Application of Synchrotron Radiation in Fundamental Research and Clinical Medicine
Abstract
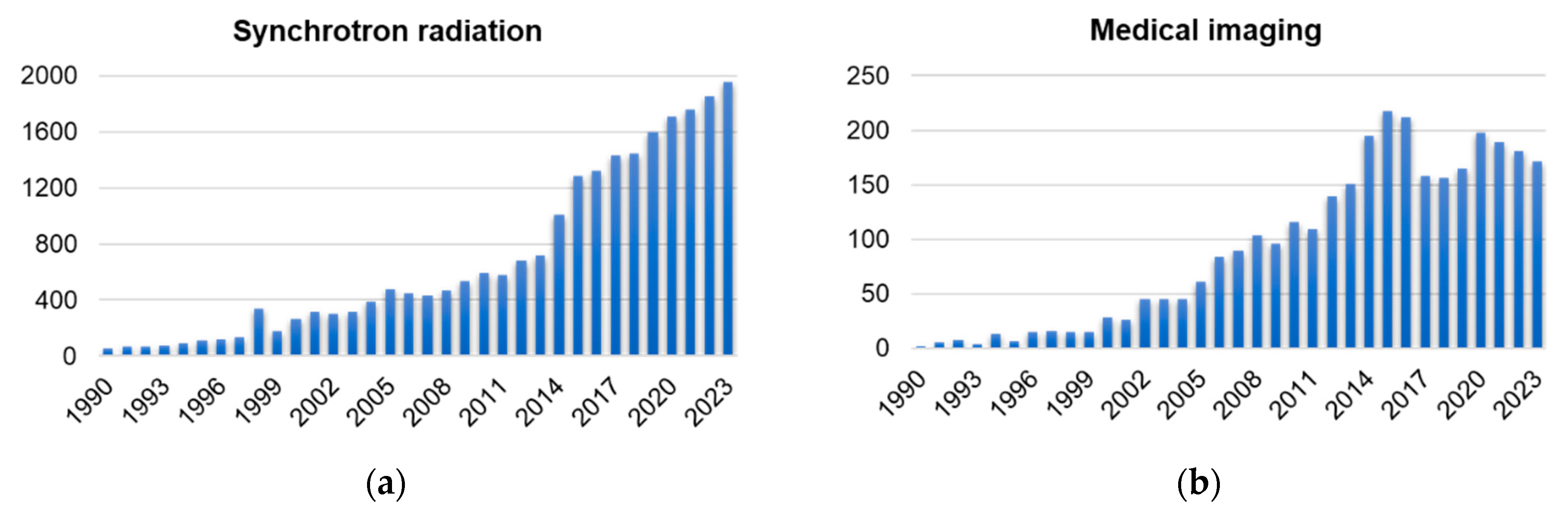
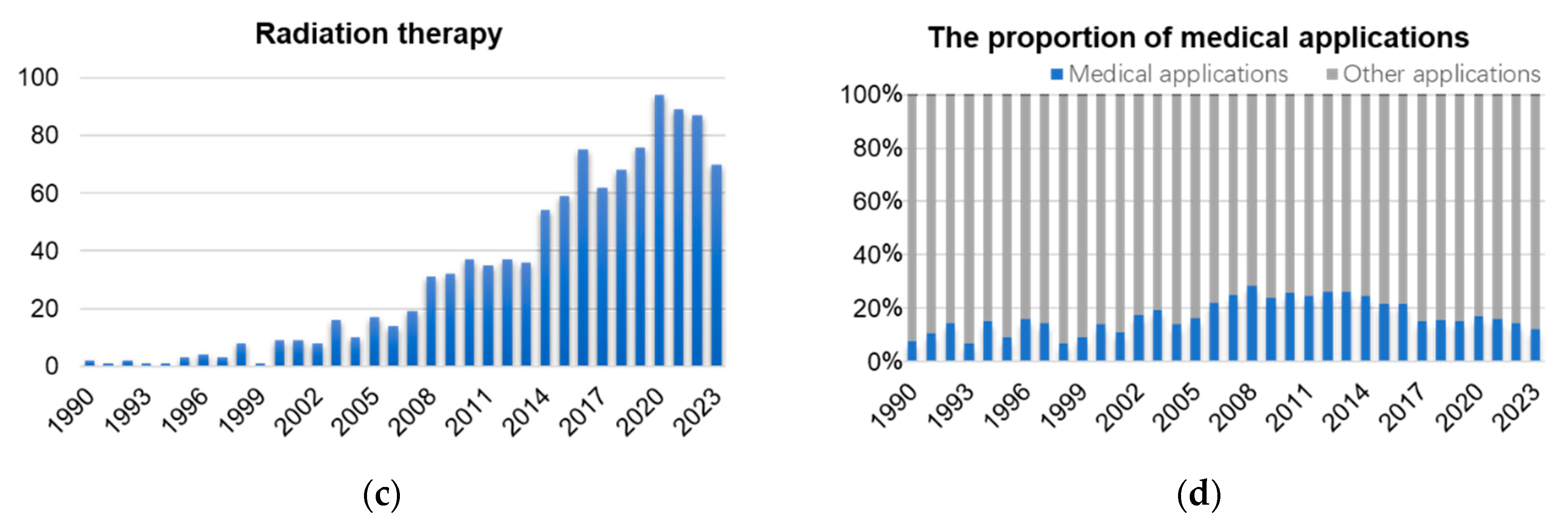
1. Introduction
2. Light Sources and Biomedical Beamlines
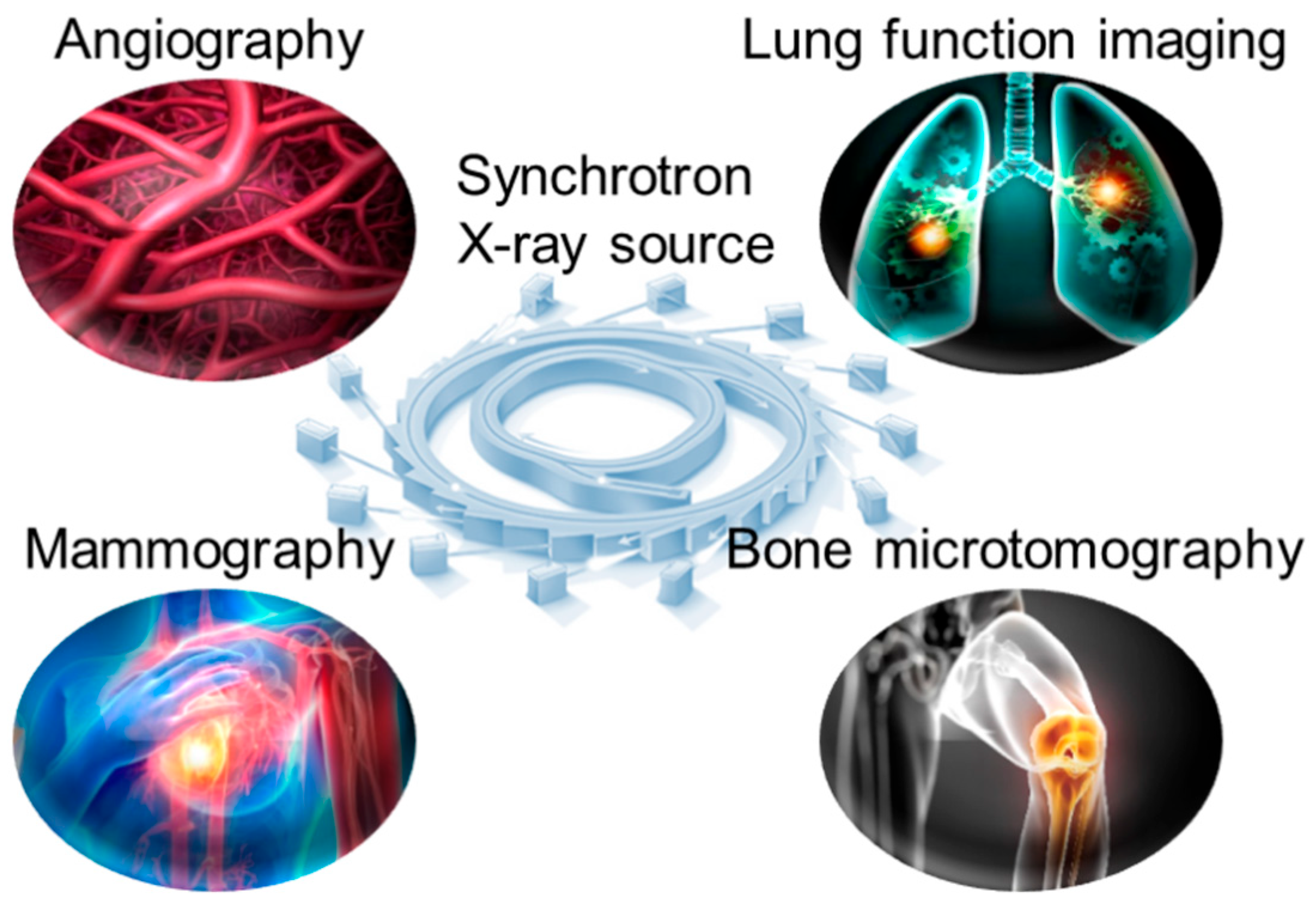
3. Synchrotron X-Ray in Medical Imaging
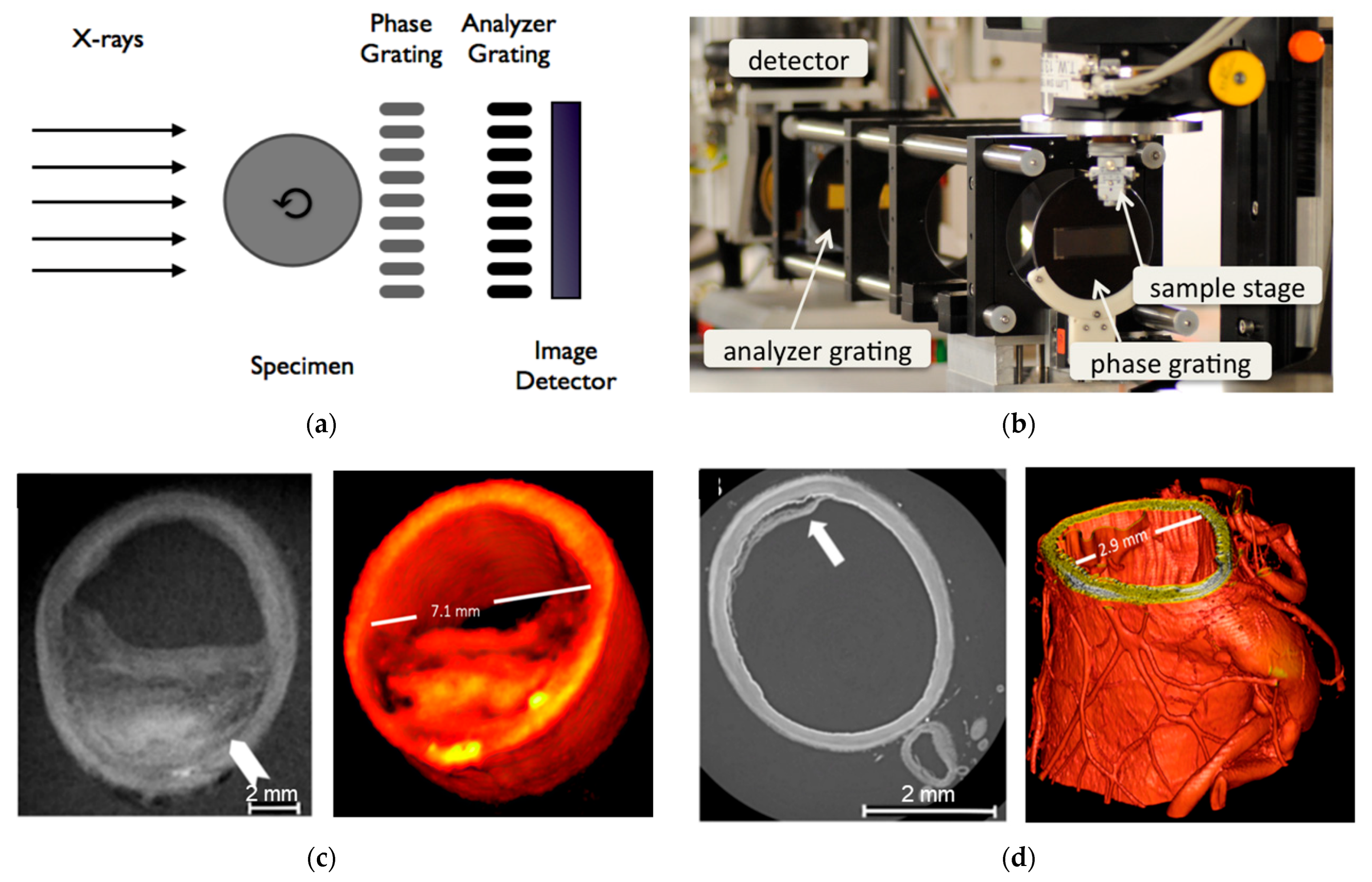
3.1. Angiography
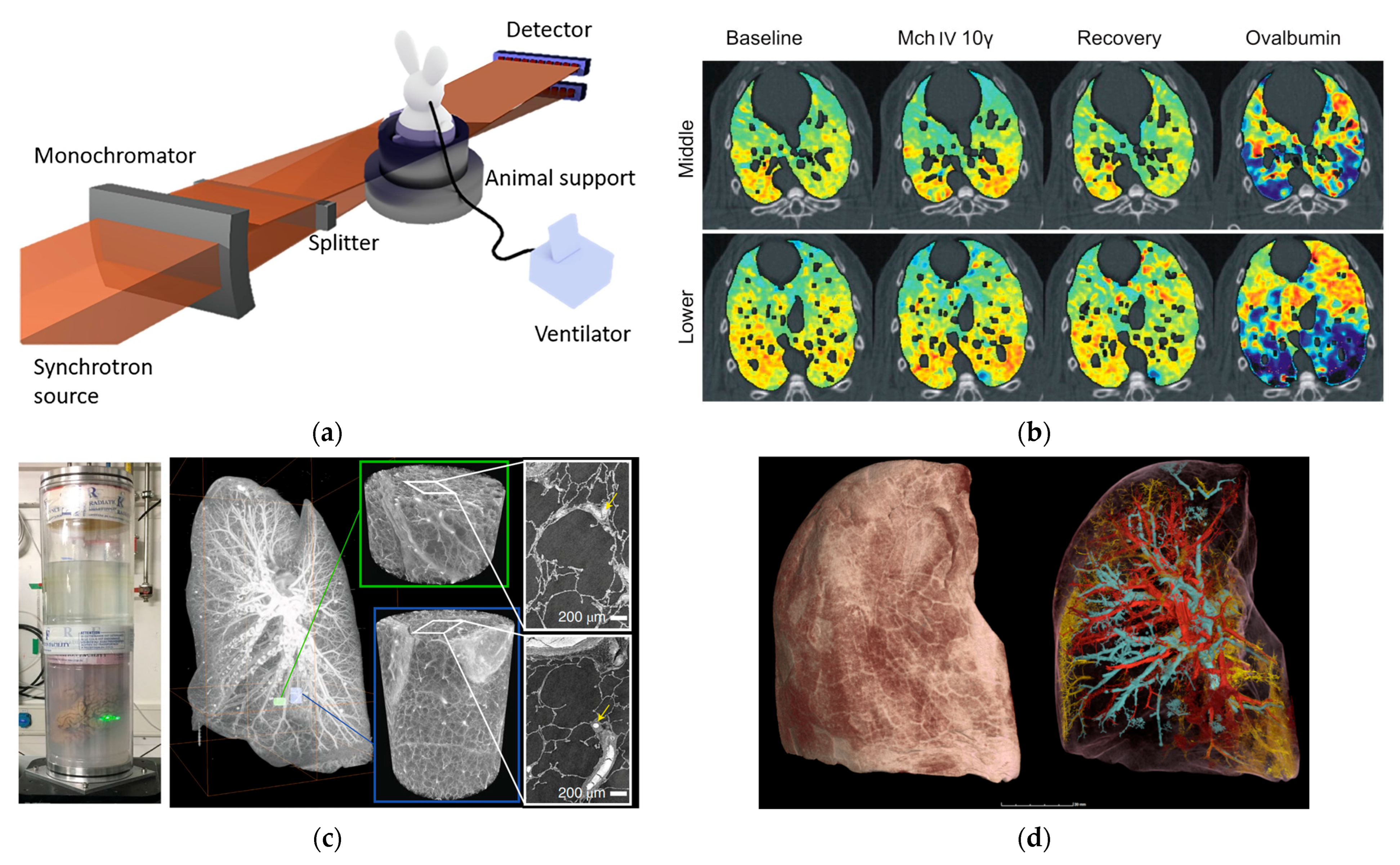
3.2. Lung Function Imaging
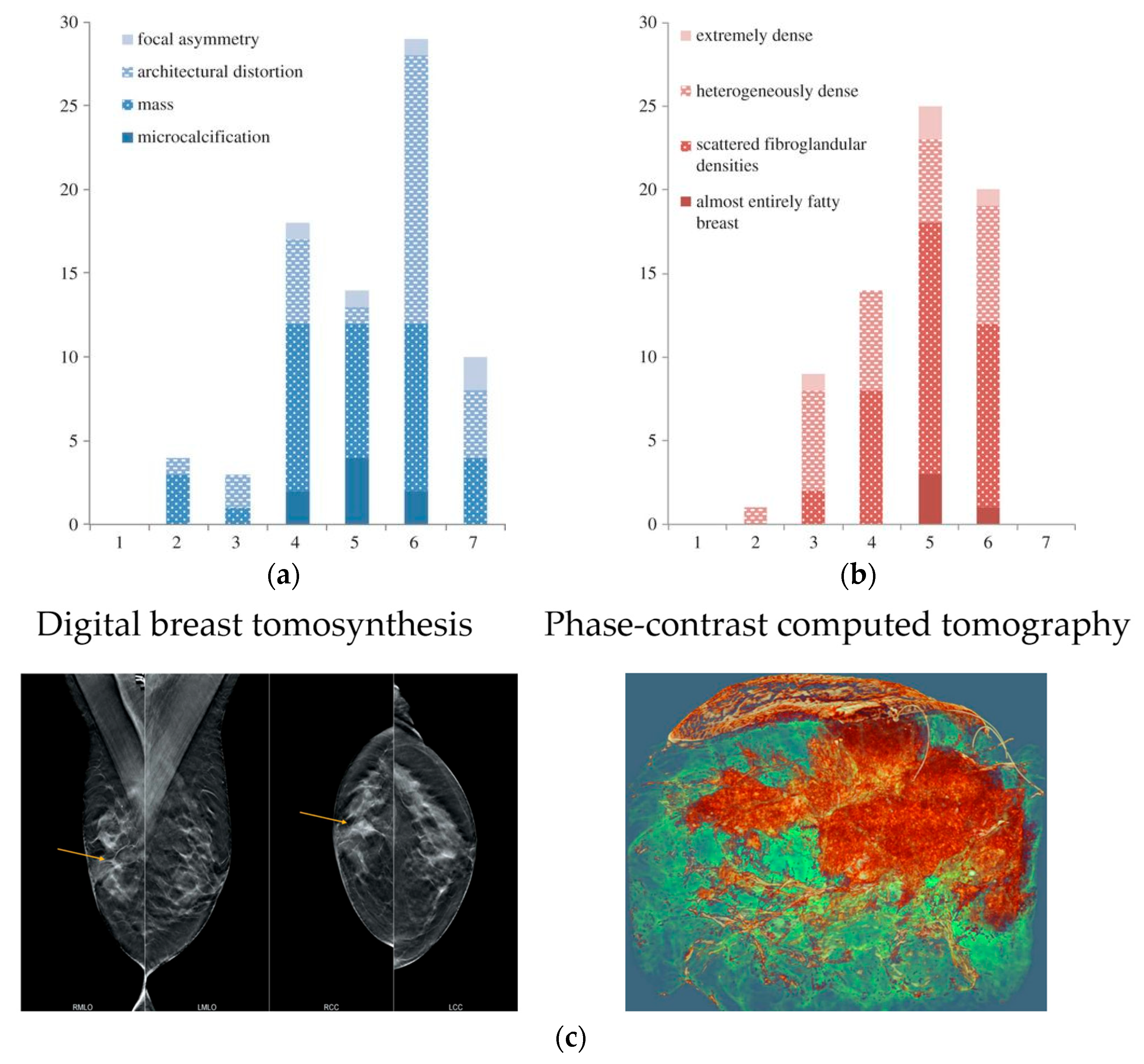
3.3. Breast Mammography
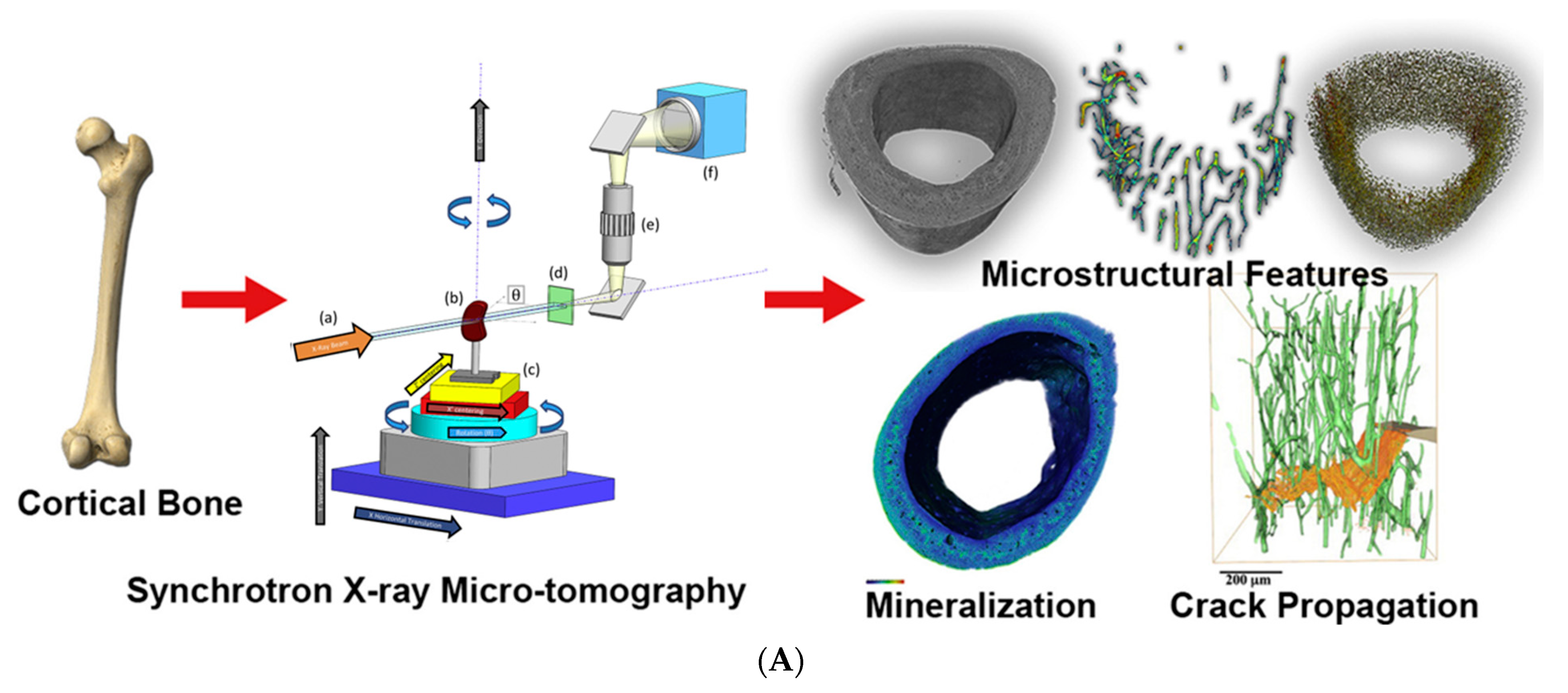
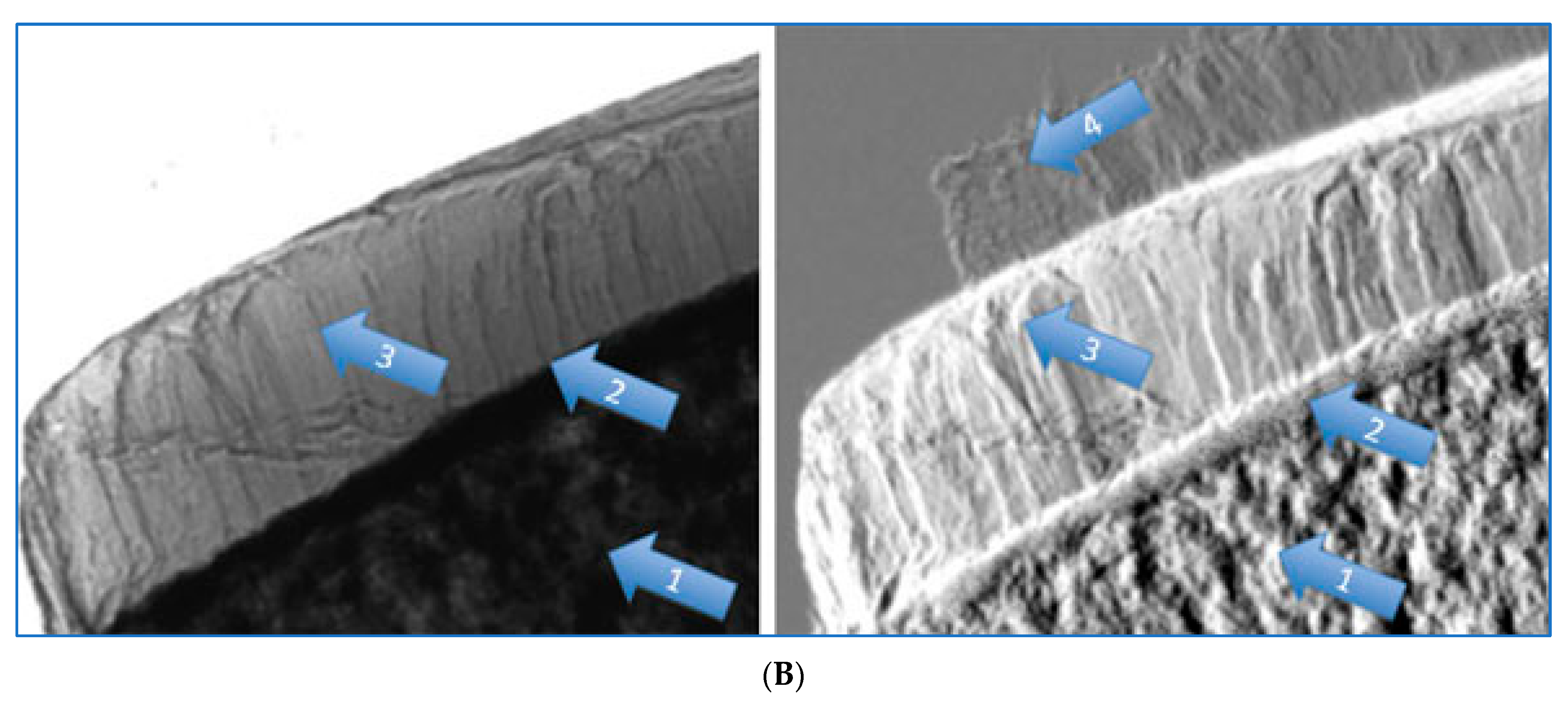
3.4. Bone Microtomography
4. Synchrotron X-Ray Therapy
4.1. Microbeam Radiotherapy
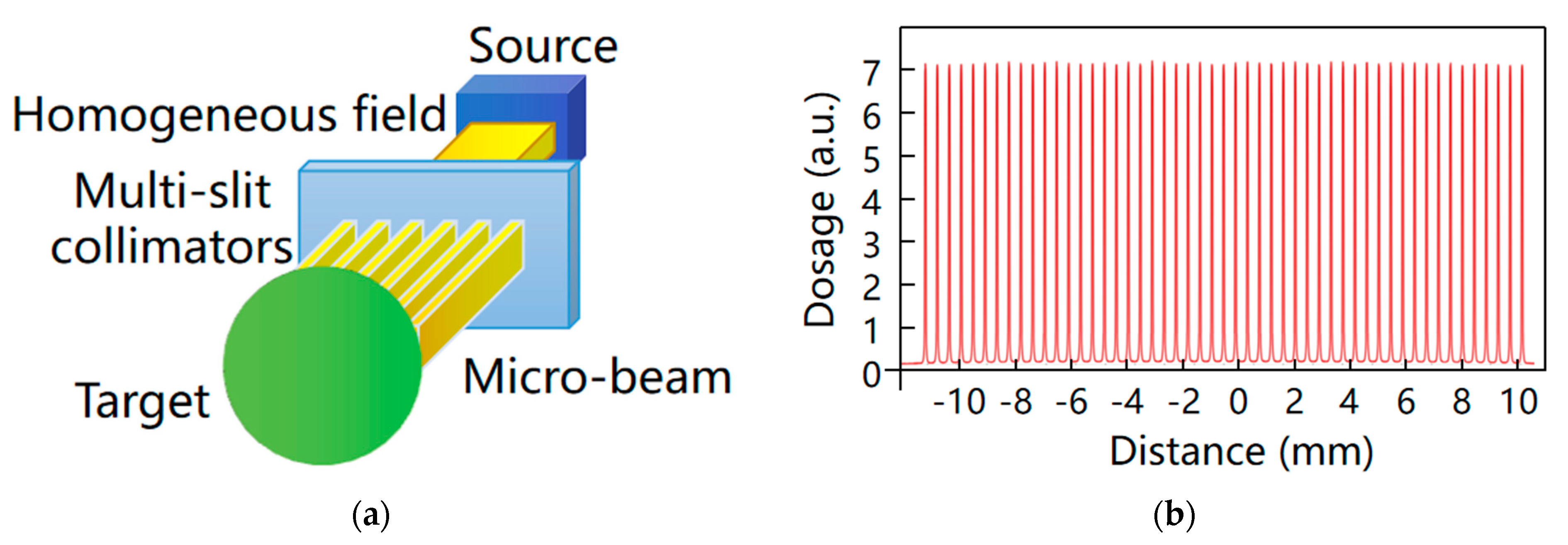
4.2. Stereotactic Radiotherapy
5. Conclusions
6. Limitations and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Suortti, P.; Thomlinson, W. Medical applications of synchrotron radiation. Phys. Med. Biol. 2003, 48, R1. [Google Scholar] [CrossRef]
- Raimondi, P.; Benabderrahmane, C.; Berkvens, P.; Biasci, J.C.; Borowiec, P.; Bouteille, J.F.; Brochard, T.; Brookes, N.B.; Carmignani, N.; Carver, L.R.; et al. The Extremely Brilliant Source storage ring of the European Synchrotron Radiation Facility. Commun. Phys. 2023, 6, 82. [Google Scholar] [CrossRef]
- Pedro, F.T.; Johan, B.; Åke, A. Future development plans for the MAX IV light source: Pushing further towards higher brightness and coherence. J. Electron Spectrosc. Relat. Phenom. 2018, 224, 8–16. [Google Scholar]
- Shin, S. New era of synchrotron radiation: Fourth-generation storage ring. AAPPS Bull. 2021, 31, 21. [Google Scholar] [CrossRef]
- Chushkin, Y.; Zontone, F. Prospects for coherent X-ray diffraction imaging at fourth-generation synchrotron sources. IUCrJ 2025, 12, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Eling, L.; Bouchet, A.; Nemoz, C.; Djonov, V.; Balosso, J.; Laissue, J.; Bräuer-Krisch, E.; Adam, J.F.; Serduc, R. Ultra high dose rate Synchrotron Microbeam Radiation Therapy. Preclinical evidence in view of a clinical transfer. Radiother. Oncol. 2019, 139, 56–61. [Google Scholar] [CrossRef]
- Romano, M.; Bravin, A.; Mittone, A.; Eckhardt, A.; Barbone, G.E.; Sancey, L.; Dinkel, J.; Bartzsch, S.; Ricke, J.; Alunni-Fabbroni, M.; et al. A Multi-Scale and Multi-Technique Approach for the Characterization of the Effects of Spatially Fractionated X-ray Radiation Therapies in a Preclinical Model. Cancers 2021, 13, 4953. [Google Scholar] [CrossRef]
- Zhao, Y.; Brun, E.; Coan, P.; Huang, Z.; Sztrókay, A.; Diemoz, P.C.; Liebhardt, S.; Mittone, A.; Gasilov, S.; Miao, J.; et al. High-resolution, low-dose phase contrast X-ray tomography for 3D diagnosis of human breast cancers. Proc. Natl. Acad. Sci. USA 2012, 109, 18290–18294. [Google Scholar] [CrossRef] [PubMed]
- Horng, A.; Stroebel, J.; Geith, T.; Milz, S.; Pacureanu, A.; Yang, Y.; Cloetens, P.; Lovric, G.; Mittone, A.; Bravin, A.; et al. Multiscale X-ray phase contrast imaging of human cartilage for investigating osteoarthritis formation. J. Biomed. Sci. 2021, 28, 42. [Google Scholar] [CrossRef]
