Preliminary Evaluation of 3D-Printed Alginate/Gelatin Scaffolds for Protein Fast Release as Suitable Devices for Personalized Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioink Development
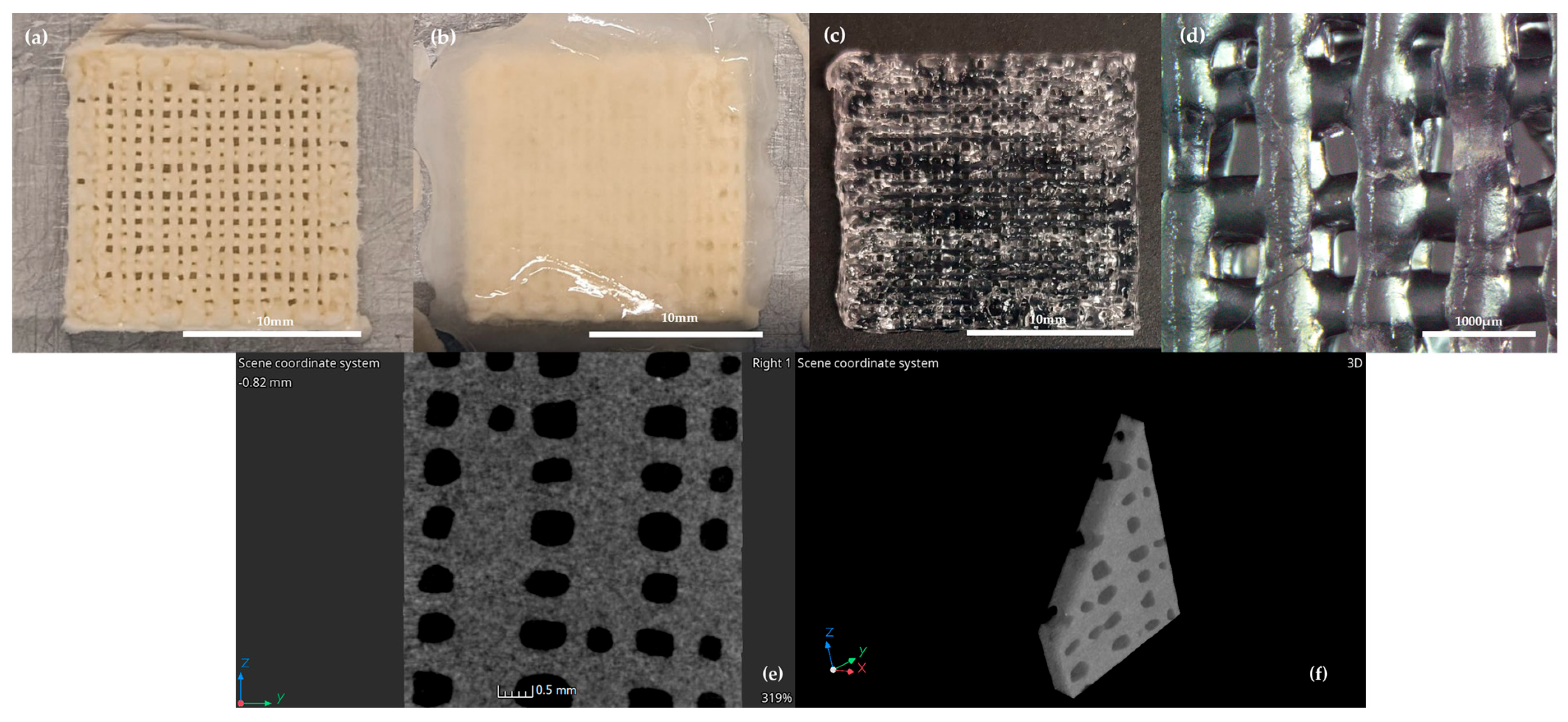
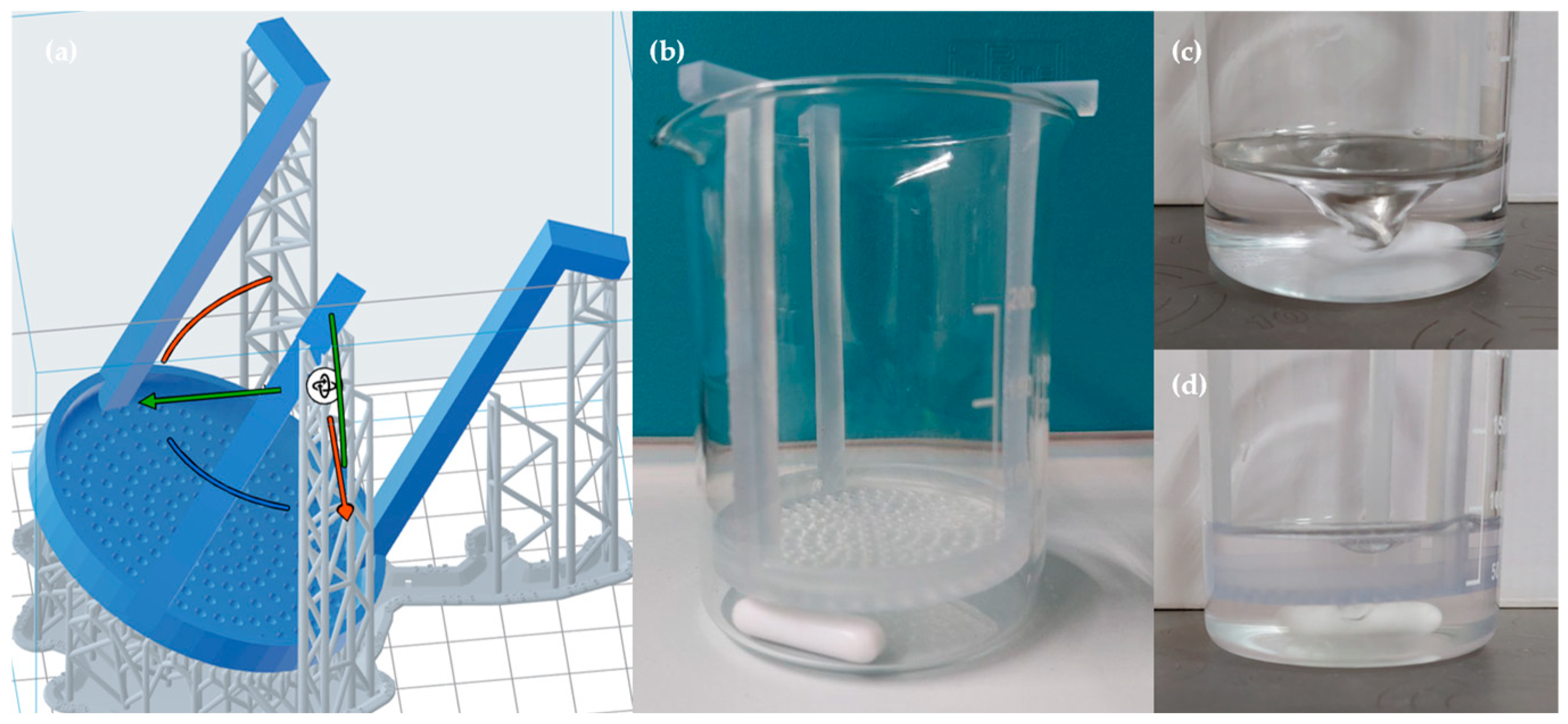
2.2. 3D Printing
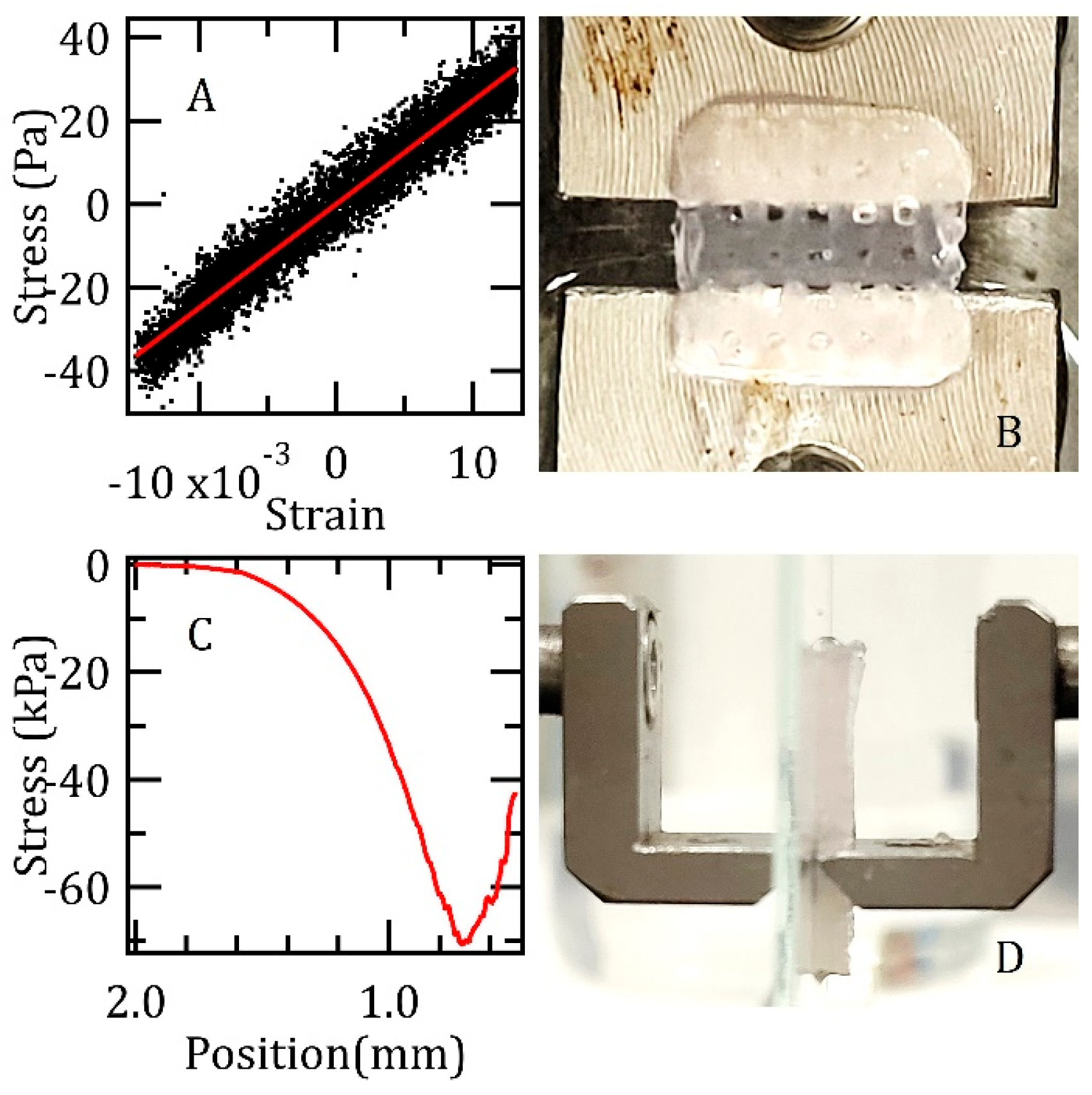
2.3. Rheological and Mechanical Tests
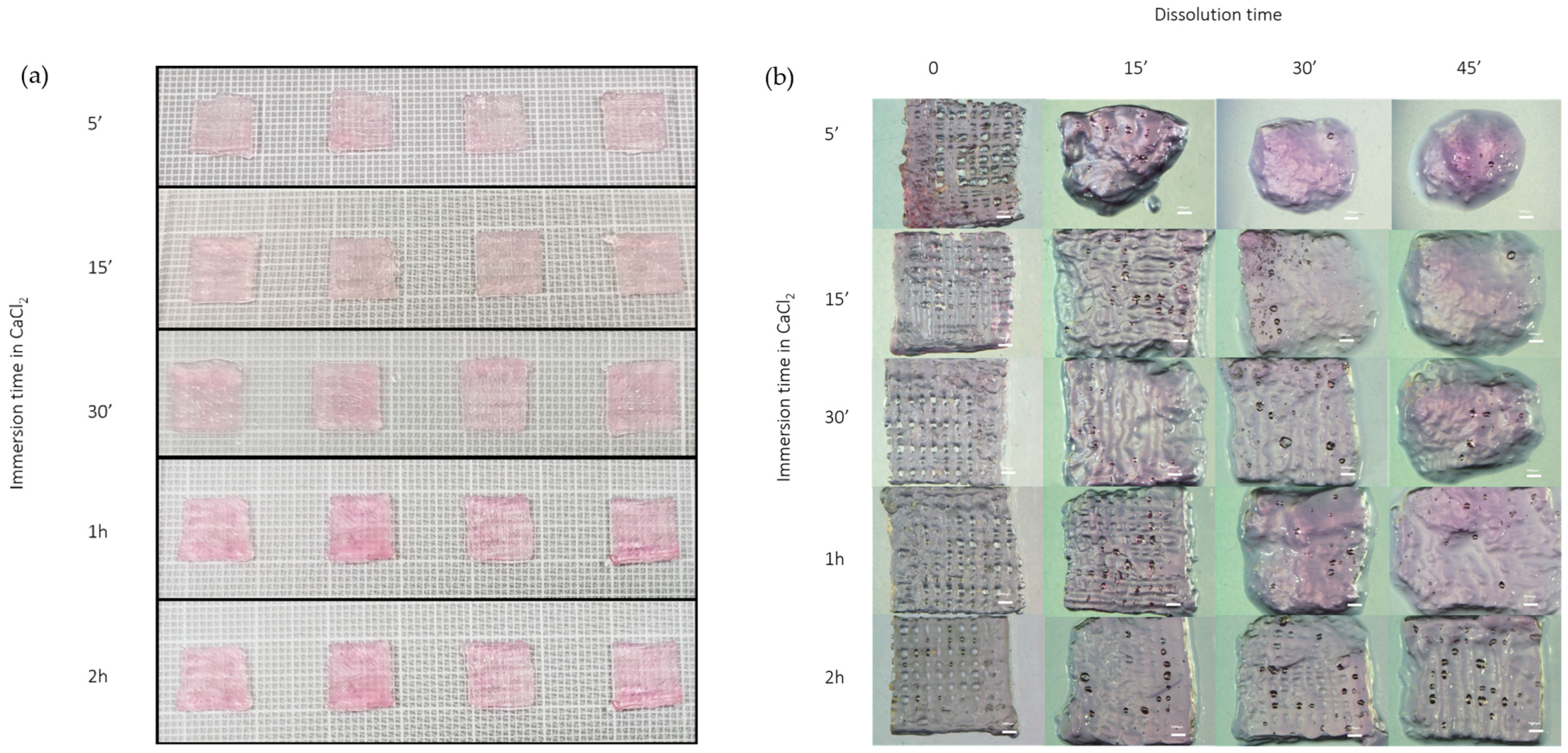
2.4. Dissolution Test
2.5. In Vitro Biological Testing
2.5.1. Cell Cultures and Samples Preparation
2.5.2. Cholesterol Efflux Assay
2.5.3. Cytotoxicity Evaluation
2.6. Statistical Analysis
3. Results
3.1. Rheological and Mechanical Characterization
3.2. Dissolution Test
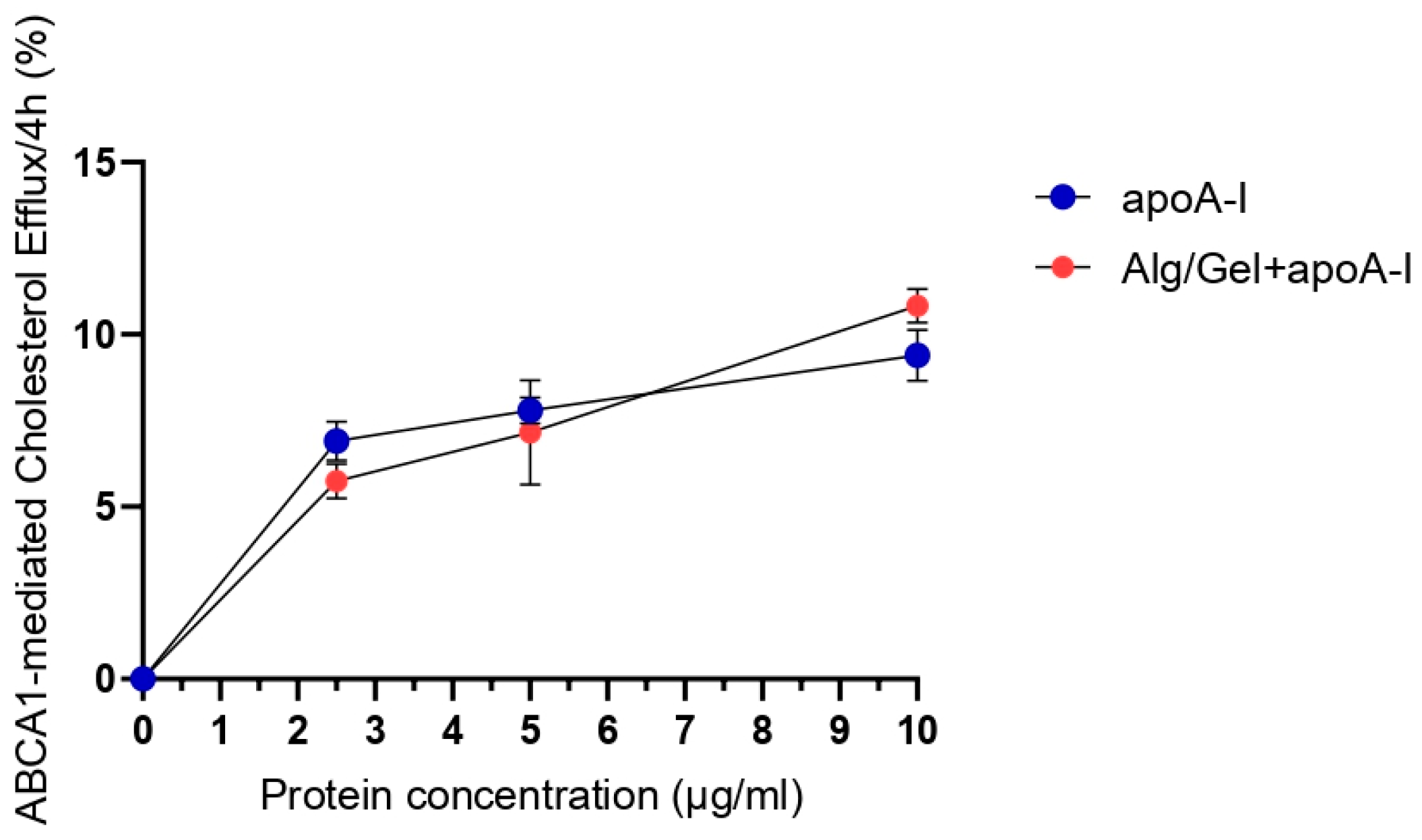
3.3. Cholesterol Efflux Assay
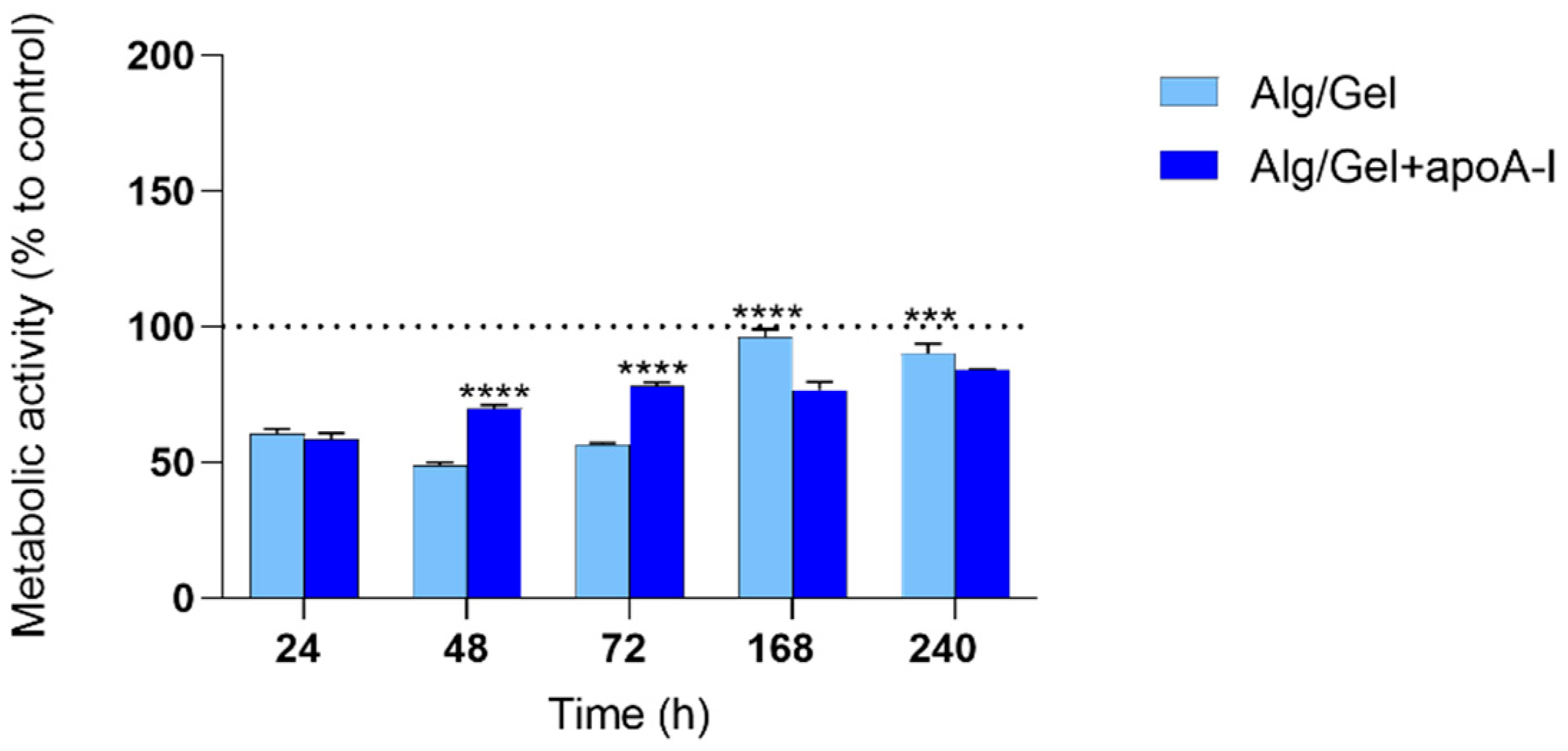
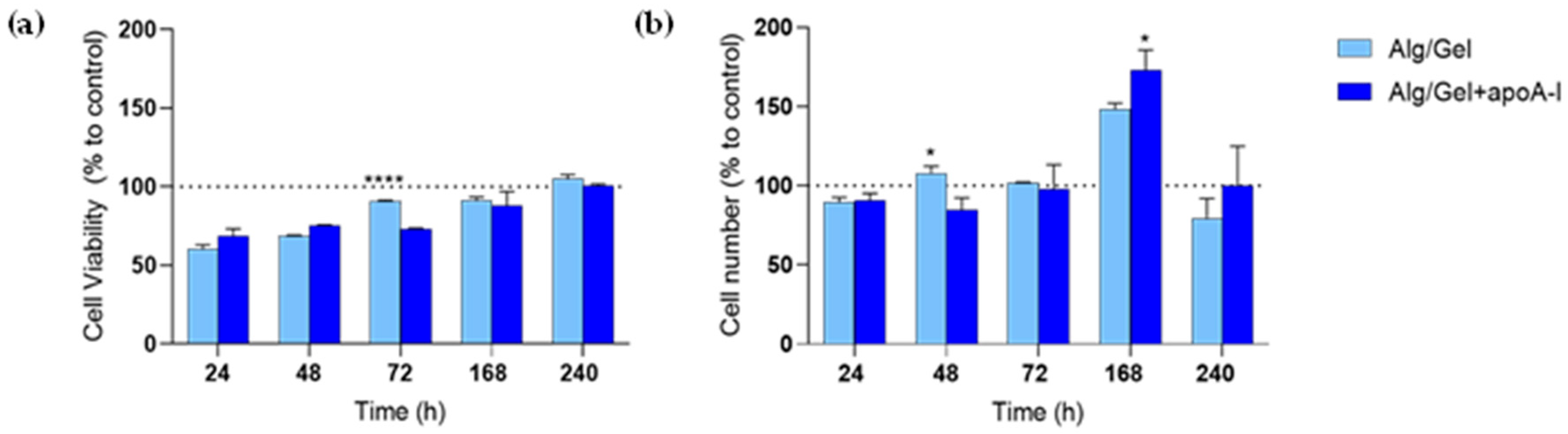
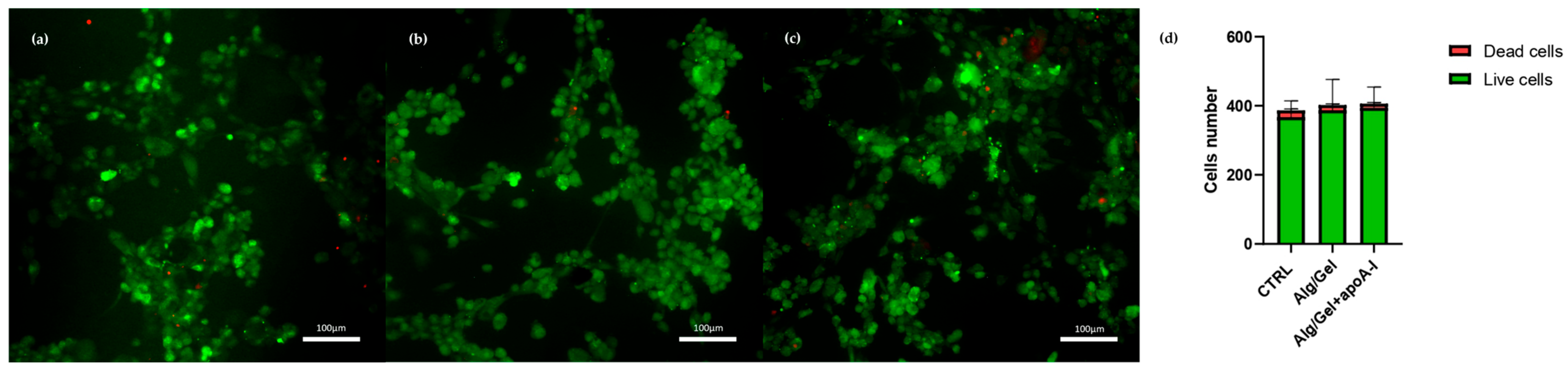
3.4. Cytotoxicity Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Ucci, A.; Perini, P.; Freyrie, A.; Schreve, M.A.; Ünlü, Ç.; Huizing, E.; Van Den Heuvel, D.A.; Kum, S.; Shishehbor, M.H.; Ferraresi, R. Endovascular and Surgical Venous Arterialization for No-Option Patients with Chronic Limb-Threatening Ischemia: A Systematic Review and Meta-Analysis. J. Endovasc. Ther. 2023, 15266028231210220. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nam, H.; Jang, J. 3D Bioprinting of Stem Cell-Laden Cardiac Patch: A Promising Alternative for Myocardial Repair. APL Bioeng. 2021, 5, 031508. [Google Scholar] [CrossRef]
