Pharmacological and Pathological Implications of Sigma-1 Receptor in Neurodegenerative Diseases
Abstract
1. Introduction
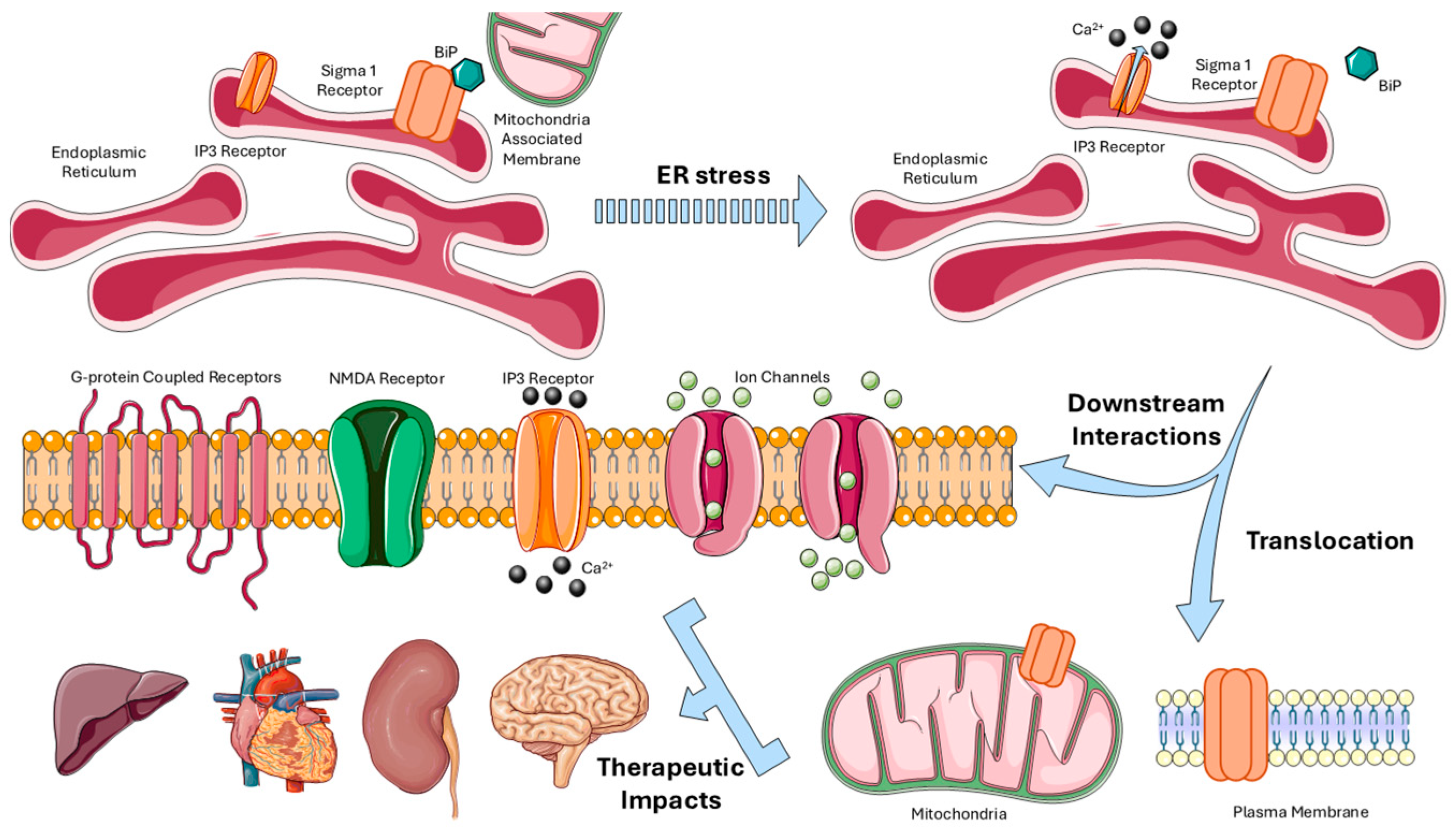
2. Sigma-1 Receptor: Structure, Functions, and Pharmacology
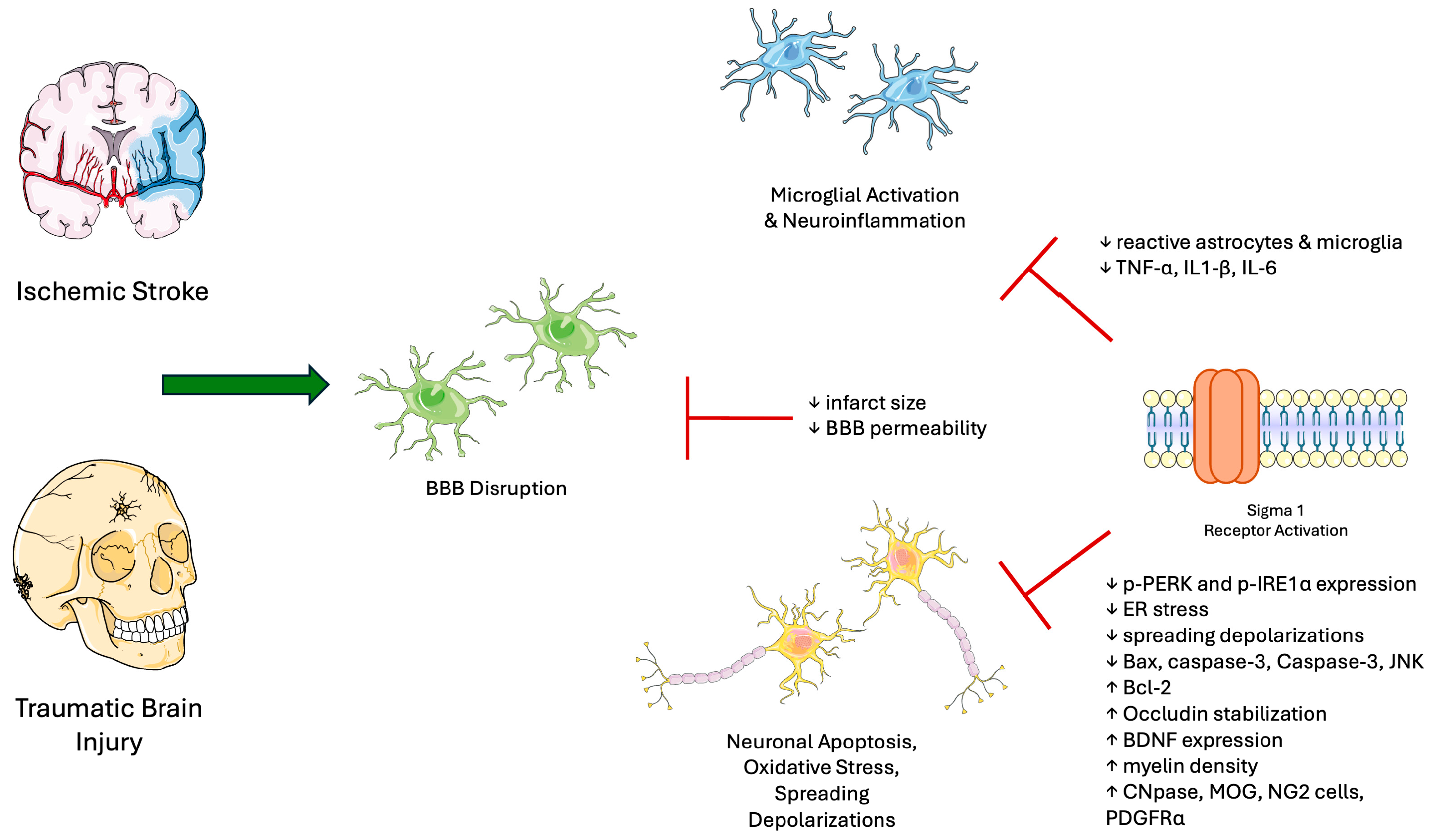
3. Sigma-1 Receptor in Nerve Injury
4. Sigma-1 Receptor in Neurodegenerative Disorders
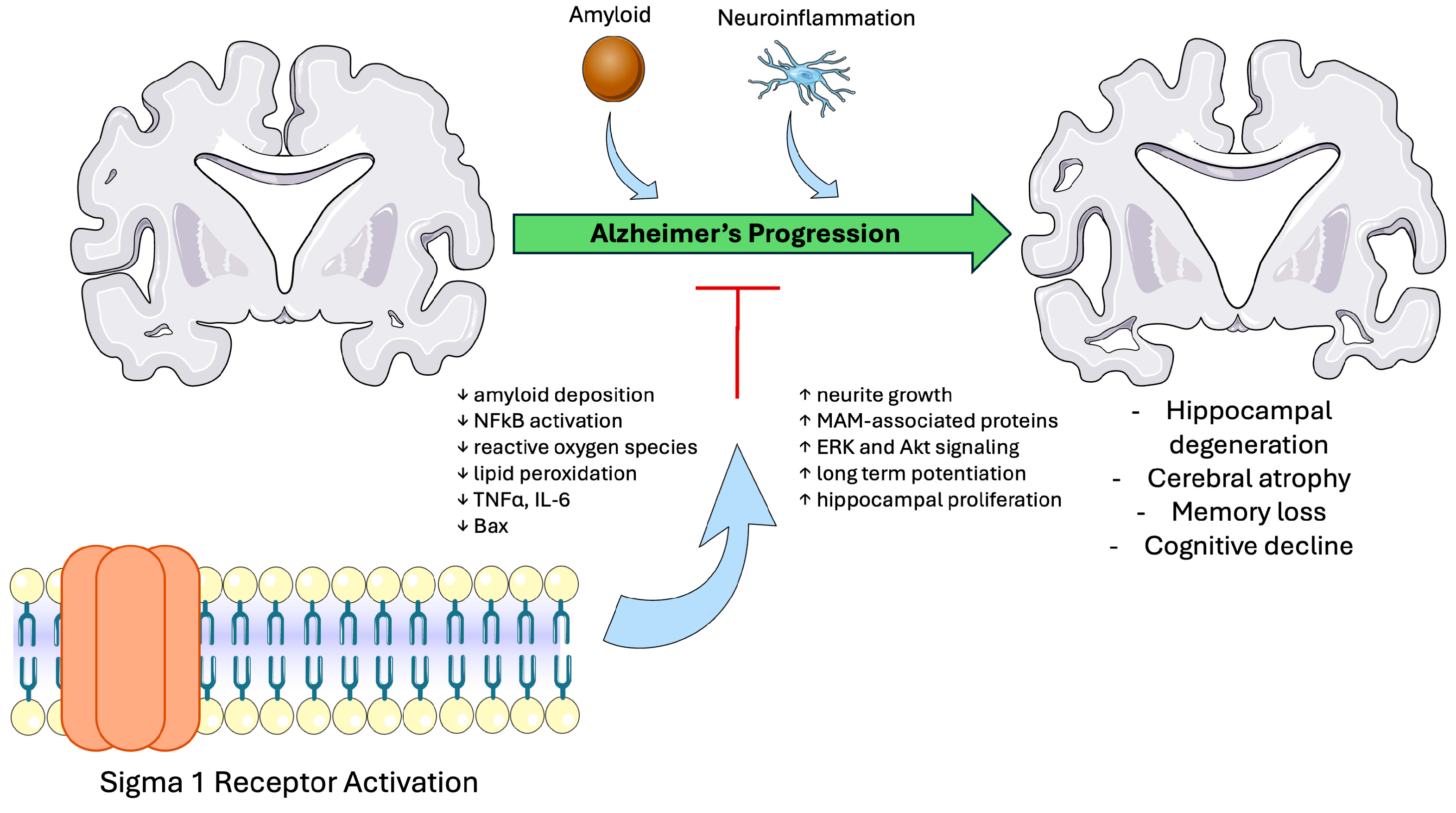
4.1. Sigma-1 Receptor in Alzheimer’s Disease
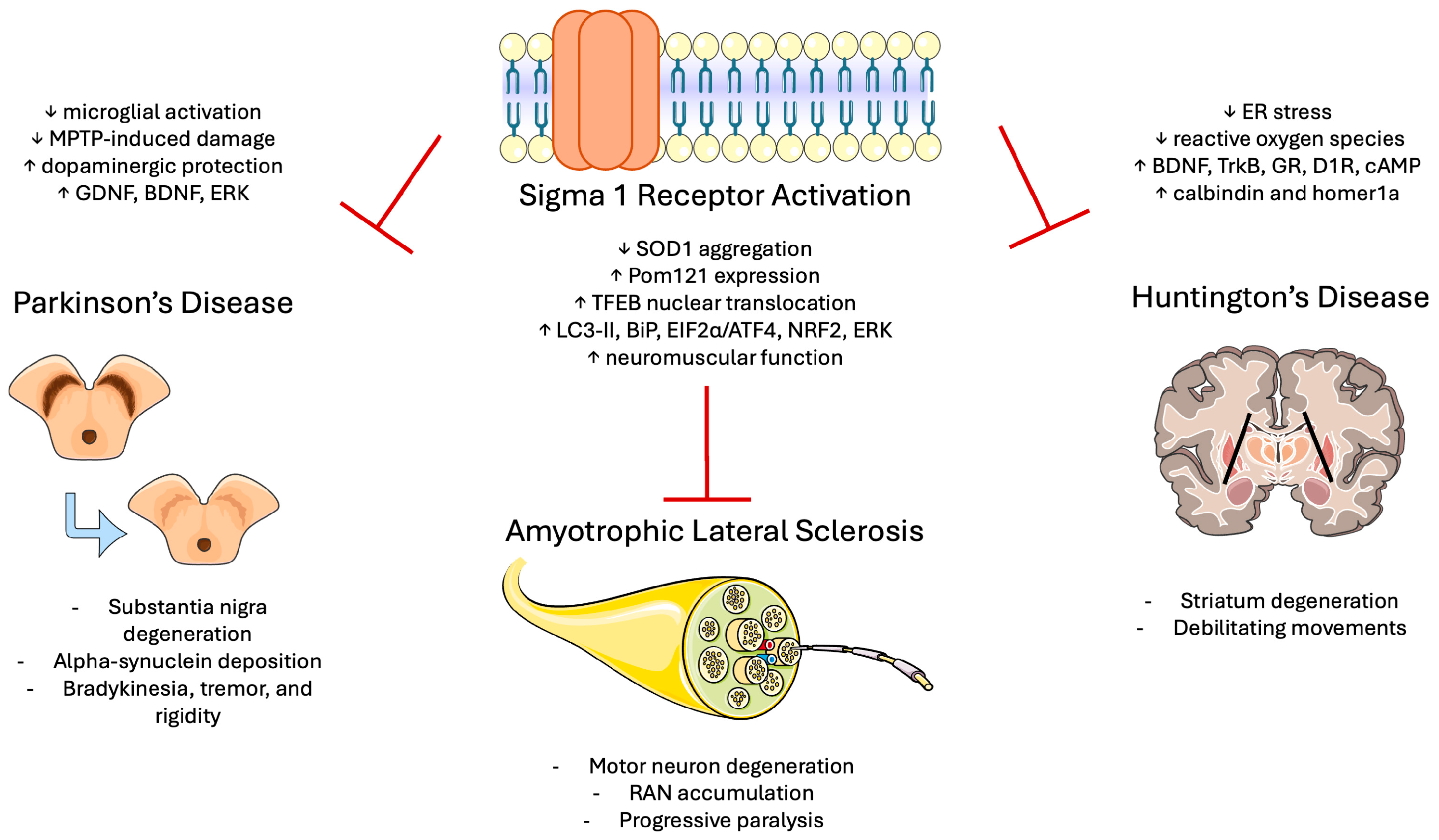
4.2. Sigma-1 Receptor in ALS
4.3. Sigma-1 Receptor in Huntington’s Disease
4.4. Sigma-1 Receptor in Parkinson’s Disease
4.5. Sigma-1 Receptor in Demyelinating Disorders
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, W.R.; Eades, C.G.; Thompson, J.A.; Huppler, R.E.; Gilbert, P.E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.W.; Cook, L. Sigma opiates and certain antipsychotic drugs mutually inhibit (+)-[3H] SKF 10,047 and [3H]haloperidol binding in guinea pig brain membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 5618–5621. [Google Scholar] [CrossRef] [PubMed]
- Su, T.P. Evidence for sigma opioid receptor: Binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther. 1982, 223, 284–290. [Google Scholar] [CrossRef]
- Quirion, R.; Bowen, W.D.; Itzhak, Y.; Junien, J.L.; Musacchio, J.M.; Rothman, R.B.; Su, T.P.; Tam, S.W.; Taylor, D.P. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992, 13, 85–86. [Google Scholar] [CrossRef]
- Hanner, M.; Moebius, F.F.; Flandorfer, A.; Knaus, H.G.; Striessnig, J.; Kempner, E.; Glossmann, H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. USA 1996, 93, 8072–8077. [Google Scholar] [CrossRef]
- Alon, A.; Schmidt, H.R.; Wood, M.D.; Sahn, J.J.; Martin, S.F.; Kruse, A.C. Identification of the gene that codes for the sigma(2) receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 7160–7165. [Google Scholar] [CrossRef] [PubMed]
- Su, T.P.; Hayashi, T. Understanding the molecular mechanism of sigma-1 receptors: Towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr. Med. Chem. 2003, 10, 2073–2080. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T. The sigma receptor: Evolution of the concept in neuropsychopharmacology. Curr. Neuropharmacol. 2005, 3, 267–280. [Google Scholar] [CrossRef]
- Palmer, C.P.; Mahen, R.; Schnell, E.; Djamgoz, M.B.; Aydar, E. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res. 2007, 67, 11166–11175. [Google Scholar] [CrossRef]
- Prasad, P.D.; Li, H.W.; Fei, Y.J.; Ganapathy, M.E.; Fujita, T.; Plumley, L.H.; Yang-Feng, T.L.; Leibach, F.H.; Ganapathy, V. Exon-intron structure, analysis of promoter region, and chromosomal localization of the human type 1 sigma receptor gene. J. Neurochem. 1998, 70, 443–451. [Google Scholar] [CrossRef]
- Kekuda, R.; Prasad, P.D.; Fei, Y.J.; Leibach, F.H.; Ganapathy, V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem. Biophys. Res. Commun. 1996, 229, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Seth, P.; Leibach, F.H.; Ganapathy, V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem. Biophys. Res. Commun. 1997, 241, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human σ1 receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, M.; Kiefer, F.; Biasini, M.; Bordoli, L.; Schwede, T. Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci. Rep. 2017, 7, 10480. [Google Scholar] [CrossRef]
- Hong, W.C. Distinct Regulation of sigma (1) Receptor Multimerization by Its Agonists and Antagonists in Transfected Cells and Rat Liver Membranes. J. Pharmacol. Exp. Ther. 2020, 373, 290–301. [Google Scholar] [CrossRef]
- Navarro, G.; Moreno, E.; Bonaventura, J.; Brugarolas, M.; Farré, D.; Aguinaga, D.; Mallol, J.; Cortés, A.; Casadó, V.; Lluís, C.; et al. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS ONE 2013, 8, e61245. [Google Scholar] [CrossRef]
- Aguinaga, D.; Medrano, M.; Vega-Quiroga, I.; Gysling, K.; Canela, E.I.; Navarro, G.; Franco, R. Cocaine Effects on Dopaminergic Transmission Depend on a Balance between Sigma-1 and Sigma-2 Receptor Expression. Front. Mol. Neurosci. 2018, 11, 17. [Google Scholar] [CrossRef]
- Fontanilla, D.; Hajipour, A.R.; Pal, A.; Chu, U.B.; Arbabian, M.; Ruoho, A.E. Probing the steroid binding domain-like I (SBDLI) of the sigma-1 receptor binding site using N-substituted photoaffinity labels. Biochemistry 2008, 47, 7205–7217. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: Roles in endoplasmic reticulum lipid compartmentalization and export. J. Pharmacol. Exp. Ther. 2003, 306, 718–725. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 14949–14954. [Google Scholar] [CrossRef] [PubMed]
- Gebreselassie, D.; Bowen, W.D. Sigma-2 receptors are specifically localized to lipid rafts in rat liver membranes. Eur. J. Pharmacol. 2004, 493, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zeng, C.; Chu, W.; Pan, F.; Rothfuss, J.M.; Zhang, F.; Tu, Z.; Zhou, D.; Zeng, D.; Vangveravong, S.; et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011, 2, 380. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, D.B. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur. J. Pharmacol. 1983, 92, 269–274. [Google Scholar] [CrossRef]
- Chu, U.B.; Ruoho, A.E. Sigma Receptor Binding Assays. Curr. Protoc. Pharmacol. 2015, 71, 1–34. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J. Pharmacol. Exp. Ther. 2003, 306, 726–733. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Aydar, E.; Palmer, C.P.; Klyachko, V.A.; Jackson, M.B. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 2002, 34, 399–410. [Google Scholar] [CrossRef]
- Su, T.P.; Hayashi, T.; Maurice, T.; Buch, S.; Ruoho, A.E. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 2010, 31, 557–566. [Google Scholar] [CrossRef]
- Brimson, J.M.; Brown, C.A.; Safrany, S.T. Antagonists show GTP-sensitive high-affinity binding to the sigma-1 receptor. Br. J. Pharmacol. 2011, 164, 772–780. [Google Scholar] [CrossRef]
- Sánchez-Blázquez, P.; Rodríguez-Muñoz, M.; Herrero-Labrador, R.; Burgueño, J.; Zamanillo, D.; Garzón, J. The calcium-sensitive Sigma-1 receptor prevents cannabinoids from provoking glutamate NMDA receptor hypofunction: Implications in antinociception and psychotic diseases. Int. J. Neuropsychopharmacol. 2014, 17, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Vilner, B.J.; John, C.S.; Bowen, W.D. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995, 55, 408–413. [Google Scholar] [PubMed]
