The Role and Function of Non-Coding RNAs in Cholangiocarcinoma Invasiveness
Abstract
1. Introduction
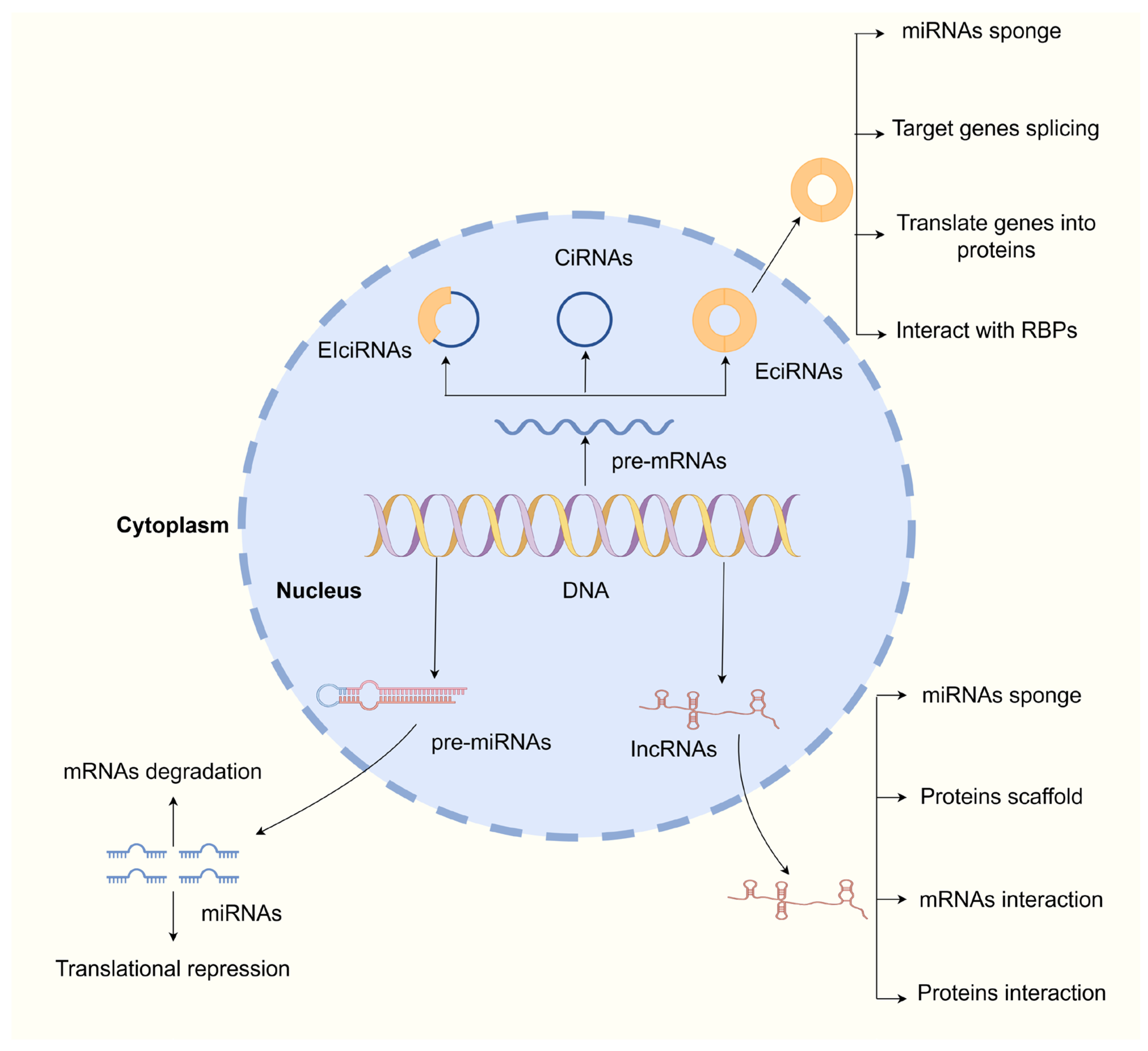
2. Overview of ncRNAs
2.1. Overview of miRNAs
2.2. Overview of lncRNAs
2.3. Overview of circRNAs
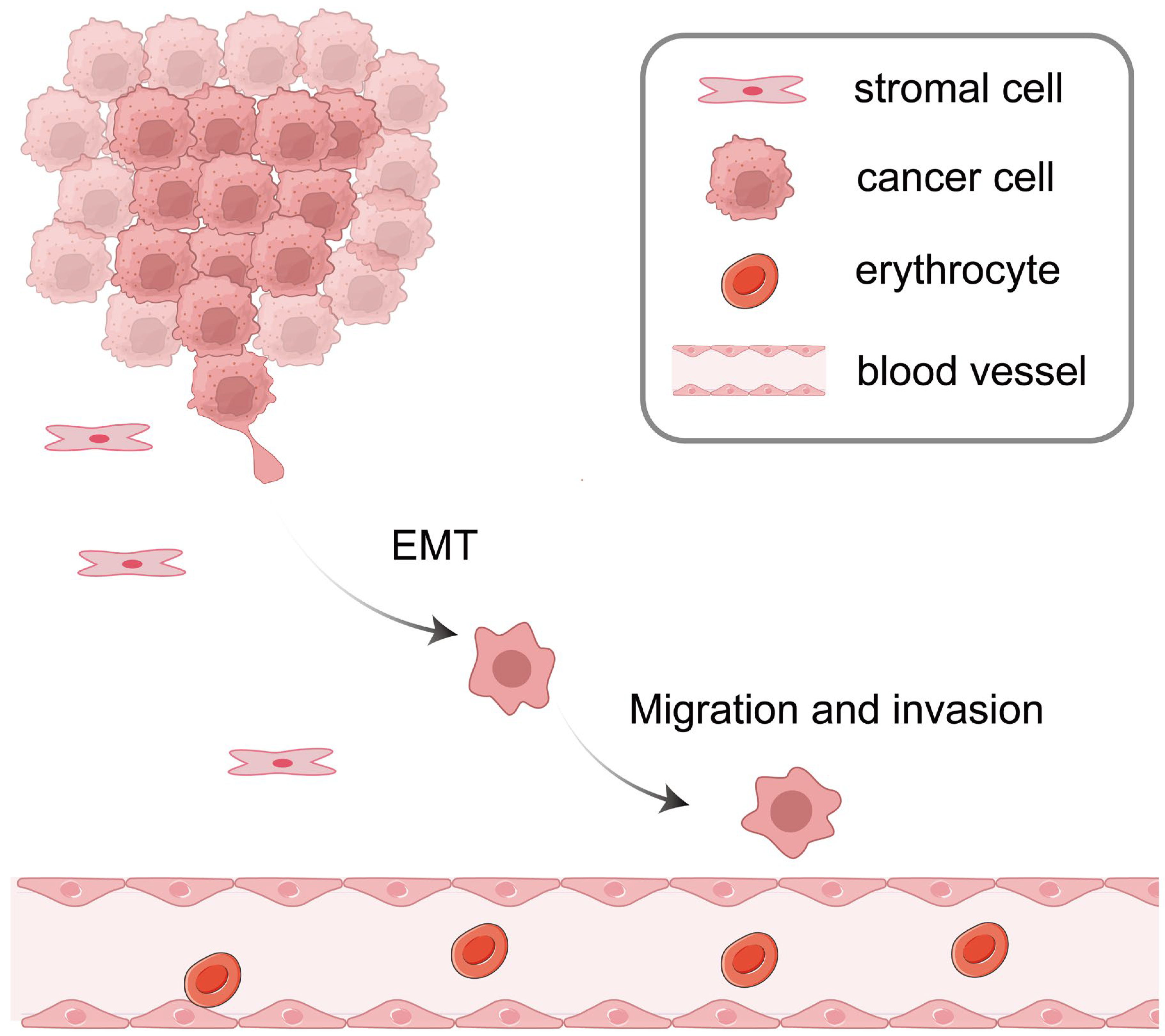
3. ncRNAs in EMT
3.1. miRNAs in EMT
3.2. lncRNAs in EMT
3.3. circRNAs in EMT
4. ncRNAs in CCA Cell Migration and Invasion
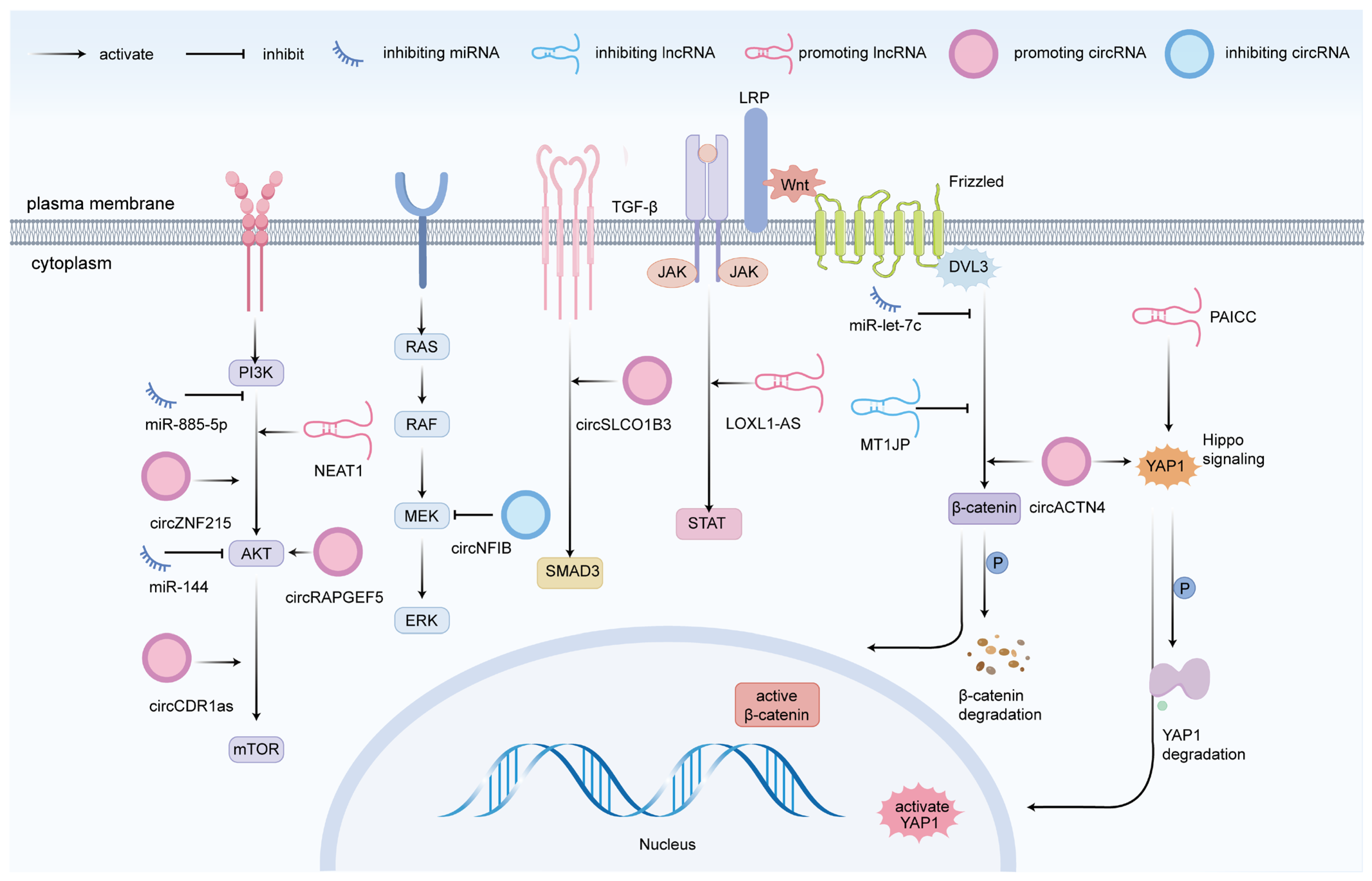
4.1. miRNAs
4.2. lncRNAs
4.3. circRNAs
5. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| piRNAs | Piwi-interacting RNAs |
| siRNAs | Small interfering RNAs |
| rRNAs | Ribosomal RNAs |
| tRNAs | Transfer RNAs |
| snRNAs | Small nuclear RNAs |
| snoRNA | Small nucleolar RNAs |
| Snail | Snail family transcriptional repressor 1 |
| Slug | Snail family transcriptional repressor 2 |
| Twist1 | Twist-related protein 1 |
| ZEB1/2 | Zinc finger E-box-binding homeobox 1/2 |
| LAMB3 | Laminin subunit beta 3 |
| CCDC6 | Coiled-coil domain containing 6 |
| BCL9L | B-cell CLL/lymphoma 9-like |
| EZH2 | Enhancer of zeste homolog 2 |
| H3K27me3 | Histone H3 lysine 27 trimethylation |
| LSD1 | Lysine-specific demethylase 1 |
| Wip1 | Wild-type p53-induced phosphatase 1 |
| NUAK1 | NUAK family kinase 1 |
| GALNT3 | N-acetylgalactosaminyl transferase-3 |
| S100A7 | S100 calcium-binding protein A7 |
| MyD88 | Myeloid differentiation primary response 8 |
| ST8SIA4 | ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 4 |
| NRP-1 | Neuropilin-1 |
| RECK | Reversion-inducing cysteine-rich protein with Kazal motifs |
| PDCD4 | Programmed cell death protein 4 |
| MTSS1 | Metastasis suppressor 1 |
| IRF1 | Interferon regulatory factor 1 |
| MEN1 | Multiple endocrine neoplasia type 1 |
| ALDOA | Aldolase A |
| SFN | Stratifin |
| GPX4 | Glutathione peroxidase 4 |
| GPAM | Glycerol-3-phosphate acyltransferase |
| E2F3 | E2F transcription factor 3 |
| COL6A3 | Collagen type VI alpha 3 chain |
| ANXA2 | Annexin A2 |
| PTP4A1 | Protein tyrosine phosphatase 4A1 |
| FBP1 | Fructose-1,6-bisphosphatase 1 |
| YAP1 | Yes-associated protein 1 |
| HOXC8 | Homeobox C8 |
| PRDX1 | Peroxiredoxin 1 |
| PTEN | Phosphatase and tensin homolog |
| SAE1 | SUMO-activating enzyme subunit 1 |
| SUMO | Small ubiquitin-like modifier |
| YBX1 | Y-Box binding protein 1 |
| FZD7 | Frizzled-7 |
References
- Moris, D.; Palta, M.; Kim, C.; Allen, P.J.; Morse, M.A.; Lidsky, M.E. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J. Clin. 2023, 73, 198–222. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Schneider, J.S.; Ben Khaled, N.; Schirmacher, P.; Seifert, C.; Frey, L.; He, Y.; Geier, A.; De Toni, E.N.; Zhang, C.; et al. Combined Hepatocellular-Cholangiocarcinoma: Biology, Diagnosis, and Management. Liver Cancer 2024, 13, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Param, N.J.; Bramel, E.R.; Sia, D. The Molecular Pathogenesis and Targeted Therapies for Cholangiocarcinoma. Surg. Pathol. Clin. 2022, 15, 529–539. [Google Scholar] [CrossRef]
- Dong, L.; Lu, D.; Chen, R.; Lin, Y.; Zhu, H.; Zhang, Z.; Cai, S.; Cui, P.; Song, G.; Rao, D.; et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell 2022, 40, 70–87.e15. [Google Scholar] [CrossRef]
- Qurashi, M.; Vithayathil, M.; Khan, S.A. Epidemiology of cholangiocarcinoma. Eur. J. Surg. Oncol. 2025, 51, 107064. [Google Scholar] [CrossRef]
- Kam, A.E.; Masood, A.; Shroff, R.T. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol. Hepatol. 2021, 6, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Badheeb, M.; Alnahar, B.W.; Almiqlash, B.; Sakr, Y.; Al-Najjar, E.; Awas, A.; Alsayed, M.; Khasawneh, B.; Alkhulaifawi, M.; et al. The Recent Trends of Systemic Treatments and Locoregional Therapies for Cholangiocarcinoma. Pharmaceuticals 2024, 17, 910. [Google Scholar] [CrossRef]
- Kiri, S.; Ryba, T. Cancer, metastasis, and the epigenome. Mol. Cancer 2024, 23, 154. [Google Scholar] [CrossRef]
- Carter, P.; Kang, Y. Tumor Heterogeneity and Cooperating Cancer Hallmarks Driven by Divergent EMT Programs. Cancer Res. 2025, 85, 12–14. [Google Scholar] [CrossRef]
- Tomecka, P.; Kunachowicz, D.; Górczyńska, J.; Gebuza, M.; Kuźnicki, J.; Skinderowicz, K.; Choromańska, A. Factors Determining Epithelial-Mesenchymal Transition in Cancer Progression. Int. J. Mol. Sci. 2024, 25, 8972. [Google Scholar] [CrossRef]
- Glaviano, A.; Lau, H.S.; Carter, L.M.; Lee, E.H.C.; Lam, H.Y.; Okina, E.; Tan, D.J.J.; Tan, W.; Ang, H.L.; Carbone, D.; et al. Harnessing the tumor microenvironment: Targeted cancer therapies through modulation of epithelial-mesenchymal transition. J. Hematol. Oncol. 2025, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; He, Q.; Yin, Y.; Xu, A.; Wu, A.; Yi, X.; Zhong, Z.; Wu, Y.; Li, X. Extracellular vesicles of Clonorchis sinensis promote the malignant phenotypes of cholangiocarcinoma via NF-κB/EMT axis. PLoS Negl. Trop. Dis. 2024, 18, e0012545. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Nakanishi, Y.; Mitsuhashi, T.; Sasaki, K.; Hatanaka, K.C.; Sasaki, M.; Nange, A.; Okumura, A.; Hayashi, M.; Yoshida, Y.; et al. CCR7 Mediates Cell Invasion and Migration in Extrahepatic Cholangiocarcinoma by Inducing Epithelial-Mesenchymal Transition. Cancers 2023, 15, 1878. [Google Scholar] [CrossRef]
