The Connectivity of the Resting Brain in Primary Open-Angle Glaucoma: A Systematic Review
Abstract
1. Introduction
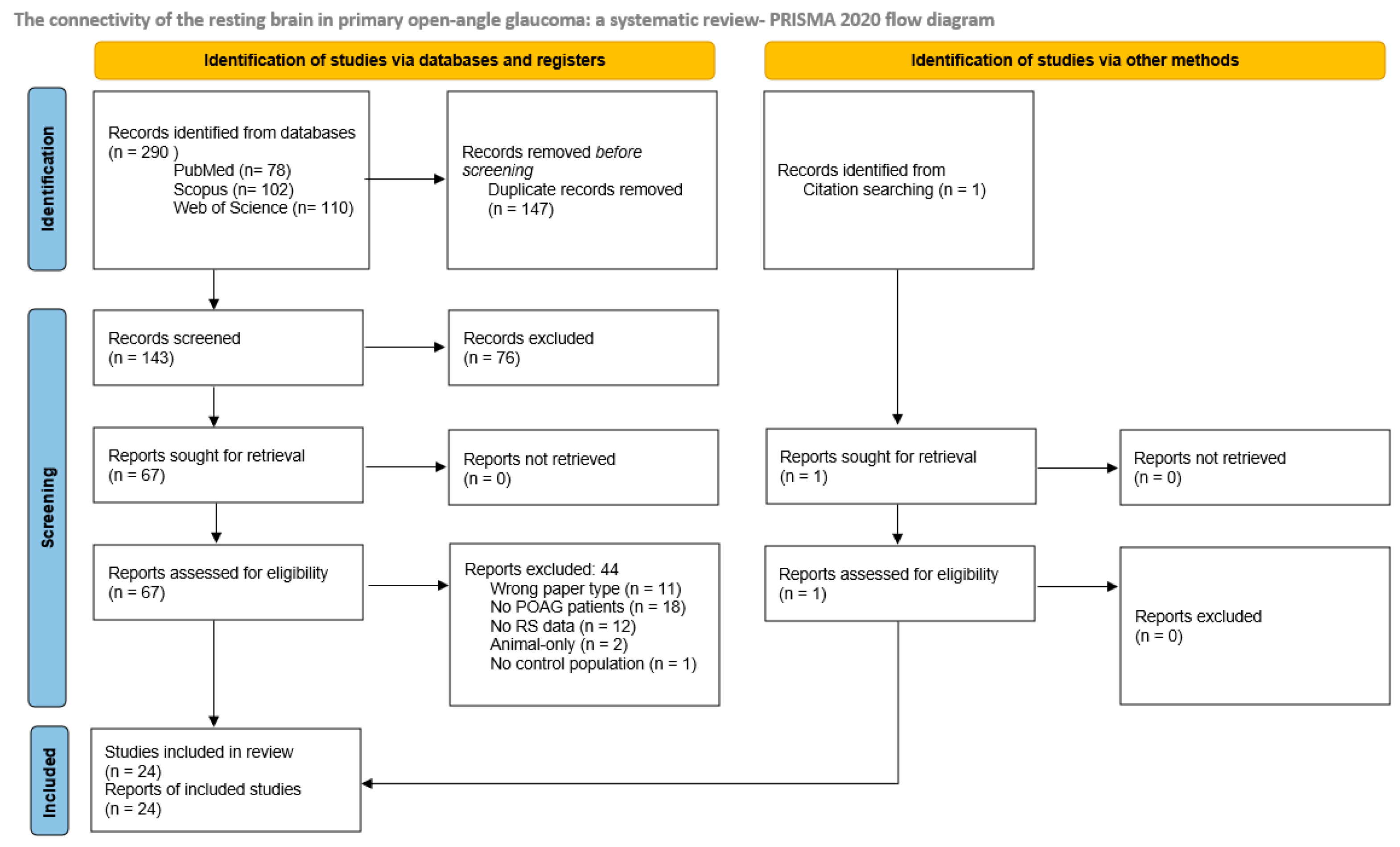
2. Materials and Methods
2.1. Registration
2.2. Search Strategy
2.3. Selection
2.4. Extraction and Analyses
3. Results
| Author, Year | Stated Aim | Subject | Method | Main Finding in POAG | Clinical Correlation |
|---|---|---|---|---|---|
| Dai et al., 2013 [22] | Analyze FC changes in visual system in POAG | 22 POAG 22 HC | Seed-based, controlled for atrophy through Voxel-Based Morphometry | ↓ positive FC b/w BA17 and R ITG, L MOG, L postCG, L preCG; b/w BA18/19 and vermis, R MTG, R STG; ↑ negative FC b/w BA17 and anterior cerebellar lobe; ↓ negative FC b/w BA17 and R MiFG, R middle cerebellar peduncle, left cerebellum, BA18/19, and R Ins | |
| Frezzoti et al., 2014 [23] | Assess white matter tracts integrity, gray matter volume changes, FC network changes, relationship to visual impairment | 13 POAG 12 HC | pICA, visual selection, voxelwise analysis | ↓ FC in exstrastriate VN (R LG), WMN (L SFG, R SMG, R LOC), DAN (b LOC, L preCG, L postCG); ↑ FC in VN (b LOC, L Fusiform), medial EN (R SFG, paracingulate, R AC) | ↓ MD—↓ FC in (b Precun, R Cun, R Calc, R MFG, R SPL) |
| Frezzotti et al., 2016 [24] | Assess if diffuse brain changes shown in advanced POAG can be detected since the early stage using multimodal MRI | 57 POAG 14 early 13 interm. 30 adv. 29 HC | pICA, voxelwise analysis | ↓ FC in VN (R Fusiform; R ITG; R LOC), WMN (R PCC; L ITG; R AG) ↑ FC in DMN (L LOC), SCN (L Putamen) | ↓ FC in LOC—↑ PSD |
| Zhou et al., 2016 [25] | Explore changes in interhemispheric FC through VMHC | 25 POAG 15 HC | VMHC (FC between symmetric interhemispheric voxels) | ↓ VMHC in Calc, Cun, Precun; ↑ VMHC in Ins, SMG, frontal gyrus, LG | ↓ VMHC in Precuneus— ↑ CDR |
| Wang, J. et al., 2016 [26] | Explore the alterations of FC and subnetwork connectivity of the VN and DMN | 25 POAG 25 HC | ICA for VN and DMN, followed by FC and FNC | ↓ FC in V1 ↓ FNC V1-V2; V1-DMN ↑ FNC V2-DMN | FNC ↑ V1-DMN—↑ MD on the left |
| Giorgio et al., 2017 [27] | Relate presence or absence of raised IOP to neurodegenerative findings in normal-tension glaucoma and POAG | 17 POAG 10 mild 2 mod. 5 severe 17 NTG 29 HC | Multimodal, including ICA, followed by voxelwise FC | ↑ FC in medial frontal ECN, VAN ↑ FNC b/w V2 and LN | — |
| Wang, Q et al., 2018a [28] | Investigate if abnormal VMHC is accompanied by anatomic connectivity changes and relate it with ophthalmic parameters | 16 POAG 6 early 4 interm. 6 adv. 19 HC | VMHC, homotopic Diffusion Tensor Imaging, correlation | ↓ zVMHC in BA17 (V1), BA18 (V2), BA19 (V3,4,5) | VMHC ↑ BA17, BA18, BA19—↑ RNFLT mean |