- Thiriet, M. Cardiovascular Disease: An Introduction. In Vasculopathies; Biomathematical and Biomechanical Modeling of the Circulatory and Ventilatory Systems; Springer International Publishing: Cham, Switzerland, 2018; Volume 8, pp. 1–90. ISBN 978-3-319-89314-3. [Google Scholar]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Moakes, C.A.; Bradbury, A.W.; Abdali, Z.; Bate, G.R.; Hall, J.; Jarrett, H.; Kelly, L.; Kigozi, J.; Lockyer, S.; Meecham, L.; et al. Vein Bypass First vs. Best Endovascular Treatment First Revascularisation Strategy for Chronic Limb-Threatening Ischaemia Due to Infra-Popliteal Disease: The BASIL-2 RCT. Health Technol. Assess. 2024, 28, 1–72. [Google Scholar] [CrossRef]
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109. [Google Scholar] [CrossRef]
- Jensen, R.V.; Hjortbak, M.V.; Bøtker, H.E. Ischemic Heart Disease: An Update. Semin. Nucl. Med. 2020, 50, 195–207. [Google Scholar] [CrossRef]
- Abbott, J.D.; Wykrzykowska, J.J.; Lenselink, C. Drug-Coated Balloons in Small Vessels. JACC Cardiovasc. Interv. 2023, 16, 1062–1064. [Google Scholar] [CrossRef]
- Mallis, P.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Future Perspectives in Small-Diameter Vascular Graft Engineering. Bioengineering 2020, 7, 160. [Google Scholar] [CrossRef]
- Favari, E.; Zanotti, I.; Zimetti, F.; Ronda, N.; Bernini, F.; Rothblat, G.H. Probucol Inhibits ABCA1-Mediated Cellular Lipid Efflux. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2345–2350. [Google Scholar] [CrossRef]
- Fox, C.A.; Moschetti, A.; Ryan, R.O. Reconstituted HDL as a Therapeutic Delivery Device. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159025. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Chi, G.; Duffy, D.; Bahit, M.C.; White, H.; Korjian, S.; Alexander, J.H.; Lincoff, A.M.; Anschuetz, G.; Girgis, I.G.; et al. ApoA-I Infusions and Burden of Ischemic Events After Acute Myocardial Infarction: Insights From the AEGIS-II Trial. J. Am. Coll. Cardiol. 2024, 84, 2185–2192. [Google Scholar] [CrossRef]
