3D Printing in Nasal Reconstruction: Application-Based Evidence on What Works, When, and Why
Abstract
1. Introduction
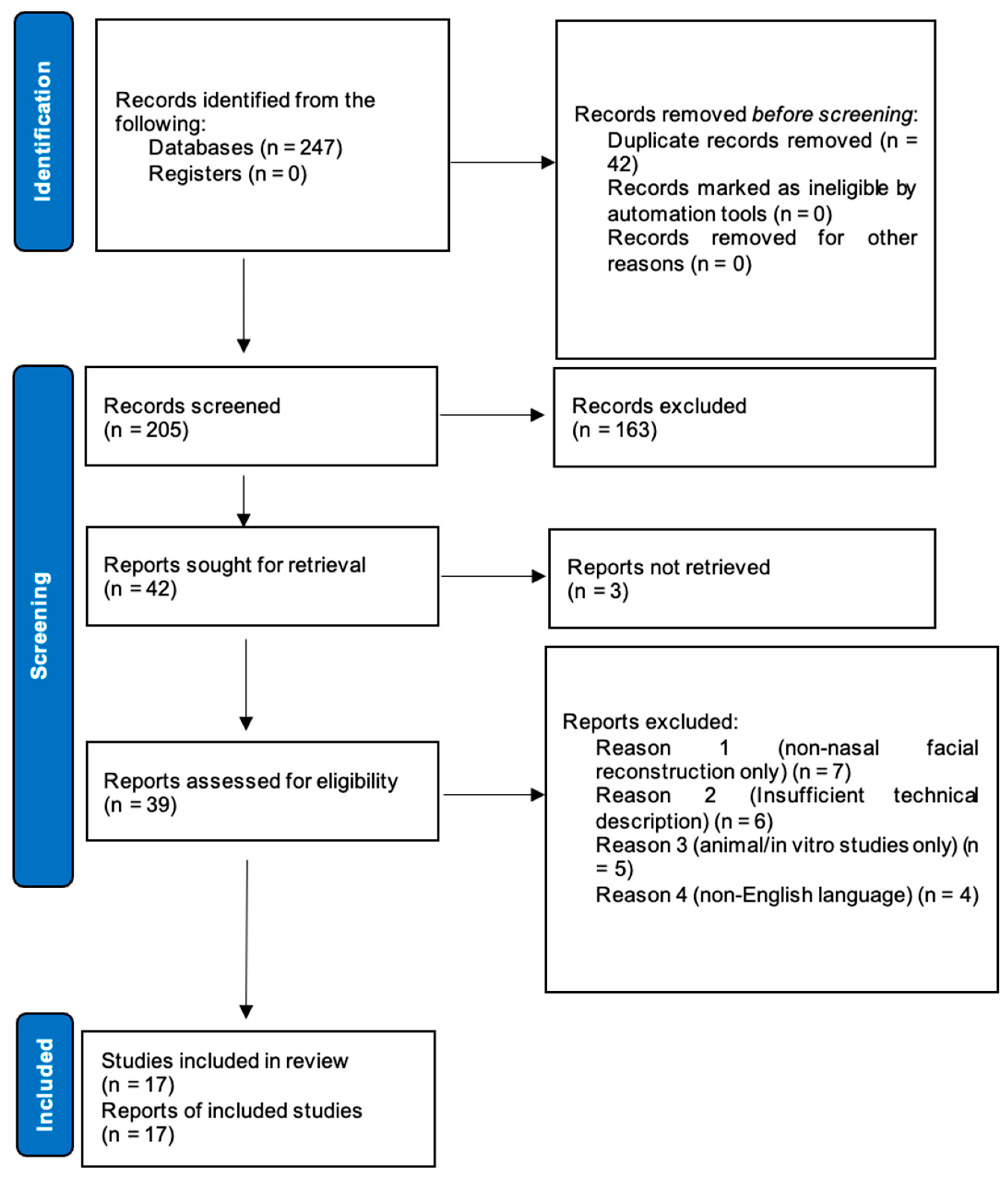
2. Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
- -
- Population: Patients undergoing nasal reconstruction.
- -
- Intervention: Application of 3D-printing technology in nasal reconstruction.
- -
- Comparator: Studies with or without comparison to conventional techniques.
- -
- Outcomes: Studies must report or discuss clinical, functional, or aesthetic outcomes, with explicit or inferred links to the two primary Level 1 problems—patient satisfaction and/or revision burden.
- -
- Study design: Primary research articles including case reports, case series, cohort studies, and clinical trials.
- -
- Language: Full text available in English.
- -
- Publication type: Peer-reviewed journal articles.
- -
- Review articles, editorials, letters, and conference abstracts.
- -
- Studies focusing exclusively on other facial reconstructions without nasal involvement.
- -
- Studies without clear description of the 3D-printing methodology.
- -
- Animal or in vitro studies without human application.
2.3. Study Selection Process
2.4. Data Extraction
- -
- Study characteristics (first author, publication year, study design, and sample size).
- -
- Patient demographics and clinical presentation.
- -
- 3D printing technique and materials.
- -
- Type of 3D-printed product (e.g., surgical guide, implant, and anatomical model).
- -
- Follow-up duration.
- -
- Clinical outcomes relevant to Level 1 (patient satisfaction scores and revision rates) and Level 2 (functional and aesthetic outcomes and complication rates).
- -
- Complications.
- -
- Comparison with conventional techniques when available.
2.5. Quality Assessment
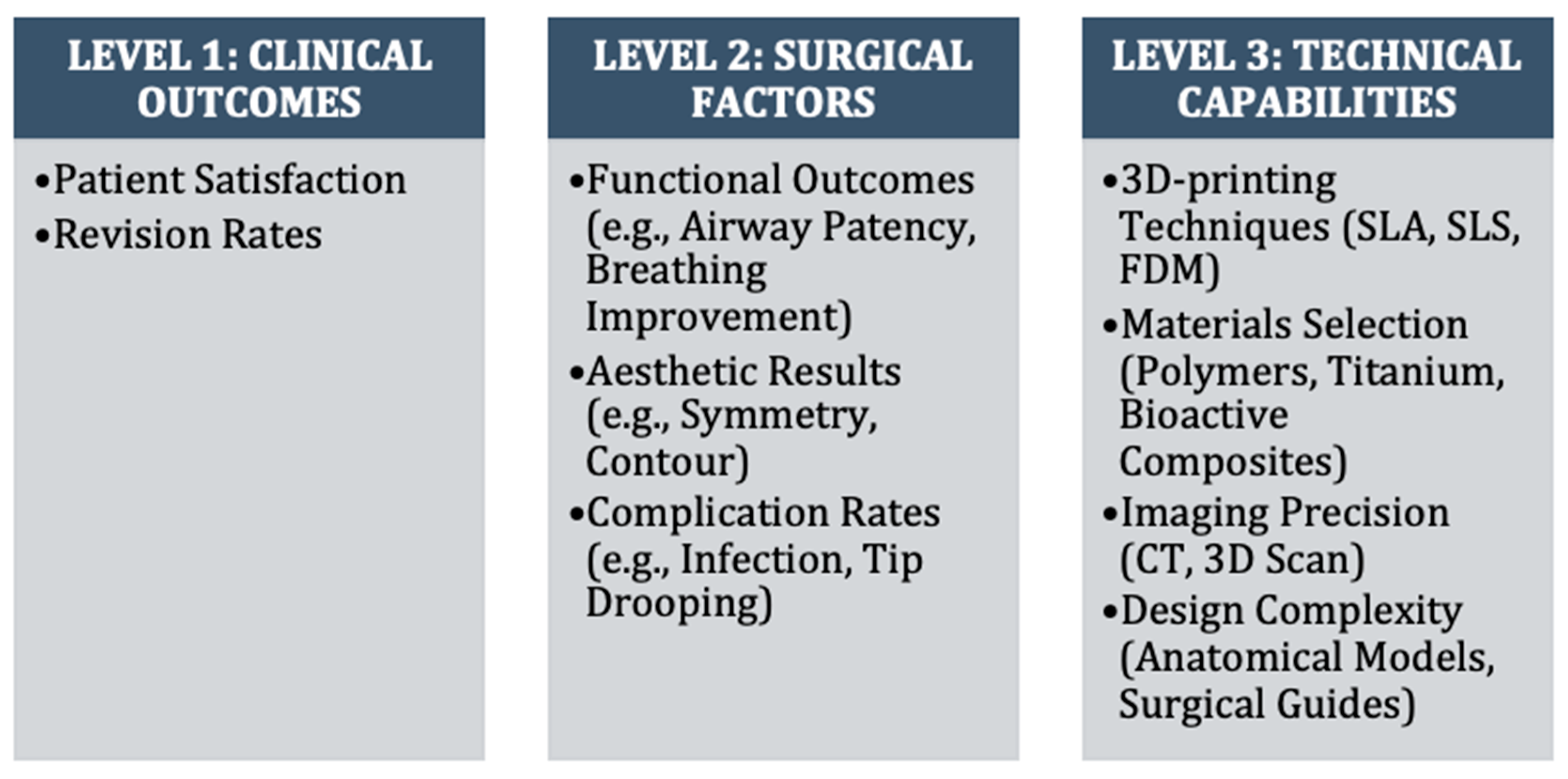
2.6. Data Synthesis
- -
- Level 1: Clinical outcomes (patient satisfaction scores, revision rates, functional outcomes, and complication rates).
