Mechanisms Underlying Hyperexcitability: Combining Mossy Fiber Sprouting and Mossy Cell Loss in Neural Network Model of the Dentate Gyrus
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of Biophysically Accurate Multicompartmental Models of Dentate Cells
2.2. Granule Cells
2.3. Mossy Cells
2.4. Basket Cells
2.5. Hilar Perforant Path-Associated Cells
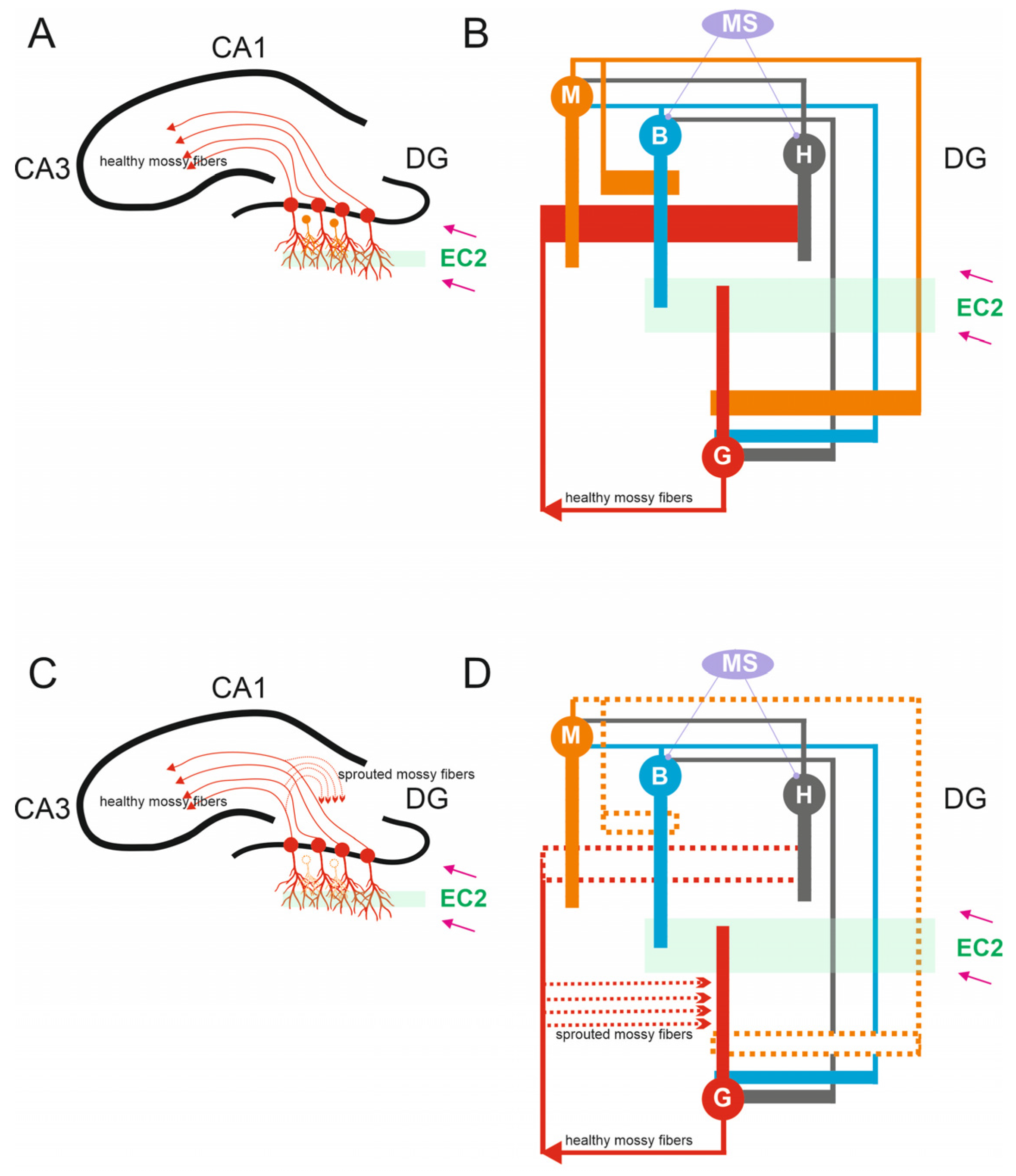
2.6. Simulating Mossy Fiber Sprouting
2.7. Mossy Cell Loss
2.8. Statistical Methods
3. Results
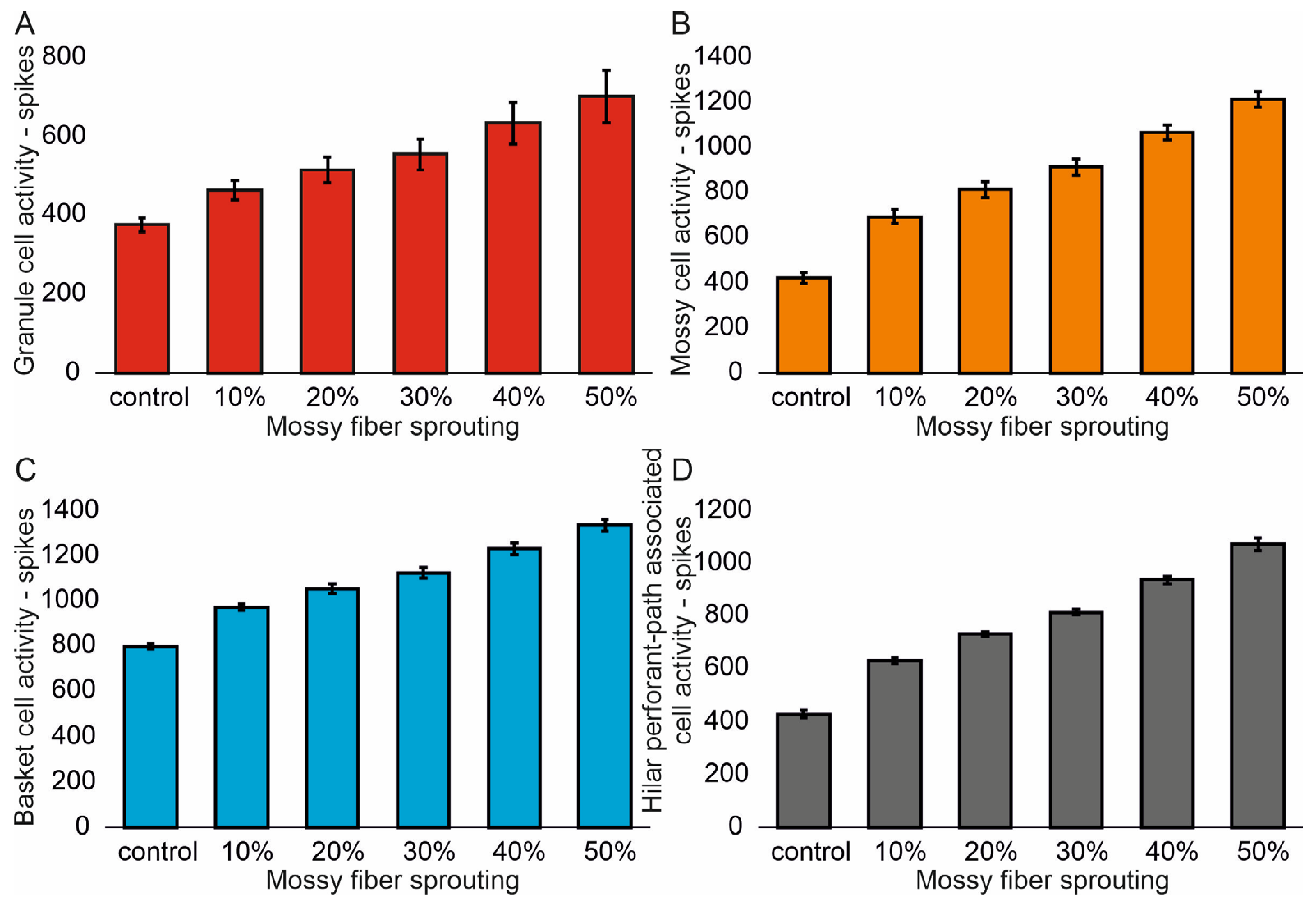
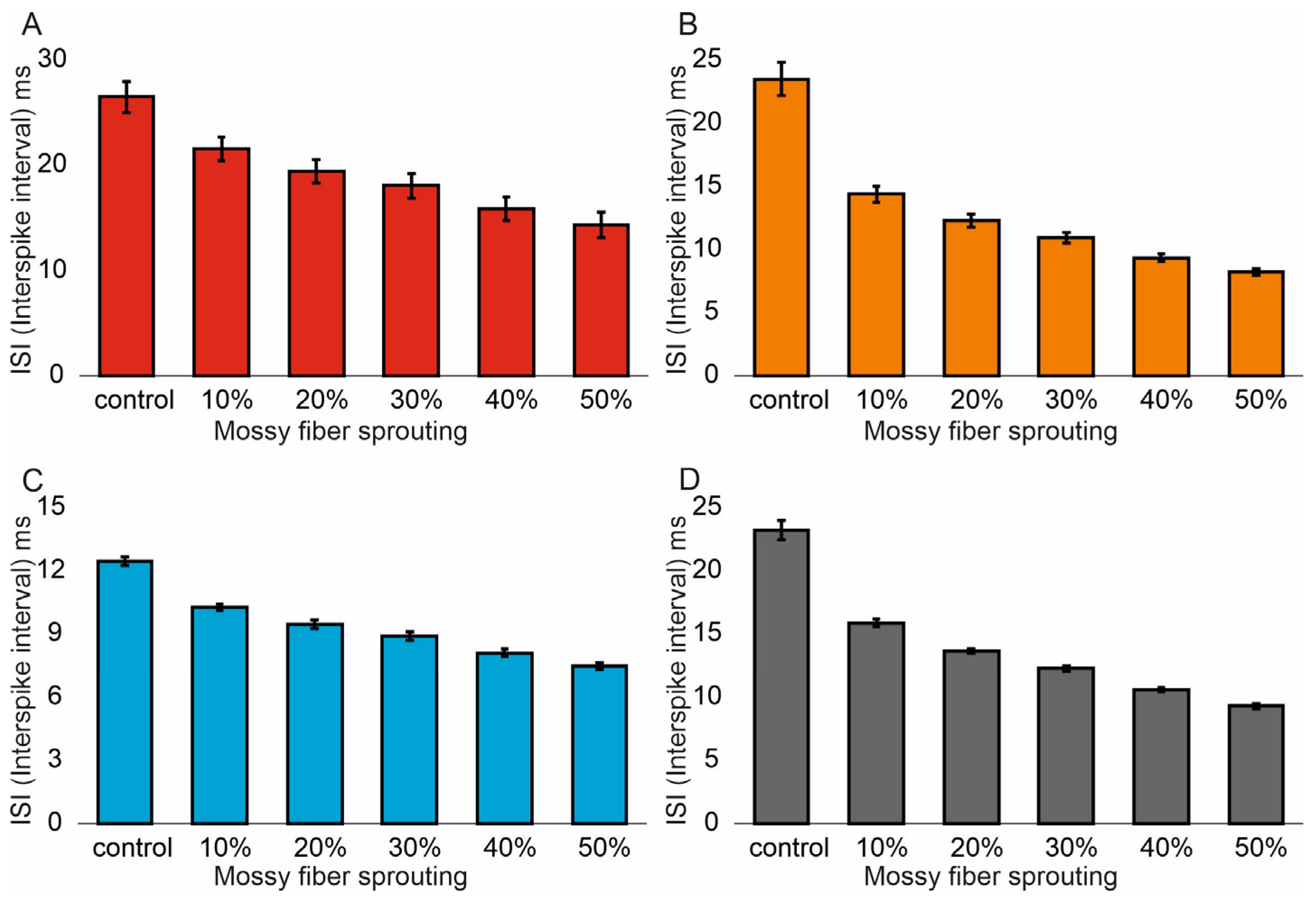
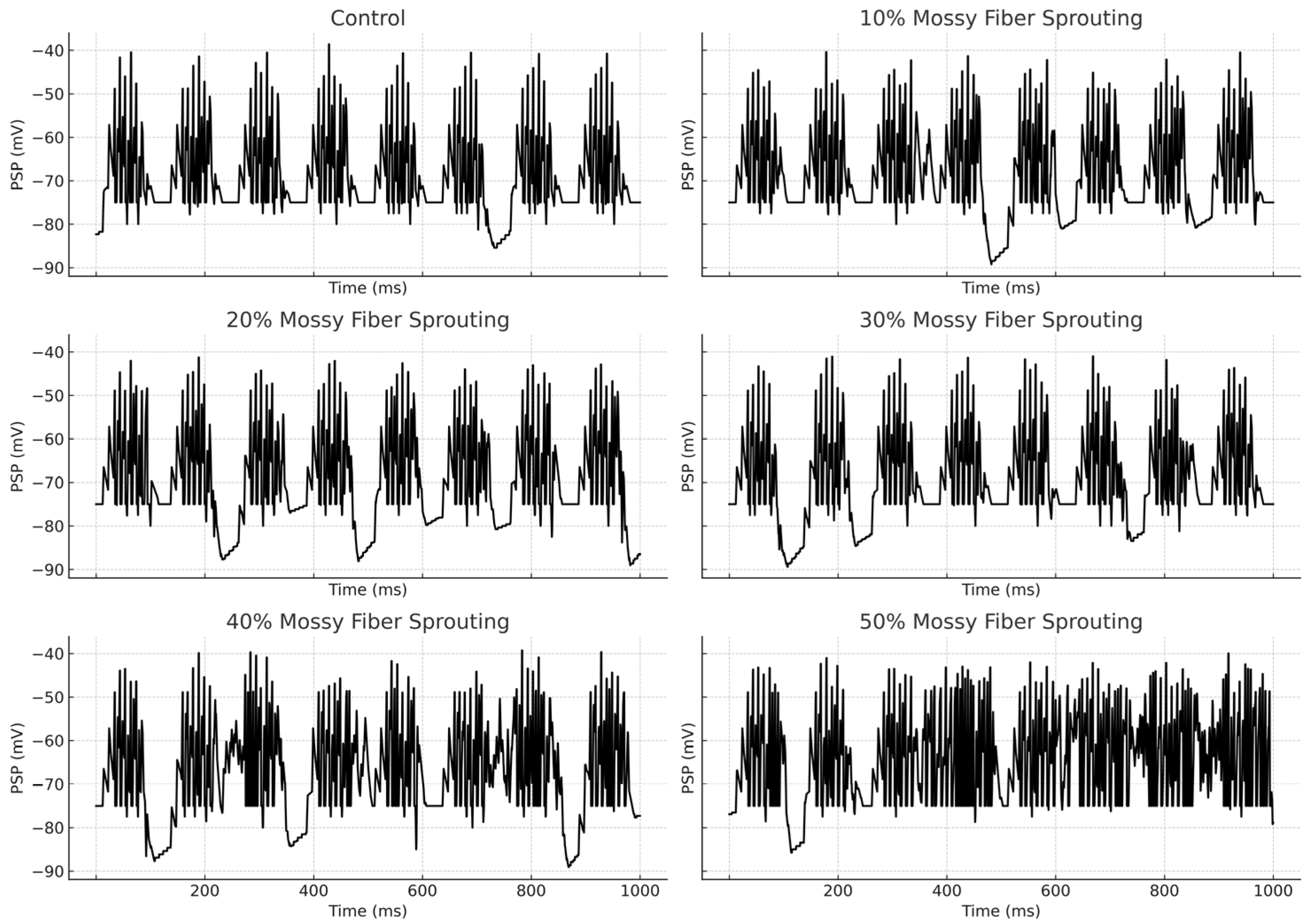
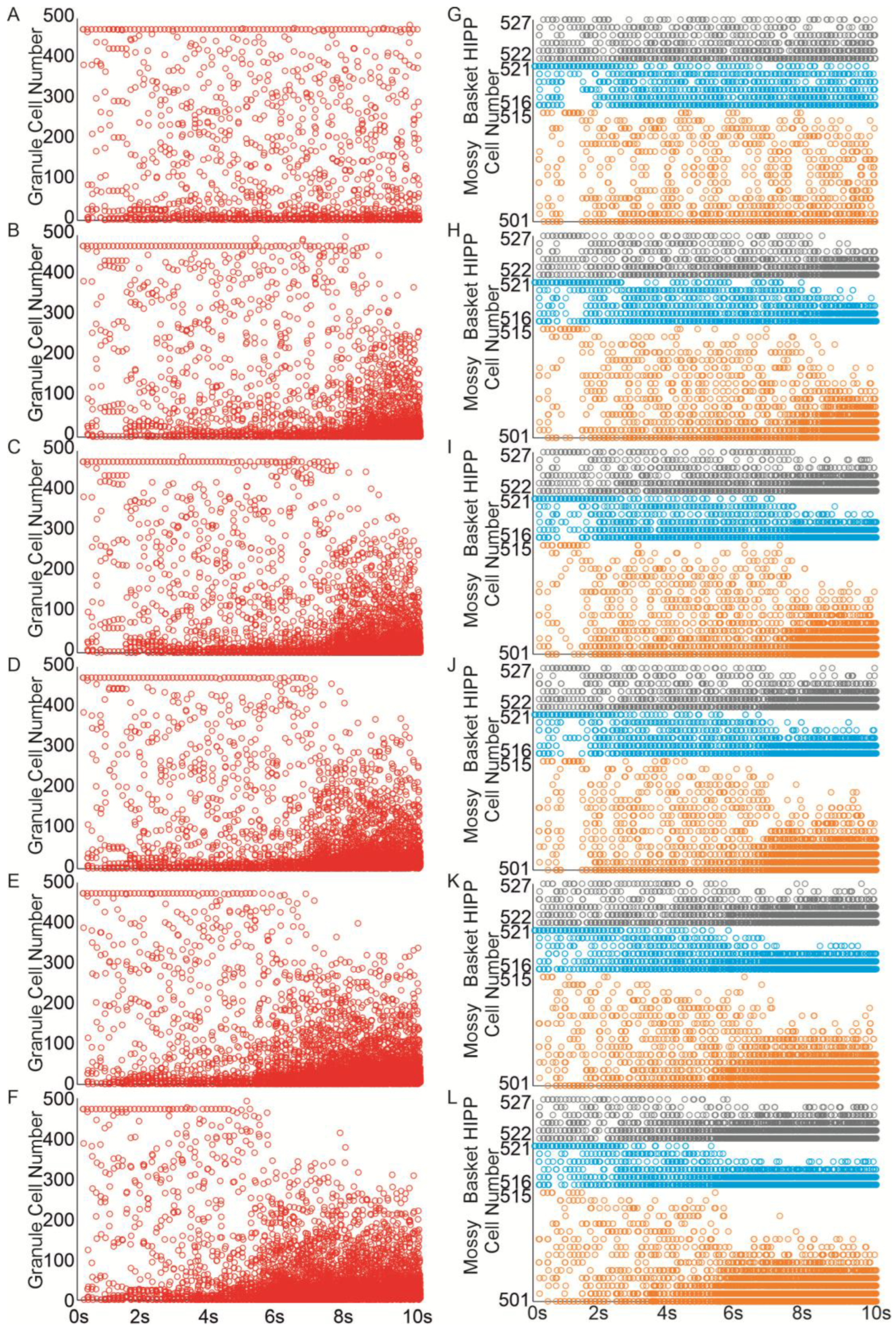
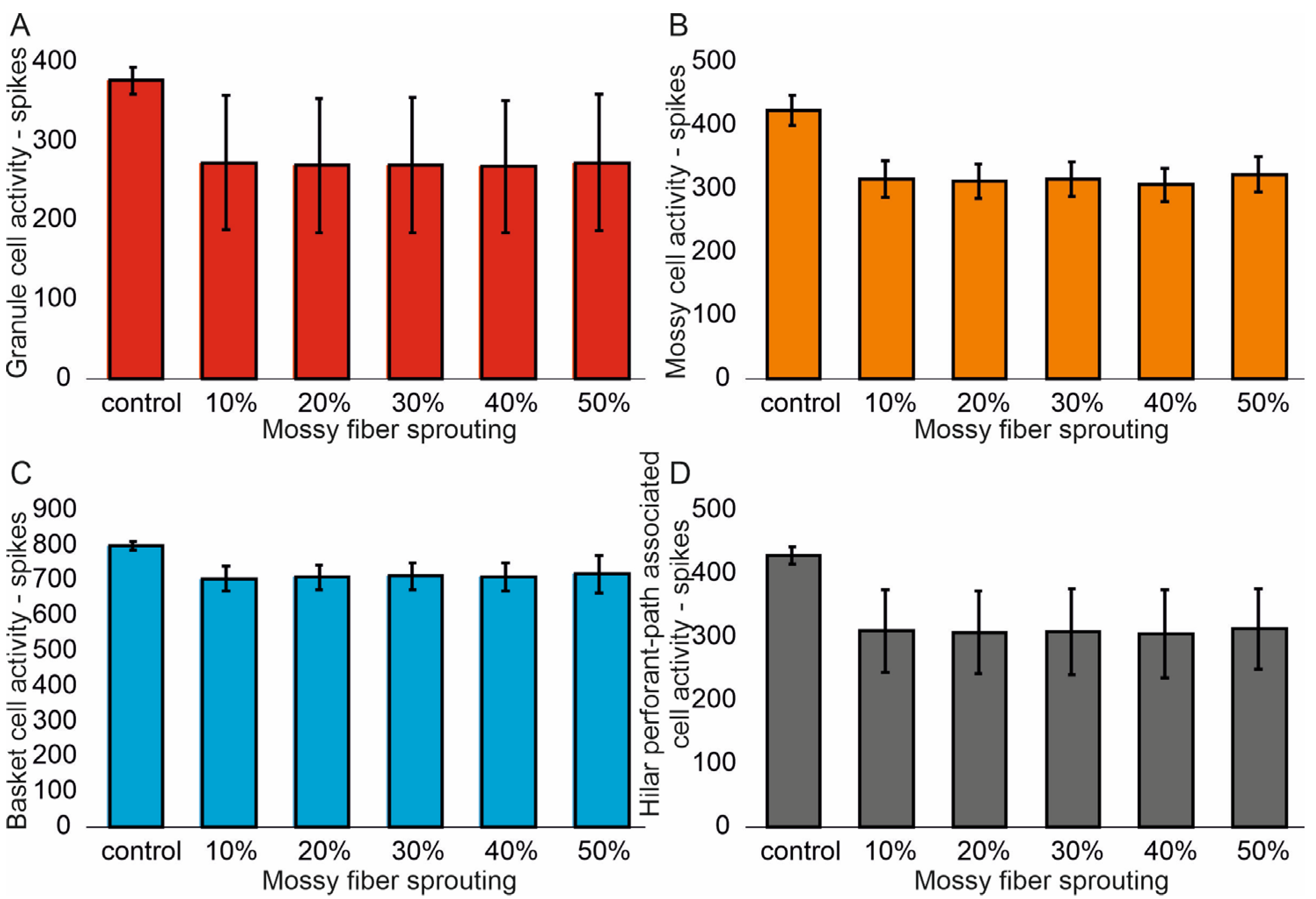
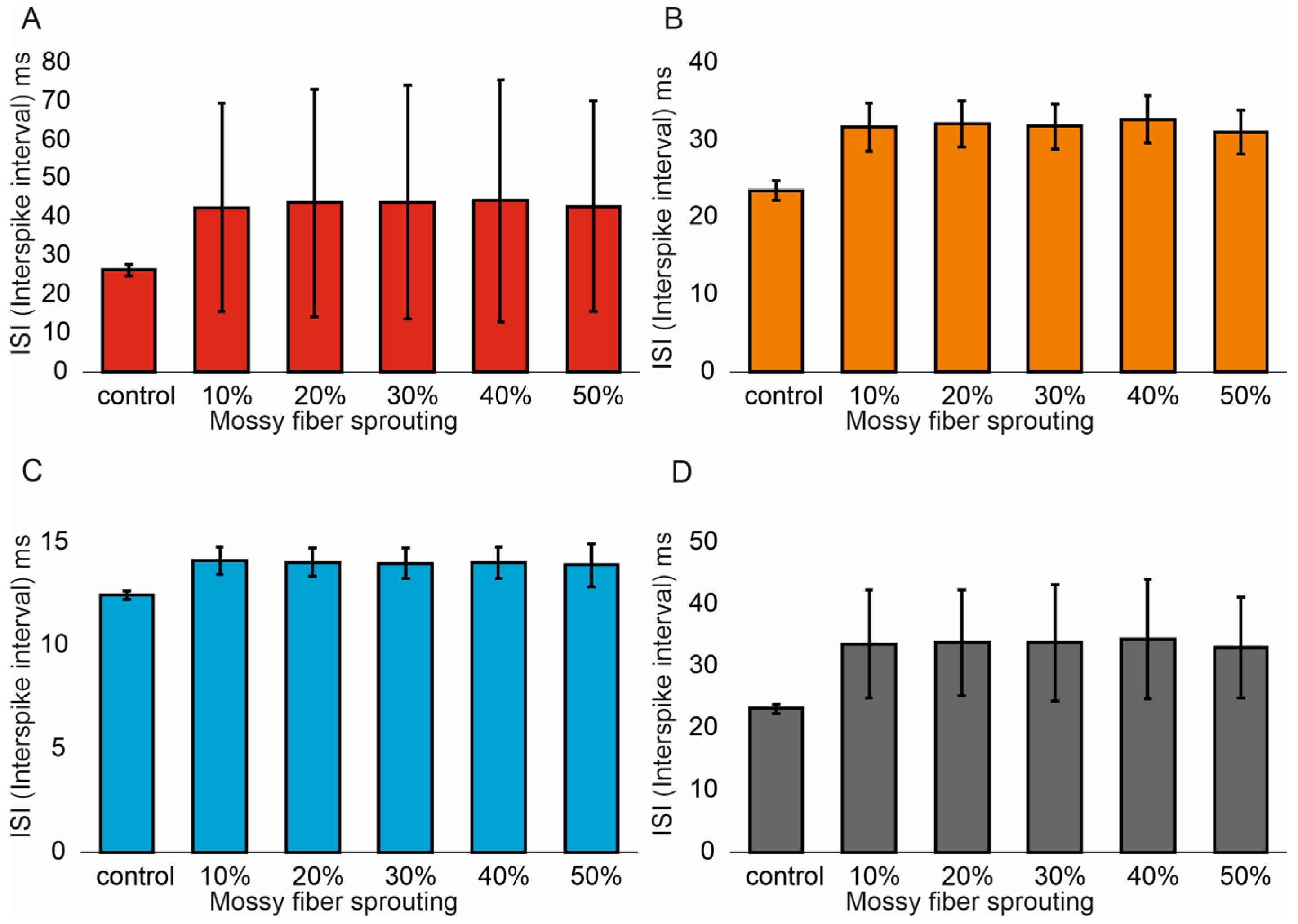
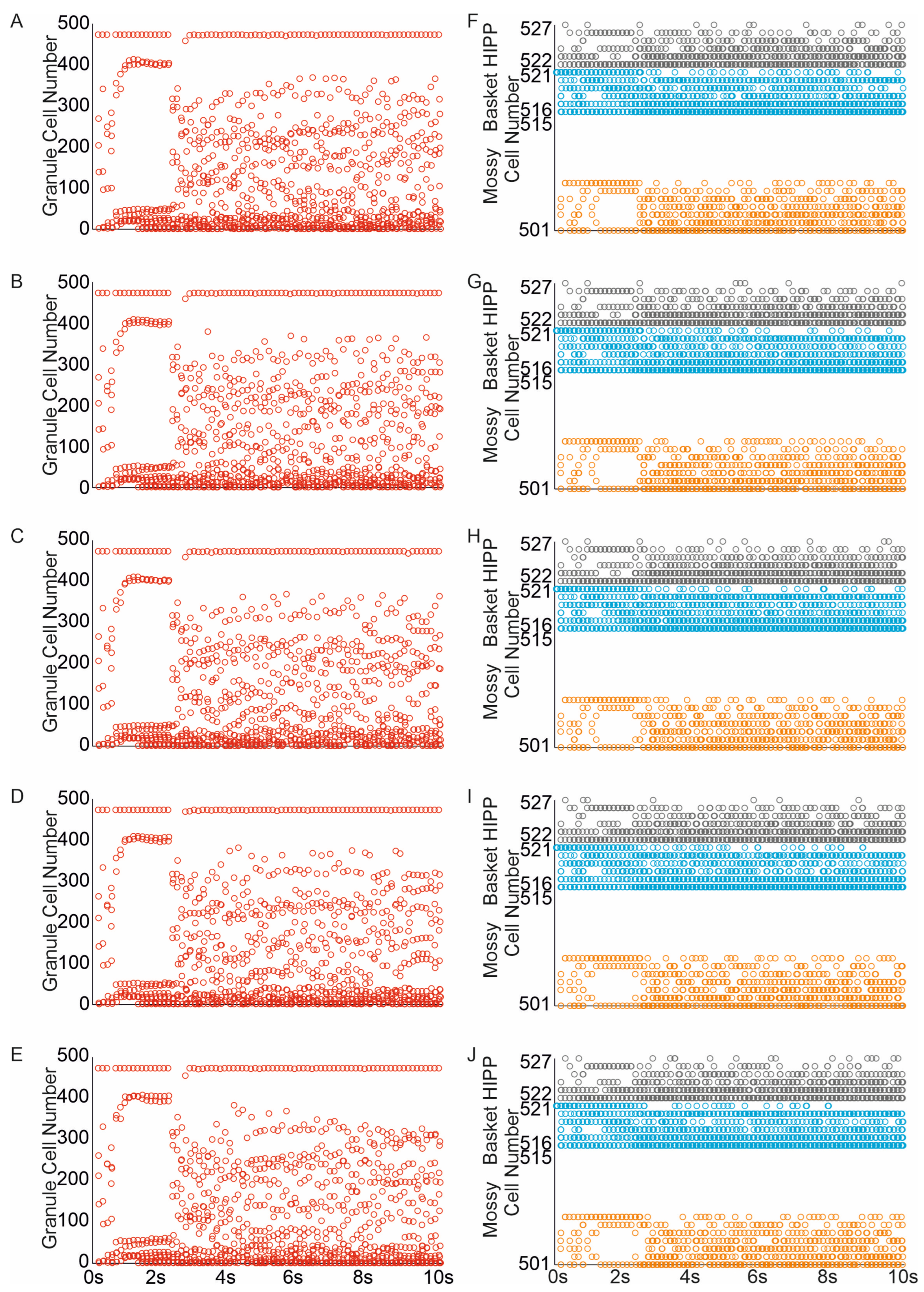
3.1. The Role of Pathological Phenomena of Mossy Fiber Sprouting
3.2. The Role of Pathological Phenomena of Mossy Fiber Sprouting and Mossy Cell Loss
4. Discussion
5. Conclusions
6. Limitations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowenstein, D.H.; Thomas, M.J.; Smith, D.H.; McIntosh, T.K. Selective vulnerability of dentate hilar neurons following traumatic brain injury: A potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992, 12, 4846–4853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toth, Z.; Hollrigel, G.S.; Gorcs, T.; Soltesz, I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J. Neurosci. 1997, 17, 8106–8117. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, V.; Bender, R.; Frotscher, M.; Ross, S.T.; Hollrigel, G.S.; Toth, Z.; Soltesz, I. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: The “irritable mossy cell” hypothesis. J. Physiol. 2000, 524, 117–134. [Google Scholar] [CrossRef]
- Frankowski, J.C.; Tierno, A.; Pavani, S.; Cao, Q.; Lyon, D.C.; Hunt, R.F. Brain-wide reconstruction of inhibitory circuits after traumatic brain injury. Nat. Commun. 2022, 13, 3417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santhakumar, V.; Ratzliff, A.D.; Jeng, J.; Toth, K.; Soltesz, I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann. Neurol. 2001, 50, 708–717. [Google Scholar] [CrossRef]
