Pharmacological Evaluation of Araliadiol as a Novel Anti-Inflammatory Agent in LPS-Induced RAW 264.7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Cell Culture for RAW 264.7 Cells
2.2. Intracellular Adenosine Triphosphate (ATP) Detection Assay
2.3. Crystal Violet Staining Assay
2.4. Cell Fractionation and Western Blot Analysis
2.5. Immunofluorescence Staining
2.6. Quantitative Reverse Transcriptase–Polymerase Chain Reaction (qRT-PCR)
2.7. Quantitation of Nitric Oxide (NO) Production
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
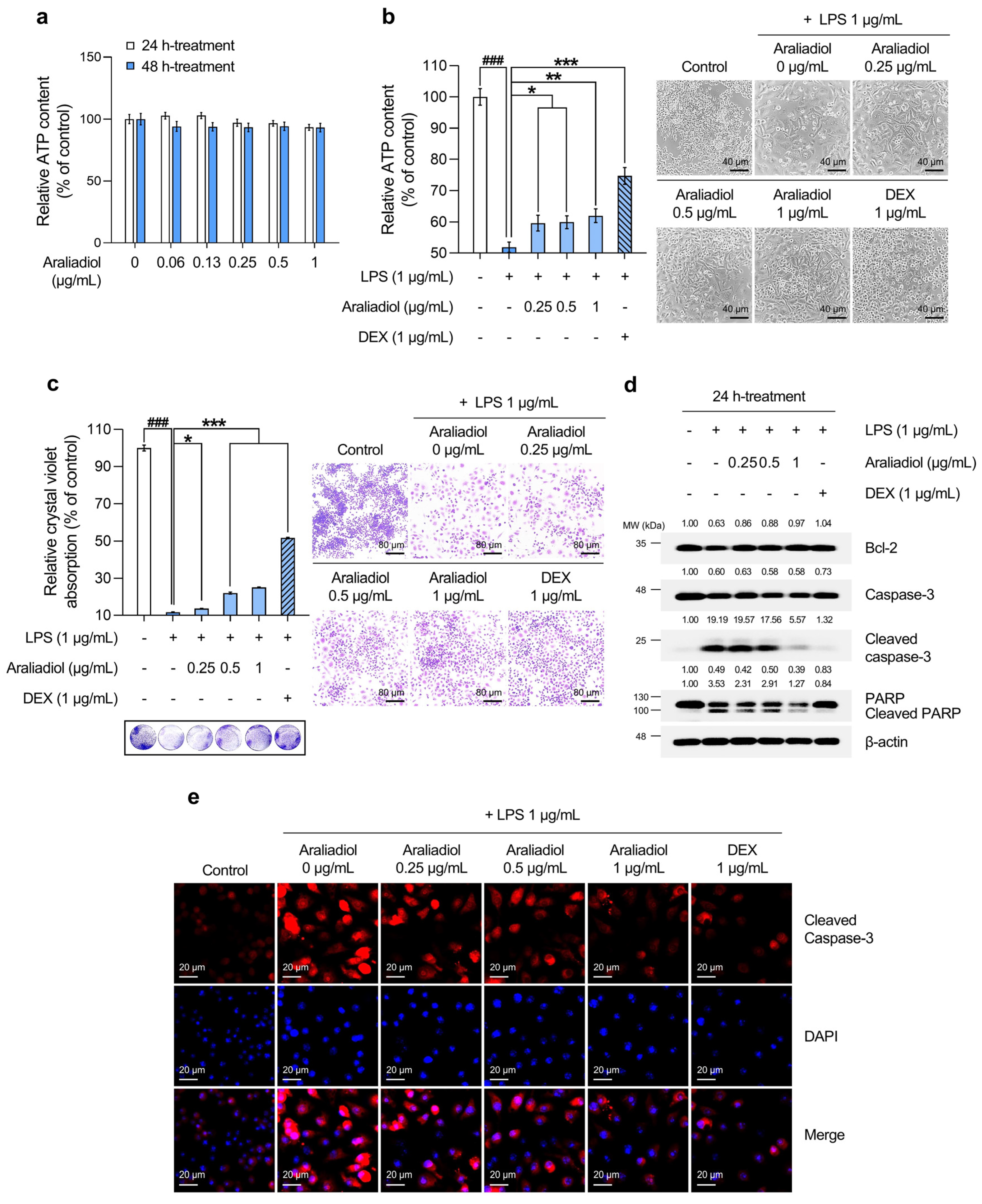
3.1. Araliadiol Attenuates Cytotoxicity and Cell Death in LPS-Induced Hyperinflammatory Responses in RAW 264.7 Cells
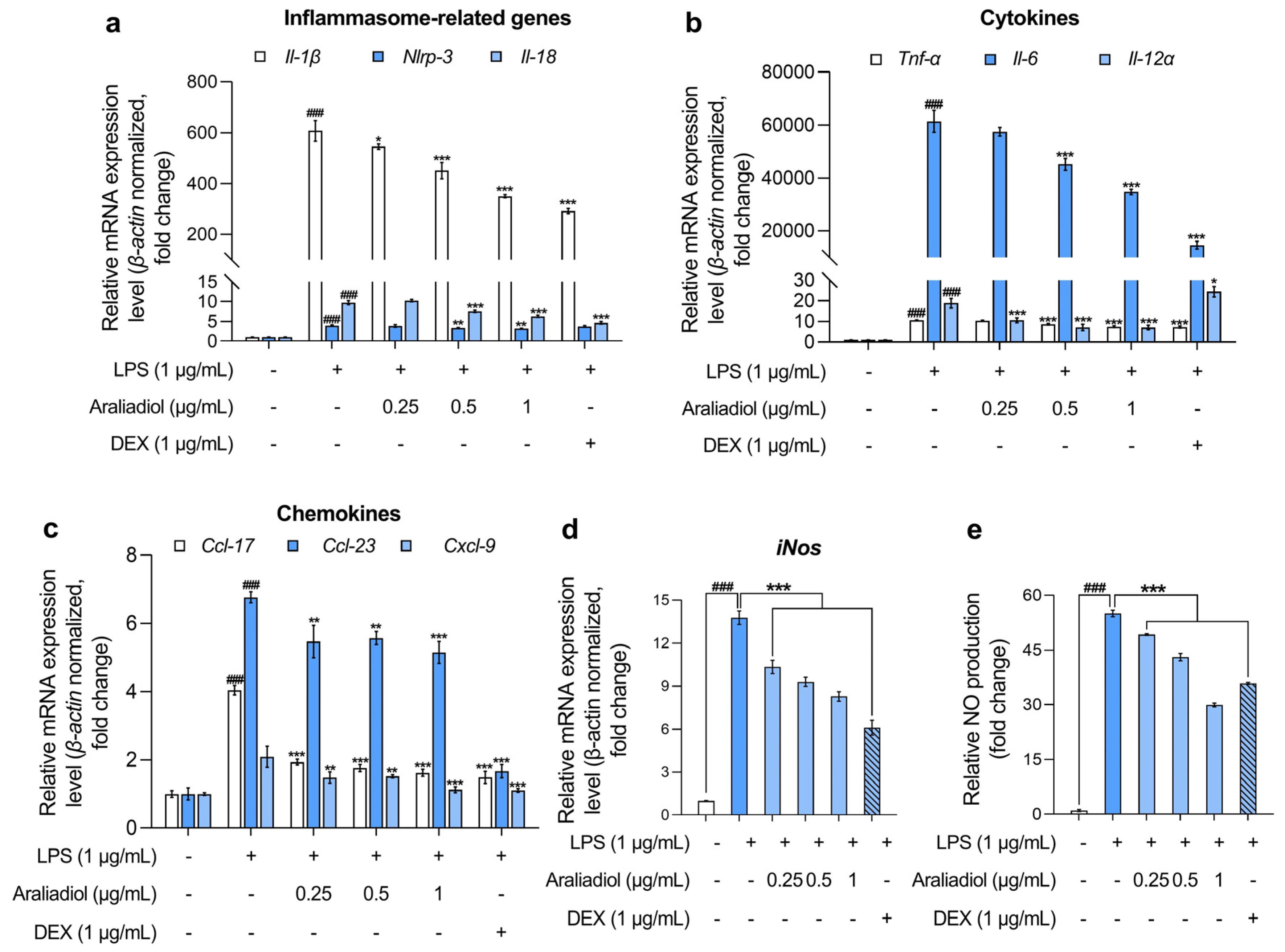
3.2. Araliadiol Downregulates the Expression of Pro-Inflammatory Mediators, Highlighting Its Therapeutic Potential in Inflammatory Diseases
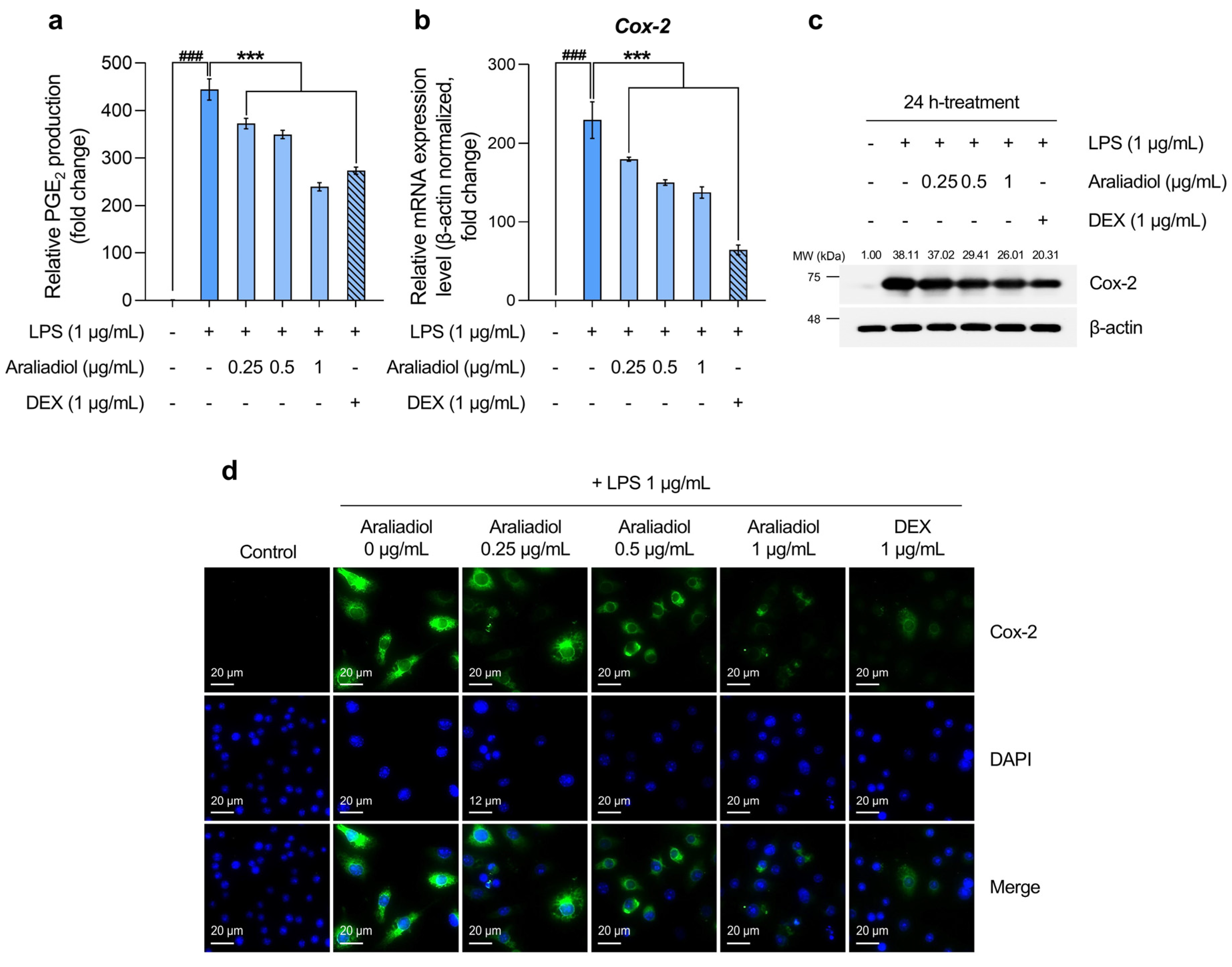
3.3. Araliadiol Has Therapeutic Potential for Alleviating Inflammatory Hyperalgesia by Downregulating Cox-2 and PGE2 Expression
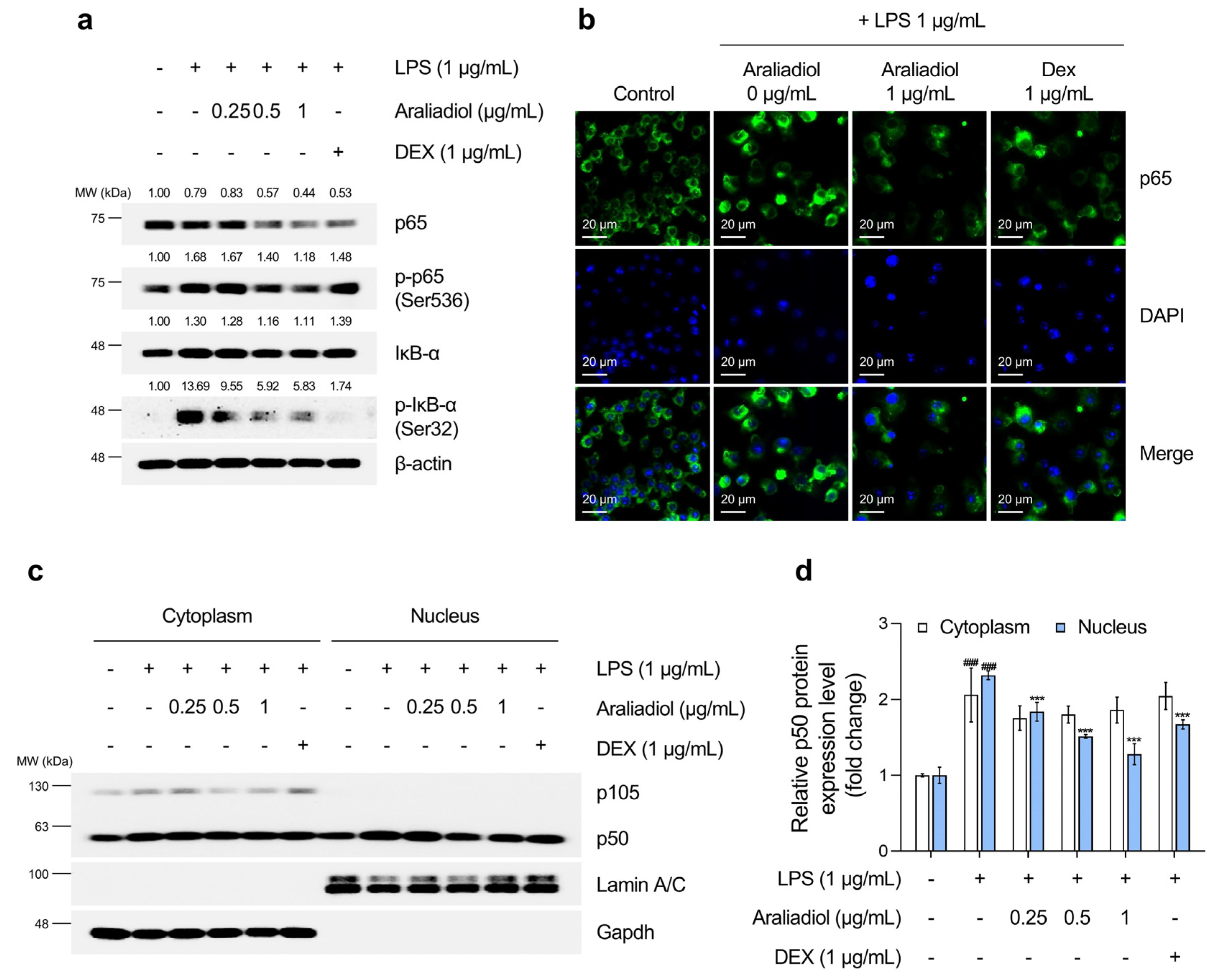
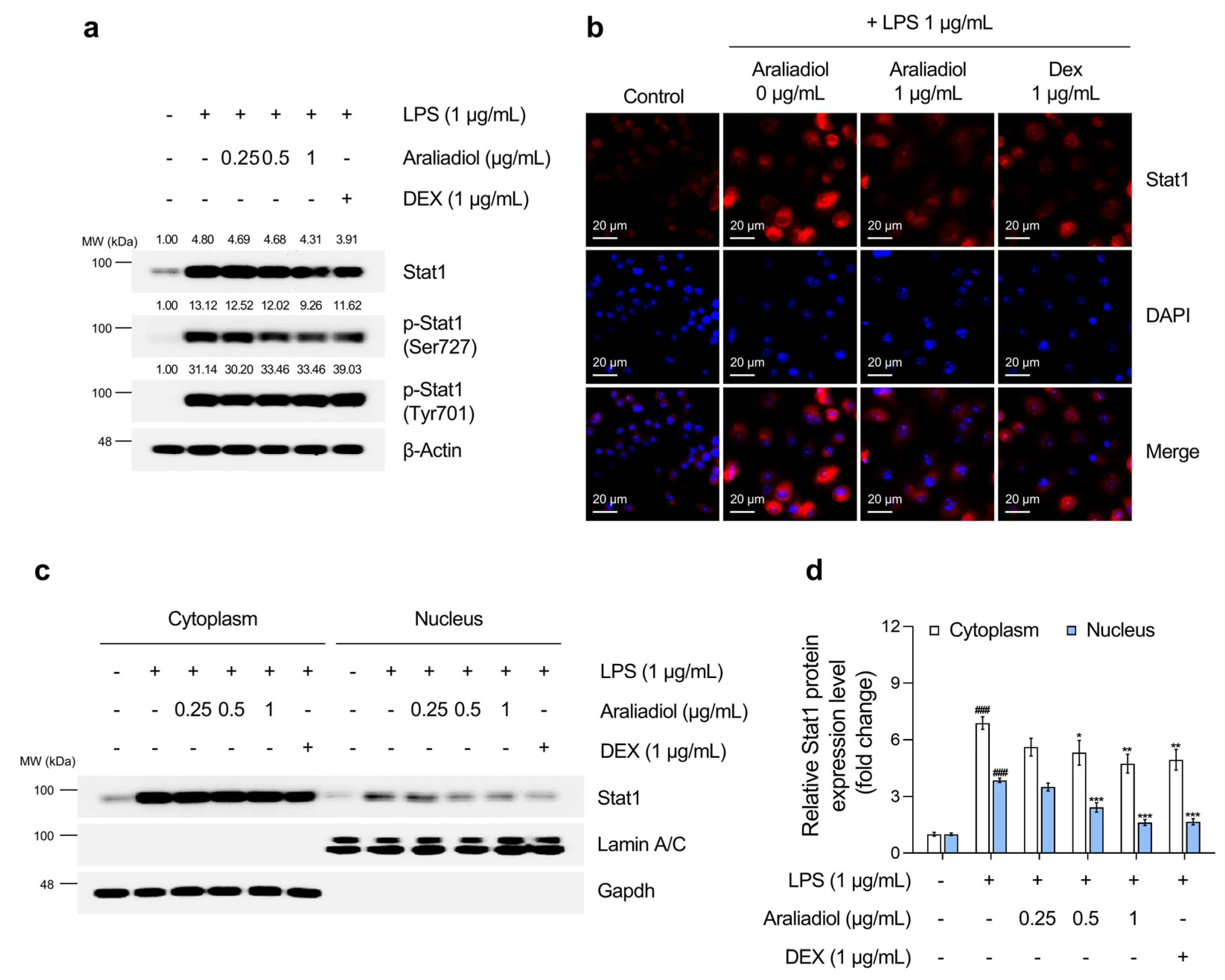
3.4. Araliadiol Suppresses Nfκb and Stat1 Signaling Pathways in LPS-Stimulated RAW 264.7 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| DEX | Dexamethasone |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| mAbs | Monoclonal antibodies |
| RA | Rheumatoid arthritis |
| IBD | Inflammatory bowel disease |
| COX | Cyclooxygenase |
| TNF | Tumor necrosis factor |
| C. asiatica | Centella asiatica |
| FBS | Fetal bovine serum |
| DPBS | Dulbecco’s phosphate-buffered saline |
| RIPA | Radio-immunoprecipitation assay |
| ANOVA | Analysis of variance |
| DAMP | Damage-associated molecular patterns |
| IAPs | Inhibitors of apoptosis proteins |
| ATP | Adenosine triphosphate |
| BCL-2 | B-cell leukemia/lymphoma 2 protein |
| PARP-1 | Poly(ADP-ribose) polymerase-1 |
| IL | Interleukin |
| NLRP | Nucleotide-binding oligomerization domain-like receptor pyrin domain containing |
| CCL | Chemokine C-C motif ligand |
| CXCL | C-X-C motif chemokine ligand |
| iNOS | Inducible nitric oxide synthase |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| TRPV1 | Transient receptor potential vanilloid 1 |
| Nav | Voltage-gated sodium channels |
| QoL | Quality of life |
| GPCR | G protein-coupled receptor |
| PGE2 | Prostaglandin E2 |
| mPGES-1 | Microsomal prostaglandin E synthase-1 |
| EP | Prostaglandin E2 receptor |
| ELISA | Enzyme-linked immunosorbent assay |
| VCAM | Vascular cell adhesion molecules |
| ICAM | Intracellular adhesion molecules |
| IκB | NF-kappa-B inhibitor |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| STAT1 | Signal transducer and activator of transcription 1 |
| WHO | World health organization |
| NASH | Non-alcoholic steatohepatitis |
References
- Keskitalo, S.; Seppänen, M.R.J.; del Sol, A.; Varjosalo, M. From rare to more common: The emerging role of omics in improving understanding and treatment of severe inflammatory and hyperinflammatory conditions. J. Allergy Clin. Immunol. 2025, 155, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids-mechanisms of action in health and disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15. [Google Scholar] [CrossRef]