- Bruno, P.; Biasci, J.C.; Detlefs, C.; Dimper, R.; Krisch, M.; Martínez-Criado, G.; Mezouar, M.; Nevo, C.; Qin, Q.; Raimondi, P.; et al. X-ray science using the ESRF—Extremely brilliant source. Eur. Phys. J. Plus 2024, 139, 928. [Google Scholar] [CrossRef]
- Jaekel, F.; Bräuer-Krisch, E.; Bartzsch, S.; Laissue, J.; Blattmann, H.; Scholz, M.; Soloviova, J.; Hildebrandt, G.; Schültke, E. Microbeam Irradiation as a Simultaneously Integrated Boost in a Conventional Whole-Brain Radiotherapy Protocol. Int. J. Mol. Sci. 2022, 23, 8319. [Google Scholar] [CrossRef] [PubMed]
- Trappetti, V.; Potez, M.; Fernandez-Palomo, C.; Volarevic, V.; Shintani, N.; Pellicioli, P.; Ernst, A.; Haberthür, D.; Fazzari, J.M.; Krisch, M.; et al. Microbeam Radiation Therapy Controls Local Growth of Radioresistant Melanoma and Treats Out-of-Field Locoregional Metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 478–493. [Google Scholar] [CrossRef]
- Murrie, R.P.; Morgan, K.S.; Maksimenko, A.; Fouras, A.; Paganin, D.M.; Hall, C.; Siu, K.K.; Parsons, D.W.; Donnelley, M. Live small-animal X-ray lung velocimetry and lung micro-tomography at the Australian Synchrotron Imaging and Medical Beamline. J. Synchrotron Radiat. 2015, 22, 1049–1055. [Google Scholar] [CrossRef]
- Martelli, S.; Perilli, E. Time-elapsed synchrotron-light microstructural imaging of femoral neck fracture. J. Mech. Behav. Biomed. Mater. 2018, 84, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Arhatari, B.; Nesterets, Y.; Taba, S.; Maksimenko, A.; Hall, C.; Stevenson, A.; Häsermann, D.; Lewis, S.; Dimmock, M.; Thompson, D.; et al. X-ray phase-contrast computed tomography for full breast mastectomy imaging at the Australian Synchrotron. Proc. SPIE 2021, 11840, 1184012. [Google Scholar] [CrossRef]
- Engels, E.; Forrester, H.; Klein, M.; Bell, C.; Balderstone, I.; Brunt, K.; Barnes, J.M.; Cameron, M.; Crosbie, C.J.; Middlrton, R.; et al. The Impact of Synchrotron Microbeam Radiation Therapy Combined With Broad Beam in a Preclinical Breast Cancer Model. Adv. Radiat. Oncol. 2025, 10, 101680. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.J.; Paino, J.; Day, L.R.; Butler, D.; Hausermann, D.; Pelliccia, D.; Crosbie, J.C. SyncMRT: A solution to image-guided synchrotron radiotherapy for quality assurance and pre-clinical trials. J. Synchrotron Radiat. 2022, 29, 1074–1084. [Google Scholar] [CrossRef]
- Iyer, J.S.; Zhu, N.; Gasilov, S.; Ladak, H.M.; Agrawal, S.K.; Stankovic, K.M. Visualizing the 3D cytoarchitecture of the human cochlea in an intact temporal bone using synchrotron radiation phase contrast imaging. Biomed. Opt. Express 2018, 9, 3757–3767. [Google Scholar] [CrossRef]
- Li, H.; Schart-Moren, N.; Rajan, G.; Shaw, J.; Rohani, S.A.; Atturo, F.; Ladak, H.M.; Rask-Andersen, H.; Agrawal, S. Vestibular Organ and Cochlear Implantation–A Synchrotron and Micro-CT Study. Front. Neurol. 2021, 12, 663722. [Google Scholar] [CrossRef]
- Panahifar, A.; Swanston, T.M.; Jake Pushie, M.; Belev, G.; Chapman, D.; Weber, L.; Cooper, D.M. Three-dimensional labeling of newly formed bone using synchrotron radiation barium K-edge subtraction imaging. Phys. Med. Biol. 2016, 61, 5077–5088. [Google Scholar] [CrossRef]
