Effects of Methionine and Glutathione on Acute Ototoxicity Induced by Amikacin and Furosemide in an Animal Model of Hearing Threshold Decrease
Abstract
1. Introduction
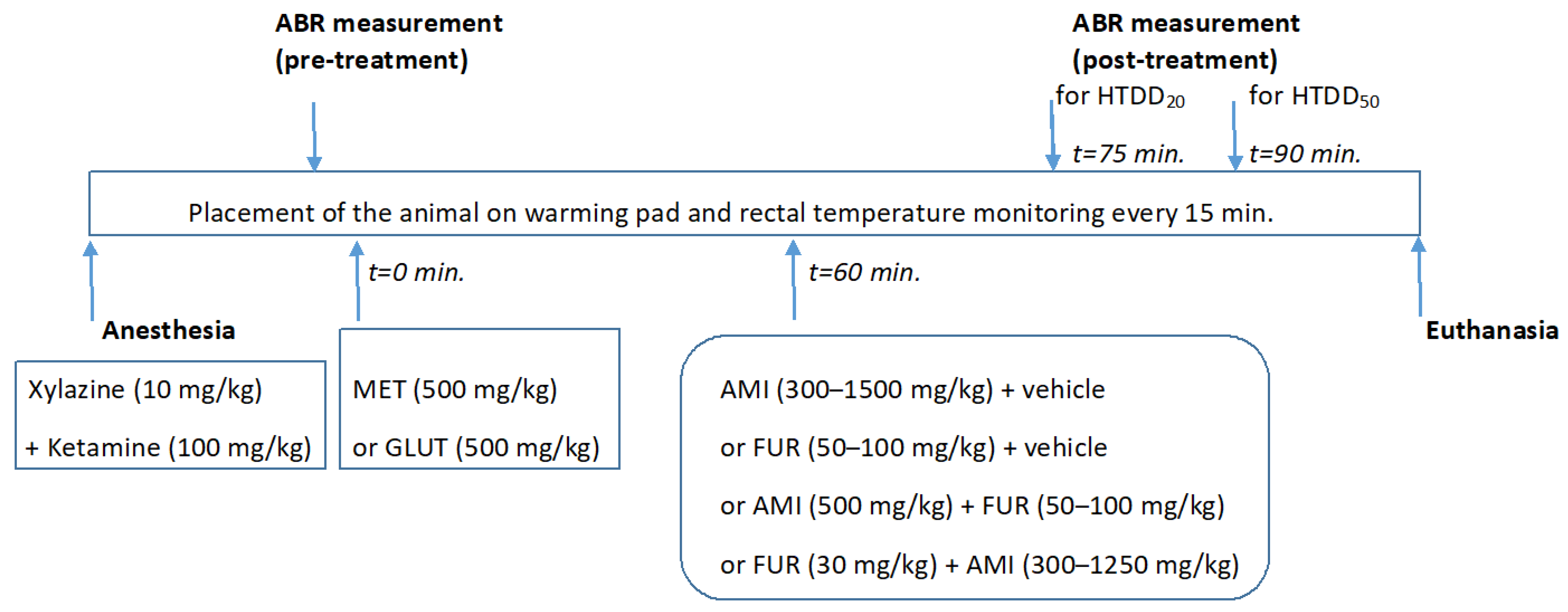
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Animal Anesthesia
2.4. Auditory Brainstem Responses (ABRs) and Hearing Threshold Detection
2.5. Animal Euthanasia
2.6. Isobolographic Transformation
2.7. Statistical Analysis
3. Results
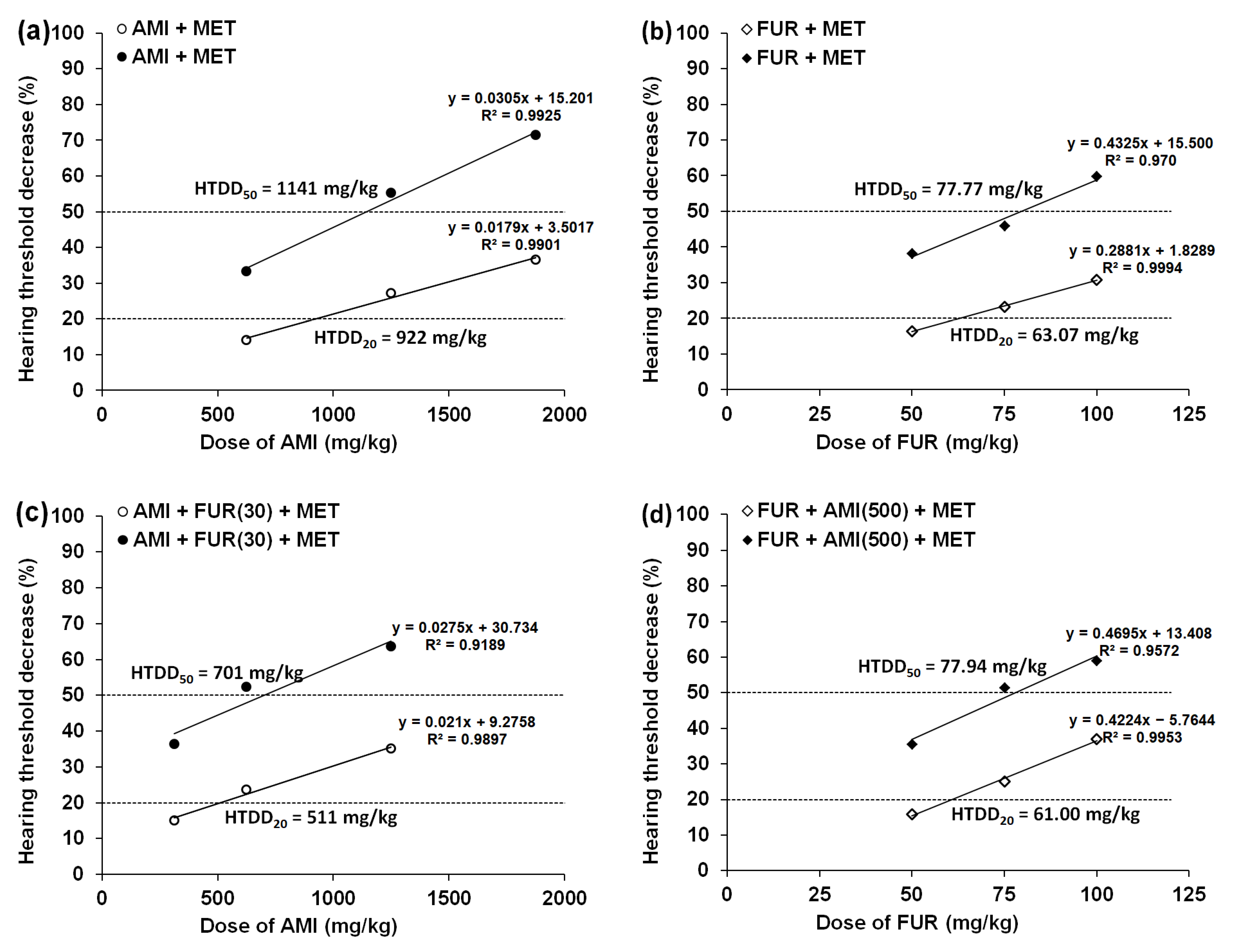
3.1. Effect of MET and GLUT on the AMI-Induced Hearing Threshold Decrease in Mice
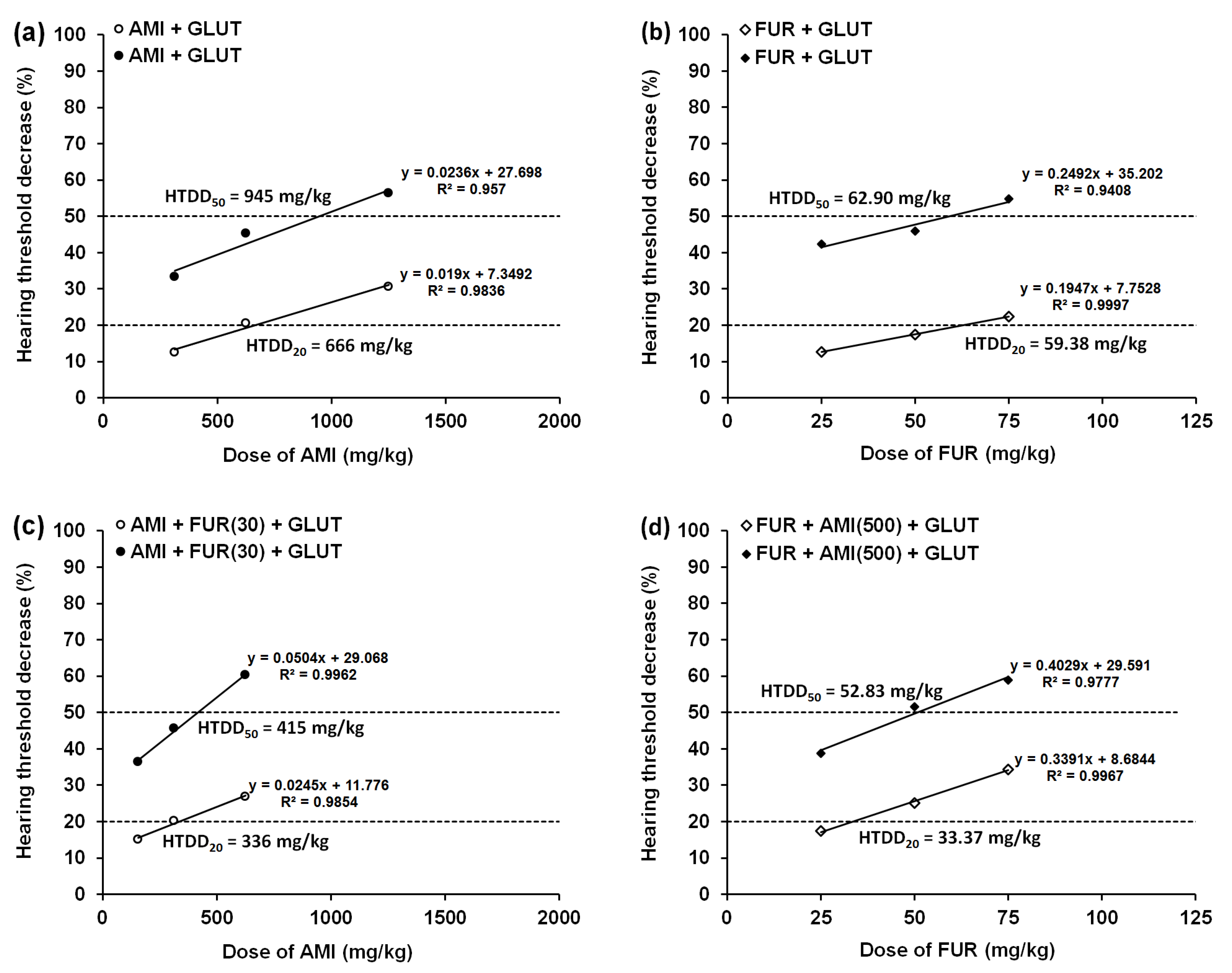
3.2. Effects of MET and GLUT on the FUR-Induced Hearing Threshold Decrease in Mice
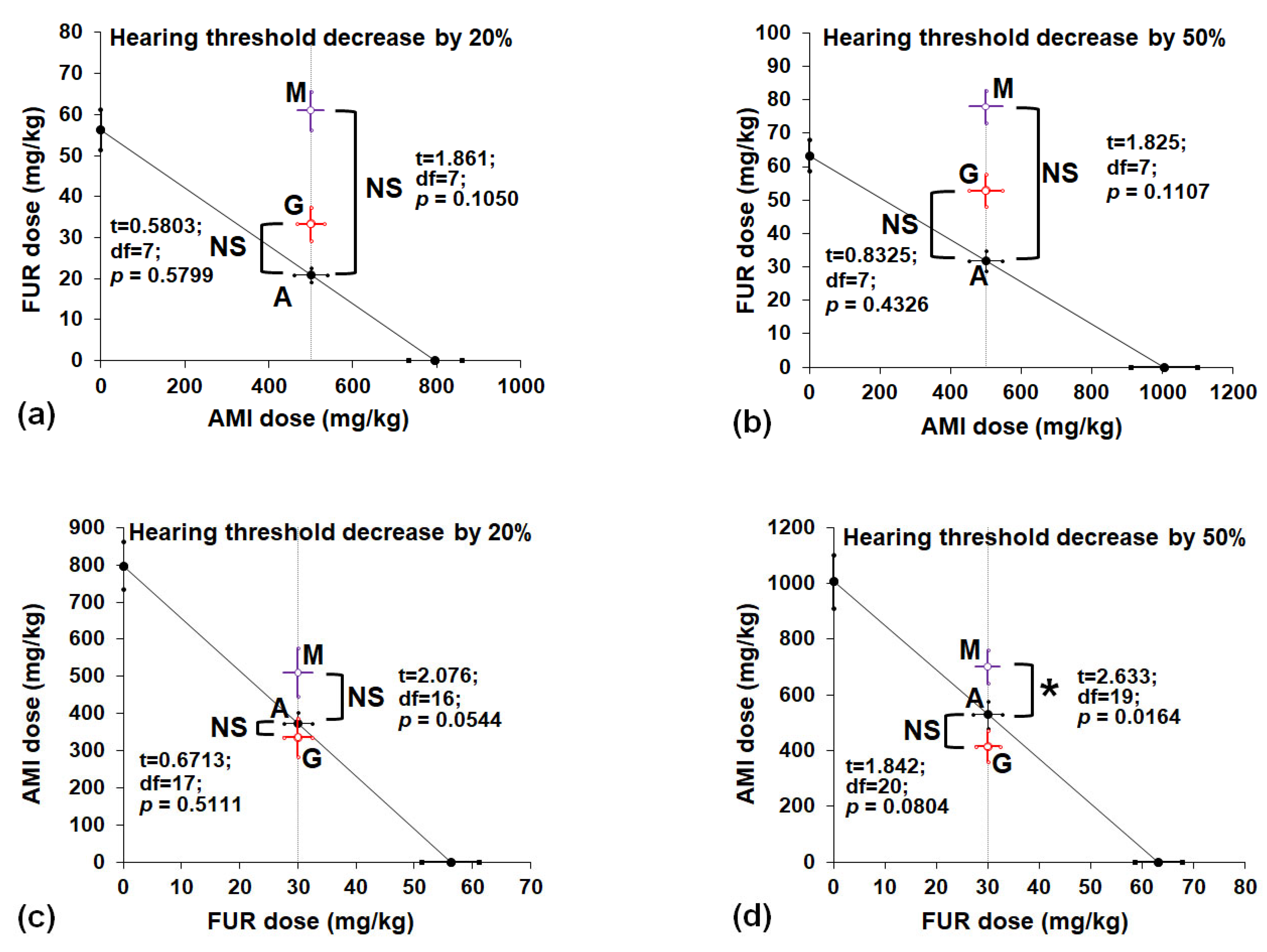
3.3. Isobolographic Interactions Between AMI, FUR, MET and GLUT in a Mouse Model of Drug-Induced Hearing Threshold Decrease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABR | Auditory Brainstem Response |
| AMI | Amikacin |
| FUR | Furosemide |
| GLUT | Glutathione |
| HTDD20 | Hearing threshold decreasing dose by 20% |
| HTDD50 | Hearing threshold decreasing dose by 50% |
| MET | Methionine |
| VEH | Vehicle |
References
- Jenkins, A.; Thomson, A.H.; Brown, N.M.; Semple, Y.; Sluman, C.; MacGowan, A.; Lovering, A.M.; Wiffen, P.J. Amikacin use and therapeutic drug monitoring in adults: Do dose regimens and drug exposures affect either outcome or adverse events? A systematic review. J. Antimicrob. Chemother. 2016, 71, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Hayward, R.S.; Harding, J.; Molloy, R.; Land, L.; Longcroft-Neal, K.; Moore, D.; Ross, J.D.C. Adverse effects of a single dose of gentamicin in adults: A systematic review. Br. J. Clin. Pharmacol. 2018, 84, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, C.; Berger, C.C.; Hartmann, J.T.; Kollmannsberger, C.; Schmoll, H.J.; Kuczyk, M.A.; Kanz, L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br. J. Cancer 1998, 77, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Forge, A.; Jagger, D.J. Structural changes in the human stria vascularis induced by aminoglycosides and loop diuretics. Hear. Res. 2022, 426, 108626. [Google Scholar] [CrossRef]
