Aberrant Development of Hippocampal GABAergic Neurons Arising from Hypothyroidism Contributes to Memory Deficits in Mice Through Maf Suppressing Mef2c
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mouse Model of Developmental Hypothyroidism
2.3. Assays for Serum Thyroid Hormones
2.4. Cell Culture
2.4.1. Primary Hippocampal Neuron Culture
2.4.2. HT22 Cell Line Culture
2.5. Novel Object Recognition (NOR) Test
2.6. Y-Maze Test
2.7. Dark Avoidance Experiment
2.8. Morris Water Maze (MWM) Test
2.9. Immunofluorescence
2.10. Quantitative Polymerase Chain Reaction (Q-PCR)
2.11. Western Blot
2.12. siRNA Knockdown and Plasmid Transfection
2.13. Quantification and Statistical Analysis
3. Results
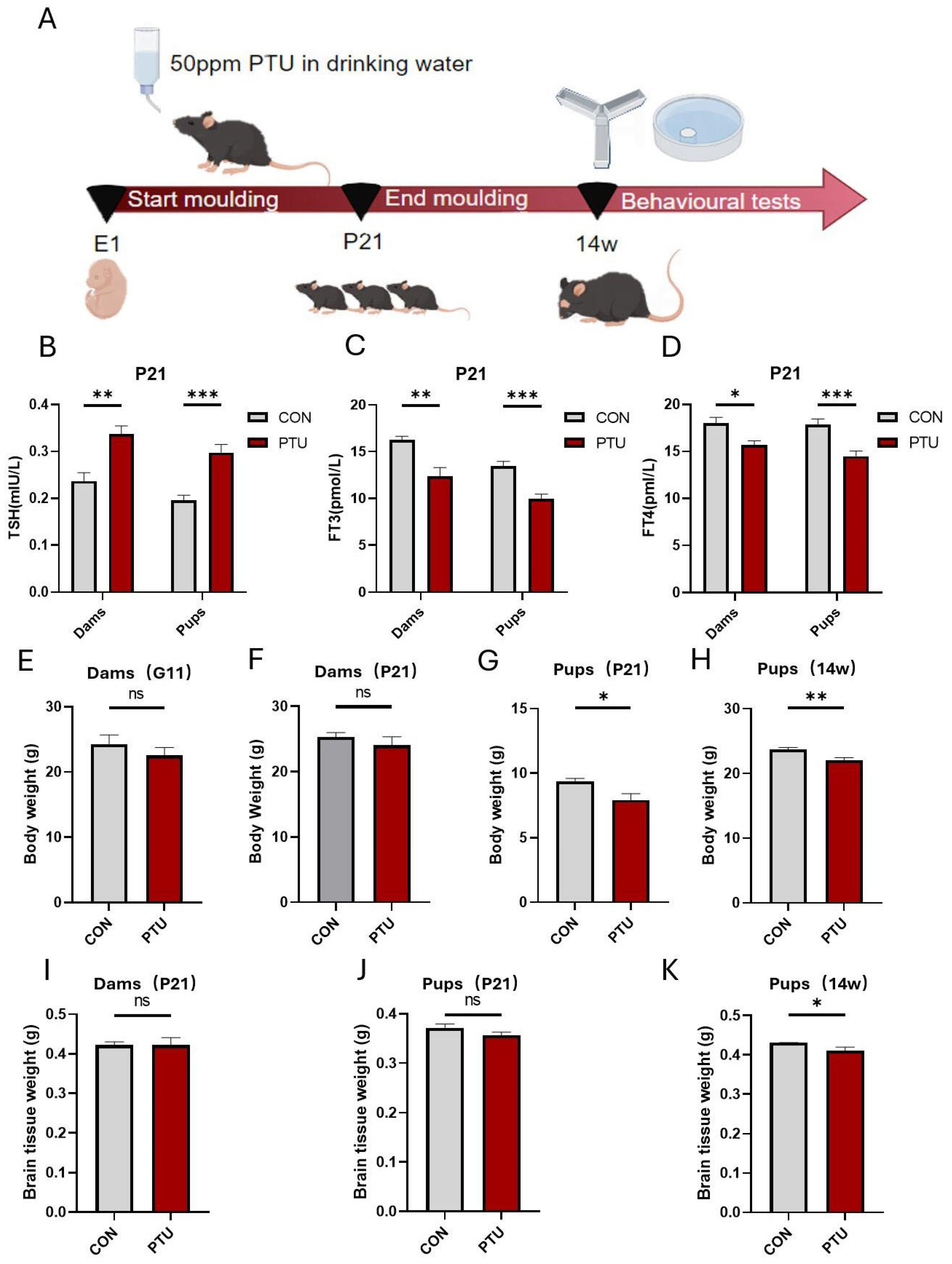
3.1. Generation and Characteristics of Developmental Hypothyroidism and Identification of Serum Hormone (FT4, FT3 and TSH) Levels in Offspring
3.2. Effect of Developmental Hypothyroidism on Cognitive and Memory Function in Offspring Mice
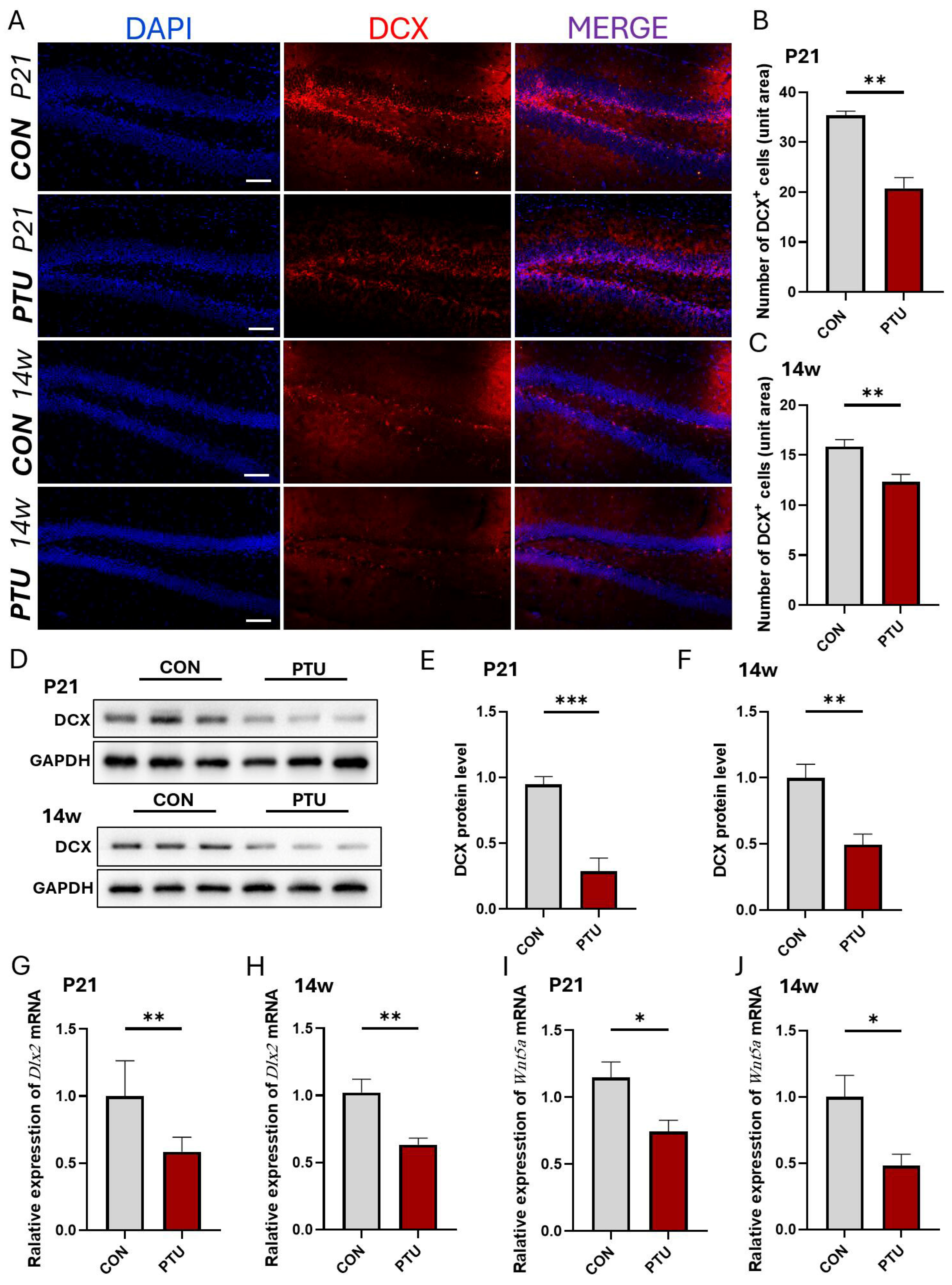
3.3. Influence of Developmental Hypothyroidism on Generation of Neuronal Precursor Cells in Offspring Hippocampus
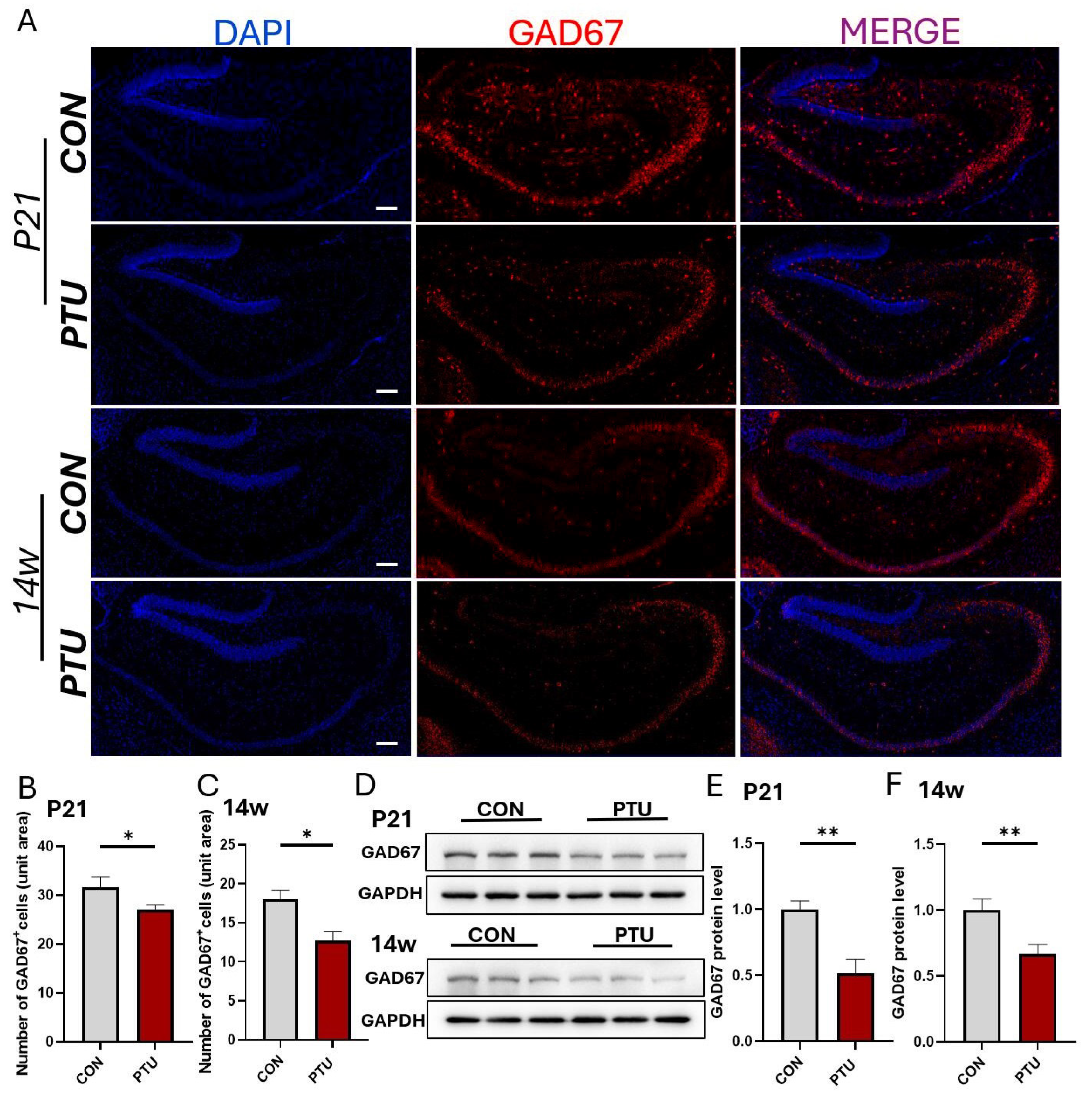
3.4. Preferential Changes in Hippocampal GABAergic Interneurons in Offspring of Hypothyroid Mice
3.5. Change in PV+ Neurons, a Subtype of GABA Interneurons, in Hippocampus of Hypothyroid Mouse Offspring
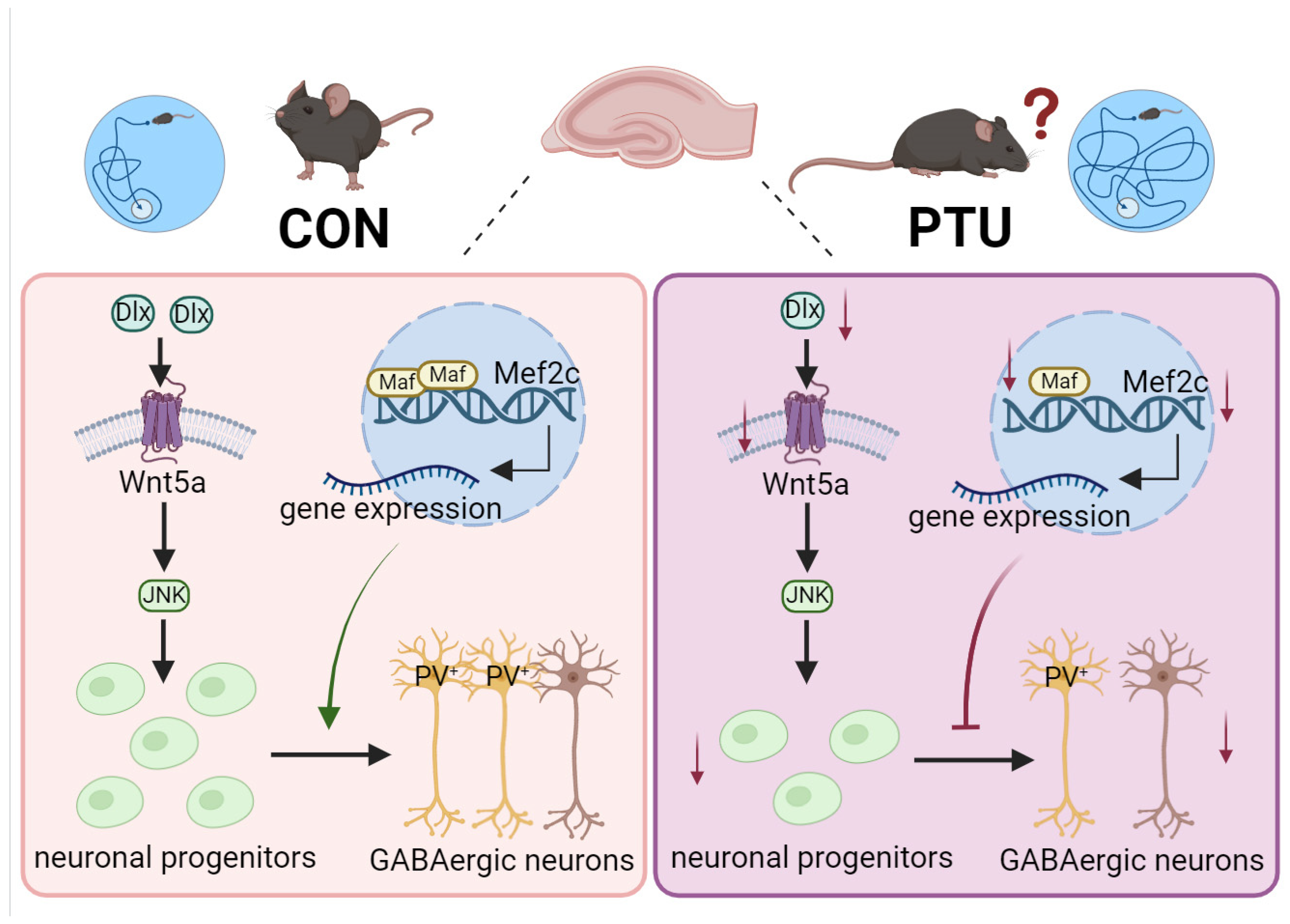
3.6. Possible Mechanism Underlying Cognitive and Memory Impairment in Offspring Arising from Abnormal Development of PV+ Interneurons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Teng, W.; Shan, Z.; Wang, S.; Li, J.; Zhu, L.; Zhou, J.; Mao, J.; Yu, X.; Li, J.; et al. The prevalence of thyroid disorders during early pregnancy in China: The benefits of universal screening in the first trimester of pregnancy. Eur. J. Endocrinol. 