- Gibson, C.M.; Duffy, D.; Korjian, S.; Bahit, M.C.; Chi, G.; Alexander, J.H.; Lincoff, A.M.; Heise, M.; Tricoci, P.; Deckelbaum, L.I.; et al. Apolipoprotein A1 Infusions and Cardiovascular Outcomes after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1560–1571. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of Alginate-Based Hydrogel Bioprinting for Application in Tissue Engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Tomić, S.L.j.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef]
- Shams, E.; Barzad, M.S.; Mohamadnia, S.; Tavakoli, O.; Mehrdadfar, A. A Review on Alginate-Based Bioinks, Combination with Other Natural Biomaterials and Characteristics. J. Biomater. Appl. 2022, 37, 355–372. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Veis, A. The Physical Chemistry of Gelatin. In International Review of Connective Tissue Research; Elsevier: Amsterdam, The Netherlands, 1965; Volume 3, pp. 113–200. ISBN 978-1-4831-6753-4. [Google Scholar]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The Bioink: A Comprehensive Review on Bioprintable Materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Mierke, C.T. Bioprinting of Cells, Organoids and Organs-on-a-Chip Together with Hydrogels Improves Structural and Mechanical Cues. Cells 2024, 13, 1638. [Google Scholar] [CrossRef]
- Foresti, R.; Rossi, S.; Pinelli, S.; Alinovi, R.; Sciancalepore, C.; Delmonte, N.; Selleri, S.; Caffarra, C.; Raposio, E.; Macaluso, G.; et al. In-Vivo Vascular Application via Ultra-Fast Bioprinting for Future 5D Personalised Nanomedicine. Sci. Rep. 2020, 10, 3205. [Google Scholar] [CrossRef]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform Inkjet Printing of Cellular Structures with Bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Grottkau, B.E.; Hui, Z.; Ran, C.; Pang, Y. Fabricating Vascularized, Anatomically Accurate Bone Grafts Using 3D Bioprinted Sectional Bone Modules, in-Situ Angiogenesis, BMP-2 Controlled Release, and Bioassembly. Biofabrication 2024, 16, 045008. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Włodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasa, B.; San Román, J. Development of Bioactive Catechol Functionalized Nanoparticles Applicable for 3D Bioprinting. Mater. Sci. Eng. C 2021, 131, 112515. [Google Scholar] [CrossRef]
- Foresti, R.; Rossi, S.; Selleri, S. Bio Composite Materials: Nano Functionalization of 4D Bio Engineered Scaffold. In Proceedings of the 2019 IEEE International Conference on BioPhotonics (BioPhotonics), Taipei, Taiwan, 20–22 May 2019; pp. 1–2. [Google Scholar]
- Hasan, M.M.; Ahmad, A.; Akter, M.Z.; Choi, Y.-J.; Yi, H.-G. Bioinks for Bioprinting Using Plant-Derived Biomaterials. Biofabrication 2024, 16, 042004. [Google Scholar] [CrossRef]
- Gerbolés, A.G.; Galetti, M.; Rossi, S.; Lo Muzio, F.P.; Pinelli, S.; Delmonte, N.; Caffarra Malvezzi, C.; Macaluso, C.; Miragoli, M.; Foresti, R. Three-Dimensional Bioprinting of Organoid-Based Scaffolds (OBST) for Long-Term Nanoparticle Toxicology Investigation. Int. J. Mol. Sci. 2023, 24, 6595. [Google Scholar] [CrossRef]
- Xia, P.; Liu, C.; Wei, X.; Guo, J.; Luo, Y. 3D-Printed Hydrogel Scaffolds with Drug- and Stem Cell-Laden Core/Shell Filaments for Cancer Therapy and Soft Tissue Repair. J. Mater. Chem. B 2024, 12, 11491–11501. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, Y.-J.; Gal, C.-W.; Sung, A.; Park, H.; Yun, H.-S. Development of an Alginate–Gelatin Bioink Enhancing Osteogenic Differentiation by Gelatin Release. Int. J. Bioprint. 2023, 9, 660. [Google Scholar] [CrossRef]