- Cardenas, D.; Turyanskaya, A.; Rauwolf, M.; Panahifar, A.; Cooper, D.; Wohl, G.R.; Streli, C.; Wobrauschek, P.; Pejović-Milić, A. Determining elemental strontium distribution in rat bones treated with strontium ranelate and strontium citrate using 2D micro-XRF and 3D dual energy K-edge subtraction synchrotron imaging. X-Ray Spectrom. 2020, 49, 424–433. [Google Scholar] [CrossRef]
- Andronowski, J.M.; Cole, M.E.; Davis, R.A.; Tubo, G.R.; Taylor, J.T.; Cooper, D.M.L. A multimodal 3D imaging approach of pore networks in the human femur to assess age-associated vascular expansion and Lacuno-Canalicular reduction. Anat. Rec. 2023, 306, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Oliva, P.; Di Trapani, V.; Arfelli, F.; Brombal, L.; Donato, S.; Golosio, B.; Longo, R.; Mettivier, G.; Rigon, L.; Taibi, A.; et al. Experimental optimization of the energy for breast-CT with synchrotron radiation. Sci. Rep. 2020, 10, 17430. [Google Scholar] [CrossRef] [PubMed]
- Baran, P.; Mayo, S.; McCormack, M.; Pacilè, S.; Tromba, G.; Dullin, C.; Zanconati, F.; Arfelli, F.; Dreossi, D.; Fox, J.; et al. High-Resolution X-Ray Phase-Contrast 3-D Imaging of Breast Tissue Specimens as a Possible Adjunct to Histopathology. IEEE Trans. Med. Imaging 2018, 37, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Fiedler, S.; Menk, R.H.; Jaekel, F.; Dreossi, D.; Casarin, K.; Tromba, G.; Bartzsch, S.; Kriesen, S.; Hildebrandt, G. Perspectives for microbeam irradiation at the SYRMEP beamline. J. Synchrotron Radiat. 2021, 28, 410–418. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kawaai, K.; Hatano, N.; Wu, Y.; Takano, H.; Momose, A.; Ishimoto, T.; Nakano, T.; Roschger, P.; Blouin, S.; et al. Hypermineralization of Hearing-Related Bones by a Specific Osteoblast Subtype. J. Bone Miner. Res. 2021, 36, 1535–1547. [Google Scholar] [CrossRef]
- Mizutani, R.; Saiga, R.; Yamamoto, Y.; Uesugi, M.; Takeuchi, A.; Uesugi, K.; Terada, Y.; Suzuki, Y.; De Andrade, V.; De Carlo, F.; et al. Structural diverseness of neurons between brain areas and between cases. Transl. Psychiatry 2021, 11, 49. [Google Scholar] [CrossRef]
- Foxley, S.; Sampathkumar, V.; De Andrade, V.; Trinkle, S.; Sorokina, A.; Norwood, K.; La Riviere, P.; Kasthuri, N. Multi-modal imaging of a single mouse brain over five orders of magnitude of resolution. Neuroimage 2021, 238, 118250. [Google Scholar] [CrossRef]
- Luo, T.; Ni, K.; Culbert, A.; Lan, G.; Li, Z.; Jiang, X.; Kaufmann, M.; Lin, W. Nanoscale Metal–Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 7334–7339. [Google Scholar] [CrossRef] [PubMed]
- Tamal, M.; Althobaiti, M.; Alomari, A.-H.; Dipty, S.T.; Suha, K.T.; Al-Hashim, M. Synchrotron X-ray Radiation (SXR) in Medical Imaging: Current Status and Future Prospects. Appl. Sci. 2022, 12, 3790. [Google Scholar] [CrossRef]