- Khan, M.O.F.; Lee, H.J. Synthesis and pharmacology of anti-inflammatory steroidal antedrugs. Chem. Rev. 2008, 108, 5131–5145. [Google Scholar] [CrossRef]
- Peppa, M.; Krania, M.; Raptis, S.A. Hypertension and other morbidities with Cushing’s syndrome associated with corticosteroids: A review. Integr. Blood Press. Control 2011, 2011, 7–16. [Google Scholar] [CrossRef]
- Curtis, J.R.; Westfall, A.O.; Allison, J.; Bijlsma, J.W.; Freeman, A.; George, V.; Kovac, S.H.; Spettell, C.M.; Saag, K.G. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006, 55, 420–426. [Google Scholar] [CrossRef]
- Díaz-González, F.; Sánchez-Madrid, F. NSAIDs: Learning new tricks from old drugs. Eur. J. Immunol. 2015, 45, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Georgouli, T.; Bird, H.; Giannoudis, P.V. Nonsteroidal anti-inflammatory drugs: Prostaglandins, indications, and side effects. Int. J. Interferon Cytokine Mediat. Res. 2011, 3, 19–27. [Google Scholar] [CrossRef]
- Bian, M.; Ma, Q.-q.; Wu, Y.; Du, H.-h.; Gong, G.-H. Small molecule compounds with good anti-inflammatory activity reported in the literature from 01/2009 to 05/2021: A review. J. Enzym. Inhib. Med. Chem. 2021, 36, 2139–2159. [Google Scholar] [CrossRef]
- Li, P.; Zheng, Y.; Chen, X. Drugs for autoimmune inflammatory diseases: From small molecule compounds to anti-TNF biologics. Front. Pharmacol. 2017, 8, 460. [Google Scholar] [CrossRef]
- Rao, P.P.N.; Kabir, S.N.; Mohamed, T. Nonsteroidal anti-inflammatory drugs (NSAIDs): Progress in small molecule drug development. Pharmaceuticals 2010, 3, 1530–1549. [Google Scholar] [CrossRef] [PubMed]
- Kotsovilis, S.; Andreakos, E. Therapeutic human monoclonal antibodies in inflammatory diseases. In Human Monoclonal Antibodies; Humana: Totowa, NJ, USA, 2014; pp. 37–59. [Google Scholar]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.P.; Lappas, C.M. The influence of safety, efficacy, and medical condition severity on natural versus synthetic drug preference. Med. Decis. Mak. 2016, 36, 1011–1019. [Google Scholar] [CrossRef]
- Lappas, C.M.; Coyne, N.; Dillard, A.J.; Meier, B.P. Do physicians prefer natural drugs? The natural versus synthetic drug bias in physicians. Eur. J. Health Psychol. 2023, 30, 40. [Google Scholar] [CrossRef]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015, 4, 27–30. [Google Scholar]
- Lupiáñez, J.A.; Rufino-Palomares, E.E. Phytochemicals: “A Small Defensive Advantage for Plants and Fungi; A Great Remedy for the Health of Mankind”. Molecules 2021, 26, 6159. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Karkush, S.I.; Ali, S.A.; Mohammed, A.A. Phytochemicals: A new arsenal in drug discovery. Int. J. Med. Sci. Dent. Health 2024, 10, 29–44. [Google Scholar] [CrossRef]
- Prakash, V.; Jaiswal, N.; Srivastava, M. A review on medicinal properties of Centella asiatica. Asian J. Pharm. Clin. Res. 2017, 10, 69–74. [Google Scholar] [CrossRef]
- Jang, J.Y.; Seong, Y.H. Anti-nociceptive and anti-inflammatory effect of an ethanol extract of the leaf and stem of Aralia cordata. Nat. Prod. Sci. 2014, 20, 301–305. [Google Scholar]