- Walter, N.G. Are non-protein coding RNAs junk or treasure?: An attempt to explain and reconcile opposing viewpoints of whether the human genome is mostly transcribed into non-functional or functional RNAs. Bioessays 2024, 46, e2300201. [Google Scholar] [CrossRef]
- Poliseno, L.; Lanza, M.; Pandolfi, P.P. Coding, or non-coding, that is the question. Cell Res. 2024, 34, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Kim, V.N. Small and long non-coding RNAs: Past, present, and future. Cell 2024, 187, 6451–6485. [Google Scholar] [CrossRef]
- Vahabi, M.; Dehni, B.; Antomás, I.; Giovannetti, E.; Peters, G.J. Targeting miRNA and using miRNA as potential therapeutic options to bypass resistance in pancreatic ductal adenocarcinoma. Cancer Metastasis Rev. 2023, 42, 725–740. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Safarzadeh, A.; Hussen, B.M.; Taheri, M.; Samsami, M. A review on the role of ncRNAs in the pathogenesis of cholangiocarcinoma. Int. J. Biol. Macromol. 2023, 225, 809–821. [Google Scholar] [CrossRef]
- Kakumani, P.K. AGO-RBP crosstalk on target mRNAs: Implications in miRNA-guided gene silencing and cancer. Transl. Oncol. 2022, 21, 101434. [Google Scholar] [CrossRef]
- Sell, M.C.; Ramlogan-Steel, C.A.; Steel, J.C.; Dhungel, B.P. MicroRNAs in cancer metastasis: Biological and therapeutic implications. Expert Rev. Mol. Med. 2023, 25, e14. [Google Scholar] [CrossRef]
- Chen, H. microRNA-Based Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2023, 25, 230. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Ojha, A.; Ju, J. Functional and Potential Therapeutic Implication of MicroRNAs in Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 17523. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hu, Y.; Liu, S. Non-coding RNAs: A promising target for early metastasis intervention. Chin. Med. J. 2023, 136, 2538–2550. [Google Scholar] [CrossRef]
- Wen, K.; Chen, X.; Gu, J.; Chen, Z.; Wang, Z. Beyond traditional translation: ncRNA derived peptides as modulators of tumor behaviors. J. Biomed. Sci. 2024, 31, 63. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.; Dimitrova, N. Transcription regulation by long non-coding RNAs: Mechanisms and disease relevance. Nat. Rev. Mol. Cell Biol. 2024, 25, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, H.; Luo, L.; Gan, M.; Liang, L.; Que, L.; Zheng, S.; Zhong, J.; Liang, L. Integrated analysis of the lncRNA-miRNA-mRNA ceRNA network in nasopharyngeal carcinoma. Transl. Cancer Res. 2024, 13, 4372–4388. [Google Scholar] [CrossRef]
- Pisignano, G.; Michael, D.C.; Visal, T.H.; Pirlog, R.; Ladomery, M.; Calin, G.A. Going circular: History, present, and future of circRNAs in cancer. Oncogene 2023, 42, 2783–2800. [Google Scholar] [CrossRef]
- Guan, L.; Hao, Q.; Shi, F.; Gao, B.; Wang, M.; Zhou, X.; Han, T.; Ren, W. Regulation of the tumor immune microenvironment by cancer-derived circular RNAs. Cell Death Dis. 2023, 14, 132. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, Y.K. Molecular mechanisms of circular RNA translation. Exp. Mol. Med. 2024, 56, 1272–1280. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Hussen, B.M.; Abdullah, S.R.; Jaafar, R.M.; Rasul, M.F.; Aroutiounian, R.; Harutyunyan, T.; Liehr, T.; Samsami, M.; Taheri, M. Circular RNAs as key regulators in cancer hallmarks: New progress and therapeutic opportunities. Crit. Rev. Oncol. Hematol. 2025, 207, 104612. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Circular RNAs: Novel Players in Cancer Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 10121. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z. IRES-mediated cap-independent translation, a path leading to hidden proteome. J. Mol. Cell Biol. 2019, 11, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Feng, Q.; Liu, H.; Du, J.; Chen, X.; Zeng, Y. Circular RNAs in cholangiocarcinoma. Cancer Lett. 2023, 553, 215980. [Google Scholar] [CrossRef]
- Li, H.; Lan, T.; Liu, H.; Liu, C.; Dai, J.; Xu, L.; Cai, Y.; Hou, G.; Xie, K.; Liao, M.; et al. IL-6-induced cGGNBP2 encodes a protein to promote cell growth and metastasis in intrahepatic cholangiocarcinoma. Hepatology 2022, 75, 1402–1419. [Google Scholar] [CrossRef]
- Zhu, B.; Zheng, J.; Hong, G.; Bai, T.; Qian, W.; Liu, J.; Hou, X. L-Fucose inhibits the progression of cholangiocarcinoma by causing microRNA-200b overexpression. Chin. Med. J. 2022, 135, 2956–2967. [Google Scholar] [CrossRef]
- Qiao, P.; Li, G.; Bi, W.; Yang, L.; Yao, L.; Wu, D. microRNA-34a inhibits epithelial mesenchymal transition in human cholangiocarcinoma by targeting Smad4 through transforming growth factor-beta/Smad pathway. BMC Cancer 2015, 15, 469. [Google Scholar] [CrossRef]