- Hao, L.; Zhao, S.; Hao, S.; He, Y.; Feng, M.; Zhou, K.; He, Y.; Yang, J.; Mao, H.; Gu, Z. Functionalized Gelatin-Alginate Based Bioink with Enhanced Manufacturability and Biomimicry for Accelerating Wound Healing. Int. J. Biol. Macromol. 2023, 240, 124364. [Google Scholar] [CrossRef]
- An, S.; Jiang, X.; Shi, J.; He, X.; Li, J.; Guo, Y.; Zhang, Y.; Ma, H.; Lu, Y.; Jiang, C. Single-Component Self-Assembled RNAi Nanoparticles Functionalized with Tumor-Targeting iNGR Delivering Abundant siRNA for Efficient Glioma Therapy. Biomaterials 2015, 53, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Rossi, S.; Pinelli, S.; Alinovi, R.; Barozzi, M.; Sciancalepore, C.; Galetti, M.; Caffarra, C.; Lagonegro, P.; Scavia, G.; et al. Highly-Defined Bioprinting of Long-Term Vascularized Scaffolds with Bio-Trap: Complex Geometry Functionalization and Process Parameters with Computer Aided Tissue Engineering. Materialia 2020, 9, 100560. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Romero-Montero, A.; Hernández-Parra, H.; Peña-Corona, S.I.; Del Prado-Audelo, M.L.; Alcalá-Alcalá, S.; Cortés, H.; Kiyekbayeva, L.; Sharifi-Rad, J.; Leyva-Gómez, G. Enhancing Chemical and Physical Stability of Pharmaceuticals Using Freeze-Thaw Method: Challenges and Opportunities for Process Optimization through Quality by Design Approach. J. Biol. Eng. 2023, 17, 35. [Google Scholar] [CrossRef]
- Matteini, P.; Ratto, F.; Rossi, F.; De Angelis, M.; Cavigli, L.; Pini, R. Hybrid Nanocomposite Films for Laser-activated Tissue Bonding. J. Biophotonics 2012, 5, 868–877. [Google Scholar] [CrossRef]
- Ratto, F.; Magni, G.; Aluigi, A.; Giannelli, M.; Centi, S.; Matteini, P.; Oberhauser, W.; Pini, R.; Rossi, F. Cyanine-Doped Nanofiber Mats for Laser Tissue Bonding. Nanomaterials 2022, 12, 1613. [Google Scholar] [CrossRef]