- Bako, P.; Gerlinger, I.; Wolpert, S.; Müller, M.; Löwenheim, H. The ototoxic effect of locally applied kanamycin and furosemide in guinea pigs. J. Neurosci. Methods 2022, 372, 109527. [Google Scholar] [CrossRef]
- Joo, Y.; Cruickshanks, K.J.; Klein, B.E.K.; Klein, R.; Hong, O.; Wallhagen, M.I. The Contribution of Ototoxic Medications to Hearing Loss Among Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 561–566. [Google Scholar] [CrossRef]
- Le, T.A.; Hiba, T.; Chaudhari, D.; Preston, A.N.; Palowsky, Z.R.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. Aminoglycoside-Related Nephrotoxicity and Ototoxicity in Clinical Practice: A Review of Pathophysiological Mechanism and Treatment Options. Adv. Ther. 2023, 40, 1357–1365. [Google Scholar] [CrossRef]
- Campbell, K. Ototoxicity: Understanding oxidative mechanisms. J. Am. Acad. Audiol. 2003, 14, 121–123. [Google Scholar] [CrossRef]
- Schmitz, H.M.; Johnson, S.B.; Santi, P.A. Kanamycin-furosemide ototoxicity in the mouse cochlea: A 3-dimensional analysis. Otolaryngol. Head Neck Surg. 2014, 150, 666–672. [Google Scholar] [CrossRef]
- Tan, W.J.T.; Song, L. Role of mitochondrial dysfunction and oxidative stress in sensorineural hearing loss. Hear. Res. 2023, 434, 108783. [Google Scholar] [CrossRef]
- Poirrier, A.L.; Pincemail, J.; Van Den Ackerveken, P.; Lefebvre, P.P.; Malgrange, B. Oxidative stress in the cochlea: An update. Curr. Med. Chem. 2010, 17, 3591–3604. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.E. Aminoglycoside ototoxicity. Drugs Today 2003, 39, 277–285. [Google Scholar] [CrossRef]
- Rybak, L.P.; Ramkumar, V. Ototoxicity. Kidney Int. 2007, 72, 931–935. [Google Scholar] [CrossRef]
- Rybak, L.P.; Whitworth, C.A. Ototoxicity: Therapeutic opportunities. Drug Discov. Today 2005, 10, 1313–1321. [Google Scholar] [CrossRef]
- Campbell, K.C.; Meech, R.P.; Klemens, J.J.; Gerberi, M.T.; Dyrstad, S.S.; Larsen, D.L.; Mitchell, D.L.; El-Azizi, M.; Verhulst, S.J.; Hughes, L.F. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear. Res. 2007, 226, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Chen, F.; Li, S.; Zhang, H. (-)-Epigallocatechin-3-gallate (EGCG) prevents aminoglycosides-induced ototoxicity via anti-oxidative and anti-apoptotic pathways. Int. J. Pediatr. Otorhinolaryngol. 2021, 150, 110920. [Google Scholar] [CrossRef]