- -
- Level 2: Intermediate factors (anatomical accuracy, structural stability, and graft integration).
- -
- Level 3: Technical aspects (3D printing methods, materials, and design approaches).
3. Importance of Precise Nasal Reconstruction
4. Advancements in 3D Printing Technology
5. Applications in Nasal Reconstruction
5.1. Preoperative Planning and Anatomical Models
5.1.1. Clinical Impact (Level 1): Limited
5.1.2. Surgical Advantages (Level 2)
5.1.3. Technical Requirements (Level 3)
5.1.4. Case Evidence
5.2. Comparative Analysis of Conventional vs. 3D Printing Approaches
5.2.1. Clinical Impact (Level 1): Stronger Evidence
5.2.2. Surgical Precision (Level 2)
5.2.3. Technical Specifications (Level 3)
5.3. Patient-Specific Implants and Frameworks
5.3.1. Clinical Impact (Level 1): Strongest Evidence
5.3.2. Structural Integration (Level 2)
5.3.3. Material and Design Requirements (Level 3)
5.4. Bioprinted Constructs and Tissue Engineering
5.4.1. Clinical Impact (Level 1): Minimal/Experimental
5.4.2. Biological Integration Challenges (Level 2)
5.4.3. Technical Development (Level 3)
5.4.4. Development Timeline
5.5. Auxiliary Applications
5.5.1. Custom Nasal Stents and Prosthetics (Level 1)
5.5.2. Digital Prosthesis Design (Level 2)
5.5.3. Material and Technical Requirements (Level 3)
5.6. Comparative Analysis Across Applications
6. Challenges and Considerations
6.1. Technological Challenges
- Challenge 1: Imaging and Scanning Limitations
- Challenge 2: Surgical Guide Precision
- Challenge 3: Material Limitations
- Challenge 4: Bioprinting Technical Constraints
6.2. Biological Challenges
- Challenge 1: Insufficient Vascularization
- Challenge 2: Material–Tissue Interface
- Challenge 3: Long-term Stability
6.3. Regulatory Challenges
- Challenge 1: Lack of Standardization
- Challenge 2: Regulatory Pathway Complexity
6.4. Ethical Considerations
- Challenge 1: Cost and Accessibility
- Challenge 2: Data Privacy and Consent
7. Future Directions
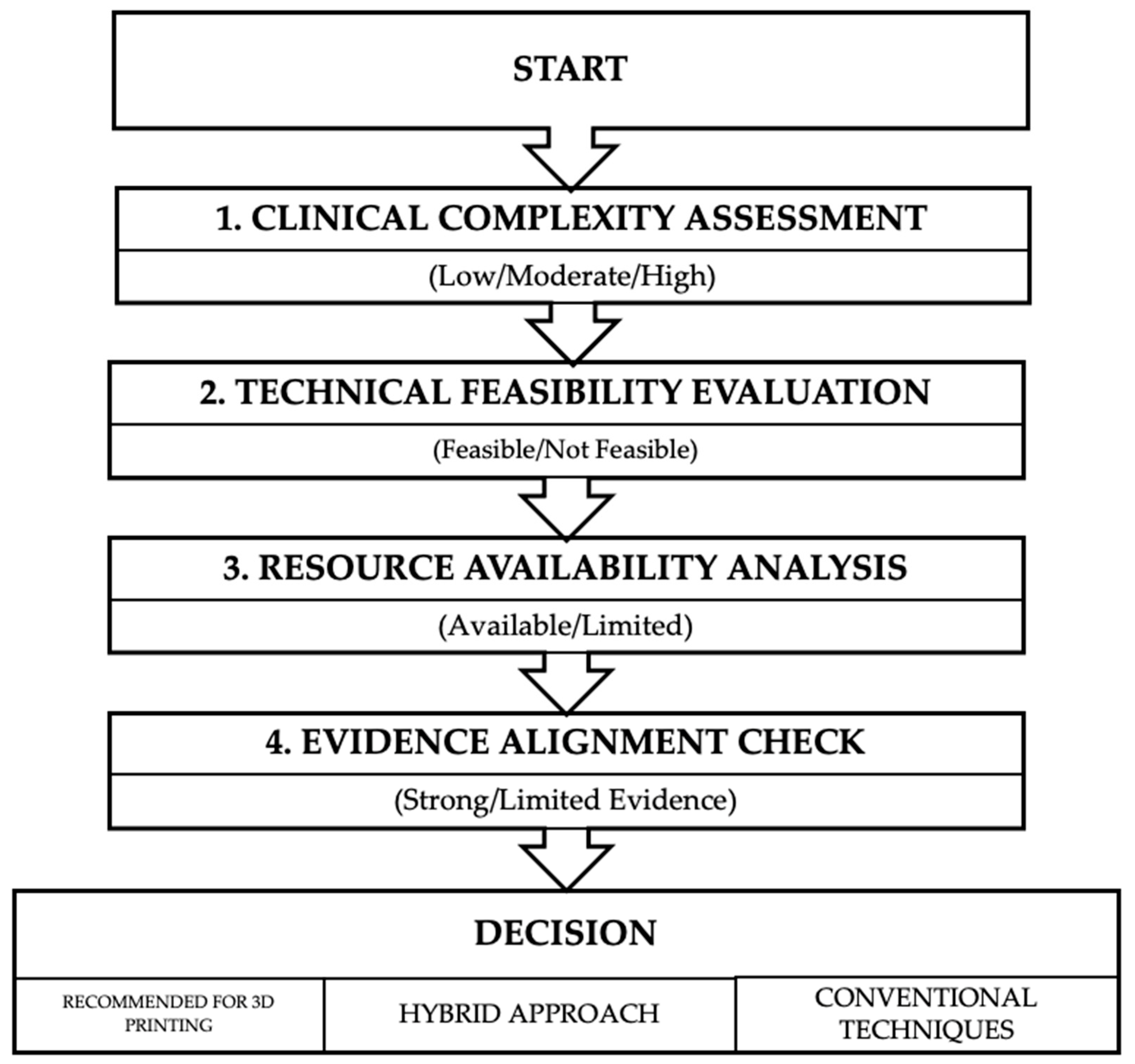
8. Clinical Guidelines and Recommendations
Hierarchical Analysis of Current Evidence
- Clinical Complexity Assessment: Categorizes cases as low, moderate, or high complexity based on specific criteria (defect size, location, tissue availability, and functional requirements).