- Golarai, G.; Greenwood, A.C.; Feeney, D.M.; Connor, J.A. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J. Neurosci. 2001, 21, 8523–8537. [Google Scholar] [CrossRef]
- Zhang, N.; Houser, C.R. Ultrastructural localization of dynorphin in the dentate gyrus in human temporal lobe epilepsy: A study of reorganized mossy fiber synapses. J. Comp. Neurol. 1999, 405, 472–490. [Google Scholar] [CrossRef]
- McIntosh, W.C.; Das, J.M. Temporal Seizure. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549852/ (accessed on 4 July 2023).
- Zhang, C.; He, Z.; Tan, Z.; Tian, F. Silencing of dentate gyrus inhibits mossy fiber sprouting and prevents epileptogenesis through NDR2 kinase in pentylenetetrazole kindling rat model of TLE. PLoS ONE 2023, 18, e0284359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Wu, X.L.; Hu, H.B.; Yang, S.N.; Zhang, Z.Y.; Fu, G.L.; Zhang, C.T.; Li, Z.M.; Wu, F.; Si, K.W.; et al. Neuronal MeCP2 in the dentate gyrus regulates mossy fiber sprouting of mice with temporal lobe epilepsy. Neurobiol. Dis. 2023, 188, 106346. [Google Scholar] [CrossRef] [PubMed]
- Sack, A.S. Adult-Born Granule Cells Contribute to Dentate Gyrus Circuit Reorganization after Traumatic Brain Injury. J. Neurosci. 2023, 43, 879–881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masukawa, L.M.; O’Connor, W.M.; Lynott, J.; Burdette, L.J.; Uruno, K.; McGonigle, P.; O’Connor, M.J. Longitudinal variation in cell density and mossy fiber reorganization in the dentate gyrus from temporal lobe epileptic patients. Brain Res. 1995, 678, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Mathern, G.W.; Pretorius, J.K.; Babb, T.L. Influence of the type of initial precipitating injury and at what age it occurs on course and outcome in patients with temporal lobe seizures. J. Neurosurg. 