| Wang, Y. et al., 2020 [29] | Assess alterations in resting-state visual networks in patients with POAG and investigate the effect of elevated IOP | 36 POAG 20 HC | ICA with spatial correlation analysis for 3 visual networks, intranetwork FC | ↓ FC b/w L Calc—lateral network ↓ FC b/w b LG—Medial network ↓ FC b/w b LG—occipital network | ↓ FC b/w L Calc—lateral network—↑ IOP |
| Wang, B et al., 2021 [30] | Evaluate the effect of elevated IOP on FC of the VN | 36 POAG 20 HC | Voxelwise FC analysis of 1 ROI | ↓ FC b/w BA17—R SFG; BA17—R Precun | ↓ FC (BA17—R SFG)—↑ IOP |
| Yang et al., 2024 [31] | Analyze brain functional abnormalities through Dynamic FC—functional stability | 70 POAG 45 HC | Dynamic FC—functional stability (Kendall’s coefficient) | ↓ Stability in early visual centers (V2, V3, V4), dorsal stream, ventral stream ↑ Stability in b IPL and R inferior frontal cortex | ↓ Stability in L early visual centers and dorsal stream—↓ MD |
3.1. Classical Functional Connectivity Techniques
| Author, Year | Stated Aim | Subject | Method | Main Finding in POAG | Clinical Correlation |
|---|---|---|---|---|---|
| Liu and Tian, 2014 [32] | To investigate regional spontaneous activity and correlate with disease severity | 21 POAG 22 HC | ALFF, correlation with HAP | ↓ ALFF in R V1; R Fusiform; R LG; R ITG; L postCG; L preCG; R posterior CL; ↑ ALFF in R MeFG and R SMA | ↑ ALFF in R SFG and ↓ ALFF in L occipital; L postCG—↑ HAP |
| Li et al., 2014 [33] | To analyze altered ALFF | 21 POAG 22 HC | ALFF and fALFF, correlation with HAP | ↓ ALFF IN R LG, R ITG, L preCG; ↑ ALFF in R MiFG, R SMA; ↓ fALLF in b Cun, R MTG, R PostCG, L PCC, R lymbic lobe; ↑ fALLF in R middle cingulate cortex, L IPL, R MiFG | ↑ Spont. activities in L Cun, b MTG ↓ in R SFG—↑ severity (HAP) |
| Yuan et al., 2018 [34] | To determine the role of the locus coeruleus–norepinephrine system in POAG in patients through ALFF and FC and experimentally in animals | 22 POAG 22 HC + animals | ALFF for LC, seed-based FC for LC, clinical correlations | ↑ ALFF in LC ↑ FC between LC and parahippocampus ↓ FC between LC and R Ins and R frontal lobe | ↑ ALFF in LC—↑ CDR; MD; ↓ RNFLT |
3.2. ALFF-Based Techniques
| Author, Year | Stated Aim | Subject | Method | Main Finding in POAG | Clinical Correlation |
|---|---|---|---|---|---|
| Song et al., 2014 [35] | To investigate spontaneous activity in POAG | 39 POAG 41 HC | ReHo, clinical correlation with visual field | ↑ ReHo in R anterior cingulate cortex, B MeFG, R anterior CL, R SFG ↓ ReHo in B Calc, R LG, B Precun, B preCG, B postCG, L IPL, L posterior CL | ↑ ReHo in SFG, L IPL, L Calc and ↓ in Precun—↑ MD |
| Wang, Y et al., 2019 [36] | To evaluate the effects of high-IOP on CNS in POAG | 36 POAG 20 HC | ReHo, clinical correlation with IOP | ↑ ReHo in L CL VIII (posterior), CL IV, CL V (anterior), L Fusiform ↓ ReHo in L MiFG | ↑ ReHo in L Fusiform and ↓ in MiFG—↑ IOP |
3.3. ReHo-Based Techniques
| Author, Year | Stated Aim | Subject | Method | Main Finding in POAG | Clinical Correlation |
|---|---|---|---|---|---|