- Bermack, J.E.; Debonnel, G. Distinct modulatory roles of sigma receptor subtypes on glutamatergic responses in the dorsal hippocampus. Synapse 2005, 55, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cobos, E.J.; Entrena, J.M.; Nieto, F.R.; Cendán, C.M.; Del Pozo, E. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr. Neuropharmacol. 2008, 6, 344–366. [Google Scholar] [CrossRef]
- Hayashi, T.; Maurice, T.; Su, T.P. Ca(2+) signaling via sigma(1)-receptors: Novel regulatory mechanism affecting intracellular Ca(2+) concentration. J. Pharmacol. Exp. Ther. 2000, 293, 788–798. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 491–496. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptor ligands: Potential in the treatment of neuropsychiatric disorders. CNS Drugs 2004, 18, 269–284. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujimoto, M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol. Pharmacol. 2010, 77, 517–528. [Google Scholar] [CrossRef]
- Carnally, S.M.; Johannessen, M.; Henderson, R.M.; Jackson, M.B.; Edwardson, J.M. Demonstration of a direct interaction between sigma-1 receptors and acid-sensing ion channels. Biophys. J. 2010, 98, 1182–1191. [Google Scholar] [CrossRef]
- Crottès, D.; Martial, S.; Rapetti-Mauss, R.; Pisani, D.F.; Loriol, C.; Pellissier, B.; Martin, P.; Chevet, E.; Borgese, F.; Soriani, O. Sig1R protein regulates hERG channel expression through a post-translational mechanism in leukemic cells. J. Biol. Chem. 2011, 286, 27947–27958. [Google Scholar] [CrossRef]
- Balasuriya, D.; Stewart, A.P.; Crottès, D.; Borgese, F.; Soriani, O.; Edwardson, J.M. The sigma-1 receptor binds to the Nav1.5 voltage-gated Na+ channel with 4-fold symmetry. J. Biol. Chem. 2012, 287, 37021–37029. [Google Scholar] [CrossRef] [PubMed]
- Kourrich, S.; Hayashi, T.; Chuang, J.Y.; Tsai, S.Y.; Su, T.P.; Bonci, A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell 2013, 152, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, E1. [Google Scholar] [CrossRef]
- Lee, R.H.C.; Lee, M.H.H.; Wu, C.Y.C.; Couto, E.S.A.; Possoit, H.E.; Hsieh, T.H.; Minagar, A.; Lin, H.W. Cerebral ischemia and neuroregeneration. Neural Regen. Res. 2018, 13, 373–385. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, E.; Johnstone, T.M.; Haider, G.; Shah, A.; Marianayagam, N.J.; Biswal, S.; Veeravagu, A. Sigma-1 receptor expression in a subpopulation of lumbar spinal cord microglia in response to peripheral nerve injury. Sci. Rep. 2023, 13, 14762. [Google Scholar] [CrossRef]
- Nardai, S.; László, M.; Szabó, A.; Alpár, A.; Hanics, J.; Zahola, P.; Merkely, B.; Frecska, E.; Nagy, Z. N,N-dimethyltryptamine reduces infarct size and improves functional recovery following transient focal brain ischemia in rats. Exp. Neurol. 2020, 327, 113245. [Google Scholar] [CrossRef]
- Zhai, M.; Liu, C.; Li, Y.; Zhang, P.; Yu, Z.; Zhu, H.; Zhang, L.; Zhang, Q.; Wang, J.; Wang, J. Dexmedetomidine inhibits neuronal apoptosis by inducing Sigma-1 receptor signaling in cerebral ischemia-reperfusion injury. Aging 2019, 11, 9556–9568. [Google Scholar] [CrossRef]
- Morihara, R.; Yamashita, T.; Liu, X.; Nakano, Y.; Fukui, Y.; Sato, K.; Ohta, Y.; Hishikawa, N.; Shang, J.; Abe, K. Protective effect of a novel sigma-1 receptor agonist is associated with reduced endoplasmic reticulum stress in stroke male mice. J. Neurosci. Res. 2018, 96, 1707–1716. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Saunders, D.E.; Clifton, A.G.; Brown, M.M. Measurement of infarct size using MRI predicts prognosis in middle cerebral artery infarction. Stroke 1995, 26, 2272–2276. [Google Scholar] [CrossRef] [PubMed]
- Schreihofer, D.A.; Dalwadi, D.; Kim, S.; Metzger, D.; Oppong-Gyebi, A.; Das-Earl, P.; Schetz, J.A. Treatment of Stroke at a Delayed Timepoint with a Repurposed Drug Targeting Sigma 1 Receptors. Transl. Stroke Res. 2024, 15, 1035–1049. [Google Scholar] [CrossRef]
- van der Heijden, K.V.; Zuiker, R.; Otto, M.E.; Bryan, C.S.; Stewart, N.; Stillwell, C.; De Kam, M.L.; van Leuken, M.B.; van Gerven, J.M.A.; Jacobs, G.E. Safety, Pharmacokinetics, and Pharmacodynamics of a 6-h N,N-Dimethyltryptamine (DMT) Infusion in Healthy Volunteers: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Transl. Sci. 2025, 18, e70234. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liang, W.; Hu, X.; Zhang, W.; Stetler, R.A.; Vosler, P.; Cao, G.; Chen, J. Neuroprotection against hypoxic-ischemic brain injury by inhibiting the apoptotic protease activating factor-1 pathway. Stroke 2010, 41, 166–172. [Google Scholar] [CrossRef]
- Katnik, C.; Garcia, A.; Behensky, A.A.; Yasny, I.E.; Shuster, A.M.; Seredenin, S.B.; Petrov, A.V.; Cuevas, J. Activation of σ1 and σ2 receptors by afobazole increases glial cell survival and prevents glial cell activation and nitrosative stress after ischemic stroke. J. Neurochem. 2016, 139, 497–509. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.B.; Zhuge, Q.; Zheng, W.; Shao, B.; Wang, B.; Sun, F.; Jin, K. Glial scar formation occurs in the human brain after ischemic stroke. Int. J. Med. Sci. 2014, 11, 344–348. [Google Scholar] [CrossRef]
- Sánchez-Blázquez, P.; Pozo-Rodrigálvarez, A.; Merlos, M.; Garzón, J. The Sigma-1 Receptor Antagonist, S1RA, Reduces Stroke Damage, Ameliorates Post-Stroke Neurological Deficits and Suppresses the Overexpression of MMP-9. Mol. Neurobiol. 2018, 55, 4940–4951. [Google Scholar] [CrossRef]
- Song, W.; Yao, Y.; Zhang, H.; Hao, X.; Zhou, L.; Song, Z.; Wei, T.; Chi, T.; Liu, P.; Ji, X.; et al. Sigma-1 Receptor Activation Improves Oligodendrogenesis and Promotes White-Matter Integrity after Stroke in Mice with Diabetic Mellitus. Molecules 2023, 28, 390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, J.; Wang, R.; Jiang, M.; Ye, Q.; Smith, A.D.; Chen, J.; Shi, Y. Macrophages reprogram after ischemic stroke and promote efferocytosis and inflammation resolution in the mouse brain. CNS Neurosci. Ther. 2019, 25, 1329–1342. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Q.; Tao, W.; Qin, P.; Chen, J.; Yang, H.; Chen, J.; Liu, H.; Dai, Q.; Zhen, X. Sigma-1 receptor-regulated efferocytosis by infiltrating circulating macrophages/microglial cells protects against neuronal impairments and promotes functional recovery in cerebral ischemic stroke. Theranostics 2023, 13, 543–559. [Google Scholar] [CrossRef]