- Kocyigit, I.; Vural, A.; Unal, A.; Sipahioglu, M.H.; Yucel, H.E.; Aydemir, S.; Yazici, C.; İlhan Sahin, M.; Oymak, O.; Tokgoz, B. Preventing amikacin related ototoxicity with N-acetylcysteine in patients undergoing peritoneal dialysis. Eur. Arch. Otorhinolaryngol. 2015, 272, 2611–2620. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, M.A.; Cho, H.J.; Oh, S.K.; Lee, I.K.; Kim, U.K.; Lee, K.Y. Galangin prevents aminoglycoside-induced ototoxicity by decreasing mitochondrial production of reactive oxygen species in mouse cochlear cultures. Toxicol. Lett. 2016, 245, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, D.; Du, T.T.; Wagner, E.L.; Choi, J.H.; Li, S.; Reed, R.; Kim, K.; Freeman, M.; Hashisaki, G.; Lukens, J.R.; et al. Necroptosis and Apoptosis Contribute to Cisplatin and Aminoglycoside Ototoxicity. J. Neurosci. 2019, 39, 2951–2964. [Google Scholar] [CrossRef]
- Lv, X.; Yang, C.; Li, X.; Liu, Y.; Yang, Y.; Jin, T.; Chen, Z.; Jia, J.; Wang, M.; Li, L. Ferroptosis and hearing loss: From molecular mechanisms to therapeutic interventions. J. Enzyme Inhib. Med. Chem. 2025, 40, 2468853. [Google Scholar] [CrossRef]
- Ding, D.; Jiang, H.; Salvi, R.J. Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear. Res. 2010, 259, 16–23. [Google Scholar] [CrossRef]
- Tokgoz, B.; Somdas, M.A.; Ucar, C.; Kocyigit, I.; Unal, A.; Sipahioglu, M.H.; Oymak, O.; Utas, C. Correlation between hearing loss and peritonitis frequency and administration of ototoxic intraperitoneal antibiotics in patients with CAPD. Ren. Fail. 2010, 32, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Tokgoz, B.; Ucar, C.; Kocyigit, I.; Somdas, M.; Unal, A.; Vural, A.; Sipahioglu, M.; Oymak, O.; Utas, C. Protective effect of N-acetylcysteine from drug-induced ototoxicity in uraemic patients with CAPD peritonitis. Nephrol. Dial. Transplant. 2011, 26, 4073–4078. [Google Scholar] [CrossRef] [PubMed]