| Zhang et al., 2015 [37] | To assess the cortical structure and cerebral blood flow changes in POAG | 23 POAG (subdivided) 29 HC | ASL-CBF in resting state, then with task, Voxel-Based Morphometry | Only in advanced disease: ↓ CBF in anterior Calc | |
| Wang, Q et al., 2018b [38] | To investigate the correlations between reduced CBF and changes in the retinas mild-to-moderate POAG through ASL-CBF | 15 (mild-to-moderate) POAG 20 HC | ASL-CBF in resting state, comparison for interhemispheric symmetricity, correlation with CDR, RNFLT, GCC | In mild and moderate disease, ↓ zCBF in L V1, L V2, R Ventral posterior area (V3v), L LOC ↑ zCBF symmetricity in V1 and V3v/VP | ↓ zCBF in R V3v/VP, R V2—↑ CDR, ↓ GCC, RNFLT |
| Wang, Q et al., 2021 [39] | To test whether disturbed neurovascular coupling in visual and higher-order cognitive cortices exists in POAG and correlates with disease stage and VF defects | 45 POAG 12 early 19 intermed. 4 advanc. 25 HC | ASL-CBF in resting state, ratio with FC strength (CBF/FCS), correlation with stage and MD | ↓ CBF/FCS in b LG; b Calc; b Rectal gyri; R STG; R ITG; R IFG ↑ CBF/FCS in R AG; R MiFG ↓ CBF in B LG; B Calc; R PostCG; R IFG; R SMG; B IPL; L SMG, B cerebellum ↑ CBF in B Rectal gyri; B MiFG; R MeFG; R SFG; R Ins | ↓ CBF/FCS in b LG—↑ stage/severity, MD defect |
| Wang, Q et al., 2024 [40] | To investigate CBF-redistributed patterns in visual and higher-order cognitive cortices and its clinical correlations | 45 POAG 23 HC | ASL-CBF, CBF connectivity (CBFC) | ↓ CBF in b LG, b Calc, R PostCG, R IPL, L cerebellar crus, R CL VI (posterior CL) ↑ CBF in R medial prefrontal gyrus, R MeFG, b MiFG, R SFG; R Ins ↓ negative CBFC in R mPFC—R ITG, R MOG Appeared negative CBFC between PostCG and R Calc, R SOG; R SFG and R ITG; L IPL and R STG Appeared positive CBFC in L MFG—R CPL, R ITG; L MFG—R CPL, R ITG; R MFG—R CPL, R MTG | ↓ CBF in b LG, b Cal and ↑ CBF in L cerebellar crus, MeFG—↑ MD |
3.4. ASL-CBF-Based Techniques
| Author, Year | Stated Aim | Subject | Method | Main Finding in POAG | Clinical Correlation |
|---|---|---|---|---|---|
| Wang J. et al., 2016a [41] | To investigate the efficiency of the functional communication change in POAG | 25 POAG 25 HC | GTA at 13% sparsity, BC, Deg, Eg, El used as topological properties at global and local levels, correlation to MD and CDR | Global GT metrics—no significant differences ↓ Disruption indices ↓ BC in L IFG, L Fusiform, L hippocampus, and R paracentral lobule ↑ BC in R MFG, L SMA, R Amygdala, R AG, L Thalamus, R Heschl’s gyrus 6 hub regions not in POAG—L IFG, B Fusiform, L Precun, L STG, R MTG 9 hub regions only in POAG—L preCG, R SFG/MiFG, R IFG, R Hyppocampus, R Amygdala, R LG, L MOG, R STG | ↓ BC of R Fusiform; ↑ BC of R LG—↓ R MD |