- Technical Feasibility Evaluation: Determines whether the required 3D technologies are available and appropriate for the specific case.
- Resource Availability Analysis: Assesses whether the necessary expertise, equipment, materials, and time are accessible.
- Evidence Alignment Check: Critically examines whether the proposed application has supporting evidence for improving Level 1 clinical outcomes in the specific clinical scenario.
- Anatomical Models and Surgical Planning: Simple FDM-printed models with ±1 mm accuracy are typically sufficient for general surgical planning, while high-precision applications like osteotomy guides require SLA/SLS methods with ±0.1–0.2 mm accuracy. The additional cost and time for ultra-high-precision printing (±0.05mm) rarely provide proportional improvements in patient satisfaction or revision rates and should be reserved for exceptional cases.
- Patient-Specific Implants: For non-load-bearing aesthetic components, PCL and PLA materials with 50–70% infill density generally provide adequate structural support. More advanced composite materials with gradient properties are only clinically necessary for implants spanning functional junctions (e.g., between rigid and flexible nasal regions) or in cases of compromised vascularization.
- Surgical Guides: Simple cutting and positioning guides typically resolve 80–90% of the precision challenges in nasal reconstruction. Complex navigational systems with real-time tracking offer diminishing Level 1 returns in most routine cases and should be prioritized for cases with significant anatomical distortion or absent landmarks.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yalamanchili, H.; Sclafani, A.; Schaefer, S.; Presti, P. The Path of Nasal Reconstruction: From Ancient India to the Present. Facial Plast. Surg. 2008, 24, 003–010. [Google Scholar] [CrossRef] [PubMed]
- Sorta-Bilajac, I.; Muzur, A. The nose between ethics and aesthetics: Sushruta’s legacy. Otolaryngol. Neck Surg. 2007, 137, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Nikparto, N.; Yari, A.; Mehraban, S.H.; Bigdelou, M.; Asadi, A.; Darehdor, A.A.; Nezaminia, S.; Khani, M.; Hakim, L.K.; Eskandari, F.; et al. The current techniques in dorsal augmentation rhinoplasty: A comprehensive review. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 16. [Google Scholar] [CrossRef]
- Schonauer, F.; D’Alessio, M.; Cavaliere, A.; Razzano, S.; D’Angelo, D. Let’s Twist Again: Nasolabial Turnover Flap for Full-thickness Aesthetical Nasal Ala Reconstruction. Plast. Reconstr. Surg.-Glob. Open 2024, 12, e6128. [Google Scholar] [CrossRef]
- Agrawal, K.S.; Agrawal, M.K.; Mehta, V.; Shende, N.; Shrivastava, P.; Singla, S. Utilizing Auricular Composite Grafts for Reconstruction of Nasal Ala Defects in Fitzpatrick Type III–V Indian Noses: Techniques and Outcomes. Aesthetic Plast. Surg. 2024. [Google Scholar] [CrossRef]
- Rahmawan, Z.R.; Dhiparedja, R.; Wicaksono, B. The Use of the V-Y Cartilage Columellar Strut Graft and Precise Skin Contouring in Nasal Reconstructive Surgery in RSPAL Dr Ramelan Hospital: A Case Report. Int. J. Sci. Adv. 2024, 5, 504–507. [Google Scholar] [CrossRef]
- Fedok, F.G.; Lee Peng, G.; Tastan, E.; Robotti, E. The Use of Costal Cartilage in Rhinoplasty. Facial Plast. Surg. Clin. N. Am. 2024, 32, 565–583. [Google Scholar] [CrossRef]
- Strohl, M.; Sweeny, L. Advances in Midface Reconstruction. Facial Plast. Surg. Clin. N. Am. 2025, 33, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomidis, A.; Tatsis, D.; Tsemberlides, K.; Papadopoulos, G.; Alexoudi, V.-A.; Kyrgidis, A.; Vahtsevanos, K. Reconstruction of the entire maxillary in an irradiated patient, with an implant retained removable prosthesis, using implants in the zygomatic bone. Hell. Arch. Oral Maxillofac. Surg. 2024, 25, 101–108. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, L. The Effect of Functional Rhinoplasty on Quality of Life: A Systematic Review and Meta-Analysis. Aesthetic Plast. Surg. 2024, 48, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Hilger, P.A.; Webster, R.C.; Hilger, J.A.; Smith, R.C. A Computerized Nasal Analysis System. Arch. Otolaryngol.-Head. Neck Surg. 1983, 109, 653–661. [Google Scholar] [CrossRef]
- Farook, T.H.; Jamayet, N.B.; Abdullah, J.Y.; Rajion, Z.A.; Alam, M.K. A systematic review of the computerized tools and digital techniques applied to fabricate nasal, auricular, orbital and ocular prostheses for facial defect rehabilitation. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 268–277. [Google Scholar] [CrossRef]
- Toriumi, D.M.; Dixon, T.K. Assessment of rhinoplasty techniques by overlay of before-and-after 3D images. Facial Plast. Surg. Clin. N. Am. 2011, 19, 711–7123. [Google Scholar] [CrossRef]
- Slavin, B.V.; Ehlen, Q.T.; Costello, J.P.; Nayak, V.V.; Bonfante, E.A.; Jalkh, E.B.B.; Runyan, C.M.; Witek, L.; Coelho, P.G. 3D Printing Applications for Craniomaxillofacial Reconstruction: A Sweeping Review. ACS Biomater. Sci. Eng. 2023, 9, 6586–6609. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Liu, H.; Liu, J.; Li, R.; Zhu, X.; Ren, M.; Wang, M.; Liu, Y.; Li, Y.; et al. Three-Dimensional Printing Strategies for Irregularly Shaped Cartilage Tissue Engineering: Current State and Challenges. Front. Bioeng. Biotechnol. 2021, 9, 777039. [Google Scholar] [CrossRef]
- Zhou, Y.; Grayson, W. Three-dimensional printing of scaffolds for facial reconstruction. MRS Bull. 2022, 47, 91–97. [Google Scholar] [CrossRef]
- Kauke-Navarro, M.; Knoedler, L.; Knoedler, S.; Deniz, C.; Stucki, L.; Safi, A.F. Balancing beauty and science: A review of facial implant materials in craniofacial surgery. Front. Surg. 2024, 11, 1348140. [Google Scholar] [CrossRef]
- Generalova, A.N.; Vikhrov, A.A.; Prostyakova, A.I.; Apresyan, S.V.; Stepanov, A.G.; Myasoedov, M.S.; Oleinikov, V.A. Polymers in 3D printing of external maxillofacial prostheses and in their retention systems. Int. J. Pharm. 2024, 657, 124181. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.C.; Adesida, A.B. Tissue Engineering Nasal Cartilage Grafts with Three-Dimensional Printing: A Comprehensive Review. Tissue Eng. Part B Rev, 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Fathi, M.; Barar, J.; Omidi, Y. Hydrogel-based scaffolds for bone and cartilage tissue engineering and regeneration. React. Funct. Polym. 2022, 177, 105313. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, S.; Ravichandran, D.; Ramanathan, A.; Sobczak, M.T.; Sacco, A.F.; Patil, D.; Thummalapalli, S.V.; Pulido, T.V.; Lancaster, J.N.; et al. 3D-Printed Polymeric Biomaterials for Health Applications. Adv. Healthc. Mater. 2025, 14, 2402571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, M.; Wu, R.; Guo, J.; Sun, A.; Li, Z.; Ye, R.; Xu, G.; Cheng, Y. From materials to clinical use: Advances in 3D-printed scaffolds for cartilage tissue engineering. Phys. Chem. Chem. Phys. 2023, 25, 24244–24263. [Google Scholar] [CrossRef]
- Nuseir, A.; Hatamleh, M.M.; Alnazzawi, A.; Al-Rabab’ah, M.; Kamel, B.; Jaradat, E. Direct 3D Printing of Flexible Nasal Prosthesis: Optimized Digital Workflow from Scan to Fit. J. Prosthodont. 2019, 28, 10–14. [Google Scholar] [CrossRef]