- Ota, Y.; Takahashi, K.; Otake, S.; Tamaki, Y.; Okada, M.; Aso, K.; Makino, Y.; Fujii, S.; Ota, T.; Haneda, M. Extracellular vesicle-encapsulated miR-30e suppresses cholangiocarcinoma cell invasion and migration via inhibiting epithelial-mesenchymal transition. Oncotarget 2018, 9, 16400–16417. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, B.; Zhang, K. miR-186 Suppresses the Progression of Cholangiocarcinoma Cells Through Inhibition of Twist1. Oncol. Res. 2019, 27, 1061–1068. [Google Scholar] [CrossRef]
- Qiu, Y.H.; Wei, Y.P.; Shen, N.J.; Wang, Z.C.; Kan, T.; Yu, W.L.; Yi, B.; Zhang, Y.J. miR-204 inhibits epithelial to mesenchymal transition by targeting slug in intrahepatic cholangiocarcinoma cells. Cell. Physiol. Biochem. 2013, 32, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Piontek, K.; Ishida, M.; Fausther, M.; Dranoff, J.A.; Fu, R.; Mezey, E.; Gould, S.J.; Fordjour, F.K.; Meltzer, S.J.; et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology 2017, 65, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, L.; Li, G.; Ma, D.; Sun, C.; Gao, S.; Zhang, P.; Gao, F. miR-221 Promotes Epithelial-Mesenchymal Transition Through Targeting PTEN and Forms a Positive Feedback Loop with β-catenin/c-Jun Signaling Pathway in Extra-Hepatic Cholangiocarcinoma. PLoS ONE 2015, 10, e0141168. [Google Scholar] [CrossRef]
- Liao, C.H.; Liu, Y.; Wu, Y.F.; Zhu, S.W.; Cai, R.Y.; Zhou, L.; Yin, X.M. microRNA-329 suppresses epithelial-to-mesenchymal transition and lymph node metastasis in bile duct cancer by inhibiting laminin subunit beta 3. J. Cell. Physiol. 2019, 234, 17786–17799. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, J.; Wang, Y.; Tang, Z.; Liu, S.; Tang, Y. MiR-19b-3p facilitates the proliferation and epithelial-mesenchymal transition, and inhibits the apoptosis of intrahepatic cholangiocarcinoma by suppressing coiled-coil domain containing 6. Arch. Biochem. Biophys. 2020, 686, 108367. [Google Scholar] [CrossRef]
- Liu, C.H.; Huang, Q.; Jin, Z.Y.; Zhu, C.L.; Liu, Z.; Wang, C. miR-21 and KLF4 jointly augment epithelial-mesenchymal transition via the Akt/ERK1/2 pathway. Int. J. Oncol. 2017, 50, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Weinberg, R.A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef]
- Yamada, S.; Fuchs, B.C.; Fujii, T.; Shimoyama, Y.; Sugimoto, H.; Nomoto, S.; Takeda, S.; Tanabe, K.K.; Kodera, Y.; Nakao, A. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery 2013, 154, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.K.; Qu, X.S.; Chen, G.; Feng, Y.; Teng, X.L.; Liu, W.X.; Cheng, Z.X.; Xu, J.; Guo, L.Q. LINC01503 promotes cell proliferation, invasion and EMT process in cholangio-carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6445–6452. [Google Scholar] [CrossRef]
- Li, J.; Guan, C.; Hu, Z.; Liu, L.; Su, Z.; Kang, P.; Jiang, X.; Cui, Y. Yin Yang 1-induced LINC00667 up-regulates pyruvate dehydrogenase kinase 1 to promote proliferation, migration and invasion of cholangiocarcinoma cells by sponging miR-200c-3p. Hum. Cell. 2021, 34, 187–200. [Google Scholar] [CrossRef]
- Gao, J.; Qin, W.; Kang, P.; Xu, Y.; Leng, K.; Li, Z.; Huang, L.; Cui, Y.; Zhong, X. Up-regulated LINC00261 predicts a poor prognosis and promotes a metastasis by EMT process in cholangiocarcinoma. Pathol. Res. Pract. 2020, 216, 152733. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Z.; Jiang, X.; Cui, Y. Overexpression of long noncoding RNA H19 indicates a poor prognosis for cholangiocarcinoma and promotes cell migration and invasion by affecting epithelial-mesenchymal transition. Biomed. Pharmacother. 2017, 92, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhao, Y.; Wang, W.; Guan, C.; Hu, Z.; Liu, L.; Jiang, X. PCAT1 induced by transcription factor YY1 promotes cholangiocarcinoma proliferation, migration and invasion by sponging miR-216a-3p to up-regulate oncogene BCL3. Biol. Chem. 2021, 402, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, X.; Zhang, B.; Peng, H.; Quan, C.; Xiao, X.; Luo, M.; Huang, Y.; Xu, D.; Huang, K.; et al. The long non-coding RNA CCAT1 promotes erlotinib resistance in cholangiocarcinoma by inducing epithelial-mesenchymal transition via the miR-181a-5p/ROCK2 axis. Am. J. Cancer Res. 2024, 14, 2852–2867. [Google Scholar] [CrossRef]
- Lu, M.; Qin, X.; Zhou, Y.; Li, G.; Liu, Z.; Geng, X.; Yue, H. Long non-coding RNA LINC00665 promotes gemcitabine resistance of Cholangiocarcinoma cells via regulating EMT and stemness properties through miR-424-5p/BCL9L axis. Cell Death Dis. 2021, 12, 72. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Zhang, F.; Wang, A.; Du, X.; Hu, P.; Zhu, Y.; Fang, Z. Long non-coding RNA CCAT2 is associated with poor prognosis in hepatocellular carcinoma and promotes tumor metastasis by regulating Snail2-mediated epithelial-mesenchymal transition. Onco Targets Ther. 2017, 10, 1191–1198. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, J.Z.; Lv, P.; Dang, Y.; Gao, J.Y.; Wang, Y. Long non-coding RNA CCAT2 promotes gastric cancer proliferation and invasion by regulating the E-cadherin and LATS2. Am. J. Cancer Res. 2016, 6, 2651–2660. [Google Scholar]