| Minosse et al., 2019 [42] | To evaluate the potential of functional network disruption indices as biomarkers of disease severity | 19 POAG 16 HC | GTA at 10% sparsity, calculation of BC, El, spectral measure of centrality, clinical correlation | Global and local GT metrics—no differences ↓ Disruption indices 2 hub regions not in POAG—R AG, L CL VII 3 hub regions only in POAG—R inferior occipital cortex, R ITG, L CL IX | Positive association between disruption indices and MD, macula ganglion cell layer thickness, RNFLT |
| Qu. et al., 2020 [43] | To generate a visual atlas based on FC from POAG patients and to prove its applicability on FC and network analysis | 36 POAG 20 HC | Parcellation of visual cortex, GTA using a data-driven atlas—Deg, E, SWI, RCI at global and nodal levels as topological properties | ↓ Deg, rich club index ↑ Nodal E, small-world index More asymmetric parcellation in visual cortices | — |
| Demaria et al., 2021 [44] | To determine network integrity in glaucoma and ways in which the VF could affect the hub function of networked brain areas | 20 POAG 24 HC | Two scans, fast eigenvector centrality mapping, hub selection (5% highest EC), correlation | Global and local functional networks—no differences Aberrant EC in Ins and MiFG in 1 of 2 scans | Aberrant EC in R LG with ↓ binocular integrated visual field |
3.5. Graph Theory-Based Techniques
4. Discussion
4.1. The Visual System
4.2. RS Changes in the Visual Cortex
4.3. RS Changes in VN Components Outside the Visual Cortices
4.4. RS Changes in Extravisual Networks
4.5. RS Changes Correlated with Clinical Measures
4.6. Lateralization of Changes
4.7. Limitations of the Studies Included
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanna, A.P. BCSC: 2023–2024. Glaucoma. Section 10; American Academy of Ophthalmology: San Francisco, CA, USA, 2023. [Google Scholar]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Malihi, M.; Filho, E.R.M.; Hodge, D.O.; Sit, A.J. Long-Term Trends in Glaucoma-Related Blindness in Olmsted County, Minnesota. Ophthalmology 2014, 121, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Hedley-Whyte, E.T.; Dreyer, E.B. Lateral Geniculate Nucleus in Glaucoma. Am. J. Ophthalmol. 1993, 116, 182–188. [Google Scholar] [CrossRef]
- Gupta, N.; Greenberg, G.; de Tilly, L.N.; Gray, B.; Polemidiotis, M.; Yücel, Y.H. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br. J. Ophthalmol. 2009, 93, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Mu, K.T.; Qi, J.P.; Wang, C.Y.; Zhu, W.Z.; Xia, L.M.; Chen, Z.Q.; Zhang, H.; Ai, F.; Morelli, J.N. Assessment of Lateral Geniculate Nucleus Atrophy with 3T MR Imaging and Correlation with Clinical Stage of Glaucoma. Am. J. Neuroradiol. 2011, 32, 1347–1353. [Google Scholar] [CrossRef]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef]
- Forster, B.B.; MacKay, A.L.; Whittall, K.P.; Kiehl, K.A.; Smith, A.M.; Hare, R.D.; Liddle, P.F. Functional magnetic resonance imaging: The basics of blood-oxygen-level dependent (BOLD) imaging. Can. Assoc. Radiol. J. = J. L’association Can. Des Radiologistes 1998, 49, 320–329. [Google Scholar]