2011, 164, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, W.; Wang, Y.; Hou, Y.; Xu, H.; Gong, J.; Xi, Q.; Chen, J. Developmental iodine deficiency and hypothyroidism impair spatial memory in adolescent rat hippocampus: Involvement of CaMKII, calmodulin and calcineurin. Neurotox. Res. 2011, 19, 81–93. [Google Scholar] [CrossRef]
- Ghassabian, A.; Bongers-Schokking, J.J.; Henrichs, J.; Jaddoe, V.W.; Visser, T.J.; Visser, W.; de Muinck Keizer-Schrama, S.M.; Hooijkaas, H.; Steegers, E.A.; Hofman, A.; et al. Maternal thyroid function during pregnancy and behavioral problems in the offspring: The generation R study. Pediatr. Res. 2011, 69, 454–459. [Google Scholar] [CrossRef]
- Navarro, D.; Alvarado, M.; Navarrete, F.; Giner, M.; Obregon, M.J.; Manzanares, J.; Berbel, P. Gestational and early postnatal hypothyroidism alters VGluT1 and VGAT bouton distribution in the neocortex and hippocampus, and behavior in rats. Front. Neuroanat. 2015, 9, 9. [Google Scholar] [CrossRef]
- Jin, T.; Wang, R.; Peng, S.; Liu, X.; Zhang, H.; He, X.; Teng, W.; Teng, X. Developmental Hypothyroidism Influences the Development of the Entorhinal-Dentate Gyrus Pathway of Rat Offspring. Endocrinol. Metab. 2022, 37, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.; Guadano-Ferraz, A.; Morte, B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid 2003, 13, 1005–1012. [Google Scholar] [CrossRef]