- Maru, D.; Malky, G.A. Current practice of ototoxicity management across the United Kingdom (UK). Int. J. Audiol. 2018, 57, S76–S88. [Google Scholar] [CrossRef]
- Ralli, M.; Rolesi, R.; Anzivino, R.; Turchetta, R.; Fetoni, A.R. Acquired sensorineural hearing loss in children: Current research and therapeutic perspectives. Acta Otorhinolaryngol. Ital. 2017, 37, 500–508. [Google Scholar] [CrossRef]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Grondin, Y.; Cotanche, D.A.; Manneberg, O.; Molina, R.; Treviño-Villarreal, J.H.; Sepulveda, R.; Clifford, R.; Bortoni, M.E.; Forsberg, S.; Labrecque, B.; et al. Pulmonary delivery of d-methionine is associated with an increase in ALCAR and glutathione in cochlear fluids. Hear. Res. 2013, 298, 93–103. [Google Scholar] [CrossRef]
- Pecha, P.P.; Almishaal, A.A.; Mathur, P.D.; Hillas, E.; Johnson, T.; Price, M.S.; Haller, T.; Yang, J.; Rajasekaran, N.S.; Firpo, M.A.; et al. Role of Free Radical Formation in Murine Cytomegalovirus-Induced Hearing Loss. Otolaryngol. Head Neck Surg. 2020, 162, 709–717. [Google Scholar] [CrossRef]
- Campbell, K.C.M.; Cosenza, N.; Meech, R.; Buhnerkempe, M.; Qin, J.; Rybak, L.; Fox, D.J. D-methionine administered as late as 36 hours post-noise exposure rescues from permanent threshold shift and dose-dependently increases serum antioxidant levels. Int. J. Audiol. 2023, 62, 151–158. [Google Scholar] [CrossRef]
- Campbell, K.C.M.; Cosenza, N.; Meech, R.; Buhnerkempe, M.; Qin, J.; Rybak, L.; Fox, D.J. D-methionine immediate and continued rescue after noise exposure does not prevent temporary threshold shift but alters cochlear and serum antioxidant levels. Int. J. Audiol. 2022, 61, 769–777. [Google Scholar] [CrossRef]
- Lockwood, D.S.; Ding, D.L.; Wang, J.; Salvi, R.J. D-Methionine attenuates inner hair cell loss in carboplatin-treated chinchillas. Audiol. Neurootol. 2000, 5, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.H.; Schacht, J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hear. Res. 2000, 142, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.J.; Cooper, M.D.; Speil, C.A.; Roberts, M.H.; Yanik, S.C.; Meech, R.P.; Hargrove, T.L.; Verhulst, S.J.; Rybak, L.P.; Campbell, K.C. d-Methionine reduces tobramycin-induced ototoxicity without antimicrobial interference in animal models. J. Cyst. Fibros. 2016, 15, 518–530. [Google Scholar] [CrossRef]