1995, 82, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mathern, G.W.; Pretorius, J.K.; Babb, T.L.; Quinn, B. Unilateral hippocampal mossy fiber sprouting and bilateral asymmetric neuron loss with episodic postictal psychosis. J. Neurosurg. 1995, 82, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, H.J.; Woolley, C.S.; Robbins, C.A.; Schwartzkroin, P.A. Kainic acid-induced mossy fiber sprouting and synapse formation in the dentate gyrus of rats. Hippocampus 2000, 10, 244–260. [Google Scholar] [CrossRef]
- Henze, D.A.; Urban, N.N.; Barrionuevo, G. The multifarious hippocampal mossy fiber pathway: A review. Neuroscience 2000, 98, 407–427. [Google Scholar] [CrossRef]
- Buckmaster, P.S.; Zhang, G.F.; Yamawaki, R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J. Neurosci. 2002, 22, 6650–6658. [Google Scholar] [CrossRef]
- Sutula, T.; Lauersdorf, S.; Lynch, M.; Jurgella, C.; Woodard, A. Deficits in radial arm maze performance in kindled rats: Evidence for long-lasting memory dysfunction induced by repeated brief seizures. J. Neurosci. 1995, 15, 8295–8301. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J.; Kusiak, A.; Cichońska, D. Memory and forgetting processes with the firing neuron model. Folia Morphol. 2018, 77, 221–233. [Google Scholar] [CrossRef]
- Świetlik, D. Simulations of Learning, Memory, and Forgetting Processes with Model of CA1 Region of the Hippocampus. Complexity 2018, 2018, 1297150. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J.; Kusiak, A.; Cichońska, D. A computational simulation of long-term synaptic potentiation inducing protocol processes with model of CA3 hippocampal microcircuit. Folia Morphol. 2018, 77, 210–220. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J.; Moryś, J.; Kusiak, A. Computer Model of Synapse Loss During an Alzheimer’s Disease-like Pathology in Hippocampal Subregions DG, CA3 and CA1—The Way to Chaos and Information Transfer. Entropy 2019, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, V.; Aradi, I.; Soltesz, I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: A network model of the dentate gyrus incorporating cell types and axonal topography. J. Neurophysiol. 