- Yabuki, Y.; Shinoda, Y.; Izumi, H.; Ikuno, T.; Shioda, N.; Fukunaga, K. Dehydroepiandrosterone administration improves memory deficits following transient brain ischemia through sigma-1 receptor stimulation. Brain Res. 2015, 1622, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Chi, T.Y.; Ji, X.F.; Liu, P.; Qi, X.X.; Zhu, L.; Wang, Z.Q.; Li, L.; Chen, L.; Zou, L.B. Sigma-1 receptor activation alleviates blood-brain barrier dysfunction in vascular dementia mice. Exp. Neurol. 2018, 308, 90–99. [Google Scholar] [CrossRef]
- Dai, L.A.; Chen, X.Y.; Li, W.J.; Yang, J.H.; Lin, M.J.; Li, X.S.; Zeng, Y.F.; Chen, S.W.; Xie, Z.L.; Zhu, Z.L.; et al. Sigma-1 Receptor and Binding Immunoglobulin Protein Interact with Ulinastatin Contributing to a Protective Effect in Rat Cerebral Ischemia/Reperfusion. World Neurosurg. 2022, 158, e488–e494. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; Jiang, Q.H.; Zhang, T.N.; Sun, H.; Shi, W.W.; Gunosewoyo, H.; Yang, F.; Tang, J.; Pang, T.; Yu, L.F. Sigma-1 Receptor Agonist TS-157 Improves Motor Functional Recovery by Promoting Neurite Outgrowth and pERK in Rats with Focal Cerebral Ischemia. Molecules 2021, 26, 1212. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H.; Travis, Z.D.; Zhang, J.H. The Stroke-Induced Blood-Brain Barrier Disruption: Current Progress of Inspection Technique, Mechanism, and Therapeutic Target. Curr. Neuropharmacol. 2020, 18, 1187–1212. [Google Scholar] [CrossRef]
- Liu, D.; Yang, L.; Liu, P.; Ji, X.; Qi, X.; Wang, Z.; Chi, T.; Zou, L. Sigma-1 receptor activation alleviates blood-brain barrier disruption post cerebral ischemia stroke by stimulating the GDNF-GFRα1-RET pathway. Exp. Neurol. 2022, 347, 113867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yu, S.; Ling, Y.; Hao, S.; Liu, J. The Protective Effects of Dexmedetomidine against Hypoxia/Reoxygenation-Induced Inflammatory Injury and Permeability in Brain Endothelial Cells Mediated by Sigma-1 Receptor. ACS Chem. Neurosci. 2021, 12, 1940–1947. [Google Scholar] [CrossRef]
- Yang, S.; Jin, H.; Zhu, Y.; Wan, Y.; Opoku, E.N.; Zhu, L.; Hu, B. Diverse Functions and Mechanisms of Pericytes in Ischemic Stroke. Curr. Neuropharmacol. 2017, 15, 892–905. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Wei, Q.; Leng, S.; Li, C.; Han, B.; Bai, Y.; Zhang, H.; Yao, H. Activation of Sigma-1 Receptor Enhanced Pericyte Survival via the Interplay Between Apoptosis and Autophagy: Implications for Blood-Brain Barrier Integrity in Stroke. Transl. Stroke Res. 2020, 11, 267–287. [Google Scholar] [CrossRef]
- Dohmen, C.; Sakowitz, O.W.; Fabricius, M.; Bosche, B.; Reithmeier, T.; Ernestus, R.I.; Brinker, G.; Dreier, J.P.; Woitzik, J.; Strong, A.J.; et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann. Neurol. 2008, 63, 720–728. [Google Scholar] [CrossRef]
- Leao, A.A. The slow voltage variation of cortical spreading depression of activity. Electroencephalogr. Clin. Neurophysiol. 1951, 3, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Í.; Varga, V.; Dvorácskó, S.; Farkas, A.E.; Körmöczi, T.; Berkecz, R.; Kecskés, S.; Menyhárt, Á.; Frank, R.; Hantosi, D.; et al. N,N-Dimethyltryptamine attenuates spreading depolarization and restrains neurodegeneration by sigma-1 receptor activation in the ischemic rat brain. Neuropharmacology 2021, 192, 108612. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Evora, P.; Castro, E.S.O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [PubMed]
- Stelfa, G.; Vavers, E.; Svalbe, B.; Serzants, R.; Miteniece, A.; Lauberte, L.; Grinberga, S.; Gukalova, B.; Dambrova, M.; Zvejniece, L. Reduced GFAP Expression in Bergmann Glial Cells in the Cerebellum of Sigma-1 Receptor Knockout Mice Determines the Neurobehavioral Outcomes after Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 11611. [Google Scholar] [CrossRef]
- Shi, M.; Liu, L.; Min, X.; Mi, L.; Chai, Y.; Chen, F.; Wang, J.; Yue, S.; Zhang, J.; Deng, Q.; et al. Activation of Sigma-1 Receptor Alleviates ER-Associated Cell Death and Microglia Activation in Traumatically Injured Mice. J. Clin. Med. 2022, 11, 2348. [Google Scholar] [CrossRef]
- Dong, H.; Ma, Y.; Ren, Z.; Xu, B.; Zhang, Y.; Chen, J.; Yang, B. Sigma-1 Receptor Modulates Neuroinflammation After Traumatic Brain Injury. Cell. Mol. Neurobiol. 2016, 36, 639–645. [Google Scholar] [CrossRef]
- Vázquez-Rosa, E.; Watson, M.R.; Sahn, J.J.; Hodges, T.R.; Schroeder, R.E.; Cintrón-Pérez, C.J.; Shin, M.K.; Yin, T.C.; Emery, J.L.; Martin, S.F.; et al. Neuroprotective Efficacy of a Sigma 2 Receptor/TMEM97 Modulator (DKR-1677) after Traumatic Brain Injury. ACS Chem. Neurosci. 2019, 10, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Sui, C.; Diao, Y.; Shi, G.; Hu, X.; Hao, Z.; Li, C.; Hao, M.; Xie, M.; Zhu, T. Activation of the sigma-1 receptor ameliorates neuronal ferroptosis via IRE1α after spinal cord injury. Brain Res. 2024, 1838, 149011. [Google Scholar] [CrossRef]
- Gaja-Capdevila, N.; Hernández, N.; Yeste, S.; Reinoso, R.F.; Burgueño, J.; Montero, A.; Merlos, M.; Vela, J.M.; Herrando-Grabulosa, M.; Navarro, X. EST79232 and EST79376, Two Novel Sigma-1 Receptor Ligands, Exert Neuroprotection on Models of Motoneuron Degeneration. Int. J. Mol. Sci. 2022, 23, 6737. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Kenny, E.M.; Fidan, E.; Yang, Q.; Anthonymuthu, T.S.; New, L.A.; Meyer, E.A.; Wang, H.; Kochanek, P.M.; Dixon, C.E.; Kagan, V.E.; et al. Ferroptosis Contributes to Neuronal Death and Functional Outcome After Traumatic Brain Injury. Crit. Care Med. 2019, 47, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Su, T.P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009, 124, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Urani, A.; Romieu, P.; Roman, F.J.; Maurice, T. Enhanced antidepressant effect of sigma(1) (sigma(1)) receptor agonists in beta(25-35)-amyloid peptide-treated mice. Behav. Brain Res. 2002, 134, 239–247. [Google Scholar] [CrossRef]
- Genovese, I.; Giamogante, F.; Barazzuol, L.; Battista, T.; Fiorillo, A.; Vicario, M.; D’Alessandro, G.; Cipriani, R.; Limatola, C.; Rossi, D.; et al. Sorcin is an early marker of neurodegeneration, Ca(2+) dysregulation and endoplasmic reticulum stress associated to neurodegenerative diseases. Cell Death Dis. 2020, 11, 861. [Google Scholar] [CrossRef]