- Xu, Y.; Yao, Y.; Qin, W.; Zhong, X.; Jiang, X.; Cui, Y. Long non-coding RNA CCAT2 promotes cholangiocarcinoma cells migration and invasion by induction of epithelial-to-mesenchymal transition. Biomed. Pharmacother. 2018, 99, 121–127. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.; Tian, F.; Nie, S.; Zhou, H.; Liu, J.; Chen, W. Up-regulated LncRNA-ATB regulates the growth and metastasis of cholangiocarcinoma via miR-200c signals. Onco Targets Ther. 2019, 12, 7561–7571. [Google Scholar] [CrossRef]
- Jiao, M.; Ning, S.; Chen, J.; Chen, L.; Jiao, M.; Cui, Z.; Guo, L.; Mu, W.; Yang, H. Long non-coding RNA ZEB1-AS1 predicts a poor prognosis and promotes cancer progression through the miR-200a/ZEB1 signaling pathway in intrahepatic cholangiocarcinoma. Int. J. Oncol. 2020, 56, 1455–1467. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Li, C.; Liu, Y.; Kang, P.; Zhong, X.; Cui, Y. LncRNA-MEG3 inhibits cell proliferation and invasion by modulating Bmi1/RNF2 in cholangiocarcinoma. J. Cell. Physiol. 2019, 234, 22947–22959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, N.; Wang, J. Hsa-circ_0058106 induces EMT and metastasis in laryngeal cancer via sponging miR-153 and inducing Twist1 nuclear translocation. Cell. Oncol. 2021, 44, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.H.; Yang, Y.C.; Zhang, R.Y.; Wang, P.; Pang, M.H.; Liang, L.Q. CircRNA_0023642 promotes migration and invasion of gastric cancer cells by regulating EMT. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2297–2303. [Google Scholar] [CrossRef]
- Deng, J.; Liao, S.; Chen, C.; Han, F.; Lei, S.; Lai, X.; Ye, K.; Han, Q.E.F.; Lu, C.; Lai, M. Specific intracellular retention of circSKA3 promotes colorectal cancer metastasis by attenuating ubiquitination and degradation of SLUG. Cell Death Dis. 2023, 14, 750. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Wang, W.; Yu, S.; Liu, L.; Sun, D.; Li, W.; Jiang, X. Upregulation of circ_0059961 suppresses cholangiocarcinoma development by modulating miR-629-5p/SFRP2 axis. Pathol. Res. Pract. 2022, 234, 153901. [Google Scholar] [CrossRef]
- Shi, T.; Morishita, A.; Kobara, H.; Masaki, T. The Role of microRNAs in Cholangiocarcinoma. Int. J. Mol. Sci. 2021, 22, 7627. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Chan, M.T.; Wu, W.K. The role of microRNAs in intrahepatic cholangiocarcinoma. J. Cell. Mol. Med. 2017, 21, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, J.; Fang, M.; Zhou, L.; Chen, Y.; Zhou, J.; Song, Y.; Kong, G.; Zhang, B.; Jiang, B.; et al. MicroRNA-129-2-3p directly targets Wip1 to suppress the proliferation and invasion of intrahepatic cholangiocarcinoma. J. Cancer 2020, 11, 3216–3224. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, B.; Li, H.; He, Z.; Lv, P.; Peng, C.; Wang, Y.; Cheng, W.; Xu, Z.; Chen, W.; et al. Wip1 is associated with tumorigenity and metastasis through MMP-2 in human intrahepatic cholangiocarcinoma. Oncotarget 2017, 8, 56672–56683. [Google Scholar] [CrossRef]
- Pan, X.; Wang, G.; Wang, B. MicroRNA-1182 and let-7a exert synergistic inhibition on invasion, migration and autophagy of cholangiocarcinoma cells through down-regulation of NUAK1. Cancer Cell Int. 2021, 21, 161. [Google Scholar] [CrossRef]
- Skalka, G.L.; Whyte, D.; Lubawska, D.; Murphy, D.J. NUAK: Never underestimate a kinase. Essays Biochem. 2024, 68, 295–307. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Y.; Tang, C.; Li, H.; Wang, B.; Yan, Q.; Hu, J.; Zou, S. MicroRNA-144 suppresses cholangiocarcinoma cell proliferation and invasion through targeting platelet activating factor acetylhydrolase isoform 1b. BMC Cancer 2014, 14, 917. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, W.; Song, H.; Lang, Q.; Pei, T. miR-885-5p inhibits proliferation and metastasis by targeting IGF2BP1 and GALNT3 in human intrahepatic cholangiocarcinoma. Mol. Carcinog. 2020, 59, 1371–1381. [Google Scholar] [CrossRef]
- Monga, S.P. β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology 2015, 148, 1294–1310. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, H.; Guo, X.J.; Feng, Y.C.; He, R.Z.; Li, X.; Yu, S.; Zhao, Y.; Shen, M.; Zhu, F.; et al. Let-7c inhibits cholangiocarcinoma growth but promotes tumor cell invasion and growth at extrahepatic sites. Cell Death Dis. 2018, 9, 249. [Google Scholar] [CrossRef]