2005, 93, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.J.; Soltesz, I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc. Natl. Acad. Sci. USA 2008, 105, 6179–6184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Almeida, A.C.; Rodrigues, A.M.; Scorza, F.A.; Cavalheiro, E.A.; Teixeira, H.Z.; Duarte, M.A.; Silveira, G.A.; Arruda, E.Z. Mechanistic hypotheses for nonsynaptic epileptiform activity induction and its transition from the interictal to ictal state—Computational simulation. Epilepsia 2008, 49, 1908–1924. [Google Scholar] [CrossRef] [PubMed]
- Dyhrfjeld-Johnsen, J.; Santhakumar, V.; Morgan, R.J.; Huerta, R.; Tsimring, L.; Soltesz, I. Topological determinants of epileptogenesis in large-scale structural and functional models of the dentate gyrus derived from experimental data. J. Neurophysiol. 2007, 97, 1566–1587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, D.; Wang, Q. Transition Dynamics of a Dentate Gyrus-CA3 Neuronal Network during Temporal Lobe Epilepsy. Front. Comput. Neurosci. 2017, 11, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Y.; Han, F.; Wang, Q. A Hippocampal-Entorhinal Cortex Neuronal Network for Dynamical Mechanisms of Epileptic Seizure. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1986–1996. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S. In silico ADME-Tox modeling: Progress and prospects. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 1147–1158. [Google Scholar] [CrossRef]
- Kumar, S.; Chowdhury, S.; Kumar, S. In silico repurposing of antipsychotic drugs for Alzheimer’s disease. BMC Neurosci. 2017, 18, 76. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J.; Kusiak, A.; Krasny, M. Virtual Therapy with the NMDA Antagonist Memantine in Hippocampal Models of Moderate to Severe Alzheimer’s Disease, in Silico Trials. Pharmaceuticals 2022, 15, 546. [Google Scholar] [CrossRef]