- Campbell, K.C.; Martin, S.M.; Meech, R.P.; Hargrove, T.L.; Verhulst, S.J.; Fox, D.J. D-methionine (D-met) significantly reduces kanamycin-induced ototoxicity in pigmented guinea pigs. Int. J. Audiol. 2016, 55, 273–278. [Google Scholar] [CrossRef]
- Campbell, K.C.; Meech, R.P.; Rybak, L.P.; Hughes, L.F. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: Mechanisms of otoprotection. J. Am. Acad. Audiol. 2003, 14, 144–156. [Google Scholar] [CrossRef]
- Vogt, W. Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic. Biol. Med. 1995, 18, 93–105. [Google Scholar] [CrossRef]
- Yin, F.; Sancheti, H.; Cadenas, E. Mitochondrial thiols in the regulation of cell death pathways. Antioxid. Redox Signal 2012, 17, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Zenner, H.P.; Keiner, S.; Zimmermann, U. Specific glutathione-SH inhibition of toxic effects of metabolized gentamicin on isolated guinea pig hair cells. Eur. Arch. Otorhinolaryngol. 1994, 251, 84–90. [Google Scholar] [CrossRef]
- Hoffman, D.W.; Whitworth, C.A.; Jones, K.L.; Rybak, L.P. Nutritional status, glutathione levels, and ototoxicity of loop diuretics and aminoglycoside antibiotics. Hear. Res. 1987, 31, 217–222. [Google Scholar] [CrossRef]
- Hoffman, D.W.; Whitworth, C.A.; Jones-King, K.L.; Rybak, L.P. Potentiation of ototoxicity by glutathione depletion. Ann. Otol. Rhinol. Laryngol. 1988, 97, 36–41. [Google Scholar] [CrossRef]
- Garetz, S.L.; Altschuler, R.A.; Schacht, J. Attenuation of gentamicin ototoxicity by glutathione in the guinea pig in vivo. Hear. Res. 1994, 77, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Nishida, I.; Takumida, M. Attenuation of aminoglycoside ototoxicity by glutathione. ORL J. Otorhinolaryngol. Relat. Spec. 1996, 58, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Lautermann, J.; Crann, S.A.; McLaren, J.; Schacht, J. Glutathione-dependent antioxidant systems in the mammalian inner ear: Effects of aging, ototoxic drugs and noise. Hear. Res. 1997, 114, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Whitworth, C.; Somani, S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope 1999, 109, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Panasiuk, A.; Zagaja, M.; Karwan, S.; Bojar, H.; Plewa, Z.; Florek-Łuszczki, M. Polygonogram and isobolographic analysis of interactions between various novel antiepileptic drugs in the 6-Hz corneal stimulation-induced seizure model in mice. PLoS ONE 2020, 15, e0234070. [Google Scholar] [CrossRef]
- Ahmadi-Noorbakhsh, S.; Farajli Abbasi, M.; Ghasemi, M.; Bayat, G.; Davoodian, N.; Sharif-Paghaleh, E.; Poormoosavi, S.M.; Rafizadeh, M.; Maleki, M.; Shirzad-Aski, H.; et al. Anesthesia and analgesia for common research models of adult mice. Lab. Anim. Res. 2022, 38, 40. [Google Scholar] [CrossRef]
- van Looij, M.A.; Liem, S.S.; van der Burg, H.; van der Wees, J.; De Zeeuw, C.I.; van Zanten, B.G. Impact of conventional anesthesia on auditory brainstem responses in mice. Hear. Res. 2004, 193, 75–82. [Google Scholar] [CrossRef]
- Verdoodt, D.; Eens, S.; Van Dam, D.; De Deyn, P.P.; Vanderveken, O.M.; Szewczyk, K.; Saldien, V.; Ponsaerts, P.; Van Rompaey, V. Effect of Oral Allylnitrile Administration on Cochlear Functioning in Mice Following Comparison of Different Anesthetics for Hearing Assessment. Front. Toxicol. 2021, 3, 641569. [Google Scholar] [CrossRef]
- Zadrożniak, M.; Szymański, M.; Łuszczki, J.J. N-Acetyl-L-cysteine Affects Ototoxicity Evoked by Amikacin and Furosemide Either Alone or in Combination in a Mouse Model of Hearing Threshold Decrease. Int. J. Mol. Sci. 2023, 24, 7596. [Google Scholar] [CrossRef]
- Zadrozniak, M.; Szymanski, M.; Luszczki, J.J. Vitamin C alleviates ototoxic effect caused by coadministration of amikacin and furosemide. Pharmacol. Rep. 2019, 71, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Domarecka, E.; Szczepek, A.J. Universal Recommendations on Planning and Performing the Auditory Brainstem Responses (ABR) with a Focus on Mice and Rats. Audiol. Res. 2023, 13, 441–458. [Google Scholar] [CrossRef]
- AVMA AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Available online: https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf (accessed on 28 May 2025).
