TAAR8 in the Brain: Implications for Dopaminergic Function, Neurogenesis, and Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of TAAR8-KO Mice by CRISPR-Cas9 Technology
2.2. TAAR8a mRNA Expression Analysis by RT-qPCR
2.3. Animals
2.4. Behavioral Tests
2.4.1. Open Field Test
2.4.2. Open Field with Novel Objects
2.4.3. Marble Burying
2.4.4. T-Maze Test
2.4.5. Light–Dark Transition Test
2.4.6. Elevated Plus Maze Test
2.4.7. Grip Strength Measurement
2.4.8. Stress-Induced Hyperthermia Test
2.4.9. Hot Plate Test
2.4.10. Forced Swimming Test
2.4.11. Prepulse Inhibition Test
2.5. Electrophysiological Recordings
2.5.1. Operating Procedure
2.5.2. Electrocorticogram and Local Field Potentials Recording
2.6. High-Performance Liquid Chromatography Measurements of the Tissue Monoamine and Metabolite Content
2.7. Immunohistochemistry
2.7.1. Perfusion and Primary Tissue Processing
2.7.2. Tyrosine Hydroxylase Immunofluorescence Staining
2.7.3. Doublecortin Immunofluorescence Staining
2.7.4. Image Processing
3. Results
3.1. Generation of tTAAR8-KO Mice by CRISPR-Cas9 Technology
3.2. Taar8a Expression Pattern in the Brain
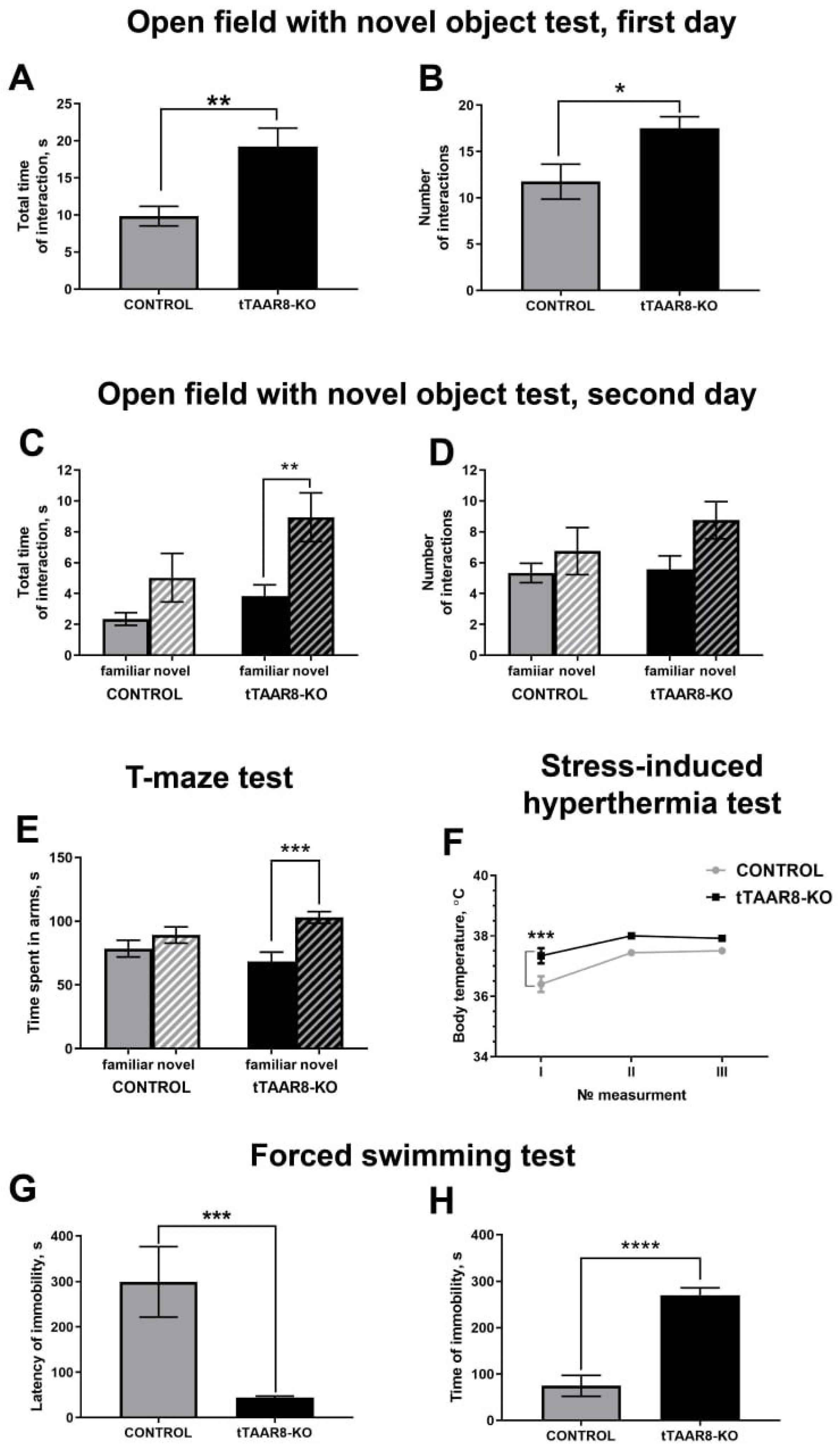
3.3. Behavioral Profile
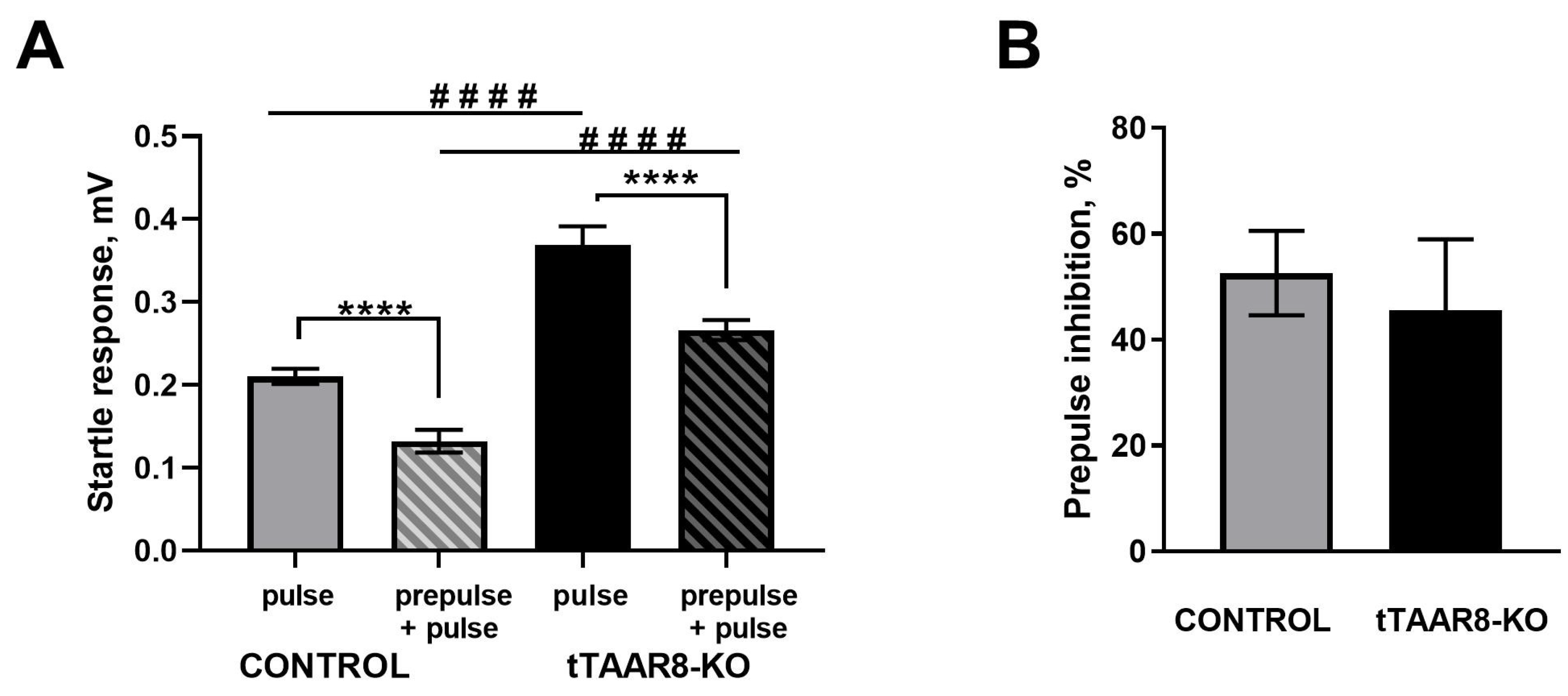
3.4. Evaluation of the Effectiveness of Sensorimotor Gating
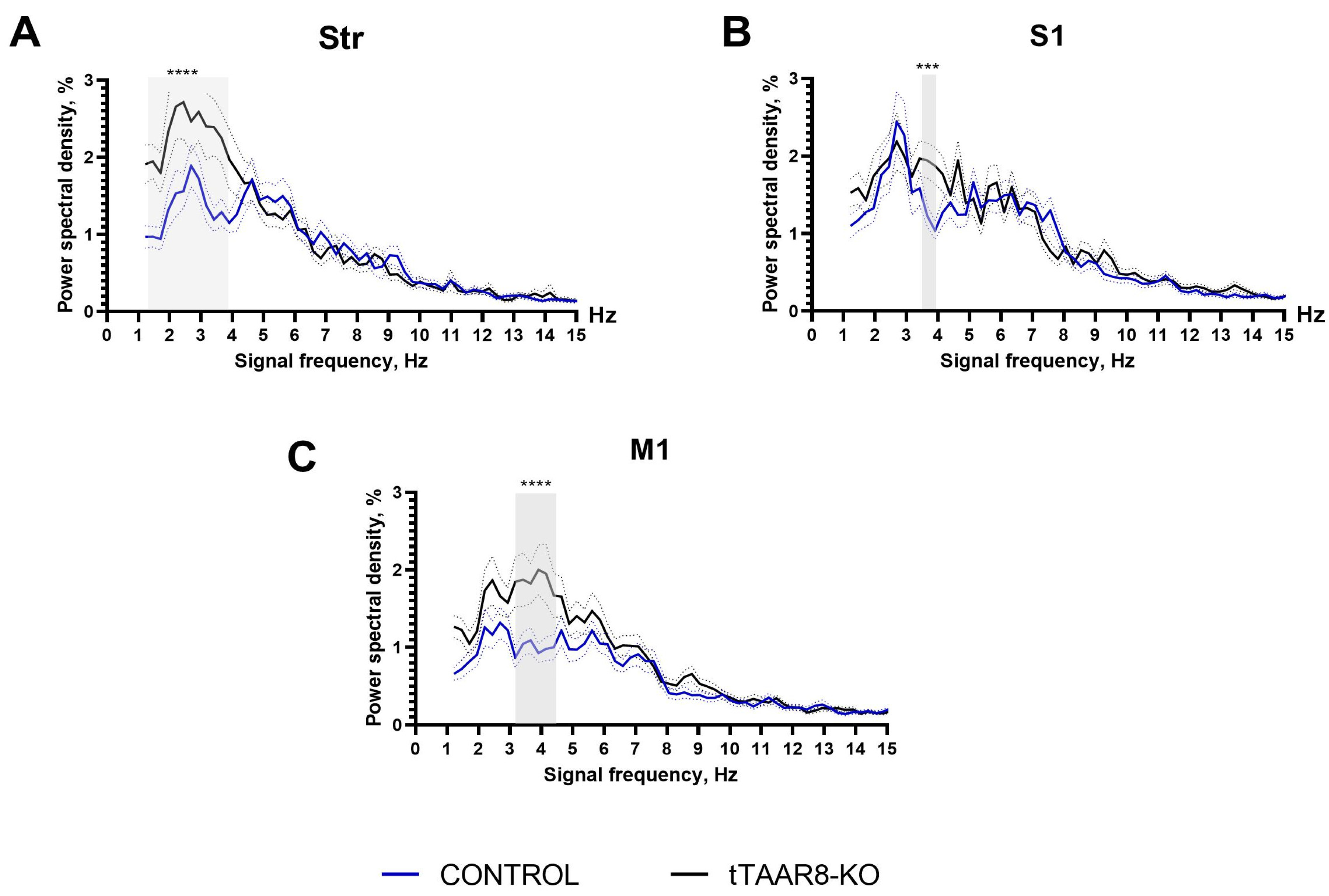
3.5. Power Spectra
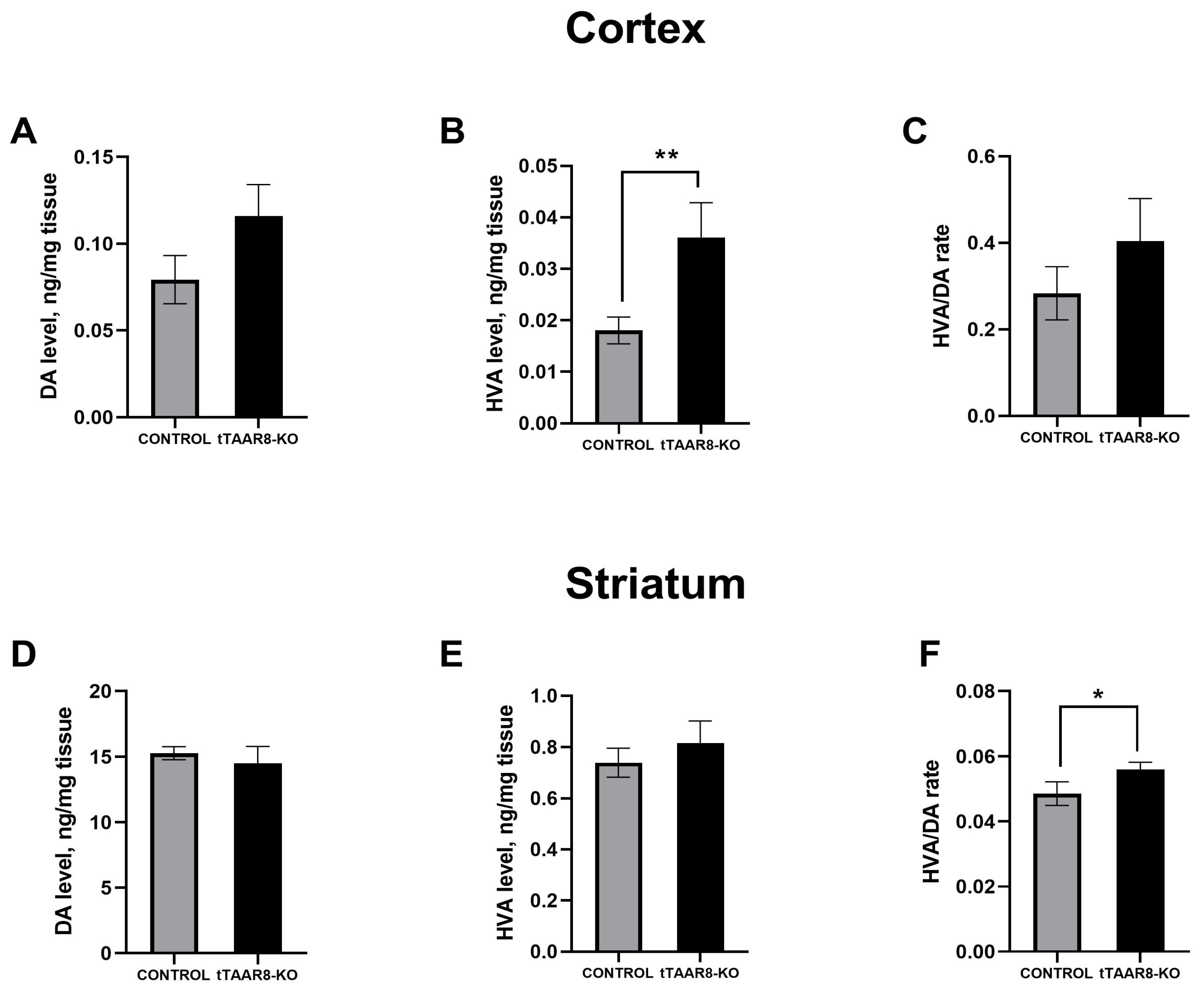
3.6. High-Performance Liquid Chromatography Study of Brain Monoamine Levels
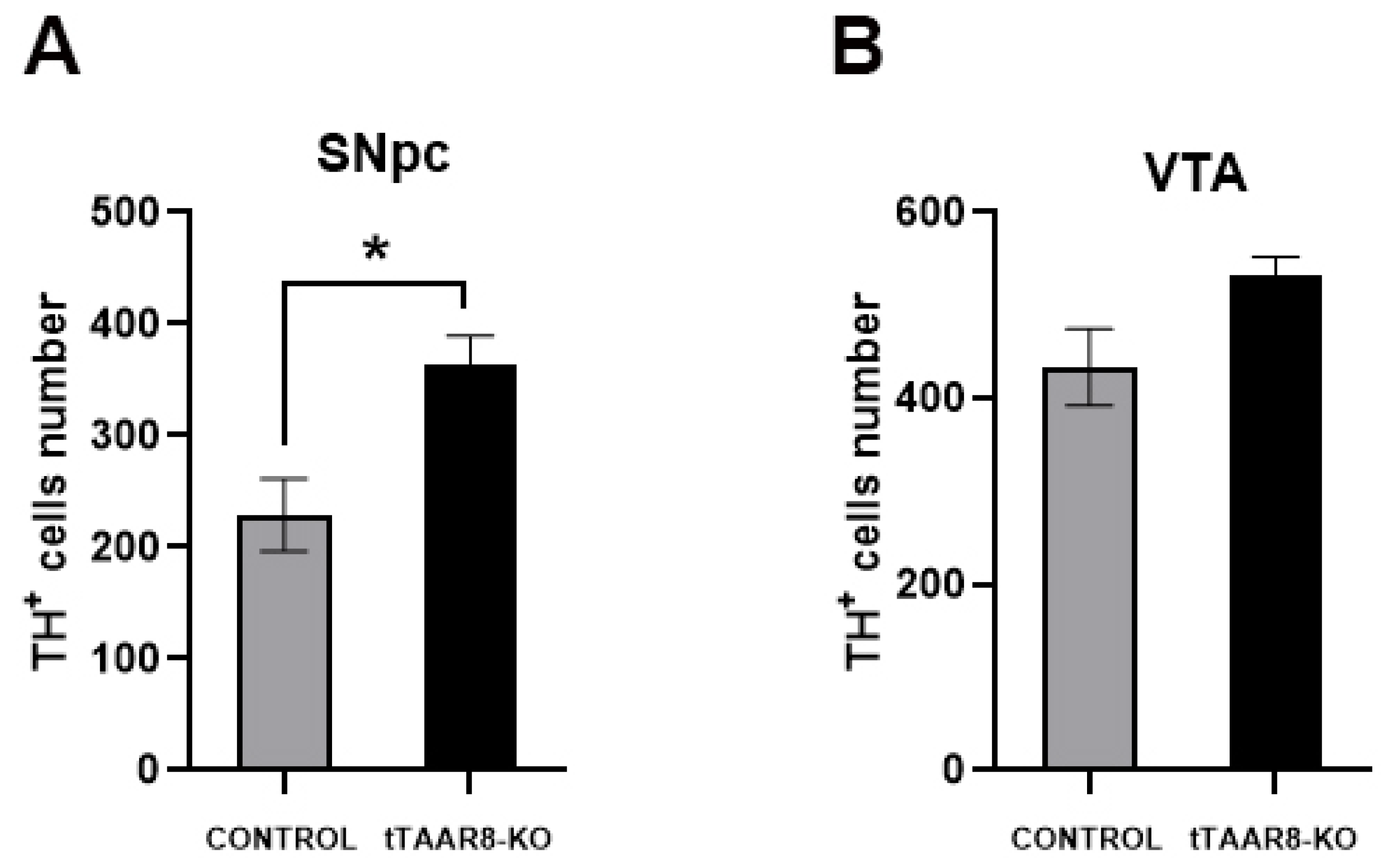
3.7. Tyrosine Hydroxylase Immunofluorescence
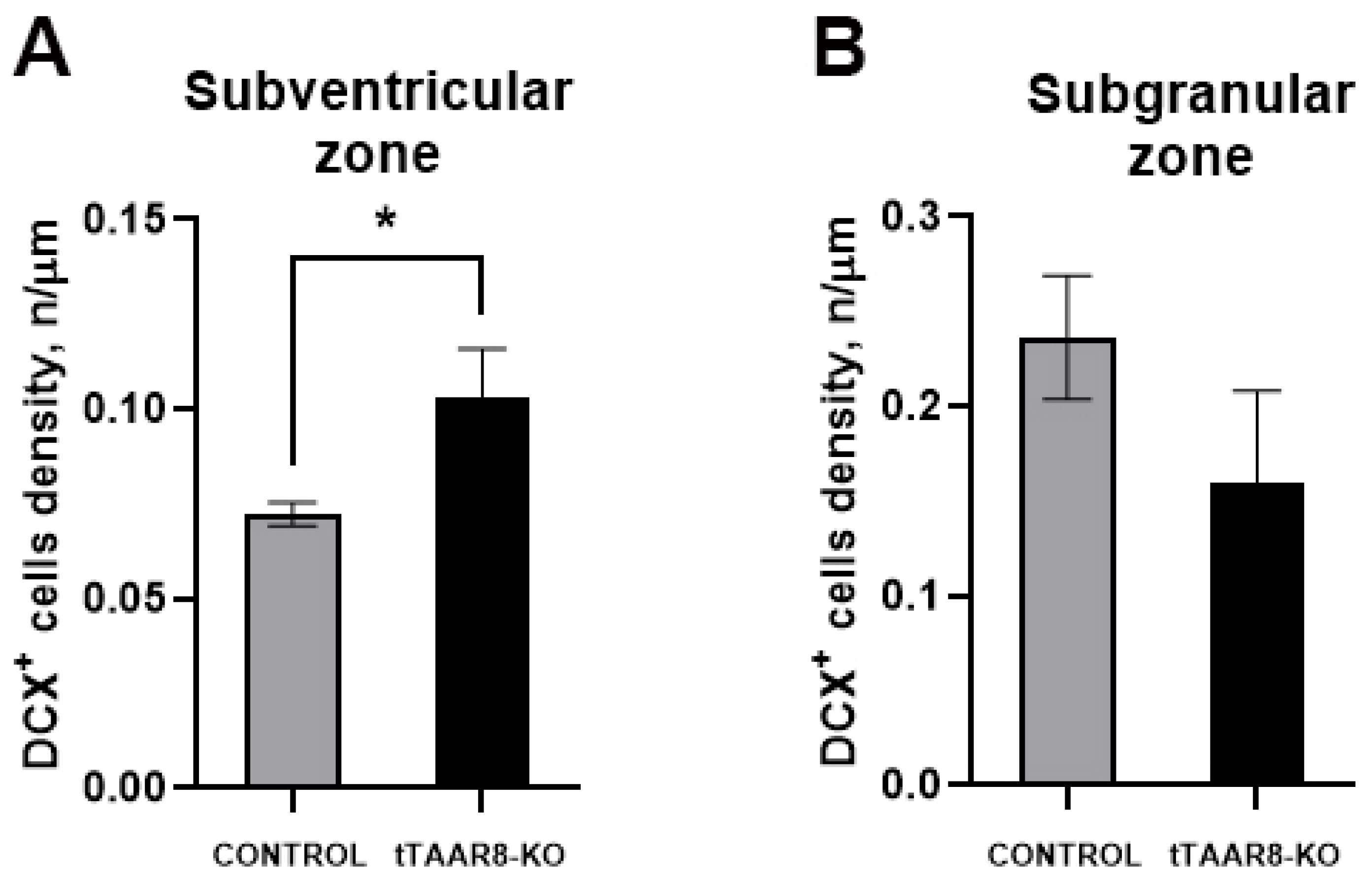
3.8. Adult Neurogenesis Analysis Immunofluorescence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | 5-hydroxytryptamine, serotonin |
| DA | dopamine |
| DCX | doublecortin |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| ECoG | electrocorticogram |
| GPCRs | G protein-coupled receptors |
| HVA | homovanillic acid |
| KO | knockout |
| LFP | local field potential |
| NE | norepinephrine |
| sgRNA | single guide RNA |
| SGZ | subgranular zone |
| SNpc | pars compacta of substantia nigra |
| SVZ | subventricular zone |
| TAARs | trace amine-associated receptors |
| TH | tyrosine hydroxylase |
| tTAAR8-KO | triple TAAR8abc–KO |
| VTA | ventral tegmental area |
References