- Fan, F.; Lu, J.; Yu, W.; Zhang, Y.; Xu, S.; Pang, L.; Zhu, B. MicroRNA-26b-5p regulates cell proliferation, invasion and metastasis in human intrahepatic cholangiocarcinoma by targeting S100A7. Oncol. Lett. 2018, 15, 386–392. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, Z.; Yang, J.; Liu, T.; Tang, Y. MicroRNA-7-5p Inhibits Migration, Invasion and Metastasis of Intrahepatic Cholangiocarcinoma by Inhibiting MyD88. J. Clin. Transl. Hepatol. 2021, 9, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Yu, G.; Liang, J.; Fan, P.; Dong, K.; Zhang, B.; Chen, X.; Zhu, H.; Chu, L. miR-144-5p and miR-451a Inhibit the Growth of Cholangiocarcinoma Cells Through Decreasing the Expression of ST8SIA4. Front. Oncol. 2020, 10, 563486. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, X.; Zhou, X.; Dong, X.; Xie, K.; Yang, C.; Jiang, H.; Sun, X.; Lu, J. Neuropilin-1 regulated by miR-320 contributes to the growth and metastasis of cholangiocarcinoma cells. Liver Int. 2018, 38, 125–135. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Tian, R.; Sun, H.; Zou, S. miR-373 negatively regulates methyl-CpG-binding domain protein 2 (MBD2) in hilar cholangiocarcinoma. Dig. Dis. Sci. 2011, 56, 1693–1701. [Google Scholar] [CrossRef]
- Wang, Y.D.; Chen, W.D.; Moore, D.D.; Huang, W. FXR: A metabolic regulator and cell protector. Cell Res. 2008, 18, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.Y.; Yu, J.H.; Zhang, W.G.; Wang, Z.D.; Dong, Q.; Tai, S.; Cui, Y.F.; Li, H. MicroRNA-421 functions as an oncogenic miRNA in biliary tract cancer through down-regulating farnesoid X receptor expression. Gene 2012, 493, 44–51. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Kitdumrongthum, S.; Metheetrairut, C.; Charoensawan, V.; Ounjai, P.; Janpipatkul, K.; Panvongsa, W.; Weerachayaphorn, J.; Piyachaturawat, P.; Chairoungdua, A. Dysregulated microRNA expression profiles in cholangiocarcinoma cell-derived exosomes. Life Sci. 2018, 210, 65–75. [Google Scholar] [CrossRef]
- Silakit, R.; Kitirat, Y.; Thongchot, S.; Loilome, W.; Techasen, A.; Ungarreevittaya, P.; Khuntikeo, N.; Yongvanit, P.; Yang, J.H.; Kim, N.H.; et al. Potential role of HIF-1-responsive microRNA210/HIF3 axis on gemcitabine resistance in cholangiocarcinoma cells. PLoS ONE 2018, 13, e0199827. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Y.; Liu, K.; Tan, L. Tumor Cell-Derived Extracellular Vesicles Promote the Growth, Metastasis and Chemoresistance in Cholangiocarcinoma by Delivering microRNA-210 to Downregulate RECK. Mol. Biotechnol. 2023, 65, 1151–1164. [Google Scholar] [CrossRef]
- Chusorn, P.; Namwat, N.; Loilome, W.; Techasen, A.; Pairojkul, C.; Khuntikeo, N.; Dechakhamphu, A.; Talabnin, C.; Chan-On, W.; Ong, C.K.; et al. Overexpression of microRNA-21 regulating PDCD4 during tumorigenesis of liver fluke-associated cholangiocarcinoma contributes to tumor growth and metastasis. Tumour Biol. 2013, 34, 1579–1588. [Google Scholar] [CrossRef]
- Yin, X.; Chai, Z.; Sun, X.; Chen, J.; Wu, X.; Yang, L.; Zhou, X.; Liu, F. Overexpression of microRNA-96 is associated with poor prognosis and promotes proliferation, migration and invasion in cholangiocarcinoma cells via MTSS1. Exp. Ther. Med. 2020, 19, 2757–2765. [Google Scholar] [CrossRef]
- Wan, P.; Chi, X.; Du, Q.; Luo, J.; Cui, X.; Dong, K.; Bing, Y.; Heres, C.; Geller, D.A. miR-383 promotes cholangiocarcinoma cell proliferation, migration, and invasion through targeting IRF1. J. Cell. Biochem. 2018, 119, 9720–9729. [Google Scholar] [CrossRef]
- Ehrlich, L.; Hall, C.; Venter, J.; Dostal, D.; Bernuzzi, F.; Invernizzi, P.; Meng, F.; Trzeciakowski, J.P.; Zhou, T.; Standeford, H.; et al. miR-24 Inhibition Increases Menin Expression and Decreases Cholangiocarcinoma Proliferation. Am. J. Pathol. 2017, 187, 570–580. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Zhang, M.; Zhang, Z.; Jia, Y.; Peng, L.; Zhu, Y.; Hu, J.; Huang, R.; Sun, X. miR-122-5p Inhibits the Proliferation, Invasion and Growth of Bile Duct Carcinoma Cells by Targeting ALDOA. Cell. Physiol. Biochem. 2018, 48, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, J.; Qi, P.; Liu, Y. Long non-coding RNA titin-antisense RNA1 contributes to growth and metastasis of cholangiocarcinoma by suppressing microRNA-513a-5p to upregulate stratifin. Bioengineered 2021, 12, 12611–12624. [Google Scholar] [CrossRef]
- Li, R.; Yan, X.; Zhong, W.; Zheng, J.; Li, X.; Liang, J.; Hu, Z.; Liu, H.; Chen, G.; Yang, Y.; et al. Stratifin promotes the malignant progression of HCC via binding and hyperactivating AKT signaling. Cancer Lett. 2024, 592, 216761. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, M.J.; Lee, J.S.; Son, J.; Kim, D.H.; Lee, J.S.; Jeong, S.K.; Chun, E.; Lee, K.Y. Stratifin (SFN) regulates lung cancer progression via nucleating the Vps34-BECN1-TRAF6 complex for autophagy induction. Clin. Transl. Med. 2022, 12, e896. [Google Scholar] [CrossRef]
- Lei, S.; Cao, W.; Zeng, Z.; Zhang, Z.; Jin, B.; Tian, Q.; Wu, Y.; Zhang, T.; Li, D.; Hu, C.; et al. JUND/linc00976 promotes cholangiocarcinoma progression and metastasis, inhibits ferroptosis by regulating the miR-3202/GPX4 axis. Cell Death Dis. 2022, 13, 967. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, D.; Li, F.; Qiu, G.; Sun, D.; Zeng, Z. Lnc-PKD2-2-3/miR-328/GPAM ceRNA Network Induces Cholangiocarcinoma Proliferation, Invasion and 5-FU Chemoresistance. Front. Oncol. 2022, 12, 871281. [Google Scholar] [CrossRef]