- Xu, Y.; Liu, J.; Zeng, Y.; Jin, S.; Liu, W.; Li, Z.; Qin, X.; Bai, Y. Traditional uses, phytochemistry, pharmacology, toxicity and quality control of medicinal genus Aralia: A review. J. Ethnopharmacol. 2022, 284, 114671. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; Bhattamisra, S.K.; Chellappan, D.K.; Candasamy, M. Actions and therapeutic potential of madecassoside and other major constituents of Centella asiatica: A review. Appl. Sci. 2021, 11, 8475. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef]
- Min, D.-H.; Yu, Y.-B.; Kim, T.-H.; Kim, H.; Lee, S. Pharmacological effects of pentacyclic triterpenoids isolated from Centella asiatica. Hortic. Environ. Biotechnol. 2024, 65, 189–197. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Lin, T.-Y.; Tseng, Y.-H.; Chu, F.-H.; Chueh, P.-J.; Kuo, Y.-H.; Wang, S.-Y. Inhibitory effect of human breast cancer cell proliferation via p21-mediated G1 cell cycle arrest by araliadiol isolated from Aralia cordata Thunb. Planta Medica 2011, 77, 164–168. [Google Scholar] [CrossRef]
- Fujimori, H.; Ohba, T.; Mikami, M.; Nakamura, S.; Ito, K.; Kojima, H.; Takahashi, T.; Iddamalgoda, A.; Shimazawa, M.; Hara, H. The protective effect of Centella asiatica and its constituent, araliadiol on neuronal cell damage and cognitive impairment. J. Pharmacol. Sci. 2022, 148, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Yeon, S.W.; Ahn, J.H.; Turk, A.; Liu, Q.; Kim, M.-O.; Hwang, B.Y.; Park, S.-Y.; Lee, M.K. Polyacetylenes from the adventitious roots of Centella asiatica with glucose uptake stimulatory activity. J. Biotechnol. 2023, 368, 53–59. [Google Scholar] [CrossRef]
- Park, S.; Park, H.W.; Seo, D.B.; Yoo, D.S.; Bae, S. In vitro hair growth-promoting effects of araliadiol via the p38/PPAR-γ signaling pathway in human hair follicle stem cells and dermal papilla cells. Front. Pharmacol. 2024, 15, 1482898. [Google Scholar] [CrossRef]
- Park, S.; Lim, Y.J.; Kim, H.S.; Shin, H.-J.; Kim, J.-S.; Lee, J.N.; Lee, J.H.; Bae, S. Phloroglucinol enhances anagen signaling and alleviates H2O2-induced oxidative stress in human dermal papilla cells. J. Microbiol. Biotechnol. 2024, 34, 812–827. [Google Scholar] [CrossRef]
- Park, S.; Han, N.; Lee, J.; Lee, J.-N.; An, S.; Bae, S. Anti-melanogenic effects of Lilium lancifolium root extract via downregulation of PKA/CREB and MAPK/CREB signaling pathways in B16F10 cells. Plants 2023, 12, 3666. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M.; Kayagaki, N. Dying cells fan the flames of inflammation. Science 2021, 374, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Anderton, H.; Wicks, I.P.; Silke, J. Cell death in chronic inflammation: Breaking the cycle to treat rheumatic disease. Nat. Rev. Rheumatol. 2020, 16, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Nicholson, D.W. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006, 6, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Anderton, H.; Rickard, J.A.; Varigos, G.A.; Lalaoui, N.; Silke, J. Inhibitor of apoptosis proteins (IAPs) limit RIPK1-mediated skin inflammation. J. Investig. Dermatol. 2017, 137, 2371–2379. [Google Scholar] [CrossRef]
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Okin, D.; Medzhitov, R. Evolution of inflammatory diseases. Curr. Biol. 2012, 22, R733–R740. [Google Scholar] [CrossRef]
- Yucel-Lindberg, T.; Båge, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef]