- Saam, T.; Herzen, J.; Hetterich, H.; Fill, S.; Willner, M.; Stockmar, M.; Achterhold, K.; Zanette, I.; Weitkamp, T.; Schüller, U.; et al. Translation of atherosclerotic plaque phase-contrast CT imaging from synchrotron radiation to a conventional lab-based X-ray source. PLoS ONE 2013, 8, e73513. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, P.; Mu, Z.; Lin, X.; Jiang, L.; Cheng, Z.; Luo, L.; Xu, Z.; Geng, J.; Wang, Y.; et al. Dynamic Detection of Thrombolysis in Embolic Stroke Rats by Synchrotron Radiation Angiography. Transl. Stroke Res. 2019, 10, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Cao, Y.; Jiang, L.; Luo, Z.; Lu, H.; Hu, J.; Wu, T. Synchrotron Radiation Imaging Reveals the Role of Estrogen in Promoting Angiogenesis After Acute Spinal Cord Injury in Rats. Spine 2018, 43, 1241–1249. [Google Scholar] [CrossRef]
- Thomlinson, W.; Gmur, N.; Chapman, D.; Garrett, R.; Lazarz, N.; Morrison, J.; Reiser, P.; Padmanabhan, V.; Ong, L.; Green, S. Venous synchrotron coronary angiography. Lancet 1991, 337, 360. [Google Scholar] [CrossRef] [PubMed]
- Dix, W.R. Intravenous coronary angiography with synchrotron radiation. Prog. Biophys. Mol. Biol. 1995, 63, 159–191. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. CMLS 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Xuan, R.; Zhao, X.; Jian, J.; Hu, D.; Qin, L.; Lv, W.; Hu, C. Phase-contrast computed tomography: A correlation study between portal pressure and three dimensional microvasculature of ex vivo liver samples from carbon tetrachloride-induced liver fibrosis in rats. Microvasc. Res. 2019, 125, 103884. [Google Scholar] [CrossRef]
- Lai, V.; Neshat, S.Y.; Rakoski, A.; Pitingolo, J.; Doloff, J.C. Drug delivery strategies in maximizing anti-angiogenesis and anti-tumor immunity. Adv. Drug Deliv. Rev. 2021, 179, 113920. [Google Scholar] [CrossRef]
- Chika, K.; Kazuyuki, H.; Chiho, T.; Toru, T.; Shonosuke, M. Large-view x-ray imaging for medical applications using the world’s only vertically polarized synchrotron radiation beam and a single asymmetric Si crystal. Phys. Med. Biol. 2023, 68, 195010. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Bayat, S.; Fardin, L.; Cercos-Pita, J.L.; Perchiazzi, G.; Bravin, A. Imaging Regional Lung Structure and Function in Small Animals Using Synchrotron Radiation Phase-Contrast and K-Edge Subtraction Computed Tomography. Front. Physiol. 2022, 13, 825433. [Google Scholar] [CrossRef] [PubMed]
- Downie, S.R.; Salome, C.M.; Verbanck, S.; Thompson, B.; Berend, N.; King, G.G. Ventilation heterogeneity is a major determinant of airway hyperresponsiveness in asthma, independent of airway inflammation. Thorax 2007, 62, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Bayat, S.; Porra, L.; Suortti, P.; Thomlinson, W. Functional lung imaging with synchrotron radiation: Methods and preclinical applications. Phys. Medica 2020, 79, 22–35. [Google Scholar] [CrossRef]
- Bayat, S.; Strengell, S.; Porra, L.; Janosi, T.Z.; Petak, F.; Suhonen, H.; Suortti, P.; Hantos, Z.; Sovijarvi, A.R.; Habre, W. Methacholine and ovalbumin challenges assessed by forced oscillations and synchrotron lung imaging. Am. J. Respir. Crit. Care Med. 2009, 180, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.L.; Tafforeau, P.; Wagner, W.L.; Jafree, D.J.; Bellier, A.; Werlein, C.; Kühnel, M.P.; Boller, E.; Walker-Samuel, S.; Robertus, J.L.; et al. Imaging intact human organs with local resolution of cellular structures using hierarchical phase-contrast tomography. Nat. Methods 2021, 18, 1532–1541. [Google Scholar] [CrossRef]