- Qassemyar, Q.; Assouly, N.; Madar, Y.; Temam, S.; Kolb, F. Total nasal reconstruction with 3D custom made porous titanium prosthesis and free thoracodorsal artery perforator flap: A case report. Microsurgery 2018, 38, 567–571. [Google Scholar] [CrossRef]
- Boyer, C.J.; Woerner, J.E.; Galea, C.; Gatlin, C.A.; Ghali, G.E.; Mills, D.K.; Weisman, J.A.; McGee, D.J.; Alexander, J.S. Personalized Bioactive Nasal Supports for Postoperative Cleft Rhinoplasty. J. Oral Maxillofac. Surg. 2018, 76, 1562.e1–1562.e5. [Google Scholar] [CrossRef]
- Zarrabi, S.; Welch, M.; Neary, J.; Kim, B.J. A Novel Approach for Total Nasal Reconstruction. J. Oral Maxillofac. Surg. 2019, 77, 1073.e1–1073.e11. [Google Scholar] [CrossRef]
- Borghi, A.; Ruggiero, F.; Tenhagen, M.; Schievano, S.; Ponniah, A.; Dunaway, D.; O’Hara, J.; Ong, J.; Britto, J.A. Design and manufacturing of a patient-specific nasal implant for congenital arhinia: Case report. JPRAS Open 2019, 21, 28–34. [Google Scholar] [CrossRef]
- Yen, C.I.; Zelken, J.A.; Chang, C.S.; Lo, L.-J.; Yang, J.-Y.; Chuang, S.-S.; Araniego, C.A.; Hsiao, Y.-C. Computer-aided design and three-dimensional printing improves symmetry in heminasal reconstruction outcomes. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1198–1206. [Google Scholar] [CrossRef]
- Jung, J.W.; Ha, D.; Kim, B.Y.; Seo, B.F.; Han, H.H.; Kim, D.H.; Rhie, J.; Kim, S.W.; Cho, D. Nasal Reconstruction Using a Customized Three-Dimensional–Printed Stent for Congenital Arhinia: Three-Year Follow-up. Laryngoscope 2019, 129, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.L.; Seelaus, R.; Robinson, B.R. Subtotal Nasal Reconstruction Using a Custom 3-Dimensional Porous Polyethylene Construct. Plast. Reconstr. Surg.-Glob. Open 2019, 7, e2568. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choi, J.Y. Surgical outcomes and complications of septal extension graft supported by 3D printed polycaprolactone plate. Laryngoscope 2020, 130, 1680–1685. [Google Scholar] [CrossRef]
- Yi, H.G.; Choi, Y.J.; Jung, J.W.; Jang, J.; Song, T.-H.; Chae, S.; Ahn, M.; Choi, T.H.; Rhie, J.-W.; Cho, D.-W. Three-dimensional printing of a patient-specific engineered nasal cartilage for augmentative rhinoplasty. J. Tissue Eng. 2019, 10, 2041731418824797. [Google Scholar] [CrossRef]
- Jung, Y.G.; Park, H.; Seo, J. Patient-Specific 3-Dimensional Printed Models for Planning Nasal Osteotomy to Correct Nasal Deformities Due to Trauma. OTO Open 2020, 4, 2473974X20924342. [Google Scholar] [CrossRef]
- Ahn, T.H.; Heo, C.Y.; Ahn, K.C. A compound osteocartilaginous graft with polycaprolactone (PCL) mesh in Asian rhinoplasty. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, e6–e7. [Google Scholar] [CrossRef]
- Salati, V.; Reinhard, A.; Broome, M. Three-Dimensional Printed Nasal Prostheses After Oncologic Rhinectomies: Workflow and Patients’ Satisfaction. J. Craniofacial Surg. 2021, 32, 2297–2300. [Google Scholar] [CrossRef]
- Brower, J.P.; Crozier, J.W.; McIntire, D.R.T.; Boyajian, M.K.; Woo, A.S. Reconstruction of a Hemirhinectomy Defect Using a Three-Dimensional Printed Custom Soft Tissue Cutting Guide. J. Craniofacial Surg. 2021, 32, e51–e52. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, H.B.; Park, D.H.; Mun, S.J. A case of nasal septal gossypiboma removal and mucosal defect reconstruction. J. Cosmet. Med. 2022, 6, 95–98. [Google Scholar] [CrossRef]
- Dupret-Bories, A.; Chabrillac, E.; Poissonnet, V.; Nolens, G.; Henriet, V.; Bouakaz, I.; Sarini, J.; Grossin, D.; Riviere, L.-D.; Vergez, S.; et al. Total nasal reconstruction in irradiated tissue, a new hope. Res. Sq. 2023, preprint. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.C.; Choi, J.W.; Kim, D.H. Efficacy and feasibility of a forehead flap surgical guide for nasal reconstruction. J. Cranio-Maxillofac. Surg. 2024, 52, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Rathee, M.; Divakar, S.; Singla, S.; Tomar, S.S. Fabrication of three-dimensionally printed polylactic acid nasal stent prosthesis for postnasal reconstruction using extraoral scanning and photogrammetry techniques: A report on two patients. J. Prosthet. Dent. 2024, 132, 840.e1–840.e6. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kurose, M.; Dehari, H.; Takashima, R.; Okuni, T.; Yorozu, A.; Tokura, T.-A.; Nakai, H.; Miyazaki, A.; Takano, K. Chondrosarcoma of the maxilla resected using anterior segmental maxillary osteotomy and an open rhinoplasty approach through a 3d-printed bone model: A case report and literature review. Acta Oto-Laryngol. Case Rep. 2024, 9, 68–75. [Google Scholar] [CrossRef]
- Graviero, G.; Guastini, L.; Mora, R.; Salzano, G.; Salzano, F.A. The role of three-dimensional CT in the evaluation of nasal structures and anomalies. Eur. Arch. Otorhinolaryngol. 2011, 268, 1163–1167. [Google Scholar] [CrossRef]
- Xie, K.; Zhu, Y.M. Interactive surgery simulation for the nose augmentation using CT data. Neural Comput. Appl. 2010, 19, 61–65. [Google Scholar] [CrossRef]
- Rhee, J.S. Role of Virtual Surgery in Preoperative Planning: Assessing the Individual Components of Functional Nasal Airway Surgery. Arch. Facial Plast. Surg. 2012, 14, 354. [Google Scholar] [CrossRef]
- Burgos, M.A.; Sanmiguel-Rojas, E.; Del Pino, C.; Sevilla-García, M.A.; Esteban-Ortega, F. New CFD tools to evaluate nasal airflow. Eur. Arch. Otorhinolaryngol. 2017, 274, 3121–3128. [Google Scholar] [CrossRef]
- Peters, F.; Mücke, M.; Möhlhenrich, S.C.; Bock, A.; Stromps, J.-P.; Kniha, K.; Hölzle, F.; Modabber, A. Esthetic outcome after nasal reconstruction with paramedian forehead flap and bilobed flap. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 740–746. [Google Scholar] [CrossRef]
- Tokgöz, E.; Carro, M.A. Cosmetic and Reconstructive Facial Plastic Surgery Related Simulation and Optimization Efforts. In Cosmetic and Reconstructive Facial Plastic Surgery; Springer Nature: Cham, Switzerland, 2023; pp. 231–256. [Google Scholar] [CrossRef]
- Tanveer, W.; Ridwan-Pramana, A.; Molinero-Mourelle, P.; Koolstra, J.H.; Forouzanfar, T. Systematic Review of Clinical Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Nasal Prostheses. Int. J. Environ. Res. Public Health 2021, 18, 3756. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, M.J.; Kang, M.K.; Kim, S.C.; Jeong, W.S.; Kim, D.H.; Lee, T.H.M.; Koh, K.S.M. Clinical Application of a Patient-Specific, Three-Dimensional Printing Guide Based on Computer Simulation for Rhinoplasty. Plast. Reconstr. Surg. 2020, 145, 365–374. [Google Scholar] [CrossRef]