- Tallarida, R.J. Drug Combinations: Tests and Analysis with Isoboles. Curr. Protocols Pharmacol. 2016, 72, 9.19.1–9.19.19. [Google Scholar] [CrossRef]
- García-Alcántara, F.; Murillo-Cuesta, S.; Pulido, S.; Bermúdez-Muñoz, J.M.; Martínez-Vega, R.; Milo, M.; Varela-Nieto, I.; Rivera, T. The expression of oxidative stress response genes is modulated by a combination of resveratrol and N-acetylcysteine to ameliorate ototoxicity in the rat cochlea. Hear. Res. 2018, 358, 10–21. [Google Scholar] [CrossRef]
- Murillo-Cuesta, S.; García-Alcántara, F.; Vacas, E.; Sistiaga, J.A.; Camarero, G.; Varela-Nieto, I.; Rivera, T. Direct drug application to the round window: A comparative study of ototoxicity in rats. Otolaryngol. Head Neck Surg. 2009, 141, 584–590. [Google Scholar] [CrossRef]
- Le Prell, C.G. Investigational Medicinal Products for the Inner Ear: Review of Clinical Trial Characteristics in ClinicalTrials.gov. J. Am. Acad. Audiol. 2021, 32, 670–694. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G.; Ojano-Dirain, C.; Rudnick, E.W.; Nelson, M.A.; DeRemer, S.J.; Prieskorn, D.M.; Miller, J.M. Assessment of nutrient supplement to reduce gentamicin-induced ototoxicity. J. Assoc. Res. Otolaryngol. 2014, 15, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Dedeoğlu, S.; Ayral, M. Protective effect of ethyl pyruvate on amikacin-induced ototoxicity in rats. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2460–2466. [Google Scholar]
- Aksoy, F.; Dogan, R.; Ozturan, O.; Eren, S.B.; Veyseller, B.; Pektas, A.; Hüseyinbas, Ö. Protective effect of trimetazidine on amikacin-induced ototoxicity in rats. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 663–669. [Google Scholar] [CrossRef]
- Dirain, C.O.; Ng, M.; Milne-Davies, B.; Joseph, J.K.; Antonelli, P.J. Evaluation of Mitoquinone for Protecting Against Amikacin-Induced Ototoxicity in Guinea Pigs. Otol. Neurotol. 2018, 39, 111–118. [Google Scholar] [CrossRef]
- Fang, J.; Wu, H.; Zhang, J.; Mao, S.; Shi, H.; Yu, D.; Chen, Z.; Su, K.; Xing, Y.; Dong, H.; et al. A reduced form of nicotinamide riboside protects the cochlea against aminoglycoside-induced ototoxicity by SIRT1 activation. Biomed. Pharmacother. 2022, 150, 113071. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wu, H.; Jie, H.; Liang, M.; Yu, D.; Feng, Y.; Balasubramanian, K.; Zheng, G.; Yang, J.; He, J. XIAP inhibits gentamicin-induced hair cell damage and ototoxicity through the caspase-3/9 pathway. Biochem. Pharmacol. 2021, 186, 114513. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, Y.; Yu, H.; Li, W.; Wu, J.; Cai, C.; He, Y. Salvianolic acid B inhibits ototoxic drug-induced ototoxicity by suppression of the mitochondrial apoptosis pathway. J. Cell Mol. Med. 2020, 24, 6883–6897. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Baek, J.I.; Lee, K.Y.; Kim, U.K. Berberine chloride protects cochlear hair cells from aminoglycoside-induced ototoxicity by reducing the accumulation of mitochondrial reactive oxygen species. Free Radic. Biol. Med. 2023, 204, 177–183. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Liu, D.; Duan, X.; Zhang, Q.; Wang, D.; Zheng, Q.; Bai, X.; Lu, Z. Naringin attenuates cisplatin- and aminoglycoside-induced hair cell injury in the zebrafish lateral line via multiple pathways. J. Cell Mol. Med. 2021, 25, 975–989. [Google Scholar] [CrossRef]
- Hong, S.; Han, E.; Park, S.; Hyun, K.; Lee, Y.; Baek, H.W.; Kim, H.J.; Rah, Y.C.; Choi, J. Protective Effects of (-)-Butaclamol Against Gentamicin-Induced Ototoxicity: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2025, 26, 4201. [Google Scholar] [CrossRef]