- Harker, S.A.; Preissner, M.; Chang, R.Y.; Trevascus, D.; Liu, C.; Wang, Y.; Chow, M.Y.T.; Cmielewski, P.; Reyne, N.; How, Y.Y.; et al. Using X-ray velocimetry to measure lung function and assess the efficacy of a pseudomonas aeruginosa bacteriophage therapy for cystic fibrosis. Sci. Rep. 2024, 14, 29727. [Google Scholar] [CrossRef]
- Arora, H.; Kernot, D.; Giron, L.; Howells, D.; Darcy, M.; Hoshino, M.; Uesugi, K.; van Loon, R.; Tanaka, G.; Sera, T. Lung disease characterised via synchrotron radiation micro-CT and digital volume correlation (DVC). TrAC Trends Anal. Chem. 2024, 172, 117588. [Google Scholar] [CrossRef]
- Jakob, R.; Stijn, E.V.; Mark, K.; Jan, C.K.; Christopher, W.; Lavinia, N.; Jan-Hendrik, M.; Thanh, Q.B.; Maximilian, A.; Danny, J.; et al. Human lung virtual histology by multi-scale x-ray phase-contrast computed tomography. Phys. Med. Biol. 2023, 68, 115014. [Google Scholar]
- Longo, R.; Tonutti, M.; Rigon, L.; Arfelli, F.; Dreossi, D.; Quai, E.; Zanconati, F.; Castelli, E.; Tromba, G.; Cova, M.A. Clinical study in phase-contrast mammography: Image-quality analysis. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130025. [Google Scholar] [CrossRef][Green Version]
- Pacilè, S.; Baran, P.; Dullin, C.; Dimmock, M.; Lockie, D.; Missbach-Guntner, J.; Quiney, H.; McCormack, M.; Mayo, S.; Thompson, D. Advantages of breast cancer visualization and characterization using synchrotron radiation phase-contrast tomography. J. Synchrotron Radiat. 2018, 25, 1460–1466. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.; Rigon, L.; Arana-Peña, L.M. Image Quality Study in Breast Tomography with Synchrotron Radiation. Rev. Cub. Fis. 2024, 41, 43–49. [Google Scholar]
- Polikarpov, M.; Vila-Comamala, J.; Wang, Z.; Pereira, A.; van Gogh, S.; Gasser, C.; Jefimovs, K.; Romano, L.; Varga, Z.; Lång, K. Towards virtual histology with X-ray grating interferometry. Sci. Rep. 2023, 13, 9049. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Bale, H.A.; Barnard, H.S.; Parkinson, D.Y.; Alliston, T.; Acevedo, C. Quantitative and qualitative bone imaging: A review of synchrotron radiation microtomography analysis in bone research. J. Mech. Behav. Biomed. Mater. 2020, 110, 103887. [Google Scholar] [CrossRef]
- Hildebrand, T.; Ma, Q.; Heyward, C.A.; Haugen, H.J.; Nogueira, L.P. Advanced soft tissue visualization in conjunction with bone structures using contrast-enhanced micro-CT. J. Med. Imaging 2024, 11, 066001. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Campi, G.; Cancedda, R.; Cedola, A. Synchrotron radiation techniques boost the research in bone tissue engineering. Acta Biomater. 2019, 89, 33–46. [Google Scholar] [CrossRef]