- Chen, H.; Lin, L.; Zou, L.; Guo, S.; Qiu, X.; Ma, K. NXT629 Ameliorates Cholesterol Gallstones in Mice Model by Improving Lipid Metabolism Disorder and Cholesterol Homeostasis Through Inhibiting the GPAM Pathway. Dig. Dis. Sci. 2025, 70, 612–621. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, L.; Wang, W.; Guan, C.; Zhao, Y.; Liu, L.; Jiang, X. Long Non-coding RNA FOXD2-AS1 Promotes Proliferation, Migration, and Invasion in Cholangiocarcinoma Through Regulating miR-760/E2F3 Axis. Dig. Dis. Sci. 2022, 67, 546–558. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, B.; Zhang, H.; Qi, Y.; Wang, Y.; Wang, W.; Wang, Y.; Wang, Y. E2F1-induced upregulation of long non-coding RNA LMCD1-AS1 facilitates cholangiocarcinoma cell progression by regulating miR-345-5p/COL6A3 pathway. Biochem. Biophys. Res. Commun. 2019, 512, 150–155. [Google Scholar] [CrossRef]
- Sun, H.B.; Zhang, G.C.; Liu, J.; Nie, C.S. Long noncoding RNA LINC00184 facilitates the proliferation, metastasis, and adenine metabolism of cholangiocarcinoma via modulating hsa-miR-23b-3p/ANXA2 axis. Environ. Toxicol. 2021, 36, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- Li, O.; Jiang, B.; Yi, W.M.; Zhang, Y.; Yang, P.Z.; Guo, C.; Sun, Z.P.; Peng, C. LncRNA NEAT1 promotes cell proliferation, migration, and invasion via the miR-186-5p/PTP4A1 axis in cholangiocarcinoma. Kaohsiung J. Med. Sci. 2021, 37, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Chen, X.; Yang, J.; Zhu, S.; Zhang, L.; Yin, Q.; Hong, Y.; Chen, H.; Chen, G.; Li, H. Long Non-Coding RNA-PAICC Promotes the Tumorigenesis of Human Intrahepatic Cholangiocarcinoma by Increasing YAP1 Transcription. Front. Oncol. 2020, 10, 595533. [Google Scholar] [CrossRef]
- To, S.Q.; Dmello, R.S.; Richards, A.K.; Ernst, M.; Chand, A.L. STAT3 Signaling in Breast Cancer: Multicellular Actions and Therapeutic Potential. Cancers 2022, 14, 429. [Google Scholar] [CrossRef]
- Yu, S.; Gao, X.; Liu, S.; Sha, X.; Zhang, S.; Zhang, X.; Sun, D.; Jiang, X. LOXL1-AS1 inhibits JAK2 ubiquitination and promotes cholangiocarcinoma progression through JAK2/STAT3 signaling. Cancer Gene Ther. 2024, 31, 552–561. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, J.; Guo, X.; Feng, Y.; Zhang, B.; Tian, L. LncRNA MT1JP plays a protective role in intrahepatic cholangiocarcinoma by regulating miR-18a-5p/FBP1 axis. BMC Cancer 2021, 21, 142. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lan, T.; Liao, H.; Feng, X.; Chen, X.; Liao, W.; Hou, G.; Xu, L.; Feng, Q.; Xie, K.; et al. CircNFIB inhibits tumor growth and metastasis through suppressing MEK1/ERK signaling in intrahepatic cholangiocarcinoma. Mol. Cancer 2022, 21, 18. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Xu, K.; Zhou, X.; Wu, K.; Yao, Y.; Liu, Z.; Chen, C.; Wang, L.; Sun, Z.; et al. N6-methyladenosine-modified circSLCO1B3 promotes intrahepatic cholangiocarcinoma progression via regulating HOXC8 and PD-L1. J. Exp. Clin. Cancer Res. 2024, 43, 119. [Google Scholar] [CrossRef]
- Liao, W.; Du, J.; Li, L.; Wu, X.; Chen, X.; Feng, Q.; Xu, L.; Chen, X.; Liao, M.; Huang, J.; et al. CircZNF215 promotes tumor growth and metastasis through inactivation of the PTEN/AKT pathway in intrahepatic cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2023, 42, 125. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Tao, L.; Cai, J.; Shen, Z.; Liu, Y.; Pan, H.; Li, S.; Ruan, Y.; Chen, T.; et al. Circ-RAPGEF5 promotes intrahepatic cholangiocarcinoma progression by stabilizing SAE1 to facilitate SUMOylation. J. Exp. Clin. Cancer Res. 2023, 42, 239. [Google Scholar] [CrossRef]
- Li, D.; Tang, Z.; Gao, Z.; Shen, P.; Liu, Z.; Dang, X. Circular RNA CDR1as Exerts Oncogenic Properties Partially through Regulating MicroRNA 641 in Cholangiocarcinoma. Mol. Cell. Biol. 2020, 40, e00042-20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, H.; Li, Z.; Li, F.; Liang, L.; Zou, Y.; Shen, H.; Li, J.; Xia, Y.; Cheng, Z.; et al. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J. Hepatol. 2022, 76, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Khosla, D.; Misra, S.; Chu, P.L.; Guan, P.; Nada, R.; Gupta, R.; Kaewnarin, K.; Ko, T.K.; Heng, H.L.; Srinivasalu, V.K.; et al. Cholangiocarcinoma: Recent Advances in Molecular Pathobiology and Therapeutic Approaches. Cancers 2024, 16, 801. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Song, J.; Kim, N.; Sim, T. Recent progress in emerging molecular targeted therapies for intrahepatic cholangiocarcinoma. RSC Med. Chem. 2025. [Google Scholar] [CrossRef]
- Fontana, R.; Mestre-Farrera, A.; Yang, J. Update on Epithelial-Mesenchymal Plasticity in Cancer Progression. Annu. Rev. Pathol. 2024, 19, 133–156. [Google Scholar] [CrossRef]
- Liaghat, M.; Ferdousmakan, S.; Mortazavi, S.H.; Yahyazadeh, S.; Irani, A.; Banihashemi, S.; Seyedi Asl, F.S.; Akbari, A.; Farzam, F.; Aziziyan, F.; et al. The impact of epithelial-mesenchymal transition (EMT) induced by metabolic processes and intracellular signaling pathways on chemo-resistance, metastasis, and recurrence in solid tumors. Cell Commun. Signal. 2024, 22, 575. [Google Scholar] [CrossRef]