- Willaert, R.V.; Opdenakker, Y.; Sun, Y.; Politis, C.; Vermeersch, H. New Technologies in Rhinoplasty: A Comprehensive Workflow for Computer-assisted Planning and Execution. Plast. Reconstr. Surg.-Glob. Open 2019, 7, e2121. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.R.; Schreiber, J.E.; Patel, A.; Tepper, O.M. 3D Printed Surgical Guides Applied in Rhinoplasty to Help Obtain Ideal Nasal Profile. Aesthetic Plast. Surg. 2021, 45, 2852–2859. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jeong, W.S.; Ock, J.; Lee, S.; Kim, N.; Choi, J.W. The Feasibility of Computer Simulations and 3-Dimensional–Printed Resection Guides for Skin Cancer Resection. J. Craniofacial Surg. 2023, 34, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bai, X.; Li, Z.; Liu, Q.; Yang, M.; Wang, X.; Lu, L. Improvement of Aesthetic and Nasal Airway in Patients With Cleft Lip Nasal Deformities: Rhinoplasty With Septal Cartilage Graft and Septoplasty. Cleft Palate Craniofacial J. 2018, 55, 554–561. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, I.H.; Yun, W.S.; Shim, J.-H.; Choi, D.; Hwang, S.H.; Kim, S.W. Long-term efficacy and safety of 3D printed implant in patients with nasal septal deformities. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 1943–1950. [Google Scholar] [CrossRef]
- Sakellaris, N.I.; Sakellari, E.I.; Kanaan, Y.; Khalil, H.; Stavrakas, M. Added value of 3D printing technology in the manufacturing of customised septal buttons: A scoping review of the literature. Am. J. Otolaryngol. 2023, 44, 103916. [Google Scholar] [CrossRef]
- Otero-Rivas, M.M.; González-Sixto, B.; Alonso-Alonso, T.; Pérez-Bustillo, A.; Valladares-Narganes, L.M.; Rodríguez-Prieto, M.Á. Titanium mesh in reconstructive surgery of the nasal pyramid. Follow-up of our 11 initial cases. Int. J. Dermatol. 2015, 54, 961–965. [Google Scholar] [CrossRef]
- Bakshi, I.; Singh Rawat, H.; Singh, S. Exploring the Impact of 3D Printing on Advancing Reconstructive Surgery: A Comprehensive Review. Int. J. Multidiscip. Res. 2024, 6, 14675. [Google Scholar] [CrossRef]
- Lan, X.; Liang, Y.; Erkut, E.J.N.; Kunze, M.; Mulet-Sierra, A.; Gong, T.; Osswald, M.; Ansari, K.; Seikaly, H.; Boluk, Y.; et al. Bioprinting of human nasoseptal chondrocytes-laden collagen hydrogel for cartilage tissue engineering. FASEB J. 2021, 35, e21191. [Google Scholar] [CrossRef]
- Lan, X.; Boluk, Y.; Adesida, A.B. 3D Bioprinting of Hyaline Cartilage Using Nasal Chondrocytes. Ann. Biomed. Eng. 2024, 52, 1816–1834. [Google Scholar] [CrossRef]
- Zuo, K.J.; Wilkes, G.H. Clinical Outcomes of Osseointegrated Prosthetic Auricular Reconstruction in Patients With a Compromised Ipsilateral Temporoparietal Fascial Flap. J. Craniofacial Surg. 2016, 27, 44–50. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94 (Suppl. 9), 153S–157S. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Meng, Q.; Xie, E.; Li, K.; Hu, J.; Chen, Q.; Li, J.; Han, F. Engineered biomimetic micro/nano-materials for tissue regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1205792. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.B.; Sah, R.L.; Masuda, K.; Watson, D. Human Septal Cartilage Tissue Engineering: Current Methodologies and Future Directions. Bioengineering 2024, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Li, T.; Wang, H.; Chen, Y. Three-Dimensional Printing of Personalized Nasal Stents for Patients With Cleft Lip. Cleft Palate Craniofacial J. 2019, 56, 521–524. [Google Scholar] [CrossRef]
- Zahid, M.J.; Mavani, P.; Awuah, W.A.; Alabdulrahman, M.; Punukollu, R.; Kundu, A.; Mago, A.; Maher, K.; Adebusoye, F.T.; Khan, T.N. Sculpting the future: A narrative review of 3D printing in plastic surgery and prosthetic devices. Health Sci. Rep. 2024, 7, e2205. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Al-Sabah, A.; Gardiner, M.D.; Combellack, E.; Hawkins, K.; Whitaker, I.S. 3D bioprinting for reconstructive surgery: Principles, applications and challenges. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1155–1170. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; An, Y. Application of digital technology in nasal reconstruction. Chin. J. Plast. Reconstr. Surg. 2021, 3, 204–208. [Google Scholar] [CrossRef]
- Vercruyssen, M.; Hultin, M.; Van Assche, N.; Svensson, K.; Naert, I.; Quirynen, M. Guided surgery: Accuracy and efficacy. Periodontol. 2000 2014, 66, 228–246. [Google Scholar] [CrossRef]
- Afshari, A.; Shahmohammadi, R.; Mosaddad, S.A.; Pesteei, O.; Hajmohammadi, E.; Rahbar, M.; Alam, M.; Abbasi, K. Free-Hand versus Surgical Guide Implant Placement. Adv. Mater. Sci. Eng. 2022, 2022, 6491134. [Google Scholar] [CrossRef]
- Zeid, N.B.; Arias, E.; Alkureishi, L.W.T. 3D Printing in Craniofacial Surgery. Plast. Aesthetic Res. 2024, 11, 34. [Google Scholar] [CrossRef]
- Thangadurai, M.; Srinivasan, S.S.; Sekar, M.P.; Sethuraman, S.; Sundaramurthi, D. Emerging perspectives on 3D printed bioreactors for clinical translation of engineered and bioprinted tissue constructs. J. Mater. Chem. B. 2024, 12, 350–381. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.; Ferguson, S.J.; Shea, K. Design and 3D Printing of Hierarchical Tissue Engineering Scaffolds Based on Mechanics and Biology Perspectives. In Proceedings of the ASME 2016 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, Charlotte, NC, USA, 21–24 August 2016; p. V007T06A002. [Google Scholar] [CrossRef]
- Zhao, X.; Li, N.; Zhang, Z.; Hong, J.; Zhang, X.; Hao, Y.; Wang, J.; Xie, Q.; Zhang, Y.; Li, H.; et al. Beyond hype: Unveiling the Real challenges in clinical translation of 3D printed bone scaffolds and the fresh prospects of bioprinted organoids. J. Nanobiotechnol. 2024, 22, 500. [Google Scholar] [CrossRef]
- Alam, M.I.; Kashyap, S.; Balaji, P.G.; Yadav, A.K.; Flora, S.J.S. 3D-Printed Medical Implants: Recent Trends and Challenges. Biomed. Mater. Devices 2024. [Google Scholar] [CrossRef]
- Van Daal, M.; De Kanter, A.F.J.; Bredenoord, A.L.; De Graeff, N. Personalized 3D printed scaffolds: The ethical aspects. New Biotechnol. 2023, 78, 116–122. [Google Scholar] [CrossRef]
- Meng, M.; Wang, J.; Huang, H.; Liu, X.; Zhang, J.; Li, Z. 3D printing metal implants in orthopedic surgery: Methods, applications and future prospects. J. Orthop. Transl. 2023, 42, 94–112. [Google Scholar] [CrossRef]
- Pettersson, A.B.V.; Ballardini, R.M.; Mimler, M.; Li, P.; Salmi, M.; Minssen, T.; Gibson, I.; Mäkitie, A. Legal issues and underexplored data protection in medical 3D printing: A scoping review. Front. Bioeng. Biotechnol. 2023, 11, 1102780. [Google Scholar] [CrossRef]
- Gilbert, F.; O’Connell, C.D.; Mladenovska, T.; Dodds, S. Print Me an Organ? Ethical and Regulatory Issues Emerging from 3D Bioprinting in Medicine. Sci. Eng. Ethics 2018, 24, 73–91. [Google Scholar] [CrossRef]
- Kalidindi, S. The role of three-dimensional (3D) printing in plastic and reconstructive surgery: Innovations and applications. Eur. J. Plast. Surg. 2024, 47, 96. [Google Scholar] [CrossRef]
- Cao, Y.; Sang, S.; An, Y.; Xiang, C.; Li, Y.; Zhen, Y. Progress of 3D Printing Techniques for Nasal Cartilage Regeneration. Aesthetic Plast. Surg. 2022, 46, 947–964. [Google Scholar] [CrossRef]
- Lane, J.C.; Black, J.S. Modeling Medical Education: The Impact of Three-Dimensional Printed Models on Medical Student Education in Plastic Surgery. J. Craniofacial Surg. 2020, 31, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Spray, T.; Austin, E.H.; Yun, T.J.; Van Arsdell, G.S. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J. Thorac. Cardiovasc. Surg. 2017, 153, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Miyaji, K.; Watanabe, R.; Suzuki, T.; Matoba, K.; Nakazono, A.; Nakamaru, Y.; Konno, A.; Psaltis, A.J.; Abe, T.; et al. Repetitive simulation training with novel 3D-printed sinus models for functional endoscopic sinus surgeries. Laryngoscope Investig. Otolaryngol. 2022, 7, 943–954. [Google Scholar] [CrossRef] [PubMed]