- Favari, E.; Thomas, M.J.; Sorci-Thomas, M.G. High-Density Lipoprotein Functionality as a New Pharmacological Target on Cardiovascular Disease: Unifying Mechanism That Explains High-Density Lipoprotein Protection Toward the Progression of Atherosclerosis. J. Cardiovasc. Pharmacol. 2018, 71, 325–331. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed]
- Neufurth, M.; Wang, X.; Schröder, H.C.; Feng, Q.; Diehl-Seifert, B.; Ziebart, T.; Steffen, R.; Wang, S.; Müller, W.E.G. Engineering a Morphogenetically Active Hydrogel for Bioprinting of Bioartificial Tissue Derived from Human Osteoblast-like SaOS-2 Cells. Biomaterials 2014, 35, 8810–8819. [Google Scholar] [CrossRef]
- Hazur, J.; Detsch, R.; Karakaya, E.; Kaschta, J.; Teßmar, J.; Schneidereit, D.; Friedrich, O.; Schubert, D.W.; Boccaccini, A.R. Improving Alginate Printability for Biofabrication: Establishment of a Universal and Homogeneous Pre-Crosslinking Technique. Biofabrication 2020, 12, 045004. [Google Scholar] [CrossRef]
- You, F.; Wu, X.; Chen, X. 3D Printing of Porous Alginate/Gelatin Hydrogel Scaffolds and Their Mechanical Property Characterization. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 299–306. [Google Scholar] [CrossRef]
- Fayyazbakhsh, F.; Khayat, M.J.; Leu, M.C. 3D-Printed Gelatin-Alginate Hydrogel Dressings for Burn Wound Healing: A Comprehensive Study. Int. J. Bioprinting 2022, 8, 618. [Google Scholar] [CrossRef]
- Donati, I.; Holtan, S.; Mørch, Y.A.; Borgogna, M.; Dentini, M.; Skjåk-Bræk, G. New Hypothesis on the Role of Alternating Sequences in Calcium−Alginate Gels. Biomacromol. 2005, 6, 1031–1040. [Google Scholar] [CrossRef]