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace Amines: Identification of a Family of Mammalian G Protein-Coupled Receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef] [PubMed]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-Methylenedioxymethamphetamine, Lysergic Acid Diethylamide, and Metabolites of the Catecholamine Neurotransmitters Are Agonists of a Rat Trace Amine Receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, L.; Ebeling, M.; Kratochwil, N.A.; Bunzow, J.R.; Grandy, D.K.; Hoener, M.C. Trace Amine-Associated Receptors Form Structurally and Functionally Distinct Subfamilies of Novel G Protein-Coupled Receptors. Genomics 2005, 85, 372–385. [Google Scholar] [CrossRef]
- Liberles, S.D. Trace Amine-associated Receptors Are Olfactory Receptors in Vertebrates. Ann. N. Y. Acad. Sci. 2009, 1170, 168–172. [Google Scholar] [CrossRef]
- Correll, C.U.; Koblan, K.S.; Hopkins, S.C.; Li, Y.; Dworak, H.; Goldman, R.; Loebel, A. Safety and Effectiveness of Ulotaront (SEP-363856) in Schizophrenia: Results of a 6-Month, Open-Label Extension Study. Npj Schizophr. 2021, 7, 63. [Google Scholar] [CrossRef]
- Højlund, M.; Correll, C.U. Ulotaront: A TAAR1/5-HT1A Agonist in Clinical Development for the Treatment of Schizophrenia. Expert Opin. Investig. Drugs 2022, 31, 1279–1290. [Google Scholar] [CrossRef]
- Kuvarzin, S.R.; Sukhanov, I.; Onokhin, K.; Zakharov, K.; Gainetdinov, R.R. Unlocking the Therapeutic Potential of Ulotaront as a Trace Amine-Associated Receptor 1 Agonist for Neuropsychiatric Disorders. Biomedicines 2023, 11, 1977. [Google Scholar] [CrossRef]
- Synan, C.; Bowen, C.; Heal, D.J.; Froger-Colléaux, C.; Beardsley, P.M.; Dedic, N.; Hopkins, S.C.; Campbell, U.; Koblan, K.S. Ulotaront, a Novel TAAR1 Agonist with 5-HT1A Agonist Activity, Lacks Abuse Liability and Attenuates Cocaine Cue-Induced Relapse in Rats. Drug Alcohol Depend. 2022, 231, 109261. [Google Scholar] [CrossRef]
- Liberles, S.D.; Buck, L.B. A Second Class of Chemosensory Receptors in the Olfactory Epithelium. Nature 2006, 442, 645–650. [Google Scholar] [CrossRef]
- Efimova, E.V.; Kozlova, A.A.; Razenkova, V.; Katolikova, N.V.; Antonova, K.A.; Sotnikova, T.D.; Merkulyeva, N.S.; Veshchitskii, A.S.; Kalinina, D.S.; Korzhevskii, D.E.; et al. Increased Dopamine Transmission and Adult Neurogenesis in Trace Amine-Associated Receptor 5 (TAAR5) Knockout Mice. Neuropharmacology 2021, 182, 108373. [Google Scholar] [CrossRef]