- Luty, A.A.; Kwok, J.B.; Dobson-Stone, C.; Loy, C.T.; Coupland, K.G.; Karlström, H.; Sobow, T.; Tchorzewska, J.; Maruszak, A.; Barcikowska, M.; et al. Sigma nonopioid intracellular receptor 1 mutations cause frontotemporal lobar degeneration-motor neuron disease. Ann. Neurol. 2010, 68, 639–649. [Google Scholar] [CrossRef]
- Al-Saif, A.; Al-Mohanna, F.; Bohlega, S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann. Neurol. 2011, 70, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, L.; Halliday, G.; Dobson-Stone, C.; Wang, Y.; Tang, H.D.; Cao, L.; Deng, Y.L.; Wang, G.; Zhang, Y.M.; et al. Genetic polymorphisms in sigma-1 receptor and apolipoprotein E interact to influence the severity of Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 765–770. [Google Scholar] [CrossRef]
- Snyder, M.A.; McCann, K.; Lalande, M.J.; Thivierge, J.P.; Bergeron, R. Sigma receptor type 1 knockout mice show a mild deficit in plasticity but no significant change in synaptic transmission in the CA1 region of the hippocampus. J. Neurochem. 2016, 138, 700–709. [Google Scholar] [CrossRef]
- Naia, L.; Ly, P.; Mota, S.I.; Lopes, C.; Maranga, C.; Coelho, P.; Gershoni-Emek, N.; Ankarcrona, M.; Geva, M.; Hayden, M.R.; et al. The Sigma-1 Receptor Mediates Pridopidine Rescue of Mitochondrial Function in Huntington Disease Models. Neurotherapeutics 2021, 18, 1017–1038. [Google Scholar] [CrossRef]
- Lisak, R.P.; Nedelkoska, L.; Benjamins, J.A. Sigma-1 receptor agonists as potential protective therapies in multiple sclerosis. J. Neuroimmunol. 2020, 342, 577188. [Google Scholar] [CrossRef]
- Francardo, V. Sigma-1 receptor: A potential new target for Parkinson’s disease? Neural Regen. Res. 2014, 9, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Kaushal, N.; Robson, M.J.; Matsumoto, R.R. Sigma receptors as potential therapeutic targets for neuroprotection. Eur. J. Pharmacol. 2014, 743, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Hegarty, E.; Sahn, J.J.; Scott, L.L.; Gökçe, S.K.; Martin, C.; Ghorashian, N.; Satarasinghe, P.N.; Iyer, S.; Sae-Lee, W.; et al. High-Content Microfluidic Screening Platform Used To Identify σ2R/Tmem97 Binding Ligands that Reduce Age-Dependent Neurodegeneration in C. elegans SC_APP Model. ACS Chem. Neurosci. 2018, 9, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, S.; Lahaye, R.A.; Vitet, H.; Scaramuzzino, C.; Virlogeux, A.; Capellano, L.; Genoux, A.; Gershoni-Emek, N.; Geva, M.; Hayden, M.R.; et al. Pridopidine rescues BDNF/TrkB trafficking dynamics and synapse homeostasis in a Huntington disease brain-on-a-chip model. Neurobiol. Dis. 2022, 173, 105857. [Google Scholar] [CrossRef]
- Geva, M.; Kusko, R.; Soares, H.; Fowler, K.D.; Birnberg, T.; Barash, S.; Wagner, A.M.; Fine, T.; Lysaght, A.; Weiner, B.; et al. Pridopidine activates neuroprotective pathways impaired in Huntington Disease. Hum. Mol. Genet. 2016, 25, 3975–3987. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef]
- Goyagi, T.; Goto, S.; Bhardwaj, A.; Dawson, V.L.; Hurn, P.D.; Kirsch, J.R. Neuroprotective effect of sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is linked to reduced neuronal nitric oxide production. Stroke 2001, 32, 1613–1620. [Google Scholar] [CrossRef]
- Vagnerova, K.; Hurn, P.D.; Bhardwaj, A.; Kirsch, J.R. Sigma 1 receptor agonists act as neuroprotective drugs through inhibition of inducible nitric oxide synthase. Anesth. Analg. 2006, 103, 430–434, table of contents. [Google Scholar] [CrossRef]
- Zampieri, D.; Fortuna, S.; Calabretti, A.; Romano, M.; Menegazzi, R.; Schepmann, D.; Wünsch, B.; Collina, S.; Zanon, D.; Mamolo, M.G. Discovery of new potent dual sigma receptor/GluN2b ligands with antioxidant property as neuroprotective agents. Eur. J. Med. Chem. 2019, 180, 268–282. [Google Scholar] [CrossRef]
- Rui, M.; Rossino, G.; Coniglio, S.; Monteleone, S.; Scuteri, A.; Malacrida, A.; Rossi, D.; Catenacci, L.; Sorrenti, M.; Paolillo, M.; et al. Identification of dual Sigma1 receptor modulators/acetylcholinesterase inhibitors with antioxidant and neurotrophic properties, as neuroprotective agents. Eur. J. Med. Chem. 2018, 158, 353–370. [Google Scholar] [CrossRef]
- Su, T.C.; Lin, S.H.; Lee, P.T.; Yeh, S.H.; Hsieh, T.H.; Chou, S.Y.; Su, T.P.; Hung, J.J.; Chang, W.C.; Lee, Y.C.; et al. The sigma-1 receptor-zinc finger protein 179 pathway protects against hydrogen peroxide-induced cell injury. Neuropharmacology 2016, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Turrigiano, G. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 2012, 4, a005736. [Google Scholar] [CrossRef]
- Pozo, K.; Goda, Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 2010, 66, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, K.; Bojarski, A.J.; Popik, P.; Malikowska-Racia, N. Novel object recognition test as an alternative approach to assessing the pharmacological profile of sigma-1 receptor ligands. Pharmacol. Rep. 2023, 75, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Sahlholm, K.; Valle-León, M.; Fernández-Dueñas, V.; Ciruela, F. Pridopidine Reverses Phencyclidine-Induced Memory Impairment. Front. Pharmacol. 2018, 9, 338. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Nakamura, Y.; Takemura, M.; Hisaoka-Nakashima, K.; Morioka, N. TLR4-TAK1-p38 MAPK pathway and HDAC6 regulate the expression of sigma-1 receptors in rat primary cultured microglia. J. Pharmacol. Sci. 2020, 144, 23–29. [Google Scholar] [CrossRef]
- Orciani, C.; Do Carmo, S.; Foret, M.K.; Hall, H.; Bonomo, Q.; Lavagna, A.; Huang, C.; Cuello, A.C. Early treatment with an M1 and sigma-1 receptor agonist prevents cognitive decline in a transgenic rat model displaying Alzheimer-like amyloid pathology. Neurobiol. Aging 2023, 132, 220–232. [Google Scholar] [CrossRef]
- Ji, J.; Gao, C.; Wang, Q.; Jia, X.; Tian, H.; Wei, Y.; Liu, Z.; Wang, Y.; Guo, L. The sigma-1 receptor-TAMM41 axis modulates neuroinflammation and attenuates memory impairment during the latent period of epileptogenesis. Anim. Model Exp. Med. 2025, 8, 197–208. [Google Scholar] [CrossRef]
- Guo, L.; Gao, T.; Jia, X.; Gao, C.; Tian, H.; Wei, Y.; Lu, W.; Liu, Z.; Wang, Y. SKF83959 Attenuates Memory Impairment and Depressive-like Behavior during the Latent Period of Epilepsy via Allosteric Activation of the Sigma-1 Receptor. ACS Chem. Neurosci. 2022, 13, 3198–3209. [Google Scholar] [CrossRef]