| Technique | Technical Specifications | Clinical Applications | Evidence Level | Clinical Outcomes (Level 1) | Selection Criteria | Resource Requirements |
|---|---|---|---|---|---|---|
| Stereolithography (SLA) | Resolution: 25–100 μm Materials: Photopolymer resins Accuracy: ±0.1 mm Process time: 3–12 h | Surgical guides for osteotomy Patient-specific implant molds Anatomical models for complex defects Template creation for forehead flaps | ★★★ (surgical guides) ★★☆ (implant molds) ★★★ (anatomical models) | Improved symmetry (Yen et al.) Reduced operative time by 20–30% Improved precision in structural framework | When dimensional accuracy <0.2 mm is required For complex osteotomies where precision directly impacts functional outcome When photorealistic models are needed for patient education | High (USD 3000–10,000/unit) Specialized equipment Post-processing required Technical expertise needed |
| Selective Laser Sintering (SLS) | Resolution: 80–120 μm Materials: Nylon, metals, ceramics Accuracy: ±0.3 mm Process time: 5–15 h | Load-bearing implants Patient-specific titanium frameworks Porous scaffolds for tissue integration | ★★☆ (metal frameworks) ★☆☆ (porous scaffolds) | Long-term stability in total reconstructions (Qassemyar et al.) Reduced donor site morbidity Success in irradiated fields (Dupret-Bories) | For structural components requiring mechanical strength >40 MPa When conventional techniques have failed For subtotal/total nasal reconstruction When donor site morbidity must be minimized | Very high (USD 5000–15,000/unit) Industrial equipment Regulatory approval pathway required Multidisciplinary team |
| Fused Deposition Modeling (FDM) | Resolution: 100–300 μm Materials: PLA, ABS, PCL Accuracy: ±0.5 mm Process time: 2–8 h | Educational models Preoperative planning for simple cases Non-load-bearing guides Prototype testing | ★★☆ (planning models) ★☆☆ (surgical guides) | Modest improvement in surgical planning Limited impact on operative time (5–15%) Unclear effect on complication rates | For initial planning when SLA precision is not critical When budget constraints exist For educational purposes When rapid prototyping (same-day) is needed | Low (USD 300–1500/unit) Desktop equipment Minimal post-processing Basic technical skills |
| PolyJet Printing | Resolution: 16–30 μm Materials: Multiple polymers simultaneously Accuracy: ±0.1 mm Process time: 4–10 h | Multi-material anatomical models Simulating tissue differences Surgical simulation with variable hardness | ★★☆ (simulation models) ★☆☆ (surgical training) | Enhanced preoperative planning Improved surgeon confidence No direct clinical outcome data | When simulating multiple tissue types is critical For complex cases requiring differentiation between bone, cartilage, and soft tissue When surgeons need tactile simulation before complex reconstruction | Very high (USD 6000–12,000/unit) Specialized equipment Multiple materials Advanced technical expertise |
| Bioprinting | Resolution: 50–300 μm Materials: Cell-laden hydrogels Viability: 60–90% cell survival Process time: 0.5–4 h | Cartilage tissue engineering Experimental nasal scaffolds Research applications only | ★☆☆ (all applications) | No Level 1 clinical data available Laboratory evidence of chondrogenic marker expression (Yi et al.) Potential for reduced immune rejection | Experimental only Not currently suitable for clinical application Consider for research protocols with IRB approval When conventional and standard 3D-printing approaches have failed | Extremely high (USD 15,000–50,000/unit) Specialized bioprinting equipment Cell culture facilities Multidisciplinary expertise required |
| Material Type | Example | Properties | Applications |
|---|---|---|---|
| Polymers | PLA, PEEK, PCL | Lightweight, biocompatible, and moldable. | Cartilage replacement; structural support. |
| Metals | Titanium | Strong, corrosion-resistant, and highly biocompatible. | Load-bearing implants for major structural deficits. |
| Bioactive Materials | Hydroxyapatite | Promotes bone integration; mimics mineral composition of human bone. | Nasal framework reconstruction requiring osseointegration. |
| Bioprinting Materials | Collagen, Hydrogels | Contains live cells; supports tissue engineering. | Future applications in regenerative nasal reconstruction. |
| Surgery Type | 3D Image Acquisition | 3D Printing Material | 3D Printing Product | Number of Patients (N) | Follow-up Period | Clinical Outcomes | Complications | Comparison with Conventional Methods | Level 1: Patient Satisfaction + Revisions | Level 2: Summetry, Complications, Functional Results | Publication Year | First Author |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct 3D printing of flexible nasal prosthesis: optimized digital workflow | CT scan | Tango Plus flexible material (26–28 Shore A hardness) | 3D-printed nasal prosthesis | 1 (27-year-old female) | 1 week | Patient reported satisfaction; excellent margin adaptation with surrounding tissues | None reported | 38% time reduction (5 h vs. 8 h); fewer clinical sessions (1 vs. 3+); superior margin precision (16 μm vs. 400 μm typical); digital storage for future reproductions | Restoration of facial aesthetics; patient satisfaction; immediate psychological benefit | Better margin adaptation; reduced treatment time; eliminated need for impression making; eliminated mold-fabrication steps | 2018 | Nuseir [26] |
| Total nasal reconstruction after failed conventional reconstruction | Facial CT scan (pre-surgical excision) | Porous titanium grade 2 (53% porosity, 860–1500 μm pore size) | Custom 3D-printed porous titanium nasal prosthesis | 1 | 24 months | Successful integration Patient resumed normal activities Patient satisfied with result No implant exposure Normal breathing after correction | Required corrective surgery at 3 months to increase nasal orifice diameter due to shortness of breath | Used after conventional methods failed twice (cartilage framework + forehead flap) Avoided need for additional cartilage harvests Alternative to episthesis, which patient refused | Restored nasal form and function Patient satisfaction Stable reconstruction at 2-year follow-up Resumption of normal activities | Accurate reconstruction based on patient’s original nasal shape Two-step approach provided better tissue integration Meshed titanium structure allowed better integration No donor site complications | 2018 | Qassemyar [27] |
| Personalized bioactive nasal supports for cleft rhinoplasty | Photogrammetry | Polylactic acid (PLA) | Bioactive nasal supports | 0 (laboratory prototype) | N/A—benchtop study | Zone of inhibition of 15.15 ± 0.99 mm (p < 0.0001) against E. coli | None in prototype testing | Replaces one-size-fits-all rubber tube retainers; adds antibiotic delivery capability; better contouring | Potential for reduced infection rates and improved healing | Potential for reduced infection rates and improved healing | 2018 | Boyer [28] |
