lesions of Maxillary and Mandibular Posterior Teeth: A Cone-Beam Computed Tomography Study. J. Imaging 2022, 8, 290. [Google Scholar] [CrossRef]
- Procop, G.W.; Church, D.; Hall, G.; Janda, W.M.; Koneman, E.W.; Schreckenberger, P.C.; Woods, G.L. Koneman’s Color Atlas and Textbook of Diagnostic Microbiolgy, 7th ed.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Rodrigues, R.C.V.; Zandi, H.; Kristoffersen, A.K.; Enersen, M.; Mdala, I.; Ørstavik, D.; Rôças, I.N.; Siqueira, J.F., Jr. Influence of the Apical Preparation Size and the Irrigant Type on Bacterial Reduction in Root Canal-treated Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1058–1063. [Google Scholar] [CrossRef]
- Amaral, R.R.; Love, R.M.; Braga, T.; Souza Côrtes, M.I.; Rachid, C.T.C.C.; Rôças, I.N.; Siqueira, J.F. Impact of root canal preparation using two single-file systems on the intra-radicular microbiome of teeth with primary apical periodontitis. Clin. Oral Investig. 2024, 28, 139. [Google Scholar] [CrossRef]
- Gesi, A.; Mareschi, P.; Doldo, T.; Ferrari, M. Apical Dimension of Root Canal Clinically Assessed with and without Periapical Lesions. Int. J. Dent. 2014, 2014, 374971. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.; Johnson, J.R.; van den Biggelaar, A.H.J.; Georgalis, L.; Geurtsen, J.; de Palacios, P.I.; Gravenstein, S.; Verstraeten, T.; Hermans, P.; Poolman, J.T. Epidemiology of Escherichia coli Bacteremia: A Systematic Literature Review. Clin. Infect. Dis. 2021, 72, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Namvar, A.E.; Bastarahang, S.; Abbasi, N.; Ghehi, G.S.; Farhadbakhtiarian, S.; Arezi, P.; Hosseini, M.; Baravati, S.Z.; Jokar, Z.; Chermahin, S.G. Clinical characteristics of Staphylococcus epidermidis: A systematic review. GMS Hyg Infect Control 2014, 9, Doc23. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.A.; Clarke, D.; Do, T.; Gilbert, S.C.; Mannocci, F.; Beighton, D. Propionibacterium acnes and Staphylococcus epidermidis isolated from refractory endodontic lesions are opportunistic pathogens. J. Clin. Microbiol. 2010, 48, 3859–3869. [Google Scholar] [CrossRef]



| P1A | P1B | |
|---|---|---|
| n (%) | 30 (100.0) | 30 (100.0) |
| Uncontaminated (%) | 26 (86.7) | 27 (90.0) |
| Contaminated (%) | 4 (13.3) | 3 (10.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoppmeier, C.M.; Classen, G.L.; Contini, S.; Rebmann, P.; Brendlen, D.; Wicht, M.J.; Barbe, A.G. Introducing a Novel Paper Point Method for Isolated Apical Sampling—The Controlled Apical Sampling Device: A Methodological Study. Biomedicines 2025, 13, 1477. https://doi.org/10.3390/biomedicines13061477
Schoppmeier CM, Classen GL, Contini S, Rebmann P, Brendlen D, Wicht MJ, Barbe AG. Introducing a Novel Paper Point Method for Isolated Apical Sampling—The Controlled Apical Sampling Device: A Methodological Study. Biomedicines. 2025; 13(6):1477. https://doi.org/10.3390/biomedicines13061477
Chicago/Turabian StyleSchoppmeier, Christoph Matthias, Gustav Leo Classen, Silvia Contini, Paul Rebmann, David Brendlen, Michael Jochen Wicht, and Anna Greta Barbe. 2025. "Introducing a Novel Paper Point Method for Isolated Apical Sampling—The Controlled Apical Sampling Device: A Methodological Study" Biomedicines 13, no. 6: 1477. https://doi.org/10.3390/biomedicines13061477
APA StyleSchoppmeier, C. M., Classen, G. L., Contini, S., Rebmann, P., Brendlen, D., Wicht, M. J., & Barbe, A. G. (2025). Introducing a Novel Paper Point Method for Isolated Apical Sampling—The Controlled Apical Sampling Device: A Methodological Study. Biomedicines, 13(6), 1477. https://doi.org/10.3390/biomedicines13061477