- Heiss, K.; Raffaele, M.; Vanella, L.; Murabito, P.; Prezzavento, O.; Marrazzo, A.; Aricò, G.; Castracani, C.C.; Barbagallo, I.; Zappalà, A.; et al. (+)-Pentazocine attenuates SH-SY5Y cell death, oxidative stress and microglial migration induced by conditioned medium from activated microglia. Neurosci. Lett. 2017, 642, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.; Beal, M.F. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Cavendish, J.Z.; Sarkar, S.N.; Colantonio, M.A.; Quintana, D.D.; Ahmed, N.; White, B.A.; Engler-Chiurazzi, E.B.; Simpkins, J.W. Mitochondrial Movement and Number Deficits in Embryonic Cortical Neurons from 3xTg-AD Mice. J. Alzheimer’s Dis. 2019, 70, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Lei, Z.G.; Chu, K.; Lam, O.J.H.; Chiang, C.Y.; Zhang, Z.J. N, N-Dimethyltryptamine, a natural hallucinogen, ameliorates Alzheimer’s disease by restoring neuronal Sigma-1 receptor-mediated endoplasmic reticulum-mitochondria crosstalk. Alzheimer’s Res. Ther. 2024, 16, 95. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Borbély, E.; Varga, V.; Szögi, T.; Schuster, I.; Bozsó, Z.; Penke, B.; Fülöp, L. Impact of Two Neuronal Sigma-1 Receptor Modulators, PRE084 and DMT, on Neurogenesis and Neuroinflammation in an Aβ(1-42)-Injected, Wild-Type Mouse Model of AD. Int. J. Mol. Sci. 2022, 23, 2514. [Google Scholar] [CrossRef]
- Maurice, T.; Volle, J.N.; Strehaiano, M.; Crouzier, L.; Pereira, C.; Kaloyanov, N.; Virieux, D.; Pirat, J.L. Neuroprotection in non-transgenic and transgenic mouse models of Alzheimer’s disease by positive modulation of σ(1) receptors. Pharmacol. Res. 2019, 144, 315–330. [Google Scholar] [CrossRef]
- Hall, H.; Iulita, M.F.; Gubert, P.; Flores Aguilar, L.; Ducatenzeiler, A.; Fisher, A.; Cuello, A.C. AF710B, an M1/sigma-1 receptor agonist with long-lasting disease-modifying properties in a transgenic rat model of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 811–823. [Google Scholar] [CrossRef]
- Fisher, A.; Bezprozvanny, I.; Wu, L.; Ryskamp, D.A.; Bar-Ner, N.; Natan, N.; Brandeis, R.; Elkon, H.; Nahum, V.; Gershonov, E.; et al. AF710B, a Novel M1/σ1 Agonist with Therapeutic Efficacy in Animal Models of Alzheimer’s Disease. Neurodegener. Dis. 2016, 16, 95–110. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Lasbleiz, C.; Peyrel, A.; Tarot, P.; Sarniguet, J.; Crouzier, L.; Cubedo, N.; Delprat, B.; Rossel, M.; Maurice, T.; Liévens, J.C. Sigma-1 receptor agonist PRE-084 confers protection against TAR DNA-binding protein-43 toxicity through NRF2 signalling. Redox. Biol. 2022, 58, 102542. [Google Scholar] [CrossRef]
- Estévez-Silva, H.M.; Cuesto, G.; Romero, N.; Brito-Armas, J.M.; Acevedo-Arozena, A.; Acebes, Á.; Marcellino, D.J. Pridopidine Promotes Synaptogenesis and Reduces Spatial Memory Deficits in the Alzheimer’s Disease APP/PS1 Mouse Model. Neurotherapeutics 2022, 19, 1566–1587. [Google Scholar] [CrossRef] [PubMed]
- Lahmy, V.; Long, R.; Morin, D.; Villard, V.; Maurice, T. Mitochondrial protection by the mixed muscarinic/σ1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Aβ25-35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Front. Cell. Neurosci. 2014, 8, 463. [Google Scholar] [CrossRef]
- Freyssin, A.; Carles, A.; Guehairia, S.; Rubinstenn, G.; Maurice, T. Fluoroethylnormemantine (FENM) shows synergistic protection in combination with a sigma-1 receptor agonist in a mouse model of Alzheimer’s disease. Neuropharmacology 2024, 242, 109733. [Google Scholar] [CrossRef] [PubMed]
- Christmann, U.; Garriga, L.; Llorente, A.V.; Diaz, J.L.; Pascual, R.; Bordas, M.; Dordal, A.; Porras, M.; Yeste, S.; Reinoso, R.F.; et al. WLB-87848, a Selective sigma(1) Receptor Agonist, with an Unusually Positioned NH Group as Positive Ionizable Moiety and Showing Neuroprotective Activity. J. Med. Chem. 2024, 67, 9150–9164. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Verma, K.; Brimson, S.; Tencomnao, T.; Brimson, J.M. Simple ammonium salt and sigma-1 receptor ligand dipentylammonium provides neuroprotective effects in cell culture and Caenorhabditis elegans models of Alzheimer’s disease. Biomed. Pharmacother. 2024, 173, 116455. [Google Scholar] [CrossRef]
- Brimson, J.M.; Safrany, S.T.; Qassam, H.; Tencomnao, T. Dipentylammonium Binds to the Sigma-1 Receptor and Protects Against Glutamate Toxicity, Attenuates Dopamine Toxicity and Potentiates Neurite Outgrowth in Various Cultured Cell Lines. Neurotox. Res. 2018, 34, 263–272. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Black, S.E.; Lotlikar, M.S.; Fenn, R.H.; Jorfi, M.; Kovacs, D.M.; Tanzi, R.E. Axonal generation of amyloid-β from palmitoylated APP in mitochondria-associated endoplasmic reticulum membranes. Cell Rep. 2021, 35, 109134. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Pokrass, M.J.; Klauer, N.R.; Nohara, H.; Su, T.P. Sigma-1 receptor regulates Tau phosphorylation and axon extension by shaping p35 turnover via myristic acid. Proc. Natl. Acad. Sci. USA 2015, 112, 6742–6747. [Google Scholar] [CrossRef]
- Maurice, T.; Strehaiano, M.; Duhr, F.; Chevallier, N. Amyloid toxicity is enhanced after pharmacological or genetic invalidation of the σ(1) receptor. Behav. Brain Res. 2018, 339, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Izzo, N.J.; Yuede, C.M.; LaBarbera, K.M.; Limegrover, C.S.; Rehak, C.; Yurko, R.; Waybright, L.; Look, G.; Rishton, G.; Safferstein, H.; et al. Preclinical and clinical biomarker studies of CT1812: A novel approach to Alzheimer’s disease modification. Alzheimer’s Dement. 2021, 17, 1365–1382. [Google Scholar] [CrossRef] [PubMed]
- Christmann, U.; Díaz, J.L.; Pascual, R.; Bordas, M.; Álvarez, I.; Monroy, X.; Porras, M.; Yeste, S.; Reinoso, R.F.; Merlos, M.; et al. Discovery of WLB-89462, a New Drug-like and Highly Selective σ(2) Receptor Ligand with Neuroprotective Properties. J. Med. Chem. 2023, 66, 12499–12519. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Sahn, J.J.; Ardestani, P.M.; Evans, A.K.; Scott, L.L.; Chan, J.Z.; Iyer, S.; Crisp, A.; Zuniga, G.; Pierce, J.T.; et al. Small molecule modulator of sigma 2 receptor is neuroprotective and reduces cognitive deficits and neuroinflammation in experimental models of Alzheimer’s disease. J. Neurochem. 2017, 140, 561–575. [Google Scholar] [CrossRef]
- An, Y.; Qi, Y.; Li, Y.; Li, Z.; Yang, C.; Jia, D. Activation of the sigma-1 receptor attenuates blood-brain barrier disruption by inhibiting amyloid deposition in Alzheimer’s disease mice. Neurosci. Lett. 2022, 774, 136528. [Google Scholar] [CrossRef]