| Total nasal reconstruction | CT scan (facial moulage scanned with CT) | Titanium | Customized titanium nasal plate | 1 (61-year-old woman) | 6 months | Successful reconstruction with desirable esthetic and functional results High patient satisfaction | None reported | Patient had previously undergone multiple failed reconstructive surgeries (temporalis flap, paramedian forehead flap, calvarial bone graft, and radial forearm flap) | Successful reconstruction after multiple conventional procedures failed Restoration of nasal form and function | Created solid retention and cantilevered nasal structure from frontal bone and orbital rims when conventional support points were absent Comprehensive 3-stage surgical approach with tissue expander | 2019 | Zarrabi [29] |
| Pediatric nasal defect correction | CT scan | Custom-made PEEK (Poly Ether Ketone) | Implant | 1 (2-year-old female) | Progressive treatment from age 2–4 | Maximum tissue expansion of 8.5 mm after first implant; 6.8 mm expansion with second implant | Infection after second implant requiring removal; skin injury at tip after first implant | Traditional approach delays reconstruction until age 20+; rhis approach provides staged reconstruction during childhood | Improved facial appearance during childhood development; psychological benefit | Progressive tissue expansion for later definitive reconstruction | 2019 | Borghi [30] |
| Semi nasal reconstruction | CT scan | PolyJet photopolymer (MED 610) | Nose contour and framework guides | 10 (3 men, 7 women) | Mean: 17.4 months (range: 7–35 months) | Statistically significant improvements in alar width and alar area symmetry (p < 0.05); reduced asymmetry compared to control group | None reported | 20–30 min reduction in operative time; only nostril asymmetry remained (vs. multiple asymmetries in control group); objective measurements showed statistical superiority | Improved aesthetic outcomes through measurably better symmetry | More precise surgical planning; reduced operative time; facilitated learning curve for surgeons | 2019 | Yen [31] |
| Nasal reconstruction using a customized 3D-printed stent | CT scan | Medical-grade silicone (Q7-4840; Dow Corning) | 3D-printed nasal stent | 1 (6-year-old male) | 3 years | Successful mucoepithelium regeneration; maintained 6 mm airway diameter; stable respiratory function without additional care | None reported | Commercial stents only designed for nostrils not nasal cavity; custom design covered both nostrils and nasal passage | Maintained respiratory function and airway patency for 3 years | Prevention of nasal passage stenosis; successful mucoepithelium regeneration | 2019 | Jung [32] |
| Subtotal nasal reconstruction | CT scan | Porous polyethylene (PPE) | 3D-printed scaffold | 1 (64-year-old male) | 22 months | “Stable and functional with excellent aesthetic appearance”; successfully integrated with surrounding tissues | None reported | Eliminated need for multiple cartilage/bone donor sites; combined multiple framework elements into single unit; reduced operative stages; more precise form and contour | Functional and aesthetic restoration of subtotal nasal defect; patient able to resume normal activities | Simplified surgical approach; precise replication of desired nasal form; reduced donor site morbidity | 2019 | Walton [33] |
| Septal extension graft supported by 3D-printed plate | CT scan | Polycaprolactone (PCL) | 3D-printed septal extension graft | 43 (20 males, 23 females; mean age 28.7 years) | Mean 14.8 months (range 12–20) | 90.7% rated excellent/good satisfaction; 65.1% without tip drooping; 30.2% mild-moderate drooping; 4.7% severe drooping | Tip stiffness (72.1%); nasal tip deviation (4.7%); infection (2.3%) | Reduced severe drooping (4.7% vs. 11.4%) and tip deviation (4.7% vs. 11.4%) compared to SEG alone; increased tip stiffness (72.1% vs. 45.5%) | Improved patient satisfaction; reduced severe tip drooping and deviation | Better structural support for tip projection | 2020 | Kim [34] |
| Augmentative rhinoplasty (preclinical) | Computer-aided design from 2D facial pictures | PCL with cartilage-derived hydrogel containing stem cells | Engineered nasal cartilage implant with octahedral interior architecture | N/A (laboratory and animal model only) | 12 weeks (animal study) | Remarkable expression of chondrogenic markers Maintenance of implant shape and structure Formation of cartilaginous tissues | None reported in animal model | Combines benefits of both autologous cartilage and synthetic implants | Potential for cartilage regeneration in the 3D-printed structure Maintained shape and structure during implantation period | Patient-specific customized design based on facial features Cell-friendly environment for tissue formation Uniform cell distribution through injection technique | 2019 | Yi [35] |
| Corrective rhinoplasty for trauma-related nasal deformities | CT scan (0.6 mm slice thickness) | Polylactic acid (PLA) | 3D-printed surgical planning model for simulated osteotomy | 11 | 6 months | Mean deviation angle improved from 11.75° to 2.08° All surgical results rated as “success” (corrected angle < 5°) Surgery performed as planned in 10/11 cases | None reported | More precise planning compared to using only 2D CT images | Successful correction of nasal deformities in all patients Significant improvement in nasal deviation angle | Precise preoperative planning of osteotomy Allowed simulation before actual surgery Valuable teaching tool for inexperienced surgeons | 2020 | Jung [36] |
| Augmentative rhinoplasty | Not clearly specified | Polycaprolactone (PCL) mesh | Compound osseocartilaginous graft with PCL mesh core | 30 | 24.4 ± 1.6 months | Nasal length improved by 5.3 ± 1.8 mm Nasal tip projection improved by 4.9 ± 1.9 mm Improved nasolabial and nasofrontal angles /30 patients satisfied Specialists rated: 6 “Excellent”, 23 “Good”, 1 “Fair” | None reported | Exceeds previously reported outcomes of conventional tip augmentations | Successful correction with high patient satisfaction (96.7%) Long-term maintenance of nasal projection | Longitudinally variable flexibility (strong base, flexible tip) Secure anchor point at anterior nasal spine Maximized augmentation of nasal length | 2020 | Ahn [37] |
| Reconstruction after oncologic rhinectomies | CT scan | Elastic resin V1 (Formlabs) with shore hardness 50A | 3D-printed nasal prostheses | 7 | 14 months (mean) | - Patient satisfaction with median scores of 8/10 for aesthetics, comfort, and security of retaining system | None specified | Significantly reduced costs and time compared to silicone prostheses; allowed rehabilitation during waiting period for definitive reconstruction | - Temporary rehabilitation during post-cancer treatment period Helped patients manage facial deformity and psychosocial impacts before definitive reconstruction | - Time-efficient workflow (mean design time: 2h48) Cost-effective solution (mean cost: USD 753 Direct printing without need for complex molds | 2021 | Salati [38] |