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007, 1, 313–323. [Google Scholar] [CrossRef]
- Pang, R.; Law, W.L.; Chu, A.C.; Poon, J.T.; Lam, C.S.; Chow, A.K.; Ng, L.; Cheung, L.W.; Lan, X.R.; Lan, H.Y.; et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 2010, 6, 603–615. [Google Scholar] [CrossRef]
- Lin, K.Y.; Ye, H.; Han, B.W.; Wang, W.T.; Wei, P.P.; He, B.; Li, X.J.; Chen, Y.Q. Genome-wide screen identified let-7c/miR-99a/miR-125b regulating tumor progression and stem-like properties in cholangiocarcinoma. Oncogene 2016, 35, 3376–3386. [Google Scholar] [CrossRef]
- Qiu, G.; Ma, D.; Li, F.; Sun, D.; Zeng, Z. lnc-PKD2-2-3, identified by long non-coding RNA expression profiling, is associated with pejorative tumor features and poor prognosis, enhances cancer stemness and may serve as cancer stem-cell marker in cholangiocarcinoma. Int. J. Oncol. 2019, 55, 45–58. [Google Scholar] [CrossRef]
- Jiang, W.; Deng, X.; Zhu, T.; Wei, Y.; Lei, Z.; Guo, M.; Yang, J. Identification of Cholangiocarcinoma Associated with Hepatolithiasis via the Combination of miRNA and Ultrasound. Cancer Manag. Res. 2020, 12, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Salem, P.E.S.; Ghazala, R.A.; El Gendi, A.M.; Emara, D.M.; Ahmed, N.M. The association between circulating MicroRNA-150 level and cholangiocarcinoma. J. Clin. Lab. Anal. 2020, 34, e23397. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Leng, K.; Yao, Y.; Kang, P.; Liao, G.; Han, Y.; Shi, G.; Ji, D.; Huang, P.; Zheng, W.; et al. A Circular RNA, Cholangiocarcinoma-Associated Circular RNA 1, Contributes to Cholangiocarcinoma Progression, Induces Angiogenesis, and Disrupts Vascular Endothelial Barriers. Hepatology 2021, 73, 1419–1435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kong, X.; Lu, J.; Wang, H.; Liu, M.; Zhao, S.; Xia, Z.; Liu, Q.; Sun, H.; Gao, X.; et al. Circulating tumor cell-derived exosome-transmitted long non-coding RNA TTN-AS1 can promote the proliferation and migration of cholangiocarcinoma cells. J. Nanobiotechnol. 2024, 22, 191. [Google Scholar] [CrossRef]
- Yang, R.; Wang, D.; Han, S.; Gu, Y.; Li, Z.; Deng, L.; Yin, A.; Gao, Y.; Li, X.; Yu, Y.; et al. MiR-206 suppresses the deterioration of intrahepatic cholangiocarcinoma and promotes sensitivity to chemotherapy by inhibiting interactions with stromal CAFs. Int. J. Biol. Sci. 2022, 18, 43–64. [Google Scholar] [CrossRef]
- Li, B.; Hao, K.; Li, M.; Wang, A.; Tang, H.; Xu, L.; Ma, C.; Du, W.; Sun, L.; Hou, X.; et al. Five miRNAs identified in fucosylated extracellular vesicles as non-invasive diagnostic signatures for hepatocellular carcinoma. Cell Rep. Med. 2024, 5, 101716. [Google Scholar] [CrossRef]
- Li, Y.K.; Yan, L.R.; Wang, A.; Jiang, L.Y.; Xu, Q.; Wang, B.G. RNA-sequencing reveals the expression profiles of tsRNAs and their potential carcinogenic role in cholangiocarcinoma. J. Clin. Lab. Anal. 2022, 36, e24694. [Google Scholar] [CrossRef]
- Hu, L.; Xie, K.; Zheng, C.; Qiu, B.; Jiang, Z.; Luo, C.; Diao, Y.; Luo, J.; Yao, X.; Shen, Y. Exosomal MALAT1 promotes the proliferation of esophageal squamous cell carcinoma through glyoxalase 1-dependent methylglyoxal removal. Noncoding RNA Res. 2024, 9, 330–340. [Google Scholar] [CrossRef]
- Xie, B.; Wang, Z.; Li, T.; Xue, J.; Zhang, C. LncRNA MALAT1 inhibits the proliferation and invasiveness of laryngeal squamous cell carcinoma Hep-2 cells by modulating miR-362-3p. Am. J. Transl. Res. 2022, 14, 3729–3740. [Google Scholar]
| Category | Length | Representative Types |
|---|---|---|
| Short ncRNAs | <50 nt | miRNAs, siRNAs, piRNAs |
| Medium ncRNAs | 50–200 nt | rRNAs, tRNAs, snRNAs, snoRNAs |
| Long ncRNAs | >200 nt | lncRNAs, circRNAs |
| ncRNA | Assessed Cell Line | Targets or Modulated Molecules | Pro/Anti | References |
|---|---|---|---|---|
| miRNA | ||||
| miR-200b | HIBEpiC, TFK-1, HuCCT-1 | STAT3 pathway | Anti-EMT | [37] |
| miR-34a | QBC939, HuCCT1 | TGF-β/Smad4 pathway | Anti-EMT | [38] |
| miR-30e | HuCCT1, HuH28, OZ | Snail, TGF-β pathway | Anti-EMT | [39] |
| miR-186 | CCLP1, SG-231, HIBEC | Twist1 | Anti-EMT | [40] |
| miR-204 | ICC-9810, RBE, HuH28, HuCCT1 | Slug | Anti-EMT | [41] |
| miR-195 | human: LX2, HuCCT1, SG231, TFK1, H69; rat:BDEne, BDEsp, RGF | Snail | Anti-EMT | [42] |
| miR-221 | QBC 939, HuCCT1 | PTEN, β-catenin/c-Jun pathway | Pro-EMT | [43] |
| miR-329 | RBE | LAMB3 | Pro-EMT | [44] |
| miR-19b-3p | HUCCT1, RBE, CCLP-1, TFK-1, HIBEpiC | CCDC6, Snail, β-catenin pathway | Pro-EMT | [45] |
| miR-21 | QBC939 | AKT/ERK1/2 pathway, Snail, Slug | Pro-EMT | [46] |
| lncRNA | ||||
| LINC01503 | RBE and QBC939 | - | Pro-EMT | [49] |
| LINC00667 | CCLP-1, QBC939, RBE, HCCC-9810, HIBEC | miR-200c-3p/PDK1 | Pro-EMT | [50] |
| LINC00261 | QBC939, RBE | - | Pro-EMT | [51] |
| H19 | QBC939, RBE | - | Pro-EMT | [52] |
| PCAT1 | RBE, HCCC-9810, CCLP-1, QBC939, HIBEC | miR-216a-3p/BCL3 | Pro-EMT | [53] |