- Macfarlane, S.; Grimmer, T.; Teo, K.; O’Brien, T.J.; Woodward, M.; Grunfeld, J.; Mander, A.; Brodtmann, A.; Brew, B.J.; Morris, P.; et al. Blarcamesine for the treatment of Early Alzheimer’s Disease: Results from the ANAVEX2-73-AD-004 Phase IIB/III trial. J. Prev. Alzheimer’s Dis. 2025, 12, 100016. [Google Scholar] [CrossRef]
- Schneider, L.S.; Thomas, R.G.; Hendrix, S.; Rissman, R.A.; Brewer, J.B.; Salmon, D.P.; Oltersdorf, T.; Okuda, T.; Feldman, H.H.; Alzheimer’s Disease Cooperative Study, T.S.G. Safety and Efficacy of Edonerpic Maleate for Patients With Mild to Moderate Alzheimer Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2019, 76, 1330–1339. [Google Scholar] [CrossRef]
- Kaur, G.; Dar, Z.A.; Bajpai, A.; Singh, R.; Bansal, R. Clinical Update on an Anti-Alzheimer Drug Candidate CT1812: A Sigma-2 Receptor Antagonist. Clin. Ther. 2024, 46, e21–e28. [Google Scholar] [CrossRef]
- Renton, A.E.; Majounie, E.; Waite, A.; Simon-Sanchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef]
- Watanabe, S.; Ilieva, H.; Tamada, H.; Nomura, H.; Komine, O.; Endo, F.; Jin, S.; Mancias, P.; Kiyama, H.; Yamanaka, K. Mitochondria-associated membrane collapse is a common pathomechanism in SIGMAR1- and SOD1-linked ALS. EMBO Mol. Med. 2016, 8, 1421–1437. [Google Scholar] [CrossRef]
- Watanabe, S.; Horiuchi, M.; Murata, Y.; Komine, O.; Kawade, N.; Sobue, A.; Yamanaka, K. Sigma-1 receptor maintains ATAD3A as a monomer to inhibit mitochondrial fragmentation at the mitochondria-associated membrane in amyotrophic lateral sclerosis. Neurobiol. Dis. 2023, 179, 106031. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.P.; De Calbiac, H.; Kabashi, E.; Barmada, S.J. Autophagy and ALS: Mechanistic insights and therapeutic implications. Autophagy 2022, 18, 254–282. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wu, H.E.; Weng, E.F.; Wu, H.C.; Su, T.P.; Wang, S.M. Fluvoxamine Exerts Sigma-1R to Rescue Autophagy via Pom121-Mediated Nucleocytoplasmic Transport of TFEB. Mol. Neurobiol. 2024, 61, 5282–5294. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Silva, H.M.; Mediavilla, T.; Giacobbo, B.L.; Liu, X.; Sultan, F.R.; Marcellino, D.J. Pridopidine modifies disease phenotype in a SOD1 mouse model of amyotrophic lateral sclerosis. Eur. J. Neurosci. 2022, 55, 1356–1372. [Google Scholar] [CrossRef]
- Gaja-Capdevila, N.; Hernández, N.; Navarro, X.; Herrando-Grabulosa, M. Sigma-1 Receptor is a Pharmacological Target to Promote Neuroprotection in the SOD1(G93A) ALS Mice. Front. Pharmacol. 2021, 12, 780588. [Google Scholar] [CrossRef]
- Zu, T.; Liu, Y.; Banez-Coronel, M.; Reid, T.; Pletnikova, O.; Lewis, J.; Miller, T.M.; Harms, M.B.; Falchook, A.E.; Subramony, S.H.; et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl. Acad. Sci. USA 2013, 110, E4968–E4977. [Google Scholar] [CrossRef]
- Lee, P.T.; Liévens, J.C.; Wang, S.M.; Chuang, J.Y.; Khalil, B.; Wu, H.E.; Chang, W.C.; Maurice, T.; Su, T.P. Sigma-1 receptor chaperones rescue nucleocytoplasmic transport deficit seen in cellular and Drosophila ALS/FTD models. Nat. Commun. 2020, 11, 5580. [Google Scholar] [CrossRef]
- Rouleau, G.A.; Clark, A.W.; Rooke, K.; Pramatarova, A.; Krizus, A.; Suchowersky, O.; Julien, J.P.; Figlewicz, D. SOD1 mutation is associated with accumulation of neurofilaments in amyotrophic lateral sclerosis. Ann. Neurol. 1996, 39, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Gradus, T.; Altman, T.; Maimon, R.; Saraf Avraham, N.; Geva, M.; Hayden, M.; Perlson, E. Targeting the Sigma-1 Receptor via Pridopidine Ameliorates Central Features of ALS Pathology in a SOD1(G93A) Model. Cell Death Dis. 2019, 10, 210. [Google Scholar] [CrossRef]
- Writing Committee for the, H.A.L.S.P.T.; Shefner, J.M.; Oskarsson, B.; Macklin, E.A.; Chibnik, L.B.; Quintana, M.; Saville, B.R.; Detry, M.A.; Vestrucci, M.; Marion, J.; et al. Pridopidine in Amyotrophic Lateral Sclerosis: The HEALEY ALS Platform Trial. JAMA 2025, 333, 1128–1137. [Google Scholar] [CrossRef]
- Martin, J.B.; Gusella, J.F. Huntington’s disease. Pathogenesis and management. N. Engl. J. Med. 1986, 315, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Carmo, C.; Naia, L.; Lopes, C.; Rego, A.C. Mitochondrial Dysfunction in Huntington’s Disease. Adv. Exp. Med. Biol. 2018, 1049, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Eddings, C.R.; Arbez, N.; Akimov, S.; Geva, M.; Hayden, M.R.; Ross, C.A. Pridopidine protects neurons from mutant-huntingtin toxicity via the sigma-1 receptor. Neurobiol. Dis. 2019, 129, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Kusko, R.; Dreymann, J.; Ross, J.; Cha, Y.; Escalante-Chong, R.; Garcia-Miralles, M.; Tan, L.J.; Burczynski, M.E.; Zeskind, B.; Laifenfeld, D.; et al. Large-scale transcriptomic analysis reveals that pridopidine reverses aberrant gene expression and activates neuroprotective pathways in the YAC128 HD mouse. Mol. Neurodegener. 2018, 13, 25. [Google Scholar] [CrossRef]
- Kalathur, R.K.; Giner-Lamia, J.; Machado, S.; Barata, T.; Ayasolla, K.R.; Futschik, M.E. The unfolded protein response and its potential role in Huntington’s disease elucidated by a systems biology approach. F1000Research 2015, 4, 103. [Google Scholar] [CrossRef]
- Shenkman, M.; Geva, M.; Gershoni-Emek, N.; Hayden, M.R.; Lederkremer, G.Z. Pridopidine reduces mutant huntingtin-induced endoplasmic reticulum stress by modulation of the Sigma-1 receptor. J. Neurochem. 2021, 158, 467–481. [Google Scholar] [CrossRef]
- Bol’shakova, A.V.; Kraskovskaya, N.A.; Gainullina, A.N.; Kukanova, E.O.; Vlasova, O.L.; Bezprozvanny, I.B. Neuroprotective Effect of σ1-Receptors on the Cell Model of Huntington’s Disease. Bull. Exp. Biol. Med. 2017, 164, 252–258. [Google Scholar] [CrossRef]
- Ryskamp, D.; Wu, J.; Geva, M.; Kusko, R.; Grossman, I.; Hayden, M.; Bezprozvanny, I. The sigma-1 receptor mediates the beneficial effects of pridopidine in a mouse model of Huntington disease. Neurobiol. Dis. 2017, 97, 46–59. [Google Scholar] [CrossRef]
- McGarry, A.; Auinger, P.; Kieburtz, K.; Geva, M.; Mehra, M.; Abler, V.; Grachev, I.D.; Gordon, M.F.; Savola, J.M.; Gandhi, S.; et al. Additional Safety and Exploratory Efficacy Data at 48 and 60 Months from Open-HART, an Open-Label Extension Study of Pridopidine in Huntington Disease. J. Huntingt. Dis. 2020, 9, 173–184. [Google Scholar] [CrossRef]