- Espinoza, S.; Sukhanov, I.; Efimova, E.V.; Kozlova, A.; Antonova, K.A.; Illiano, P.; Leo, D.; Merkulyeva, N.; Kalinina, D.; Musienko, P.; et al. Trace Amine-Associated Receptor 5 Provides Olfactory Input Into Limbic Brain Areas and Modulates Emotional Behaviors and Serotonin Transmission. Front. Mol. Neurosci. 2020, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Carnicelli, V.; Santoro, A.; Sellari-Franceschini, S.; Berrettini, S.; Zucchi, R. Expression of Trace Amine-Associated Receptors in Human Nasal Mucosa. Chemosens. Percept. 2010, 3, 99–107. [Google Scholar] [CrossRef]
- Johnson, M.A.; Tsai, L.; Roy, D.S.; Valenzuela, D.H.; Mosley, C.; Magklara, A.; Lomvardas, S.; Liberles, S.D.; Barnea, G. Neurons Expressing Trace Amine-Associated Receptors Project to Discrete Glomeruli and Constitute an Olfactory Subsystem. Proc. Natl. Acad. Sci. USA 2012, 109, 13410–13415. [Google Scholar] [CrossRef] [PubMed]
- Katolikova, N.V.; Vaganova, A.N.; Shafranskaya, D.D.; Efimova, E.V.; Malashicheva, A.B.; Gainetdinov, R.R. Expression Pattern of Trace Amine-Associated Receptors during Differentiation of Human Pluripotent Stem Cells to Dopaminergic Neurons. Int. J. Mol. Sci. 2023, 24, 15313. [Google Scholar] [CrossRef]
- Li, Q.; Liberles, S.D. Odor Sensing by Trace Amine-Associated Receptors. In Chemosensory Transduction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 67–80. ISBN 978-0-12-801694-7. [Google Scholar]
- Izquierdo, C.; Gómez-Tamayo, J.C.; Nebel, J.-C.; Pardo, L.; Gonzalez, A. Identifying Human Diamine Sensors for Death Related Putrescine and Cadaverine Molecules. PLoS Comput. Biol. 2018, 14, e1005945. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.M.; Lemon, J.K.; Fluegge, D.; Pashkovski, S.L.; Korzan, W.J.; Datta, S.R.; Spehr, M.; Fendt, M.; Liberles, S.D. Detection and Avoidance of a Carnivore Odor by Prey. Proc. Natl. Acad. Sci. USA 2011, 108, 11235–11240. [Google Scholar] [CrossRef]
- Ferrero, D.M.; Wacker, D.; Roque, M.A.; Baldwin, M.W.; Stevens, R.C.; Liberles, S.D. Agonists for 13 Trace Amine-Associated Receptors Provide Insight into the Molecular Basis of Odor Selectivity. ACS Chem. Biol. 2012, 7, 1184–1189. [Google Scholar] [CrossRef]