- Leirisalo-Repo, M. The present knowledge of the inflammatory process and the inflammatory mediators. Pharmacol. Toxicol. 1994, 75, 1–3. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G.; Grosser, T. Prostanoids and inflammatory pain. Prostaglandins Other Lipid Mediat. 2013, 104, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.D.; Christensen, A.D.; Tewari, D.; McMahon, S.B.; Hamilton, J.A. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018, 39, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, P.; Roine, R.P.; Kalso, E.; Heiskanen, T. Worse health-related quality of life, impaired functioning and psychiatric comorbidities are associated with excess mortality in patients with severe chronic pain. Eur. J. Pain 2022, 26, 1135–1146. [Google Scholar] [CrossRef]
- van Amerongen, G.; de Boer, M.W.; Groeneveld, G.J.; Hay, J.L. A literature review on the pharmacological sensitivity of human evoked hyperalgesia pain models. Br. J. Clin. Pharmacol. 2016, 82, 903–922. [Google Scholar] [CrossRef]
- Su, Y.-S.; Sun, W.-H.; Chen, C.-C. Molecular mechanism of inflammatory pain. World J. Anesthesiol. 2014, 3, 71–81. [Google Scholar] [CrossRef]
- Kawabata, A. Prostaglandin E2 and pain—An update. Biol. Pharm. Bull. 2011, 34, 1170–1173. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nozaki-Taguchi, N. Analysis of the effects of cyclooxygenase (COX)-1 and COX-2 in spinal nociceptive transmission using indomethacin, a non-selective COX inhibitor, and NS-398, a COX-2 selective inhibitor. Brain Res. 1996, 739, 104–110. [Google Scholar] [CrossRef]
- Myers, L.K.; Kang, A.H.; Postlethwaite, A.E.; Rosloniec, E.F.; Morham, S.G.; Shlopov, B.V.; Goorha, S.; Ballou, L.R. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000, 43, 2687–2693. [Google Scholar] [CrossRef]
- Trebino, C.E.; Stock, J.L.; Gibbons, C.P.; Naiman, B.M.; Wachtmann, T.S.; Umland, J.P.; Pandher, K.; Lapointe, J.-M.; Saha, S.; Roach, M.L.; et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl. Acad. Sci. USA 2003, 100, 9044–9049. [Google Scholar] [CrossRef]
- Clark, P.; Rowland, S.E.; Denis, D.; Mathieu, M.-C.; Stocco, R.; Poirier, H.; Burch, J.; Han, Y.; Audoly, L.; Therien, A.G.; et al. MF498 [N-{[4-(5,9-Diethoxy-6-oxo-6,8-dihydro-7H-pyrrolo[3,4-g]quinolin-7-yl)-3-methylbenzyl]sulfonyl}-2-(2-methoxyphenyl)acetamide], a Selective E Prostanoid Receptor 4 Antagonist, Relieves Joint Inflammation and Pain in Rodent Models of Rheumatoid and Osteoarthritis. J. Pharmacol. Exp. Ther. 2008, 325, 425–434. [Google Scholar]
- Giuliano, F.; Warner, T.D. Origins of prostaglandin E2: Involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J. Pharmacol. Exp. Ther. 2002, 303, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The human transcription factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, X.; Liu, Y.; Tang, S.; Miao, J.; Zhou, Q.; Chen, S. IL-1β-induced NF-κB activation down-regulates miR-506 expression to promotes osteosarcoma cell growth through JAG1. Biomed. Pharmacother. 2017, 95, 1147–1155. [Google Scholar] [CrossRef]
- Babiuch, K.; Kuśnierz-Cabala, B.; Kęsek, B.; Okoń, K.; Darczuk, D.; Chomyszyn-Gajewska, M. Evaluation of proinflammatory, NF-kappaB dependent cytokines: IL-1α, IL-6, IL-8, and TNF-α in tissue specimens and saliva of patients with oral squamous cell carcinoma and oral potentially malignant disorders. J. Clin. Med. 2020, 9, 867. [Google Scholar] [CrossRef]