- Joshi, H.; Jha, B.K. Generalized Diffusion Characteristics of Calcium Model with Concentration and Memory of Cells: A Spatiotemporal Approach. Iran. J. Sci. Technol. Trans. Sci. 2022, 46, 309–322. [Google Scholar] [CrossRef]
- Upadhyay, D.; Joshi, H. Mathematical modeling of local calcium signaling in neurons using artificial neural networks. Discret. Contin. Dyn. Syst.—S 2025, 18, 1392–1415. [Google Scholar] [CrossRef]
- Patton, P.E.; McNaughton, B. Connection matrix of the hippocampal formation: I. The dentate gyrus. Hippocampus 1995, 5, 245–286. [Google Scholar] [CrossRef] [PubMed]
- Aradi, I.; Holmes, W.R. Role of multiple calcium and calcium-dependent conductances in regulation of hippocampal dentate granule cell excitability. J. Comput. Neurosci. 1999, 6, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Bartos, M.; Vida, I.; Frotscher, M.; Geiger, J.R.; Jonas, P. Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J. Neurosci. 2001, 21, 2687–2698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buckmaster, P.S.; Strowbridge, B.W.; Kunkel, D.D.; Schmiege, D.L.; Schwartzkroin, P.A. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus 1992, 2, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Buzsáki, G. Interneurons of the hippocampus. Hippocampus 1996, 6, 347–470. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.R.; Lübke, J.; Roth, A.; Frotscher, M.; Jonas, P. Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 1997, 18, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Saraga, F.; Wu, C.P.; Zhang, L.; Skinner, F.K. Active dendrites and spike propagation in multi-compartment models of oriens-lacunosum/moleculare hippocampal interneurons. J. Physiol. 2003, 552 Pt 3, 673–689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lübke, J.; Frotscher, M.; Spruston, N. Specialized electrophysiological properties of anatomically identified neurons in the hilar region of the rat fascia dentata. J. Neurophysiol. 1998, 79, 1518–1534. [Google Scholar] [CrossRef] [PubMed]