- Muehleman, C.; Majumdar, S.; Sema Issever, A.; Arfelli, F.; Menk, R.-H.; Rigon, L.; Heitner, G.; Reime, B.; Metge, J.; Wagner, A.; et al. X-ray detection of structural orientation in human articular cartilage. Osteoarthr. Cartil. 2004, 12, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Coan, P.; Bamberg, F.; Diemoz, P.C.; Bravin, A.; Timpert, K.; Mützel, E.; Raya, J.G.; Adam-Neumair, S.; Reiser, M.F.; Glaser, C. Characterization of osteoarthritic and normal human patella cartilage by computed tomography X-ray phase-contrast imaging: A feasibility study. Investig. Radiol. 2010, 45, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Schmeltz, M.; Ivanovic, A.; Schlepütz, C.M.; Wimmer, W.; Remenschneider, A.K.; Caversaccio, M.; Stampanoni, M.; Anschuetz, L.; Bonnin, A. The human middle ear in motion: 3D visualization and quantification using dynamic synchrotron-based X-ray imaging. Commun. Biol. 2024, 7, 157. [Google Scholar] [CrossRef]
- Kim, S.; Jang, S.; Lee, O. Simultaneous visualization of micro-damage in cortical bone, trabecular bone, and intracortical vasculature for diagnosing osteoporosis: An animal model synchrotron imaging. Microsc. Res. Techniq. 2024, 87, 695–704. [Google Scholar] [CrossRef]
- Bouchet, A.; Serduc, R.; Laissue, J.A.; Djonov, V. Effects of microbeam radiation therapy on normal and tumoral blood vessels. Phys. Med. 2015, 31, 634–641. [Google Scholar] [CrossRef]
- Trappetti, V.; Fazzari, J.M.; Fernandez-Palomo, C.; Scheidegger, M.; Volarevic, V.; Martin, O.A.; Djonov, V.G. Microbeam Radiotherapy-A Novel Therapeutic Approach to Overcome Radioresistance and Enhance Anti-Tumour Response in Melanoma. Int. J. Mol. Sci. 2021, 22, 7755. [Google Scholar] [CrossRef]
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron Microbeam Radiation Therapy as a New Approach for the Treatment of Radioresistant Melanoma: Potential Underlying Mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, A.; Lemasson, B.; Christen, T.; Potez, M.; Rome, C.; Coquery, N.; Le Clec’h, C.; Moisan, A.; Bräuer-Krisch, E.; Leduc, G.; et al. Synchrotron microbeam radiation therapy induces hypoxia in intracerebral gliosarcoma but not in the normal brain. Radiother. Oncol. 2013, 108, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef] [PubMed]
- Eling, L.; Bouchet, A.; Ocadiz, A.; Adam, J.-F.; Kershmiri, S.; Elleaume, H.; Krisch, M.; Verry, C.; Laissue, J.A.; Balosso, J.; et al. Unexpected Benefits of Multiport Synchrotron Microbeam Radiation Therapy for Brain Tumors. Cancers 2021, 13, 936. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Bouchet, A.; Potez, M.; Coquery, N.; Rome, C.; Lemasson, B.; Bräuer-Krisch, E.; Rémy, C.; Laissue, J.; Barbier, E.L.; Djonov, V.; et al. Permeability of Brain Tumor Vessels Induced by Uniform or Spatially Microfractionated Synchrotron Radiation Therapies. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1174–1182. [Google Scholar] [CrossRef]
- Pelliccia, D.; Poole, C.M.; Livingstone, J.; Stevenson, A.W.; Smyth, L.M.L.; Rogers, P.A.W.; Hausermann, D.; Crosbie, J.C. Image guidance protocol for synchrotron microbeam radiation therapy. J. Synchrotron Radiat. 2016, 23, 566–573. [Google Scholar] [CrossRef]
- Livingstone, J.; Stevenson, A.W.; Häusermann, D.; Adam, J.-F. Experimental optimisation of the X-ray energy in microbeam radiation therapy. Phys. Med. 2018, 45, 156–161. [Google Scholar] [CrossRef]