- D’Souza, R.D.; Meier, A.M.; Bista, P.; Wang, Q.; Burkhalter, A. Recruitment of inhibition and excitation across mouse visual cortex depends on the hierarchy of interconnecting areas. eLife 2016, 5, e19332. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Mihalas, S.; Hirokawa, K.E.; Whitesell, J.D.; Choi, H.; Bernard, A.; Bohn, P.; Caldejon, S.; Casal, L.; Cho, A.; et al. Hierarchical organization of cortical and thalamic connectivity. Nature 2019, 575, 195–202. [Google Scholar] [CrossRef]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal GABAergic Inhibitory Interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- Yeh, C.Y.; Asrican, B.; Moss, J.; Quintanilla, L.J.; He, T.; Mao, X.; Casse, F.; Gebara, E.; Bao, H.; Lu, W.; et al. Mossy Cells Control Adult Neural Stem Cell Quiescence and Maintenance through a Dynamic Balance between Direct and Indirect Pathways. Neuron 2018, 99, 493–510 e494. [Google Scholar] [CrossRef]
- Liu, Z.; Neff, R.A.; Berg, D.K. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science 2006, 314, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, F.; Robain, O.; Jardin, L.; Ben-Ari, Y. Distribution of GABAergic neurons in late fetal and early postnatal rat hippocampus. Brain Res. Dev. Brain Res. 1989, 50, 177–187. [Google Scholar] [CrossRef]
- Alvarez, D.D.; Giacomini, D.; Yang, S.M.; Trinchero, M.F.; Temprana, S.G.; Buttner, K.A.; Beltramone, N.; Schinder, A.F. A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science 2016, 354, 459–465. [Google Scholar] [CrossRef]
- Ma, D.; Sun, C.; Manne, R.; Guo, T.; Bosc, C.; Barry, J.; Magliery, T.; Andrieux, A.; Li, H.; Gu, C. A cytoskeleton-membrane interaction conserved in fast-spiking neurons controls movement, emotion, and memory. Mol. Psychiatry 2023, 28, 3994–4010. [Google Scholar] [CrossRef] [PubMed]