| Semi nasal reconstruction (hemirhinectomy defect) | 3D photo using VECTRA M5 imaging system | Tango Polyjet Material | Flap size contour guide | 1 | Not specified | Successful reconstruction with restored nasal appearance (implied) | None reported | Improved predictability compared to conventional forehead flap design, which requires significant surgeon artistry and experience | Made complex nasal reconstruction more accessible with consistent results Reduced reliance on surgeon artistry | Created patient-specific soft tissue cutting guide Converted 3D structure into 2D template for flap design Improved surgical efficiency and predictability | 2021 | Brower [39] |
| Nasal septal gossypiboma removal and mucosal defect reconstruction | Contrast-enhanced paranasal sinus CT | Not applicable (not a 3D printing case) | Not applicable | 1 | 8 months | Complete resolution of nasal obstruction and foreign body sensation Well-healed nasal septum at 6 months Small (2 mm) septal perforation noted at 8 months No saddle nose deformity | Severe adhesion between gossypiboma and septal mucosa resulted in significant mucosal defect (3.0 cm × 2.0 cm) | Posterior-based inferior turbinate flap used to reconstruct the defective septum, avoiding synthetic materials | Relief of obstructive symptoms Successful removal of retained surgical material Preservation of nasal structure | Endoscopic approach minimized additional trauma Use of natural tissue for reconstruction prevented foreign body reactions Successful management of a large septal defect | 2022 | Kim [40] |
| Total nasal reconstruction in irradiated tissue | CT scan | Hydroxyapatite (bioceramic) with photopolymerizable resin | Custom 3D-printed integrated biomaterial bio prosthesis | 1 | 12 months | Complete integration of bioprosthesis High patient satisfaction with cosmetic outcome Quality of life score (EQ-5D VAS) improved from 40/100 to 100/100 Improved sense of smell Patient able to resume social activities | 5 mm exposure on tips of nasal alae requiring minor surgical revision | Previous conventional reconstructions (scapula free flap, forehead flap with costal cartilage) had failed in this patient due to tissue “melting” in irradiated field | Successful total nasal reconstruction after multiple conventional techniques failed Dramatic improvement in quality of life Restoration of nasal function and appearance | Two-stage approach using patient’s arm as bioreactor promoted integration in irradiated tissue Limited donor site morbidity 3D-printed porous structure allowed for tissue ingrowth | 2023 | Dupret-Bories [41] |
| Semi nasal reconstruction | 3D scan | Unknown | forehead flap surgical guide | 2024 | Park [42] | |||||||
| Nasal stent prosthesis | Extraoral scanning and photogrammetry | PLA | Nasal stent | 2024 | Rathee [43] | |||||||
| Open rhinoplasty in chondrosarcoma case | CT and MRI scanning | 3D-printed bone model | Implantable structures | 2024 | Yamamoto [44] |
| Parameter | Conventional Techniques | 3D-Printing Approaches | Key Differentiating Factors |
|---|---|---|---|
| Preoperative Planning | Relies on 2D imaging and surgeon experience; limited ability to simulate outcomes | Patient-specific 3D models; virtual surgical planning; outcome simulation | 3D approaches provide tangible models for planning and patient education; conventional approaches rely more heavily on surgeon experience |
| Structural Accuracy | Dependent on intraoperative judgment; may require multiple adjustments | Precise based on preoperative imaging; may not adapt to intraoperative findings | 3D approaches offer higher initial precision but less adaptability; technical limitations in imaging-to-printing fidelity remain |
| Tissue Compatibility | Well-established biological integration of autologous tissues | Variable based on material selection; promising but limited long-term data | Conventional approaches have stronger evidence for long-term integration; 3D approaches still addressing biocompatibility challenges |
| Surgical Learning Curve | Steep learning curve; highly dependent on surgical expertise | Initial technical learning curve; potentially reduces reliance on advanced surgical skills | 3D approaches may standardize certain procedures but require new technical competencies |
| Resource Requirements | Lower initial technology costs; higher operating room time | Higher technology/equipment costs; potentially reduced OR time | Cost–benefit analysis remains unclear; depends on healthcare system infrastructure |
| Customization Capability | Limited by available donor tissue; reliant on intraoperative modification | Highly customizable preoperatively; less adaptable intraoperatively | 3D approaches offer greater preoperative customization but may struggle with intraoperative adjustments |
| Clinical Evidence Base | Extensive long-term outcomes data; well-documented complications | Limited long-term data; mostly case reports and small series | Conventional approaches have significantly stronger evidence base, particularly for Level 1 outcomes |
| Application | Level 3 (Technical) | Level 2 (Surgical) | Level 1 (Clinical) | Evidence Quality |
|---|---|---|---|---|
| Preoperative modeling | High-accuracy anatomical models (±0.2 mm) | Reduced planning time (20–30%); improved surgical precision | Limited evidence for reduced complication rates; some evidence for improved aesthetic outcomes | Moderate |
| Surgical guides | Precise cutting and positioning guides (±0.3 mm) | Reduced operative time (15–30%); more accurate implementation of surgical plan | Emerging evidence for improved symmetry; limited evidence for reduced revision rates | Moderate–Low |
| Patient-specific implants | Exact anatomical fit (±0.5 mm); customized mechanical properties | Reduced need for intraoperative modification; simplified surgical technique | Isolated case reports of successful outcomes; no comparative studies with conventional techniques | Low |
| Bioprinted scaffolds | Controlled porosity and architecture; cell compatibility | Laboratory evidence of tissue integration | No clinical evidence of superior long-term outcomes | Very Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, R.; Al-Musaileem, N.; Raman, K.S.; Al-Majid, D.; Solomon, P.; Rival, R. 3D Printing in Nasal Reconstruction: Application-Based Evidence on What Works, When, and Why. Biomedicines 2025, 13, 1434. https://doi.org/10.3390/biomedicines13061434
Chowdhury R, Al-Musaileem N, Raman KS, Al-Majid D, Solomon P, Rival R. 3D Printing in Nasal Reconstruction: Application-Based Evidence on What Works, When, and Why. Biomedicines. 2025; 13(6):1434. https://doi.org/10.3390/biomedicines13061434
Chicago/Turabian StyleChowdhury, Raisa, Nisreen Al-Musaileem, Karanvir S. Raman, Dana Al-Majid, Philip Solomon, and Richard Rival. 2025. "3D Printing in Nasal Reconstruction: Application-Based Evidence on What Works, When, and Why" Biomedicines 13, no. 6: 1434. https://doi.org/10.3390/biomedicines13061434
APA StyleChowdhury, R., Al-Musaileem, N., Raman, K. S., Al-Majid, D., Solomon, P., & Rival, R. (2025). 3D Printing in Nasal Reconstruction: Application-Based Evidence on What Works, When, and Why. Biomedicines, 13(6), 1434. https://doi.org/10.3390/biomedicines13061434