- Petcharunpaisan, S.; Ramalho, J.; Castillo, M. Arterial spin labeling in neuroimaging. World J. Radiol. 2010, 2, 384–398. [Google Scholar] [CrossRef]
- Hahn, A.; Lanzenberger, R.; Kasper, S. Making Sense of Connectivity. Int. J. Neuropsychopharmacol. 2018, 22, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.M.; Smith, S.M.; Beckmann, C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, R.M.; Womelsdorf, T.; Allen, E.A.; Bandettini, P.A.; Calhoun, V.D.; Corbetta, M.; Della Penna, S.; Duyn, J.H.; Glover, G.H.; Gonzalez-Castillo, J.; et al. Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage 2013, 80, 360–378. [Google Scholar] [CrossRef]
- Yu-Feng, Z.; Yong, H.; Chao-Zhe, Z.; Qing-Jiu, C.; Man-Qiu, S.; Meng, L.; Li-Xia, T.; Tian-Zi, J.; Yu-Feng, W. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007, 29, 83–91. [Google Scholar] [CrossRef]
- Golestani, A.M.; Kwinta, J.B.; Khatamian, Y.B.; Chen, J.J. The Effect of Low-Frequency Physiological Correction on the Reproducibility and Specificity of Resting-State fMRI Metrics: Functional Connectivity, ALFF, and ReHo. Front. Neurosci. 2017, 11, 546. [Google Scholar] [CrossRef]
- Zang, Y.; Jiang, T.; Lu, Y.; He, Y.; Tian, L. Regional homogeneity approach to fMRI data analysis. NeuroImage 2004, 22, 394–400. [Google Scholar] [CrossRef]
- Farahani, F.V.; Karwowski, W.; Lighthall, N.R. Application of Graph Theory for Identifying Connectivity Patterns in Human Brain Networks: A Systematic Review. Front. Neurosci. 2019, 13, 585. [Google Scholar] [CrossRef] [PubMed]
- Garaci, F.; Altobelli, S.; Toschi, N.; Mancino, R.; Nucci, C.; Schillaci, O.; Floris, R. Brain imaging in glaucoma from clinical studies to clinical practice. Prog. Brain Res. 2015, 221, 159–175. [Google Scholar]
- Nuzzi, R.; Dallorto, L.; Rolle, T. Changes of Visual Pathway and Brain Connectivity in Glaucoma: A Systematic Review. Front. Neurosci. 2018, 12, 363. [Google Scholar] [CrossRef]
- Sujanthan, S.; Shmuel, A.; Mendola, J.D. Resting-state functional MRI of the visual system for characterization of optic neuropathy. Front. Hum. Neurosci. 2022, 16, 943618. [Google Scholar] [CrossRef]
- Dai, H.; Morelli, J.N.; Ai, F.; Yin, D.; Hu, C.; Xu, D.; Li, Y. Resting-state functional MRI: Functional connectivity analysis of the visual cortex in primary open-angle glaucoma patients. Hum. Brain Mapp. 2013, 34, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Frezzotti, P.; Giorgio, A.; Motolese, I.; De Leucio, A.; Iester, M.; Motolese, E.; Federico, A.; De Stefano, N. Structural and Functional Brain Changes beyond Visual System in Patients with Advanced Glaucoma. PLoS ONE 2014, 9, e105931. [Google Scholar] [CrossRef]