- Pagano, J.; Landi, S.; Stefanoni, A.; Nardi, G.; Albanesi, M.; Bauer, H.F.; Pracucci, E.; Schon, M.; Ratto, G.M.; Boeckers, T.M.; et al. Shank3 deletion in PV neurons is associated with abnormal behaviors and neuronal functions that are rescued by increasing GABAergic signaling. Mol. Autism 2023, 14, 28. [Google Scholar] [CrossRef]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef]
- Yang, Y.; Cvekl, A. Large Maf Transcription Factors: Cousins of AP-1 Proteins and Important Regulators of Cellular Differentiation. Einstein J. Biol. Med. 2007, 23, 2–11. [Google Scholar] [CrossRef]
- Hong, E.; Yik, J.; Amanatullah, D.F.; Di Cesare, P.E.; Haudenschild, D.R. c-Maf Transcription Factor Regulates ADAMTS-12 Expression in Human Chondrogenic Cells. Cartilage 2013, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Pai, E.L.; Chen, J.; Fazel Darbandi, S.; Cho, F.S.; Chen, J.; Lindtner, S.; Chu, J.S.; Paz, J.T.; Vogt, D.; Paredes, M.F.; et al. Maf and Mafb control mouse pallial interneuron fate and maturation through neuropsychiatric disease gene regulation. eLife 2020, 9, e54903. [Google Scholar] [CrossRef]
- Allaway, K.C.; Gabitto, M.I.; Wapinski, O.; Saldi, G.; Wang, C.Y.; Bandler, R.C.; Wu, S.J.; Bonneau, R.; Fishell, G. Genetic and epigenetic coordination of cortical interneuron development. Nature 2021, 597, 693–697. [Google Scholar] [CrossRef]
- Harrington, A.J.; Raissi, A.; Rajkovich, K.; Berto, S.; Kumar, J.; Molinaro, G.; Raduazzo, J.; Guo, Y.; Loerwald, K.; Konopka, G.; et al. MEF2C regulates cortical inhibitory and excitatory synapses and behaviors relevant to neurodevelopmental disorders. eLife 2016, 5, e20059. [Google Scholar] [CrossRef]