- Doe, B.; Brown, E.; Boroviak, K. Generating CRISPR/Cas9-Derived Mutant Mice by Zygote Cytoplasmic Injection Using an Automatic Microinjector. Methods Protoc. 2018, 1, 5. [Google Scholar] [CrossRef]
- Marshall, O.J. PerlPrimer: Cross-Platform, Graphical Primer Design for Standard, Bisulphite and Real-Time PCR. Bioinformatics 2004, 20, 2471–2472. [Google Scholar] [CrossRef]
- Chiellini, G.; Frascarelli, S.; Ghelardoni, S.; Carnicelli, V.; Tobias, S.C.; DeBarber, A.; Brogioni, S.; Ronca-Testoni, S.; Cerbai, E.; Grandy, D.K.; et al. Cardiac Effects of 3-Iodothyronamine: A New Aminergic System Modulating Cardiac Function. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Murtazina, R.Z.; Zhukov, I.S.; Korenkova, O.M.; Popova, E.A.; Kuvarzin, S.R.; Efimova, E.V.; Kubarskaya, L.G.; Batotsyrenova, E.G.; Zolotoverkhaya, E.A.; Vaganova, A.N.; et al. Genetic Deletion of Trace-Amine Associated Receptor 9 (TAAR9) in Rats Leads to Decreased Blood Cholesterol Levels. Int. J. Mol. Sci. 2021, 22, 2942. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Pelevin, A.; Kurzina, N.; Zavialov, V.; Volnova, A. A Custom Solution for Acoustic Startle Response Setup with Spike2-Based Data Acquisition Interface. Methods Protoc. 2023, 6, 57. [Google Scholar] [CrossRef]
- Efimova, E.V.; Kuvarzin, S.R.; Mor, M.S.; Katolikova, N.V.; Shemiakova, T.S.; Razenkova, V.; Ptukha, M.; Kozlova, A.A.; Murtazina, R.Z.; Smirnova, D.; et al. Trace Amine-Associated Receptor 2 Is Expressed in the Limbic Brain Areas and Is Involved in Dopamine Regulation and Adult Neurogenesis. Front. Behav. Neurosci. 2022, 16, 847410. [Google Scholar] [CrossRef]
- Mühlhaus, J.; Dinter, J.; Nürnberg, D.; Rehders, M.; Depke, M.; Golchert, J.; Homuth, G.; Yi, C.-X.; Morin, S.; Köhrle, J.; et al. Analysis of Human TAAR8 and Murine Taar8b Mediated Signaling Pathways and Expression Profile. Int. J. Mol. Sci. 2014, 15, 20638–20655. [Google Scholar] [CrossRef]
- Katolikova, N.V.; Vaganova, A.N.; Efimova, E.V.; Gainetdinov, R.R. Expression of Trace Amine-Associated Receptors in the Murine and Human Hippocampus Based on Public Transcriptomic Data. Cells 2022, 11, 1813. [Google Scholar] [CrossRef]
- Alnefeesi, Y.; Tamura, J.K.; Lui, L.M.W.; Jawad, M.Y.; Ceban, F.; Ling, S.; Nasri, F.; Rosenblat, J.D.; McIntyre, R.S. Trace Amine-Associated Receptor 1 (TAAR1): Potential Application in Mood Disorders: A Systematic Review. Neurosci. Biobehav. Rev. 2021, 131, 192–210. [Google Scholar] [CrossRef]