- Sutula, T.; Zhang, P.; Lynch, M.; Sayin, U.; Golarai, G.; Rod, R. Synaptic and axonal remodeling of mossy fibers in the hilus and supragranular region of the dentate gyrus in kainate-treated rats. J. Comp. Neurol. 1998, 390, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.; Dudek, F.E. Chronic seizures and collateral sprouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res. 1988, 474, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Mello, L.E.; Cavalheiro, E.A.; Tan, A.M.; Kupfer, W.R.; Pretorius, J.K.; Babb, T.L.; Finch, D.M. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: Cell loss and mossy fiber sprouting. Epilepsia 1993, 34, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.M.; Evenson, D.A.; Nadler, J.V. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: Visualization after retrograde transport of biocytin. J. Comp. Neurol. 1995, 352, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Ratzliff, A.d.H.; Santhakumar, V.; Howard, A.; Soltesz, I. Mossy cells in epilepsy: Rigor mortis or vigor mortis? Trends Neurosci. 2002, 25, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Ratzliff, A.d.H.; Howard, A.L.; Santhakumar, V.; Osapay, I.; Soltesz, I. Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: Implications for epileptogenesis. J. Neurosci. 2004, 24, 2259–2269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sierra, A.; Gröhn, O.; Pitkänen, A. Imaging microstructural damage and plasticity in the hippocampus during epileptogenesis. Neuroscience 2015, 309, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Ren, B.X.; Tang, F.R. Neurogenesis in the Hippocampus of Patients with Temporal Lobe Epilepsy. Curr. Neurol. Neurosci. Rep. 2016, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R.; Ikegaya, Y. Mossy fiber sprouting as a potential therapeutic target for epilepsy. Curr. Neurovasc. Res. 2004, 1, 3–10, Erratum in Curr. Neurovasc. Res. 2004, 1, 191. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R. Dentate Circuitry as a Model to Study Epileptogenesis. Biol. Pharm. Bull. 2016, 39, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Engel, J., Jr.; Eliashiv, D.S.; García, H.H. Update on Cysticercosis Epileptogenesis: The Role of the Hippocampus. Curr. Neurol. Neurosci. Rep. 2016, 16, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Traub, R.D.; Knowles, W.D.; Miles, R.; Wong, R.K. Models of the cellular mechanism underlying propagation of epileptiform activity in the CA2-CA3 region of the hippocampal slice. Neuroscience 1987, 21, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.D.; Jefferys, J.G.; Whittington, M.A. Enhanced NMDA conductance can account for epileptiform activity induced by low Mg2+ in the rat hippocampal slice. J. Physiol. 1994, 478 Pt 3, 379–393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- França, K.L.; de Almeida, A.C.; Infantosi, A.F.; Duarte, M.A.; da Silveira, G.A.; Scorza, F.A.; Arida, R.M.; Cavalheiro, E.A.; Rodrigues, A.M. Enhanced synaptic connectivity in the dentate gyrus during epileptiform activity: Network simulation. Comput. Intell. Neurosci. 2013, 2013, 949816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathern, G.W.; Bertram, E.H., 3rd; Babb, T.L.; Pretorius, J.K.; Kuhlman, P.A.; Spradlin, S.; Mendoza, D. In contrast to kindled seizures, the frequency of spontaneous epilepsy in the limbic status model correlates with greater aberrant fascia dentata excitatory and inhibitory axon sprouting, and increased staining for N-methyl-D-aspartate, AMPA and GABA(A) receptors. Neuroscience 1997, 77, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Nadler, J.V. The recurrent mossy fiber pathway of the epileptic brain. Neurochem. Res. 2003, 28, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Houser, C.R. Neuronal loss and synaptic reorganization in temporal lobe epilepsy. Adv. Neurol. 1999, 79, 743–761. [Google Scholar] [PubMed]