- Goldberg, Y.P.; Navon-Perry, L.; Cruz-Herranz, A.; Chen, K.; Hecker-Barth, G.; Spiegel, K.; Cohen, Y.; Niethammer, M.; Tan, A.M.; Schuring, H.; et al. The Safety Profile of Pridopidine, a Novel Sigma-1 Receptor Agonist for the Treatment of Huntington’s Disease. CNS Drugs 2025, 39, 485–498. [Google Scholar] [CrossRef]
- Alexoudi, A.; Alexoudi, I.; Gatzonis, S. Parkinson’s disease pathogenesis, evolution and alternative pathways: A review. Rev. Neurol. 2018, 174, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wang, L.; Zhang, T.; Zhang, B.; Chen, L. Sigma-1 receptor knockout increases α-synuclein aggregation and phosphorylation with loss of dopaminergic neurons in substantia nigra. Neurobiol. Aging 2017, 59, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Limegrover, C.S.; Yurko, R.; Izzo, N.J.; LaBarbera, K.M.; Rehak, C.; Look, G.; Rishton, G.; Safferstein, H.; Catalano, S.M. Sigma-2 receptor antagonists rescue neuronal dysfunction induced by Parkinson’s patient brain-derived α-synuclein. J. Neurosci. Res. 2021, 99, 1161–1176. [Google Scholar] [CrossRef]
- Vetel, S.; Foucault-Fruchard, L.; Tronel, C.; Buron, F.; Vergote, J.; Bodard, S.; Routier, S.; Sérrière, S.; Chalon, S. Neuroprotective and anti-inflammatory effects of a therapy combining agonists of nicotinic α7 and σ1 receptors in a rat model of Parkinson’s disease. Neural Regen. Res. 2021, 16, 1099–1104. [Google Scholar] [CrossRef]
- Wang, M.; Wan, C.; He, T.; Han, C.; Zhu, K.; Waddington, J.L.; Zhen, X. Sigma-1 receptor regulates mitophagy in dopaminergic neurons and contributes to dopaminergic protection. Neuropharmacology 2021, 196, 108360. [Google Scholar] [CrossRef]
- Francardo, V.; Geva, M.; Bez, F.; Denis, Q.; Steiner, L.; Hayden, M.R.; Cenci, M.A. Pridopidine Induces Functional Neurorestoration Via the Sigma-1 Receptor in a Mouse Model of Parkinson’s Disease. Neurotherapeutics 2019, 16, 465–479. [Google Scholar] [CrossRef]
- Pandey, S.; Srivanitchapoom, P. Levodopa-induced Dyskinesia: Clinical Features, Pathophysiology, and Medical Management. Ann. Indian Acad. Neurol. 2017, 20, 190–198. [Google Scholar] [CrossRef]
- Johnston, T.H.; Geva, M.; Steiner, L.; Orbach, A.; Papapetropoulos, S.; Savola, J.M.; Reynolds, I.J.; Ravenscroft, P.; Hill, M.; Fox, S.H.; et al. Pridopidine, a clinic-ready compound, reduces 3,4-dihydroxyphenylalanine-induced dyskinesia in Parkinsonian macaques. Mov. Disord. 2019, 34, 708–716. [Google Scholar] [CrossRef]
- Fox, S.H.; Metman, L.V.; Nutt, J.G.; Brodsky, M.; Factor, S.A.; Lang, A.E.; Pope, L.E.; Knowles, N.; Siffert, J. Trial of dextromethorphan/quinidine to treat levodopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2017, 32, 893–903. [Google Scholar] [CrossRef]
- Sun, Y.; Sukumaran, P.; Singh, B.B. Sigma1 Receptor Inhibits TRPC1-Mediated Ca(2+) Entry That Promotes Dopaminergic Cell Death. Cell. Mol. Neurobiol. 2021, 41, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Sha, S.; Zhou, L.; Wang, C.; Yin, J.; Chen, L. Sigma-1 receptor deficiency reduces MPTP-induced parkinsonism and death of dopaminergic neurons. Cell Death Dis. 2015, 6, e1832. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, Y. Globoid cell leucodystrophy (Krabbe’s disease): Deficiency of galactocerebroside beta-galactosidase. Proc. Natl. Acad. Sci. USA 1970, 66, 302–309. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Valsami, G.; Dev, K.K. The Effect of Donepezil Hydrochloride in the Twitcher Mouse Model of Krabbe Disease. Mol. Neurobiol. 2024, 61, 8688–8701. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.G.; Bundey, S.E. Wolfram (DIDMOAD) syndrome. J. Med. Genet. 1997, 34, 838–841. [Google Scholar] [CrossRef]
- Fischer, T.T.; Nguyen, L.D.; Ehrlich, B.E. Boosting ER-mitochondria calcium transfer to treat Wolfram syndrome. Cell Calcium 2022, 104, 102572. [Google Scholar] [CrossRef]
- Crouzier, L.; Danese, A.; Yasui, Y.; Richard, E.M.; Liévens, J.C.; Patergnani, S.; Couly, S.; Diez, C.; Denus, M.; Cubedo, N.; et al. Activation of the sigma-1 receptor chaperone alleviates symptoms of Wolfram syndrome in preclinical models. Sci. Transl. Med. 2022, 14, eabh3763. [Google Scholar] [CrossRef] [PubMed]
- Atzmon, A.; Herrero, M.; Sharet-Eshed, R.; Gilad, Y.; Senderowitz, H.; Elroy-Stein, O. Drug Screening Identifies Sigma-1-Receptor as a Target for the Therapy of VWM Leukodystrophy. Front. Mol. Neurosci. 2018, 11, 336. [Google Scholar] [CrossRef]
- Noyes, K.; Weinstock-Guttman, B. Impact of diagnosis and early treatment on the course of multiple sclerosis. Am. J. Manag. Care 2013, 19, s321–s331. [Google Scholar]
- Collina, S.; Rui, M.; Stotani, S.; Bignardi, E.; Rossi, D.; Curti, D.; Giordanetto, F.; Malacrida, A.; Scuteri, A.; Cavaletti, G. Are sigma receptor modulators a weapon against multiple sclerosis disease? Future Med. Chem. 2017, 9, 2029–2051. [Google Scholar] [CrossRef]
- Behensky, A.A.; Katnik, C.; Yin, H.; Cuevas, J. Activation of Sigma Receptors with Afobazole Modulates Microglial, but Not Neuronal, Apoptotic Gene Expression in Response to Long-Term Ischemia Exposure. Front. Neurosci. 2019, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Naaz, S.; Ozair, E. Dexmedetomidine in current anaesthesia practice- a review. J. Clin. Diagn. Res. 2014, 8, GE01-04. [Google Scholar] [CrossRef] [PubMed]
- Kenche, H.; Singh, M.; Smith, J.; Shen, K. Neuronal mitochondrial dysfunction in a cellular model of circadian rhythm disruption is rescued by donepezil. Biochem. Biophys. Res. Commun. 2021, 567, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 6, CD001190. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, X.F.; Chi, T.Y.; Liu, P.; Jin, G.; Chen, L.; Zou, L.B. Sigma-1 receptor in brain ischemia/reperfusion: Possible role in the NR2A-induced pathway to regulate brain-derived neurotrophic factor. J. Neurol. Sci. 2017, 376, 166–175. [Google Scholar] [CrossRef]
- Ryskamp, D.; Wu, L.; Wu, J.; Kim, D.; Rammes, G.; Geva, M.; Hayden, M.; Bezprozvanny, I. Pridopidine stabilizes mushroom spines in mouse models of Alzheimer’s disease by acting on the sigma-1 receptor. Neurobiol. Dis. 2019, 124, 489–504. [Google Scholar] [CrossRef]
- Kimura, T.; Hong Nguyen, P.T.; Ho, S.A.; Tran, A.H.; Ono, T.; Nishijo, H. T-817MA, a neurotrophic agent, ameliorates the deficits in adult neurogenesis and spatial memory in rats infused i.c.v. with amyloid-beta peptide. Br. J. Pharmacol. 2009, 157, 451–463. [Google Scholar] [CrossRef]