| LINC00665 | HuCCT1, HuH28, SNU-1196, SNU-1079, SNU-308, SNU-245, SNU-478, SNU-869, HEK293T | miR-424-5p/BCL9L, Wnt/β-Catenin pathway | Pro-EMT | [54] |
| CCAT1 | HCC-9810, RBE | miR-181a-5p/ROCK2 | Pro-EMT | [55] |
| CCAT2 | RBE, HCCC-9810, QBC939, CCLP-1, Huh-28, HuCCT1, HIBEC | - | Pro-EMT | [58] |
| ATB | BEC, HUCCT1, RBE, TFK1, Huh-28 | miR-200c, ZEB1, ZEB2 | Pro-EMT | [59] |
| ZEB1-AS1 | HuH28, HuCCT1, RBE, CCLP-1, HCCC-9810, HIBEC | miR-200a, ZEB1 | Pro-EMT | [60] |
| MEG3 | QBC939, TFK-1, HCCC-9810, RBE, CCLP-1, HIBEC | Snail’ | Anti-EMT | [61] |
| circRNA | ||||
| circ_0059961 | CCLP-1, QBC939, HIBEC | miR-629-5p/SFRP2 | Anti-EMT | [65] |
| miRNA | ||||
| miR-129-2-3p | QBC-939, RBE, BEC | Wip1 | Anti-migration and invasion | [68] |
| miR-1182andmiR-let-7a | HIBEPIC, CCC-5, HCC-9810, Huh28 | NUAK1 | Anti-migration and invasion | [70] |
| miR-144 | HCCC-9810, CCLP1, HuCC-T1, RBE | LIS1, AKT pathway | Anti-migration and invasion | [72] |
| miR-885-5p | HuCCT1, RBE, Huh28 | GALNT3, PI3K/AKT pathway | Anti-migration and invasion | [73] |
| miR-let-7c | TFK-1, HUCCT-1 | EZH2, DVL3/β-catenin pathway | Anti-migration and invasion | [75] |
| miR-26b-5p | RBE, HCCC-9810 | S100A7 | Anti-migration and invasion | [76] |
| miR-7-5p | HCCC-9810, HuCCT1, QBC-939, RBE, HIBEC | MyD88 | Anti-migration and invasion | [77] |
| miR-144-5p and miR-451a | HuCCT-1, HCCC 9810, RBE, TFK-1 | ST8SIA4 | Anti-migration and invasion | [78] |
| miR-320 | CCLP-1, QBC939, HIBEC | NRP-1 | Anti-migration and invasion | [79] |
| miR-373 | QBC939, HIBEpic | MBD2 | Anti-migration and invasion | [80] |
| miR-421 | HCCC-9180, SSP25, RBE, GBC-SD, HEK293T | FXR | Pro-migration and invasion | [82] |
| miR-210 | KKU-213, KKU-055, KKU-100 | RECK | Pro-migration and invasion | [86] |
| miR-21 | M213, M214, KKU100, M055, M139, M156, OCA17, MMNK1 | PDCD4 | Pro-migration and invasion | [87] |
| miR-96 | HuCCT1, HuH28, RBE, HIBEC | MTSS1 | Pro-migration and invasion | [88] |
| miR-383 | RBE, HuCCT1, QBC939, CCLP, HIBEpic | IRF1 | Pro-migration and invasion | [89] |
| miR-24 | Mz-ChA-1, TFK-1, SG231, CCLP-1, HuCC-T1, HuH-28 | MEN1 | Pro-migration and invasion | [90] |
| miR-122-5p | HIBEpiC, QBC939, LIPF155C, LICCF, CCLP1, RBE, HEK293T | ALDOA | Pro-migration and invasion | [91] |
| lncRNA | ||||
| TTN-AS1 | HIBEC, TFK-1, CCLP, HCCC-9810, HUCCT1 | miR-513a-5p/SFN | Pro-migration and invasion | [93] |
| LINC00976 | HIBEC, HuCCT1, HCCC-9810, QBC939, HuH28, RBE | miR-3202/GPX4 | Pro-migration and invasion | [96] |
| PKD2-2-3 | HuH28, HuCCT1, RBE, TFK1, HIBEpiC | miR-328/GPAM | Pro-migration and invasion | [97] |
| FOXD2-AS1 | HIBEC, CCLP-1, QBC939, HuCCT1, RBE | miR-760/E2F3 | Pro-migration and invasion | [99] |
| LMCD1-AS1 | RBE, KMBC, QBC939, HCCC-9810, HuCCT1, HIBEC | miR-345-5p/COL6A3 | Pro-migration and invasion | [100] |
| LINC00184 | KMBC, HuCCT1, QBC939, HIBEC | miR-23b-3p/ANXA2 | Pro-migration and invasion | [101] |
| NEAT1 | HuCCT1, RBE, HCCC-9810, HCCCT-1 | miR186-5p/PTP4A1, PI3K/AKT pathway | Pro-migration and invasion | [102] |
| PAICC | QBC-939, HUCCT-1, HCCC-9810, HIBEC | miR-141-3p and miR-27a-3p/YAP1, Hippo pathway | Pro-migration and invasion | [103] |
| LOXL1-AS1 | HIBEC, CCLP-1, QBC939, RBE, HuCCT1 | miR-324-3p/ABCA1, JAK2/STAT3 pathway | Pro-migration and invasion | [105] |
| MT1JP | HCCC-9810, RBE, HUCCT1 | miR-18a-5p/FBP1, Wnt/β-catenin pathway | Anti-migration and invasion | [106] |
| circRNA | ||||
| circNFIB | HuCCT1, HCCC9810, RBE | MEK1/ERK2 pathway | Anti-migration and invasion | [107] |
| circSLCO1B3 | RBE, CCLP1, HCCC, HIBEpic | miR-502-5p/HOXC8, TGF-β/SMAD3 pathway | Pro-migration and invasion | [108] |
| circZNF215 | HuCCT1, RBE, HCCC9810 | PRDX1, PTEN, PI3K/AKT pathway | Pro-migration and invasion | [109] |
| circRAPGEF5 | RBE, CCLP1, 9810, HUCCT1, HIBEC | miR-3185/SAE1, AKT pathway | Pro-migration and invasion | [110] |
| circCDR1as | HIBEpiC, HCCC-9810, RBE, HIBEpiC | miR-641/AKT3/mTOR | Pro-migration and invasion | [111] |
| circACTN4 | HIBEpiC, RBE, QBC939, FRH0201 | miR-424-5p/YAP1, Hippo pathway, Wnt/β-catenin pathway | Pro-migration and invasion | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Wei, F.; Zhang, Y.; He, W.; Yan, H.; Wu, J. The Role and Function of Non-Coding RNAs in Cholangiocarcinoma Invasiveness. Biomedicines 2025, 13, 1369. https://doi.org/10.3390/biomedicines13061369
Meng Y, Wei F, Zhang Y, He W, Yan H, Wu J. The Role and Function of Non-Coding RNAs in Cholangiocarcinoma Invasiveness. Biomedicines. 2025; 13(6):1369. https://doi.org/10.3390/biomedicines13061369
Chicago/Turabian StyleMeng, Yu, Fang Wei, Ye Zhang, Wenting He, Haijiao Yan, and Jun Wu. 2025. "The Role and Function of Non-Coding RNAs in Cholangiocarcinoma Invasiveness" Biomedicines 13, no. 6: 1369. https://doi.org/10.3390/biomedicines13061369
APA StyleMeng, Y., Wei, F., Zhang, Y., He, W., Yan, H., & Wu, J. (2025). The Role and Function of Non-Coding RNAs in Cholangiocarcinoma Invasiveness. Biomedicines, 13(6), 1369. https://doi.org/10.3390/biomedicines13061369