- Shemiakova, T.S.; Efimova, E.V.; Gainetdinov, R.R. TAARs as Novel Therapeutic Targets for the Treatment of Depression: A Narrative Review of the Interconnection with Monoamines and Adult Neurogenesis. Biomedicines 2024, 12, 1263. [Google Scholar] [CrossRef]
- Khan, M.Z.; Nawaz, W. The Emerging Roles of Human Trace Amines and Human Trace Amine-Associated Receptors (hTAARs) in Central Nervous System. Biomed. Pharmacother. 2016, 83, 439–449. [Google Scholar] [CrossRef]
- Vaganova, A.N.; Katolikova, N.V.; Murtazina, R.Z.; Kuvarzin, S.R.; Gainetdinov, R.R. Public Transcriptomic Data Meta-Analysis Demonstrates TAAR6 Expression in the Mental Disorder-Related Brain Areas in Human and Mouse Brain. Biomolecules 2022, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Vaganova, A.N.; Murtazina, R.Z.; Shemyakova, T.S.; Prjibelski, A.D.; Katolikova, N.V.; Gainetdinov, R.R. Pattern of TAAR5 Expression in the Human Brain Based on Transcriptome Datasets Analysis. Int. J. Mol. Sci. 2021, 22, 8802. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, G.; Erba, P.; Carnicelli, V.; Manfredi, C.; Frascarelli, S.; Ghelardoni, S.; Mariani, G.; Zucchi, R. Distribution of Exogenous [125I]-3-Iodothyronamine in Mouse in Vivo: Relationship with Trace Amine-Associated Receptors. J. Endocrinol. 2012, 213, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, P.A. An Overview of the Tasks Used to Test Working Memory in Rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef]
- Adriaan Bouwknecht, J.; Olivier, B.; Paylor, R.E. The Stress-Induced Hyperthermia Paradigm as a Physiological Animal Model for Anxiety: A Review of Pharmacological and Genetic Studies in the Mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef]
- Dinter, J.; Mühlhaus, J.; Wienchol, C.L.; Yi, C.-X.; Nürnberg, D.; Morin, S.; Grüters, A.; Köhrle, J.; Schöneberg, T.; Tschöp, M.; et al. Inverse Agonistic Action of 3-Iodothyronamine at the Human Trace Amine-Associated Receptor 5. PLoS ONE 2015, 10, e0117774. [Google Scholar] [CrossRef]
- Khajavi, N.; Mergler, S.; Biebermann, H. 3-Iodothyronamine, a Novel Endogenous Modulator of Transient Receptor Potential Melastatin 8? Front. Endocrinol. 2017, 8, 198. [Google Scholar] [CrossRef]