- Lizama, B.N.; North, H.A.; Pandey, K.; Williams, C.; Duong, D.; Cho, E.; Di Caro, V.; Ping, L.; Blennow, K.; Zetterberg, H.; et al. An interim exploratory proteomics biomarker analysis of a phase 2 clinical trial to assess the impact of CT1812 in Alzheimer’s disease. Neurobiol. Dis. 2024, 199, 106575. [Google Scholar] [CrossRef]
- Grundman, M.; Morgan, R.; Lickliter, J.D.; Schneider, L.S.; DeKosky, S.; Izzo, N.J.; Guttendorf, R.; Higgin, M.; Pribyl, J.; Mozzoni, K.; et al. A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer’s disease. Alzheimer’s Dement. 2019, 5, 20–26. [Google Scholar] [CrossRef]
- Vijverberg, E.; de Haan, W.; Scheijbeler, E.; Hamby, M.E.; Catalano, S.; Scheltens, P.; Grundman, M.; Caggiano, A.O. A Pilot Electroencephalography Study of the Effect of CT1812 Treatment on Synaptic Activity in Patients with Mild to Moderate Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2024, 11, 1809–1817. [Google Scholar] [CrossRef]
| Ligand | Sigma Receptor Target | Disease | Mechanism of Action | Developmental Status | Ref. |
|---|---|---|---|---|---|
| AF710B | Sigma 1 Agonist | Alzheimer’s Disease | ↓ cognitive impairment ↓ amyloid plaques ↓ inflammatory cytokines | Preclinical | [108,118] |
| Aniline Derivatives | Sigma 1 Agonist | Stroke | ↓ p-PERK and p-IRE1α expression ↓ ER stress | Preclinical | [49] |
| Afobazole | Sigma 1 Agonist | Stroke | ↓ Bax and caspase-3 ↑ Bcl-2 ↓ neuronal death ↓ reactive astrocytes | Preclinical | [55,181] |
| Blarcamesine | Sigma 1 Agonist | Alzheimer’s Disease, Multiple Sclerosis | MS: ↑ oligodendrogliosis ↓ apoptosis and excitotoxicity ↓ reactive oxygen species and quinolinic acid | Phase IIb/III (Alzheimer’s Disease) Preclinical (Multiple Sclerosis) | [90,136] |
| Dexmedetomidine | Sigma 1 Agonist | Stroke | ↓ BBB permeability ↓ neuronal damage ↓ CHOP, Caspase-3, and JNK ↑ Occludin stabilization | Approved (Sedation) | [48,67,182] |
| Dipentylammonium | Sigma 1 Agonist | Alzheimer’s Disease | ↑ neurite growth length ↓ excitotoxicity ↓ NFkB activation | Preclinical | [126,127] |
| Donepezil | Sigma 1 Agonist | General Neurodegeneration | ↑ oxidative respiration ↑ mitochondrial membrane potentials | Approved (Alzheimer’s Disease) | [183,184] |
| Fluvoxamine | Sigma 1 Agonist | ALS | ↑ chaperone activity ↑ Pom121 expression ↑ TFEB nuclear translocation ↑ LC3-II expression | Preclinical | [143] |
| N,N-Dimethyltryptamine | Sigma 1 Agonist | Alzheimer’s Disease, Stroke | Alzheimer’s Disease: ↓ cognitive impairment ↓ amyloid plaques ↑ MAM-associated proteins Stroke: ↓ apoptosis and ferroptosis ↓ infarct size ↑ BDNF expression ↓ TNF-α, IL1-β, IL-6 | Phase I (Alzheimer’s Disease) Preclinical (Stroke) | [47,53,72,114] |
| Oxeladin | Sigma 1 Agonist | Stroke | ↑ neurologic function ↓ infarct size ↑ BDNF expression | Preclinical | [52] |
| OZP002 | Sigma 1 Agonist | Alzheimer’s Disease | ↓ cognitive impairment ↓ reactive oxygen species and lipid peroxidation ↓ Bax, TNFα, IL-6 ↓ reactive gliosis ↑ synaptophysin and choline acetyltransferase | Preclinical | [117] |
| (+)-Pentazocine | Sigma 1 Agonist | General Neurodegeneration | ↓ microglial recruitment ↓ GAD, SOD, and p65 | Preclinical | [111] |
| PRE-084 | Sigma 1 Agonist | ALS, Alzheimer’s Disease, Parkinson’s Disease, Stroke | ALS: ↑ neuromuscular function ↑ BiP, EIF2α/ATF4, NRF2 Alzheimer’s Disease: ↑ hippocampal proliferation ↓ reactive gliosis ↓ microglial activation Parkinson’s Disease: ↑ dopaminergic protection ↓ microglial activation ↓ MPTP-induced damage Stroke: ↓ spreading depolarizations ↑ myelin density ↑ CNpase, MOG, NG2 cells, PDGFRα ↓ BBB permeability ↓ learning impairments ↑ BDNF, NR2A, CaMKIV, and TORC1 | Preclinical | [58,62,72,116,121,145,165,166,185] |
| Pridopidine | Sigma 1 Agonist | ALS, Alzheimer’s Disease, Parkinson’s Disease, Huntington’s Disease | ALS: ↑ neuromuscular function ↑ ERK ↓ SOD1 aggregation Alzheimer’s Disease: ↓ excitotoxicity ↑ synapses and dendritic spines ↑ ERK and Akt signaling ↑ long term potentiation Parkinson’s Disease: ↑ dopaminergic protection ↑ GDNF, BDNF, ERK Huntington’s Disease: ↑ BDNF, TrkB, GR, D1R, cAMP ↓ ER stress ↓ reactive oxygen species ↑ calbindin and homer1a | Phase III (Huntington’s Disease) Phase II/III (ALS) Preclinical (Alzheimer’s Disease, Parkinson’s Disease) | [89,94,122,144,149,154,156,158,160,167,169,186] |
| T-817MA | Sigma 1 Agonist | Alzheimer’s Disease | ↓ cognitive impairment ↑ hippocampal proliferation | Phase IIa | [137,187] |
| TS-157 | Sigma 1 Agonist | Stroke | ↑ neurite outgrowth ↑ ERK signaling ↑ motor recovery | Preclinical | [64] |
| Ulinastatin | Sigma 1 Agonist | Stroke | ↑ motor recovery | Preclinical | [63] |
| WLB-87848 | Sigma 1 Agonist | Alzheimer’s Disease | ↓ cognitive impairment ↑ neuron viability | Preclinical | [125] |
| BD1063 | Sigma 1 Antagonist | ALS | ↑ neuromuscular function | Preclinical | [145] |
| WLB-89462 | Sigma 2 Agonist | Alzheimer’s Disease | ↓ cognitive impairment | Preclinical | [133] |
| CT1812 | Sigma 2 Antagonist | Alzheimer’s Disease | ↓ cognitive impairment ↓ amyloid plaques ↓ phosphorylated tau fragments | Phase II | [132,138,188,189,190] |
| S1RA | Sigma 2 Antagonist | Stroke | ↑ stroke recovery ↓ reactive gliosis ↓ MMP-9 expression | Preclinical | [57] |
| SAS-0132 | Sigma 2 Antagonist | Alzheimer’s Disease | ↓ cognitive impairment | Preclinical | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drewes, N.; Fang, X.; Gupta, N.; Nie, D. Pharmacological and Pathological Implications of Sigma-1 Receptor in Neurodegenerative Diseases. Biomedicines 2025, 13, 1409. https://doi.org/10.3390/biomedicines13061409
Drewes N, Fang X, Gupta N, Nie D. Pharmacological and Pathological Implications of Sigma-1 Receptor in Neurodegenerative Diseases. Biomedicines. 2025; 13(6):1409. https://doi.org/10.3390/biomedicines13061409
Chicago/Turabian StyleDrewes, Noah, Xiangwei Fang, Nikhil Gupta, and Daotai Nie. 2025. "Pharmacological and Pathological Implications of Sigma-1 Receptor in Neurodegenerative Diseases" Biomedicines 13, no. 6: 1409. https://doi.org/10.3390/biomedicines13061409
APA StyleDrewes, N., Fang, X., Gupta, N., & Nie, D. (2025). Pharmacological and Pathological Implications of Sigma-1 Receptor in Neurodegenerative Diseases. Biomedicines, 13(6), 1409. https://doi.org/10.3390/biomedicines13061409