- Frezzotti, P.; Giorgio, A.; Toto, F.; De Leucio, A.; De Stefano, N. Early changes of brain connectivity in primary open angle glaucoma. Hum. Brain Mapp. 2016, 37, 4581–4596. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, J.; Li, T.; Wang, N.; Xian, J.; He, H. Abnormal interhemispheric resting-state functional connectivity in primary open-angle glaucoma. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE Engineering in Medicine and Biology Society, Annual International Conference, Orlando, FL, USA, 16–20 August 2016; Volume 2016, pp. 4055–4058. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Zhou, P.; Wang, N.; Xian, J.; He, H. Altered functional connectivity within and between the default model network and the visual network in primary open-angle glaucoma: A resting-state fMRI study. Brain Imaging Behav. 2017, 11, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Zhang, J.; Costantino, F.; De Stefano, N.; Frezzotti, P. Diffuse brain damage in normal tension glaucoma. Hum. Brain Mapp. 2018, 39, 532–541. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Wang, H.; Zhang, X.; Qu, X.; Wang, Y.; Li, T.; Wang, N.; Xian, J. Reduced Functional and Anatomic Interhemispheric Homotopic Connectivity in Primary Open-Angle Glaucoma: A Combined Resting State-fMRI and DTI Study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1861–1868. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, W.; Xie, Y.; Zhou, J.; Yan, T.; Han, W.; Qiu, J. Functional Alterations in Resting-State Visual Networks in High-Tension Glaucoma: An Independent Component Analysis. Front. Hum. Neurosci. 2020, 14, 330. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yan, T.; Zhou, J.; Xie, Y.; Qiu, J.; Wang, Y.; Lu, W. Altered fMRI-derived functional connectivity in patients with high-tension glaucoma. J. Neuroradiol. 2021, 48, 94–98. [Google Scholar] [CrossRef]
- Yang, B.; Su, M.; Wang, Q.; Qu, X.; Wang, H.; Chen, W.; Sun, Y.; Li, T.; Wang, Y.; Wang, N.; et al. Altered stability of dynamic brain functional architecture in primary open-angle glaucoma: A surface-based resting-state fMRI study. Brain Imaging Behav. 2024, 18, 44–56. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, J. Amplitude of low frequency fluctuation in primary open angle glaucoma: A resting state fMRI study. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE Engineering in Medicine and Biology Society, Annual International Conference, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 6706–6709. [Google Scholar] [CrossRef]
- Li, T.; Liu, Z.; Li, J.; Liu, Z.; Tang, Z.; Xie, X.; Yang, D.; Wang, N.; Tian, J.; Xian, J. Altered amplitude of low-frequency fluctuation in primary open-angle glaucoma: A resting-state FMRI study. Investig. Ophthalmol. Vis. Sci. 2014, 56, 322–329. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, Y.; Song, Y.; Zhao, Y.; Yan, X.; Chen, W.; Li, M.; Gong, J.; Mu, K.; Wang, J.; et al. Morphological and Functional Changes of Locus Coeruleus in Patients with Primary Open-Angle Glaucoma and Animal Models. Neuroscience 2018, 372, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Mu, K.; Wang, J.; Lin, F.; Chen, Z.; Yan, X.; Hao, Y.; Zhu, W.; Zhang, H. Altered spontaneous brain activity in primary open angle glaucoma: A resting-state functional magnetic resonance imaging study. PLoS ONE 2014, 9, e89493. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, W.; Yan, T.; Zhou, J.; Xie, Y.; Yuan, J.; Liu, G.; Teng, Y.; Han, W.; Chen, D.; et al. Functional MRI reveals effects of high intraocular pressure on central nervous system in high-tension glaucoma patients. Acta Ophthalmol. 2019, 97, e341–e348. [Google Scholar] [CrossRef]
- Zhang, S.D.; Wang, B.; Xie, Y.; Zhu, S.H.; Thomas, R.; Qing, G.P.; Zhang, C.; Wang, N.L. Retinotopic Changes in the Gray Matter Volume and Cerebral Blood Flow in the Primary Visual Cortex of Patients With Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6171–6178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, W.W.; Qu, X.X.; Wang, H.Z.; Wang, Y.; Zhang, X.; Li, T.; Wang, N.L.; Xian, J.F. Reduced Cerebral Blood Flow in the Visual Cortex and Its Correlation With Glaucomatous Structural Damage to the Retina in Patients With Mild to Moderate Primary Open-angle Glaucoma. J. Glaucoma 2018, 27, 816–822. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, X.; Chen, W.; Wang, H.; Huang, C.; Li, T.; Wang, N.; Xian, J. Altered coupling of cerebral blood flow and functional connectivity strength in visual and higher order cognitive cortices in primary open angle glaucoma. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2021, 41, 901–913. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, X.; Wang, H.; Chen, W.; Sun, Y.; Li, T.; Chen, J.; Wang, Y.; Wang, N.; Xian, J. Arterial spin labeling reveals disordered cerebral perfusion and cerebral blood flow-based functional connectivity in primary open-angle glaucoma. Brain Imaging Behav. 2024, 18, 231–242. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Wang, N.; Xian, J.; He, H. Graph theoretical analysis reveals the reorganization of the brain network pattern in primary open angle glaucoma patients. Eur. Radiol. 2016, 26, 3957–3967. [Google Scholar] [CrossRef] [PubMed]
- Minosse, S.; Garaci, F.; Martucci, A.; Lanzafame, S.; Di Giuliano, F.; Picchi, E.; Cesareo, M.; Mancino, R.; Guerrisi, M.; Pistolese, C.A.; et al. Primary Open Angle Glaucoma Is Associated With Functional Brain Network Reorganization. Front. Neurol. 2019, 10, 1134. [Google Scholar] [CrossRef]
- Qu, H.Y.; Wang, Y.; Yan, T.Q.; Zhou, J.; Lu, W.Z.; Qiu, J.F. Data-Driven Parcellation Approaches Based on Functional Connectivity of Visual Cortices in Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2020, 61, 33. [Google Scholar] [CrossRef]
- Demaria, G.; Invernizzi, A.; Ombelet, D.; Carvalho, J.C.; Renken, R.J.; Cornelissen, F.W. Binocular Integrated Visual Field Deficits Are Associated With Changes in Local Network Function in Primary Open-Angle Glaucoma: A Resting-State fMRI Study. Front. Aging Neurosci. 2021, 13, 744139. [Google Scholar] [CrossRef]