- Shinohara, K.; Kondoh, T.; Nariyama, N.; Fujita, H.; Washio, M.; Aoki, Y. Optimization of X-ray microplanar beam radiation therapy for deep-seated tumors by a simulation study. J. X-Ray Sci. Technol. 2014, 22, 395–406. [Google Scholar] [CrossRef]
- Smyth, L.M.L.; Day, L.R.; Woodford, K.; Rogers, P.A.W.; Crosbie, J.C.; Senthi, S. Identifying optimal clinical scenarios for synchrotron microbeam radiation therapy: A treatment planning study. Phys. Med. 2019, 60, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.; Li, N.; Davis, J.; Paino, J.; Cameron, M.; Dipuglia, A.; Vogel, S.; Valceski, M.; Khochaiche, A.; O’Keefe, A.; et al. Toward personalized synchrotron microbeam radiation therapy. Sci. Rep. 2020, 10, 8833. [Google Scholar] [CrossRef] [PubMed]
- Petasecca, M.; Cullen, A.; Fuduli, I.; Espinoza, A.; Porumb, C.; Stanton, C.; Aldosari, A.H.; Bräuer-Krisch, E.; Requardt, H.; Bravin, A.; et al. X-Tream: A novel dosimetry system for Synchrotron Microbeam Radiation Therapy. J. Instrum. 2012, 7, P07022. [Google Scholar] [CrossRef]
- Davis, J.A.; Paino, J.R.; Dipuglia, A.; Cameron, M.; Siegele, R.; Pastuovic, Z.; Petasecca, M.; Perevertaylo, V.; Rosenfeld, A.; Lerch, M. Characterisation and evaluation of a PNP strip detector for synchrotron microbeam radiation therapy. Biomed. Phys. Eng. Express 2018, 4, 044002. [Google Scholar] [CrossRef]
- Archer, J.; Li, E.; Davis, J.; Cameron, M.; Rosenfeld, A.; Lerch, M. High spatial resolution scintillator dosimetry of synchrotron microbeams. Sci. Rep. 2019, 9, 6873. [Google Scholar] [CrossRef]
- Eling, L.; Verry, C.; Balosso, J.; Flandin, I.; Kefs, S.; Bouchet, A.; Adam, J.F.; Laissue, J.A.; Serduc, R. Neurologic Changes Induced by Whole-Brain Synchrotron Microbeam Irradiation: 10-Month Behavioral and Veterinary Follow-Up. Int. J. Radiat. Oncol. 2024, 120, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Di Manici, I.; Reyes-Herrera, J.; Day, L.; Del Río, M.S.; Krisch, M.; Pellicioli, P. Synchrotron X-ray spectra characterisation for radiation therapy applications at the ESRF-ID17 biomedical beamline. Phys. Scr. 2024, 99, 065021. [Google Scholar] [CrossRef]
- Soliman, Y.S.; Capron, M.; Pontoni, D.; Krisch, M.; Pellicioli, P. Study of a gel dosimeter based on Ag nanoparticles for applications in radiation therapy with synchrotron X-rays at ultrahigh dose rate compared to 60Co γ-rays. Radiat. Phys. Chem. 2025, 227, 112351. [Google Scholar] [CrossRef]
- Bräuer-Krisch, E.; Adam, J.F.; Alagoz, E.; Bartzsch, S.; Crosbie, J.; DeWagter, C.; Dipuglia, A.; Donzelli, M.; Doran, S.; Fournier, P.; et al. Medical physics aspects of the synchrotron radiation therapies: Microbeam radiation therapy (MRT) and synchrotron stereotactic radiotherapy (SSRT). Phys. Med. 2015, 31, 568–583. [Google Scholar] [CrossRef]
- Edouard, M.; Broggio, D.; Prezado, Y.; Estève, F.; Elleaume, H.; Adam, J.F. Treatment plans optimization for contrast-enhanced synchrotron stereotactic radiotherapy. Med. Phys. 2010, 37, 2445–2456. [Google Scholar] [CrossRef]