- Zallocchi, M.; Vijayakumar, S.; Fleegel, J.; Batalkina, L.; Brunette, K.E.; Shukal, D.; Chen, Z.; Devuyst, O.; Liu, H.; He, D.Z.Z.; et al. Piplartine attenuates aminoglycoside-induced TRPV1 activity and protects from hearing loss in mice. Sci. Transl. Med. 2024, 16, eadn2140. [Google Scholar] [CrossRef]
- Gaffney, P.J.; Shetty, K.R.; Yuksel, S.; Kaul, V.F. Antioxidant Therapies in the Treatment of Aminoglycoside-Induced Ototoxicity: A Meta-Analysis. Laryngoscope 2025, 135, 1278–1286. [Google Scholar] [CrossRef]
- Lindeborg, M.M.; Jung, D.H.; Chan, D.K.; Mitnick, C.D. Prevention and management of hearing loss in patients receiving ototoxic medications. Bull. World Health Organ. 2022, 100, 789–796a. [Google Scholar] [CrossRef]
- Zhu, X.; Vasilyeva, O.N.; Kim, S.; Jacobson, M.; Romney, J.; Waterman, M.S.; Tuttle, D.; Frisina, R.D. Auditory efferent feedback system deficits precede age-related hearing loss: Contralateral suppression of otoacoustic emissions in mice. J. Comp. Neurol. 2007, 503, 593–604. [Google Scholar] [CrossRef]
| Treatment | HTDD20 (mg/kg) | HTDD50 (mg/kg) |
|---|---|---|
| AMI+VEH a | 797 ± 64 | 1006 ± 95 |
| AMI+GLUT(500) | 666 ± 60 (↓ 16%) | 945 ± 88 (↓ 6%) |
| AMI+MET(500) | 922 ± 78 # (↑ 16%) | 1141 ± 91 (↑ 13%) |
| One-way ANOVA | F(2;15) = 3.555 | F(2;15) = 1.201 |
| p = 0.0545 | p = 0.3283 | |
| AMI+FUR(30)+VEH a | 377 ± 58 | 428 ± 56 |
| AMI+FUR(30)+GLUT(500) | 336 ± 51 (↓ 11%) | 415 ± 56 (↓ 3%) |
| AMI+FUR(30)+MET(500) | 511 ± 65 (↑ 36%) | 701 ± 60 *,## (↑ 64%) |
| One-way ANOVA | F(2;15) = 2.466 | F(2;15) = 7.926 |
| p = 0.1186 | p = 0.0045 |
| Treatment | HTDD20 (mg/kg) | HTDD50 (mg/kg) |
|---|---|---|
| FUR+VEH a | 56.25 ± 4.89 | 63.24 ± 4.68 |
| FUR+GLUT(500) | 59.38 ± 4.92 (↑ 6%) | 62.90 ± 4.83 (↓ 0.5%) |
| FUR+MET(500) | 63.07 ± 5.02 (↑ 12%) | 77.77 ± 5.01 (↑ 23%) |
| One-way ANOVA | F(2;15) = 0.477 | F(2;15) = 3.074 |
| p = 0.6298 | p = 0.0761 | |
| FUR+AMI(500)+VEH a | 37.35 ± 4.10 | 46.73 ± 4.39 |
| FUR+AMI(500)+GLUT(500) | 33.37 ± 4.09 (↓ 11%) | 52.83 ± 4.79 (↑ 13%) |
| FUR+AMI(500)+MET(500) | 61.00 ± 4.72 **, ## (↑ 63%) | 77.94 ± 4.93 ***,## (↑ 67%) |
| One-way ANOVA | F(2;15) = 11.99 | F(2;15) = 12.34 |
| p = 0.0008 | p = 0.0007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadrożniak, M.; Szymański, M.; Luszczki, J.J. Effects of Methionine and Glutathione on Acute Ototoxicity Induced by Amikacin and Furosemide in an Animal Model of Hearing Threshold Decrease. Biomedicines 2025, 13, 1476. https://doi.org/10.3390/biomedicines13061476
Zadrożniak M, Szymański M, Luszczki JJ. Effects of Methionine and Glutathione on Acute Ototoxicity Induced by Amikacin and Furosemide in an Animal Model of Hearing Threshold Decrease. Biomedicines. 2025; 13(6):1476. https://doi.org/10.3390/biomedicines13061476
Chicago/Turabian StyleZadrożniak, Marek, Marcin Szymański, and Jarogniew J. Luszczki. 2025. "Effects of Methionine and Glutathione on Acute Ototoxicity Induced by Amikacin and Furosemide in an Animal Model of Hearing Threshold Decrease" Biomedicines 13, no. 6: 1476. https://doi.org/10.3390/biomedicines13061476
APA StyleZadrożniak, M., Szymański, M., & Luszczki, J. J. (2025). Effects of Methionine and Glutathione on Acute Ototoxicity Induced by Amikacin and Furosemide in an Animal Model of Hearing Threshold Decrease. Biomedicines, 13(6), 1476. https://doi.org/10.3390/biomedicines13061476