- de Prati, A.C.; Ciampa, A.R.; Cavalieri, E.; Zaffini, R.; Darra, E.; Menegazzi, M.; Suzuki, H.; Mariotto, S. STAT1 as a new molecular target of anti-inflammatory treatment. Curr. Med. Chem. 2005, 12, 1819–1828. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Role of the NF-kB pathway in the pathogenesis of human disease states. Curr. Mol. Med. 2001, 1, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Miklossy, G.; Hilliard, T.S.; Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 2013, 12, 611–629. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Balakin, K.V.; Lavrovsky, Y. Small molecule inhibitors of NF-κB and JAK/STAT signal transduction pathways as promising anti-inflammatory therapeutics. Mini Rev. Med. Chem. 2011, 11, 55–78. [Google Scholar] [CrossRef]
- Xu, A.; Deng, F.; Chen, Y.; Kong, Y.; Pan, L.; Liao, Q.; Rao, Z.; Xie, L.; Yao, C.; Li, S.; et al. NF-κB pathway activation during endothelial-to-mesenchymal transition in a rat model of doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2020, 130, 110525. [Google Scholar] [CrossRef]
- Serasanambati, M.; Chilakapati, S.R. Function of nuclear factor kappa B (NF-kB) in human diseases—A review. South Indian J. Biol. Sci. 2016, 2, 368–387. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Faugere, M.; Micoulaud-Franchi, J.-A.; Faget-Agius, C.; Lançon, C.; Cermolacce, M.; Richieri, R. Quality of life is associated with chronic inflammation in depression: A cross-sectional study. J. Affect. Disord. 2018, 227, 494–497. [Google Scholar] [CrossRef]
- Russell, A.S.; Gulliver, W.P.; Irvine, E.J.; Albani, S.; Dutz, J.P. Quality of life in patients with immune-mediated inflammatory diseases. J. Rheumatol. Suppl. 2011, 88, 7–19. [Google Scholar] [CrossRef]
- Jacobs, P.; Bissonnette, R.; Guenther, L.C. Socioeconomic burden of immune-mediated inflammatory diseases–Focusing on work productivity and disability. J. Rheumatol. Suppl. 2011, 88, 55–61. [Google Scholar] [CrossRef]
- Aswad, M.; Rayan, M.; Abu-Lafi, S.; Falah, M.; Raiyn, J.; Abdallah, Z.; Rayan, A. Nature is the best source of anti-inflammatory drugs: Indexing natural products for their anti-inflammatory bioactivity. Inflamm. Res. 2018, 67, 67–75. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Gosslau, A.; Li, S.; Ho, C.T.; Chen, K.Y.; Rawson, N.E. The importance of natural product characterization in studies of their anti-inflammatory activity. Mol. Nutr. Food Res. 2011, 55, 74–82. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Swanson, P.E.; Knudson, C.M.; Chang, K.C.; Cobb, J.P.; Osborne, D.F.; Zollner, K.M.; Buchman, T.G.; Korsmeyer, S.J.; Karl, I.E. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 1999, 162, 4148–4156. [Google Scholar] [CrossRef] [PubMed]
- Thapaliya, S.; Wree, A.; Povero, D.; Inzaugarat, M.E.; Berk, M.; Dixon, L.; Papouchado, B.G.; Feldstein, A.E. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig. Dis. Sci. 2014, 59, 1197–1206. [Google Scholar] [CrossRef]
- D’Lima, D.; Hermida, J.; Hashimoto, S.; Colwell, C.; Lotz, M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006, 54, 1814–1821. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Kalafateli, M.; Tsounis, E.P.; Triantos, C. Exploring the role of IL-1β in inflammatory bowel disease pathogenesis. Front. Med. 2024, 11, 1307394. [Google Scholar] [CrossRef] [PubMed]