- Cho, J.Y.; Rumschlag, J.A.; Tsvetkov, E.; Proper, D.S.; Lang, H.; Berto, S.; Assali, A.; Cowan, C.W. MEF2C Hypofunction in GABAergic Cells Alters Sociability and Prefrontal Cortex Inhibitory Synaptic Transmission in a Sex-Dependent Manner. Biol. Psychiatry Glob. Open Sci. 2024, 4, 100289. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Xu, H.; Dong, J.; Wei, W.; Wang, Y.; Shan, Z.; Teng, W.; Xi, Q.; Chen, J. Developmental iodine deficiency delays the maturation of newborn granule neurons associated with downregulation of p35 in postnatal rat hippocampus. Environ. Toxicol. 2014, 29, 847–855. [Google Scholar] [CrossRef]
- Sawin, S.; Brodish, P.; Carter, C.S.; Stanton, M.E.; Lau, C. Development of cholinergic neurons in rat brain regions: Dose-dependent effects of propylthiouracil-induced hypothyroidism. Neurotoxicol. Teratol. 1998, 20, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Dong, J.; Wang, Y.; Xu, H.; Wei, W.; Zhong, J.; Liu, W.; Xi, Q.; Chen, J. Developmental iodine deficiency and hypothyroidism impair neural development, up-regulate caveolin-1 and down-regulate synaptophysin in rat hippocampus. J. Neuroendocrinol. 2010, 22, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Modzelewski, K.L.; Law, A.C.; Walkey, A.J.; Pearce, E.N.; Bosch, N.A. Comparison of Propylthiouracil vs Methimazole for Thyroid Storm in Critically Ill Patients. JAMA Netw. Open 2023, 6, e238655. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Klootwijk, W.; Morvan Dubois, G.; Destree, O.; Darras, V.M.; Van der Geyten, S.; Demeneix, B.; Visser, T.J. Characterization of recombinant Xenopus laevis type I iodothyronine deiodinase: Substitution of a proline residue in the catalytic center by serine (Pro132Ser) restores sensitivity to 6-propyl-2-thiouracil. Endocrinology 2006, 147, 3519–3529. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, K.; Asai, M.; Hara, T. High-Fat Diet Enhances Working Memory in the Y-Maze Test in Male C57BL/6J Mice with Less Anxiety in the Elevated Plus Maze Test. Nutrients 2020, 12, 2036. [Google Scholar] [CrossRef]
- Lu, L.; Shi, Y.; Wei, B.; Li, W.; Yu, X.; Zhao, Y.; Yu, D.; Sun, M. YTHDF3 modulates the Cbln1 level by recruiting BTG2 and is implicated in the impaired cognition of prenatal hypoxia offspring. iScience 2024, 27, 108703. [Google Scholar] [CrossRef]
- Yu, X.; Guo, J.; Song, Y.; Wei, B.; Shi, Y.; Zhao, Y.; Zhao, Z.; Gao, Q.; Wang, B.; Sun, M. HDAC1/2/3-mediated downregulation of neurogranin is involved in cognitive impairment in offspring exposed to maternal subclinical hypothyroidism. FASEB J. 2024, 38, e23736. [Google Scholar] [CrossRef]
- Roque, P.S.; Thorn Perez, C.; Hooshmandi, M.; Wong, C.; Eslamizade, M.J.; Heshmati, S.; Brown, N.; Sharma, V.; Lister, K.C.; Goyon, V.M.; et al. Parvalbumin interneuron loss mediates repeated anesthesia-induced memory deficits in mice. J. Clin. Investig. 2023, 133, e159344. [Google Scholar] [CrossRef] [PubMed]
- Nucera, C.; Muzzi, P.; Tiveron, C.; Farsetti, A.; La Regina, F.; Foglio, B.; Shih, S.C.; Moretti, F.; Della Pietra, L.; Mancini, F.; et al. Maternal thyroid hormones are transcriptionally active during embryo-foetal development: Results from a novel transgenic mouse model. J. Cell Mol. Med. 2010, 14, 2417–2435. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Wang, L.; Kimura, M.; Abe, H.; Mizukami, S.; Yoshida, T.; Shibutani, M. Developmental hypothyroidism abolishes bilateral differences in sonic hedgehog gene control in the rat hippocampal dentate gyrus. Toxicol. Sci. 2015, 144, 128–137. [Google Scholar] [CrossRef]