- Gómez-Nieto, R.; Hormigo, S.; López, D.E. Prepulse Inhibition of the Auditory Startle Reflex Assessment as a Hallmark of Brainstem Sensorimotor Gating Mechanisms. Brain Sci. 2020, 10, 639. [Google Scholar] [CrossRef]
- Willott, J.F.; Tanner, L.; O’Steen, J.; Johnson, K.R.; Bogue, M.A.; Gagnon, L. Acoustic Startle and Prepulse Inhibition in 40 Inbred Strains of Mice. Behav. Neurosci. 2003, 117, 716–727. [Google Scholar] [CrossRef]
- Shoji, H.; Miyakawa, T. Relationships between the Acoustic Startle Response and Prepulse Inhibition in C57BL/6J Mice: A Large-Scale Meta-Analytic Study. Mol. Brain 2018, 11, 42. [Google Scholar] [CrossRef]
- Frohlich, J.; Toker, D.; Monti, M.M. Consciousness among Delta Waves: A Paradox? Brain 2021, 144, 2257–2277. [Google Scholar] [CrossRef] [PubMed]
- Vyazovskiy, V.V.; Olcese, U.; Hanlon, E.C.; Nir, Y.; Cirelli, C.; Tononi, G. Local Sleep in Awake Rats. Nature 2011, 472, 443–447. [Google Scholar] [CrossRef]
- Dzirasa, K.; Ribeiro, S.; Costa, R.; Santos, L.M.; Lin, S.-C.; Grosmark, A.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G.; Nicolelis, M.A.L. Dopaminergic Control of Sleep–Wake States. J. Neurosci. 2006, 26, 10577–10589. [Google Scholar] [CrossRef]
- LaDage, L.D. Environmental Change, the Stress Response, and Neurogenesis. Integr. Comp. Biol. 2015, 55, 372–383. [Google Scholar] [CrossRef]
- Lupo, G.; Gioia, R.; Nisi, P.S.; Biagioni, S.; Cacci, E. Molecular Mechanisms of Neurogenic Aging in the Adult Mouse Subventricular Zone. J. Exp. Neurosci. 2019, 13, 117906951982904. [Google Scholar] [CrossRef]
- Cameron, H.A.; Glover, L.R. Adult Neurogenesis: Beyond Learning and Memory. Annu. Rev. Psychol. 2015, 66, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Surget, A.; Belzung, C. Adult Hippocampal Neurogenesis Shapes Adaptation and Improves Stress Response: A Mechanistic and Integrative Perspective. Mol. Psychiatry 2022, 27, 403–421. [Google Scholar] [CrossRef]
- Nunez-Parra, A.; Pugh, V.; Araneda, R.C. Regulation of Adult Neurogenesis by Behavior and Age in the Accessory Olfactory Bulb. Mol. Cell. Neurosci. 2011, 47, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Goto, T.; Waeber, C.; Bhide, P.G. Dopamine Modulates Cell Cycle in the Lateral Ganglionic Eminence. J. Neurosci. 2003, 23, 2840–2850. [Google Scholar] [CrossRef]