- Chang, G.H.; Azar, D.A.; Lyle, C.; Chitalia, V.C.; Shazly, T.; Kolachalama, V.B. Intrinsic Coating Morphology Modulates Acute Drug Transfer in Drug-Coated Balloon Therapy. Sci. Rep. 2019, 9, 6839. [Google Scholar] [CrossRef] [PubMed]
- Shazly, T.; Torres, W.M.; Secemsky, E.A.; Chitalia, V.C.; Jaffer, F.A.; Kolachalama, V.B. Understudied Factors in Drug-coated Balloon Design and Evaluation: A Biophysical Perspective. Bioeng. Transl. Med. 2023, 8, e10370. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Pan, M.; Zhao, R.; Deng, J.; Wu, Y. Recent Advances, Challenges and Perspectives in Enantioselective Release. J. Control. Release 2020, 324, 156–171. [Google Scholar] [CrossRef]
- Wei, C.; He, P.; He, L.; Ye, X.; Cheng, J.; Wang, Y.; Li, W.; Liu, Y. Structure Characterization and Biological Activities of a Pectic Polysaccharide from Cupule of Castanea Henryi. Int. J. Biol. Macromol. 2018, 109, 65–75. [Google Scholar] [CrossRef]
- Gross, K.A.; Young, C.J.; Beck, M.A.; Keebaugh, E.W.; Bronts, T.J.; Saber-Samandari, S.; Riley, D.P. Characterization and Dissolution of Functionalized Amorphous Calcium Phosphate Biolayers Using Single-Splat Technology. Acta Biomater. 2011, 7, 2270–2275. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, Y.; Chen, H.; Su, Y.; Cheng, Y.; Xu, J.; Sun, H.; Song, K. A 3D Bioprinted Antibacterial Hydrogel Dressing of Gelatin/Sodium Alginate Loaded with Ciprofloxacin Hydrochloride. Biotechnol. J. 2024, 19, 2400209. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Liu, D.; Ji, L.; Tong, X.; Pan, B.; Han, J.-Y.; Huang, Y.; Chen, Y.E.; Pennathur, S.; Zhang, Y.; Zheng, L. Human Apolipoprotein A-I Induces Cyclooxygenase-2 Expression and Prostaglandin I-2 Release in Endothelial Cells through ATP-Binding Cassette Transporter A1. Am. J. Physiol.-Cell Physiol. 2011, 301, C739–C748. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liu, Y.; Kessler, P.S.; Vaughan, A.M.; Oram, J.F. The Macrophage Cholesterol Exporter ABCA1 Functions as an Anti-Inflammatory Receptor. J. Biol. Chem. 2009, 284, 32336–32343. [Google Scholar] [CrossRef]