- Amano, I.; Takatsuru, Y.; Khairinisa, M.A.; Kokubo, M.; Haijima, A.; Koibuchi, N. Effects of Mild Perinatal Hypothyroidism on Cognitive Function of Adult Male Offspring. Endocrinology 2018, 159, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Gine, E.; Echeverry-Alzate, V.; Lopez-Moreno, J.A.; Lopez-Jimenez, A.; Torres-Romero, D.; Perez-Castillo, A.; Santos, A. Developmentally-induced hypothyroidism alters the expression of Egr-1 and Arc genes and the sensitivity to cannabinoid agonists in the hippocampus. Possible implications for memory and learning. Mol. Cell Endocrinol. 2013, 365, 119–128. [Google Scholar] [CrossRef]
- Li, Y.; Long, S.; Yu, J.; Feng, J.; Meng, S.; Li, Y.; Zhao, L.; Yu, Y. Preoperative Sleep Deprivation Exacerbates Anesthesia/Surgery-induced Abnormal GABAergic Neurotransmission and Neuronal Damage in the Hippocampus in Aged Mice. Mol. Neurobiol. 2025, 1–14. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Turovskaya, M.V.; Kononov, A.V.; Zinchenko, V.P. Short-term episodes of hypoxia induce posthypoxic hyperexcitability and selective death of GABAergic hippocampal neurons. Exp. Neurol. 2013, 250, 1–7. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Plotnikov, E.Y.; Turovsky, E.A. Neuronal Calcium Sensor-1 Protects Cortical Neurons from Hyperexcitation and Ca(2+) Overload during Ischemia by Protecting the Population of GABAergic Neurons. Int. J. Mol. Sci. 2022, 23, 5675. [Google Scholar] [CrossRef]
- Ognjanovski, N.; Schaeffer, S.; Wu, J.; Mofakham, S.; Maruyama, D.; Zochowski, M.; Aton, S.J. Parvalbumin-expressing interneurons coordinate hippocampal network dynamics required for memory consolidation. Nat. Commun. 2017, 8, 15039. [Google Scholar] [CrossRef]