- Dhimolea, E. Canakinumab. In Proceedings of the MAbs, Toronto, ON, Canada, 10–14 May 2010; pp. 3–13. [Google Scholar]
- Wendling, D.; Prati, C. Paradoxical effects of anti-TNF-α agents in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 159–169. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, C.L. TNF-alpha inhibitors: Current indications. Indian J. Crit. Care Med. 2007, 11, 139–148. [Google Scholar] [CrossRef]
- Li, P.; Schwarz, E.M. The TNF-α transgenic mouse model of inflammatory arthritis. Springer Semin. Immunopathol. 2003, 25, 19–33. [Google Scholar] [CrossRef]
- Piguet, P.F.; Grau, G.E.; Vesin, C.; Loetscher, H.; Gentz, R.; Lesslauer, W. Evolution of collagen arthritis in mice is arrested by treatment with anti-tumour necrosis factor (TNF) antibody or a recombinant soluble TNF receptor. Immunology 1992, 77, 510–514. [Google Scholar] [PubMed]
- Verstockt, B.; Salas, A.; Sands, B.E.; Abraham, C.; Leibovitzh, H.; Neurath, M.F.; Vande Casteele, N.; Alimentiv Translational Research Consortium (ATRC). IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 433–446. [Google Scholar] [CrossRef]
- Ataie-Kachoie, P.; Pourgholami, M.H.; Morris, D.L. Inhibition of the IL-6 signaling pathway: A strategy to combat chronic inflammatory diseases and cancer. Cytokine Growth Factor Rev. 2013, 24, 163–173. [Google Scholar] [CrossRef]
- Mannon, P.J.; Fuss, I.J.; Mayer, L.; Elson, C.O.; Sandborn, W.J.; Present, D.; Dolin, B.; Goodman, N.; Groden, C.; Hornung, R.L. Anti–interleukin-12 antibody for active Crohn’s disease. N. Engl. J. Med. 2004, 351, 2069–2079. [Google Scholar] [CrossRef]
- Kuan, W.P.; Tam, L.-S.; Wong, C.-K.; Ko, F.W.; Li, T.; Zhu, T.; Li, E.K. CXCL 9 and CXCL 10 as Sensitive markers of disease activity in patients with rheumatoid arthritis. J. Rheumatol. 2010, 37, 257–264. [Google Scholar] [CrossRef]
- Lupancu, T.J.; Eivazitork, M.; Hamilton, J.A.; Achuthan, A.A.; Lee, K.M.C. CCL17/TARC in autoimmunity and inflammation—Not just a T-cell chemokine. Immunol. Cell Biol. 2023, 101, 600–609. [Google Scholar] [CrossRef]
- Rioja, I.; Hughes, F.J.; Sharp, C.H.; Warnock, L.C.; Montgomery, D.S.; Akil, M.; Wilson, A.G.; Binks, M.H.; Dickson, M.C. Potential novel biomarkers of disease activity in rheumatoid arthritis patients: CXCL13, CCL23, transforming growth factor α, tumor necrosis factor receptor superfamily member 9, and macrophage colony-stimulating factor. Arthritis Rheum. 2008, 58, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Nijjar, J.S.; Abbott-Banner, K.; Alvarez, Y.; Aston, N.; Bass, D.; Bentley, J.H.; Ellis, J.; Ellson, C.; Emery, E.C.; Feeney, M.; et al. Efficacy, safety and tolerability of GSK3858279, an anti-CCL17 monoclonal antibody and analgesic, in healthy volunteers and patients with knee osteoarthritis pain: A phase I, randomised, double-blind, placebo-controlled, proof-of-mechanism and proof-of-concept study. Ann. Rheum. Dis. 2025, 84, 856–865. [Google Scholar]
- Elia, G.; Guglielmi, G. CXCL9 chemokine in ulcerative colitis. La Clin. Ter. 2018, 169, e235–e241. [Google Scholar]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Stichtenoth, D.O.; Frölich, J.C. Nitric oxide and inflammatory joint diseases. Br. J. Rheumatol. 1998, 37, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Möller, B.; Villiger, P.M. Inhibition of IL-1, IL-6, and TNF-α in immune-mediated inflammatory diseases. Springer Semin. Immunopathol. 2006, 27, 391–408. [Google Scholar] [CrossRef]