- Kandel, E.R.; Koester, J.D.; Mack, S.H.; Siegelbaum, S.A. Principles of Neural Science, 6th ed.; McGraw Hill LLC: New York, NY, USA, 2021. [Google Scholar]
- Goodale, M.A.; Milner, A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Mishkin, M.; Ungerleider, L.G.; Macko, K.A. Object vision and spatial vision: Two cortical pathways. Trends Neurosci. 1983, 6, 414–417. [Google Scholar] [CrossRef]
- Yücel, Y.; Gupta, N. Glaucoma of the brain: A disease model for the study of transsynaptic neural degeneration. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 173, pp. 465–478. [Google Scholar]
- Palejwala, A.H.; O’Connor, K.P.; Milton, C.K.; Anderson, C.; Pelargos, P.; Briggs, R.G.; Conner, A.K.; O’Donoghue, D.L.; Glenn, C.A.; Sughrue, M.E. Anatomy and white matter connections of the fusiform gyrus. Sci. Rep. 2020, 10, 13489. [Google Scholar] [CrossRef] [PubMed]
- Grill-Spector, K.; Kourtzi, Z.; Kanwisher, N. The lateral occipital complex and its role in object recognition. Vis. Res. 2001, 41, 1409–1422. [Google Scholar] [CrossRef]
- Zhang, P.; Wen, W.; Sun, X.; He, S. Selective reduction of fMRI responses to transient achromatic stimuli in the magnocellular layers of the LGN and the superficial layer of the SC of early glaucoma patients. Hum. Brain Mapp. 2016, 37, 558–569. [Google Scholar] [CrossRef]
- Skottun, B.C. The magnocellular system versus the dorsal stream. Front. Hum. Neurosci. 2014, 8, 786. [Google Scholar] [CrossRef][Green Version]
- Menon, V. 20 years of the default mode network: A review and synthesis. Neuron 2023, 111, 2469–2487. [Google Scholar] [CrossRef]
- Hodapp, E.; Parrish, R.K.; Anderson, D.R. Clinical Decisions in Glaucoma; Mosby: Maryland Heights, MI, USA, 1993. [Google Scholar]
- Ferreira, L.K.; Busatto, G.F. Resting-state functional connectivity in normal brain aging. Neurosci. Biobehav. Rev. 2013, 37, 384–400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velkov, N.; Kandilarova, S.; Stoyanov, D. The Connectivity of the Resting Brain in Primary Open-Angle Glaucoma: A Systematic Review. Biomedicines 2025, 13, 1402. https://doi.org/10.3390/biomedicines13061402
Velkov N, Kandilarova S, Stoyanov D. The Connectivity of the Resting Brain in Primary Open-Angle Glaucoma: A Systematic Review. Biomedicines. 2025; 13(6):1402. https://doi.org/10.3390/biomedicines13061402
Chicago/Turabian StyleVelkov, Nikola, Sevdalina Kandilarova, and Drozdstoy Stoyanov. 2025. "The Connectivity of the Resting Brain in Primary Open-Angle Glaucoma: A Systematic Review" Biomedicines 13, no. 6: 1402. https://doi.org/10.3390/biomedicines13061402
APA StyleVelkov, N., Kandilarova, S., & Stoyanov, D. (2025). The Connectivity of the Resting Brain in Primary Open-Angle Glaucoma: A Systematic Review. Biomedicines, 13(6), 1402. https://doi.org/10.3390/biomedicines13061402