- O’Keeffe, G.C.; Barker, R.A.; Caldwell, M.A. Dopaminergic Modulation of Neurogenesis in the Subventricular Zone of the Adult Brain. Cell Cycle 2009, 8, 2888–2894. [Google Scholar] [CrossRef]
- Hussain, A.; Saraiva, L.R.; Korsching, S.I. Positive Darwinian Selection and the Birth of an Olfactory Receptor Clade in Teleosts. Proc. Natl. Acad. Sci. USA 2009, 106, 4313–4318. [Google Scholar] [CrossRef]
- Eyun, S. Accelerated Pseudogenization of Trace Amine-associated Receptor Genes in Primates. Genes Brain Behav. 2019, 18, e12543. [Google Scholar] [CrossRef]
- Guo, L.; Dai, W.; Xu, Z.; Liang, Q.; Miller, E.T.; Li, S.; Gao, X.; Baldwin, M.W.; Chai, R.; Li, Q. Evolution of Brain-Expressed Biogenic Amine Receptors into Olfactory Trace Amine-Associated Receptors. Mol. Biol. Evol. 2022, 39, msac006. [Google Scholar] [CrossRef] [PubMed]
- Dieris, M.; Kowatschew, D.; Korsching, S.I. Olfactory Function in the Trace Amine-Associated Receptor Family (TAARs) Evolved Twice Independently. Sci. Rep. 2021, 11, 7807. [Google Scholar] [CrossRef]
- Eyun, S.; Moriyama, H.; Hoffmann, F.G.; Moriyama, E.N. Molecular Evolution and Functional Divergence of Trace Amine–Associated Receptors. PLoS ONE 2016, 11, e0151023. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Nishida, M. Evolution of Trace Amine–Associated Receptor (TAAR) Gene Family in Vertebrates: Lineage-Specific Expansions and Degradations of a Second Class of Vertebrate Chemosensory Receptors Expressed in the Olfactory Epithelium. Mol. Biol. Evol. 2007, 24, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Bugda Gwilt, K.; González, D.P.; Olliffe, N.; Oller, H.; Hoffing, R.; Puzan, M.; El Aidy, S.; Miller, G.M. Actions of Trace Amines in the Brain-Gut-Microbiome Axis via Trace Amine-Associated Receptor-1 (TAAR1). Cell. Mol. Neurobiol. 2019, 40, 191–201. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, F.; Ruiz-Pérez, M.V.; Urdiales, J.L.; Medina, M.A. Pharmacological Potential of Biogenic Amine–Polyamine Interactions beyond Neurotransmission. Br. J. Pharmacol. 2013, 170, 4–16. [Google Scholar] [CrossRef]
- Del Rio, B.; Fernandez, M.; Redruello, B.; Ladero, V.; Alvarez, M.A. New Insights into the Toxicological Effects of Dietary Biogenic Amines. Food Chem. 2024, 435, 137558. [Google Scholar] [CrossRef]