- Xia, F.; Richards, B.A.; Tran, M.M.; Josselyn, S.A.; Takehara-Nishiuchi, K.; Frankland, P.W. Parvalbumin-positive interneurons mediate neocortical-hippocampal interactions that are necessary for memory consolidation. eLife 2017, 6, e27868. [Google Scholar] [CrossRef]
- Kataoka, K. Multiple mechanisms and functions of maf transcription factors in the regulation of tissue-specific genes. J. Biochem. 2007, 141, 775–781. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Zeng, X.; Cai, Y.; Chen, H.; Yang, H. Aberrant Development of Hippocampal GABAergic Neurons Arising from Hypothyroidism Contributes to Memory Deficits in Mice Through Maf Suppressing Mef2c. Biomedicines 2025, 13, 1436. https://doi.org/10.3390/biomedicines13061436
Wu M, Zeng X, Cai Y, Chen H, Yang H. Aberrant Development of Hippocampal GABAergic Neurons Arising from Hypothyroidism Contributes to Memory Deficits in Mice Through Maf Suppressing Mef2c. Biomedicines. 2025; 13(6):1436. https://doi.org/10.3390/biomedicines13061436
Chicago/Turabian StyleWu, Mengyan, Xingdong Zeng, Yongle Cai, Haonan Chen, and Hao Yang. 2025. "Aberrant Development of Hippocampal GABAergic Neurons Arising from Hypothyroidism Contributes to Memory Deficits in Mice Through Maf Suppressing Mef2c" Biomedicines 13, no. 6: 1436. https://doi.org/10.3390/biomedicines13061436
APA StyleWu, M., Zeng, X., Cai, Y., Chen, H., & Yang, H. (2025). Aberrant Development of Hippocampal GABAergic Neurons Arising from Hypothyroidism Contributes to Memory Deficits in Mice Through Maf Suppressing Mef2c. Biomedicines, 13(6), 1436. https://doi.org/10.3390/biomedicines13061436