- Obeid, L.; Deman, P.; Tessier, A.; Balosso, J.; Estève, F.; Adam, J.F. Absolute perfusion measurements and associated iodinated contrast agent time course in brain metastasis: A study for contrast-enhanced radiotherapy. J. Cereb. Blood Flow Metab. 2014, 34, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.F.; Biston, M.C.; Rousseau, J.; Boudou, C.; Charvet, A.M.; Balosso, J.; Estève, F.; Elleaume, H. Heavy element enhanced synchrotron stereotactic radiotherapy as a promising brain tumour treatment. Phys. Med. 2008, 24, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Samalens, L.; Courivaud, C.; Adam, J.F.; Barbier, E.L.; Serduc, R.; Depaulis, A. Innovative minimally invasive options to treat drug-resistant epilepsies. Rev. Neurol. 2024, 180, 599–607. [Google Scholar] [CrossRef] [PubMed]
| Medical Beamlines | Country | Specification of Device | Featured Applications |
|---|---|---|---|
| European Synchrotron Radiation Facility (ID17) | France | Source: Wiggler Energy range: 25–185 keV Beam size: Min (H × V): 10.0 mm × 51.0 µm Max (H × V): 150.0 mm × 7.0 mm Flux: 2 × 1014 ph/s (at 33 keV) | |
| Australian Synchrotron (IMBL) | Australia | Source: Superconducting multipole wiggler Energy range: 25–250 keV Max beam size (H × V): 50 cm × 4 cm bandwidth < 10−3 Flux: 3.39 × 1012 ph/s (at 22.1 keV) | |
| Canadian Light Source (BMIT) | Canada | 05 B1-1 beamline Source: Bending magnet Energy range: 12.6–40 keV Beam size (H × V): 200 mm × 4 mm Flux density: 109 ph/s/mm2 (mono) 1012 ph/s/mm2 (pink) 05ID-2 beamline Source: Superconducting wiggler Energy range: 28–140 keV Beam size (H × V): 160 mm × 10 mm Flux density: 5 × 10 ph/s/mm2 |
|
| Elettra Sincrotrone Trieste (SYRMEP) | Italy | Source: Bending magnet Energy range: 8–40 keV Beam size (H × V): 160 mm × 5 mm Flux density: 2 × 108 ph/s/mm2 (at 20 keV) |
|
| Super Photon ring-8 GeV (BL20B2, XU) | Japan | Source: Bending magnet Energy range: 5–113 keV Beam size 1 (H × V): 75 mm × 5 mm Beam size 2 (H × V): 300 mm × 20 mm Flux density: 3.6 × 108 ph/s/mm2 (at 40 keV) | |
| Advanced Photon Source (2-BM-A, B) | America | Source: Bending magnet Energy range: 11–35 keV Beam size (H × V): 25 mm × 4 mm Flux: 1 × 1012 ph/s (at 17 keV) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, C.; Zhang, J.; Li, Y.; Xie, M.; Sun, D. Application of Synchrotron Radiation in Fundamental Research and Clinical Medicine. Biomedicines 2025, 13, 1419. https://doi.org/10.3390/biomedicines13061419
Xiao C, Zhang J, Li Y, Xie M, Sun D. Application of Synchrotron Radiation in Fundamental Research and Clinical Medicine. Biomedicines. 2025; 13(6):1419. https://doi.org/10.3390/biomedicines13061419
Chicago/Turabian StyleXiao, Chao, Jinde Zhang, Yang Li, Mingyuan Xie, and Dongbai Sun. 2025. "Application of Synchrotron Radiation in Fundamental Research and Clinical Medicine" Biomedicines 13, no. 6: 1419. https://doi.org/10.3390/biomedicines13061419
APA StyleXiao, C., Zhang, J., Li, Y., Xie, M., & Sun, D. (2025). Application of Synchrotron Radiation in Fundamental Research and Clinical Medicine. Biomedicines, 13(6), 1419. https://doi.org/10.3390/biomedicines13061419