- Masukawa, L.M.; Burdette, L.J.; McGonigle, P.; Wang, H.; O’Connor, W.; Sperling, M.R.; O’Connor, M.J.; Uruno, K. Physiological and anatomical correlates of the human dentate gyrus: Consequences or causes of epilepsy. Adv. Neurol. 1999, 79, 781–794. [Google Scholar] [PubMed]
- Bernstein, H.L.; Lu, Y.L.; Botterill, J.J.; Duffy, Á.M.; LaFrancois, J.J.; Scharfman, H.E. Field EPSPs of Dentate Gyrus Granule Cells Studied by Selective Optogenetic Activation of Hilar Mossy Cells in Hippocampal Slices. Hippocampus 2025, 35, e23652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butler, C.R.; Westbrook, G.L.; Schnell, E. Adaptive Mossy Cell Circuit Plasticity after Status Epilepticus. J. Neurosci. 2022, 42, 3025–3036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Physiological Property | Granule Cell | Mossy Cell | Basket Cell | Hilar Perforant Path-Associated Cell |
|---|---|---|---|---|
| RP, mV | −75 | −60 | −60 | −70 |
| TP, mV | −49 | −48 | −45 | −50 |
| TR, ms | 1.5 | 1.5 | 1.5 | 1.5 |
| EPSPa, mV | 4.7 | 4 | 7.5 | 6 |
| IPSPa, mV | −5 | −6 | −4 | −6 |
| CaT, mV | −68 | −68 | x | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świetlik, D. Mechanisms Underlying Hyperexcitability: Combining Mossy Fiber Sprouting and Mossy Cell Loss in Neural Network Model of the Dentate Gyrus. Biomedicines 2025, 13, 1416. https://doi.org/10.3390/biomedicines13061416
Świetlik D. Mechanisms Underlying Hyperexcitability: Combining Mossy Fiber Sprouting and Mossy Cell Loss in Neural Network Model of the Dentate Gyrus. Biomedicines. 2025; 13(6):1416. https://doi.org/10.3390/biomedicines13061416
Chicago/Turabian StyleŚwietlik, Dariusz. 2025. "Mechanisms Underlying Hyperexcitability: Combining Mossy Fiber Sprouting and Mossy Cell Loss in Neural Network Model of the Dentate Gyrus" Biomedicines 13, no. 6: 1416. https://doi.org/10.3390/biomedicines13061416
APA StyleŚwietlik, D. (2025). Mechanisms Underlying Hyperexcitability: Combining Mossy Fiber Sprouting and Mossy Cell Loss in Neural Network Model of the Dentate Gyrus. Biomedicines, 13(6), 1416. https://doi.org/10.3390/biomedicines13061416