- Schirbel, A.; Reichert, A.; Roll, S.; Baumgart, D.C.; Büning, C.; Wittig, B.; Wiedenmann, B.; Dignass, A.; Sturm, A. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 3168. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-R.; Amaya, F.; Barrett, L.; Wang, H.; Takada, J.; Samad, T.A.; Woolf, C.J. Prostaglandin E2 Receptor EP4 Contributes to Inflammatory Pain Hypersensitivity. J. Pharmacol. Exp. Ther. 2006, 319, 1096–1103. [Google Scholar] [CrossRef]
- Sarkar, S.; Hobson, A.R.; Hughes, A.; Growcott, J.; Woolf, C.J.; Thompson, D.G.; Aziz, Q. The prostaglandin E2 receptor-1 (EP-1) mediates acid-induced visceral pain hypersensitivity in humans. Gastroenterology 2003, 124, 18–25. [Google Scholar] [CrossRef]
- Grösch, S.; Niederberger, E.; Geisslinger, G. Investigational drugs targeting the prostaglandin E2 signaling pathway for the treatment of inflammatory pain. Expert Opin. Investig. Drugs 2017, 26, 51–61. [Google Scholar] [CrossRef]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Kawada, T.; Han, I.-S.; Kim, B.-S.; Goto, T.; Takahashi, N.; Fushiki, T.; Kurata, T.; Yu, R. Capsaicin inhibits the production of tumor necrosis factor α by LPS-stimulated murine macrophages, RAW 264.7: A PPARγ ligand-like action as a novel mechanism. FEBS Lett. 2004, 572, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-A.; Cheon, H.G. Activating transcription factor-3 induction is involved in the anti-inflammatory action of berberine in RAW264.7 murine macrophages. Korean J. Physiol. Pharmacol. 2016, 20, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Hossen, I.; Zhang, K.; Wu, H.; Xiao, J.; Huang, M.; Cao, Y. Epigallocatechin gallate (EGCG) inhibits lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells via modulating nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) signaling pathway. Food Sci. Nutr. 2023, 11, 4634–4650. [Google Scholar] [CrossRef]
- Mu, M.M.; Chakravortty, D.; Sugiyama, T.; Koide, N.; Takahashi, K.; Mori, I.; Yoshida, T.; Yokochi, T. The inhibitory action of quercetin on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. J. Endotoxin Res. 2001, 7, 431–438. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Lee, J.H.; Seok, J.H.; Hong, J.H.; Lee, Y.S.; Lim, J.H.; Kim, Y.M.; Hur, G.M. Acetaminophen Inhibits iNOS Gene Expression in RAW 264.7 Macrophages: Differential Regulation of NF-κB by Acetaminophen and Salicylates. Biochem. Biophys. Res. Commun. 2000, 272, 758–764. [Google Scholar] [CrossRef]
| Antigen | Host | Clonality (Species Reactivity) | Dilution | Manufacturer (Cat. Number) |
|---|---|---|---|---|
| Bcl-2 | Rabbit | Monoclonal (Human, Mouse) | 1:1000 | CST (#3498) |
| Caspase-3 | Rabbit | Polyclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#9662) |
| Cleaved caspase-3 | Rabbit | Monoclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#9664) |
| PARP | Rabbit | Polyclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#9542) |
| β-actin | Mouse | Monoclonal (Human, Mouse, Rat, …) | 1:1000 | Santa Cruz (#sc-47778) |
| Cox-2 | Rabbit | Monoclonal (Human, Mouse, Rat) | 1:1000 | CST (#12282) |