- Revel, F.G.; Moreau, J.-L.; Gainetdinov, R.R.; Bradaia, A.; Sotnikova, T.D.; Mory, R.; Durkin, S.; Zbinden, K.G.; Norcross, R.; Meyer, C.A.; et al. TAAR1 Activation Modulates Monoaminergic Neurotransmission, Preventing Hyperdopaminergic and Hypoglutamatergic Activity. Proc. Natl. Acad. Sci. USA 2011, 108, 8485–8490. [Google Scholar] [CrossRef]

| N | Oligonucleotide | Sequence | Purpose |
|---|---|---|---|
| 1 | sg RNA_taar8abc | 5′-GCGGCCTCTAATACGACTCACTATAGGG GCCCCGGGTCATCCTGTACAGTTTTAGAGCTAGAAATAGCA-3′ | Common sgRNA for three genes: Taar8a, Taar8b, Taar8c |
| 2 | TAAR8_F | 5′-CTTCTAGCAGGACAGAAATCCC-3′ | Common forward primer for PCR genotyping |
| 3 | TAAR8A_R | 5′-AGAAAGCAACAGTATGCTTACATAC-3′ | The reverse primer for PCR genotyping, specific for each gene |
| TAAR8B_R | 5′-CCAGGATAAACTAGAAGCTTAGAAAC-3′ | ||
| TAAR8C_R | 5′-TGAGATCACAGGGACCATCAG-3′ | ||
| 4 | Pr_taar8abc_R_sgABC | 5′-ATCTCCAAAGTACCAGCAGCTC-3′ | Common reverse primer for sequencing each gene: Taar8a, Taar8b, Taar8c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shemiakova, T.S.; Markina, A.A.; Efimova, E.V.; Murtazina, R.Z.; Volnova, A.B.; Veshchitskii, A.A.; Leonova, E.I.; Gainetdinov, R.R. TAAR8 in the Brain: Implications for Dopaminergic Function, Neurogenesis, and Behavior. Biomedicines 2025, 13, 1391. https://doi.org/10.3390/biomedicines13061391
Shemiakova TS, Markina AA, Efimova EV, Murtazina RZ, Volnova AB, Veshchitskii AA, Leonova EI, Gainetdinov RR. TAAR8 in the Brain: Implications for Dopaminergic Function, Neurogenesis, and Behavior. Biomedicines. 2025; 13(6):1391. https://doi.org/10.3390/biomedicines13061391
Chicago/Turabian StyleShemiakova, Taisiia S., Alisa A. Markina, Evgeniya V. Efimova, Ramilya Z. Murtazina, Anna B. Volnova, Aleksandr A. Veshchitskii, Elena I. Leonova, and Raul R. Gainetdinov. 2025. "TAAR8 in the Brain: Implications for Dopaminergic Function, Neurogenesis, and Behavior" Biomedicines 13, no. 6: 1391. https://doi.org/10.3390/biomedicines13061391
APA StyleShemiakova, T. S., Markina, A. A., Efimova, E. V., Murtazina, R. Z., Volnova, A. B., Veshchitskii, A. A., Leonova, E. I., & Gainetdinov, R. R. (2025). TAAR8 in the Brain: Implications for Dopaminergic Function, Neurogenesis, and Behavior. Biomedicines, 13(6), 1391. https://doi.org/10.3390/biomedicines13061391