| p65 | Rabbit | Monoclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#8242) |
| Phospho-p65 (Ser536) | Rabbit | Monoclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#3033) |
| Iκb-α | Rabbit | Polyclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#9242) |
| Phospho-Iκb-α (Ser32) | Rabbit | Monoclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#2859) |
| p50/p105 | Rabbit | Monoclonal (Human, Mouse, Rat) | 1:1000 | CST (#13586) |
| Lamin A/C | Mouse | Monoclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#4777) |
| GAPDH | Rabbit | Monoclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#5174) |
| Stat1 | Rabbit | Monoclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#14994) |
| Phospho-Stat1 (Ser727) | Rabbit | Polyclonal (Human, Mouse, Rat, …) | 1:1000 | CST (#9177) |
| Phospho-Stat1 (Tyr701) | Rabbit | Monoclonal (Human, Mouse) | 1:1000 | CST (#9167) |
| Target mRNA | Sequences of Primer | Amplicons (bp) |
|---|---|---|
| β-actin | F: 5′-GTATGGAATCCTGTGGCATC-3′ | 322 |
| R: 5′-AAGCACTTGCGGTGCACGAT-3′ | ||
| Il-1β | F: 5′-GCCCATCCTCTGTGACTCAT-3′ | 230 |
| R: 5′-AGGCCACAGGTATTTTGTCG-3′ | ||
| Il-6 | F: 5′-GAGGATACCACTCCCAACAGACC-3′ | 141 |
| R: 5′-AAGTGCATCATCGTTGTTCATACA-3′ | ||
| Tnf-α | F: 5′-CTACTCCTCAGAGCCCCCAG-3′ | 231 |
| R: 5′-TGACCACTCTCCCTTTGCAG-3′ | ||
| Cox-2 | F: 5′-GAAGTCTTTGGTCTGGTGCCTG-3′ | 133 |
| R: 5′-GTCTGCTGGTTTGGAATAGTTGC-3′ | ||
| Il-18 | F: 5′-CCATGCTTTCTGGACTCCTGCC-3′ | 133 |
| R: 5′-CCATTGTTCCTGGGCCAAGAGG-3′ | ||
| Nlrp3 | F: 5′-GCCCTTGGAGACACAGGACTCA-3′ | 224 |
| R: 5′-CCCTGCTGTTTCAGCACCTCAC-3′ | ||
| iNos | F: 5′-CGAAACGCTTCACTTCCAA-3′ | 51 |
| R: 5′-TGAGCCTATATTGCTGTGGCT-3′ | ||
| Cxcl-9 | F: 5′-ATGAAGTCCGCTGTTCTTTTCC-3′ | 386 |
| R: 5′-GTCTCTTATGTAGTCTTCCTTG-3′ | ||
| Il-12a | F: 5′-CGGGACCAAACCAGCACATTGA-3′ | 105 |
| R: 5′-GCAGCTCCCTCTTGTTGTGGAA-3′ | ||
| Ccl-17 | F: 5′-ATGCCATCGTGTTTCTGACTGT-3′ | 99 |
| R: 5′-GCCTTGGGTTTTTCACCAATC-3′ | ||
| Ccl-22 | F: 5′-AAGCCTGGCGTTGTTTTGAT-3′ | 99 |
| R: 5′-TCCCTAGGACAGTTTATGGAGTAGCT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Cho, S.; Shin, H.-J.; Baek, S.; Gwon, H.-I.; Lee, J.; Yoo, D.S.; Park, H.W.; Seo, D.B.; Bae, S. Pharmacological Evaluation of Araliadiol as a Novel Anti-Inflammatory Agent in LPS-Induced RAW 264.7 Cells. Biomedicines 2025, 13, 1408. https://doi.org/10.3390/biomedicines13061408
Park S, Cho S, Shin H-J, Baek S, Gwon H-I, Lee J, Yoo DS, Park HW, Seo DB, Bae S. Pharmacological Evaluation of Araliadiol as a Novel Anti-Inflammatory Agent in LPS-Induced RAW 264.7 Cells. Biomedicines. 2025; 13(6):1408. https://doi.org/10.3390/biomedicines13061408
Chicago/Turabian StylePark, Seokmuk, Suhyeon Cho, Hee-Jae Shin, Seyeol Baek, Hye-In Gwon, Jungmin Lee, Dae Sung Yoo, Han Woong Park, Dae Bang Seo, and Seunghee Bae. 2025. "Pharmacological Evaluation of Araliadiol as a Novel Anti-Inflammatory Agent in LPS-Induced RAW 264.7 Cells" Biomedicines 13, no. 6: 1408. https://doi.org/10.3390/biomedicines13061408
APA StylePark, S., Cho, S., Shin, H.-J., Baek, S., Gwon, H.-I., Lee, J., Yoo, D. S., Park, H. W., Seo, D. B., & Bae, S. (2025). Pharmacological Evaluation of Araliadiol as a Novel Anti-Inflammatory Agent in LPS-Induced RAW 264.7 Cells. Biomedicines, 13(6), 1408. https://doi.org/10.3390/biomedicines13061408