- Yang, P.; Xie, F.; Zhu, L.; Selvaraj, J.N.; Zhang, D.; Cai, J. Fabrication of Chitin-fibrin Hydrogels to Construct the 3D Artificial Extracellular Matrix Scaffold for Vascular Regeneration and Cardiac Tissue Engineering. J. Biomed. Mater. Res. A 2024, 112, 2257–2272. [Google Scholar] [CrossRef]
| 25 °C | 30 °C | 37 °C | |
| Rotor | R6 | R6 | R3 |
| RPM | 100 | 200 | 100 |
| Tolerance interval (%) | 51.5–52.5 | 56–58 | 47 |
| cP (mPa) | 5000 °C ± 100 | 2800 °C ± 100 | 460 °C ± 20 |
| Specimen Nr | 1 | 2 | 3 |
|---|---|---|---|
| Young’s Modulus (KPa) | 2.51 ± 0.03 | 2.50 ± 0.05 | 2.57 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghezzi, B.; Foresti, R.; Scialoia, L.P.; Botti, M.; Mersanne, A.; Ratto, F.; Rossi, F.; Martini, C.; Perini, P.; Favari, E.; et al. Preliminary Evaluation of 3D-Printed Alginate/Gelatin Scaffolds for Protein Fast Release as Suitable Devices for Personalized Medicine. Biomedicines 2025, 13, 1365. https://doi.org/10.3390/biomedicines13061365
Ghezzi B, Foresti R, Scialoia LP, Botti M, Mersanne A, Ratto F, Rossi F, Martini C, Perini P, Favari E, et al. Preliminary Evaluation of 3D-Printed Alginate/Gelatin Scaffolds for Protein Fast Release as Suitable Devices for Personalized Medicine. Biomedicines. 2025; 13(6):1365. https://doi.org/10.3390/biomedicines13061365
Chicago/Turabian StyleGhezzi, Benedetta, Ruben Foresti, Luisa Pia Scialoia, Maddalena Botti, Arianna Mersanne, Fulvio Ratto, Francesca Rossi, Chiara Martini, Paolo Perini, Elda Favari, and et al. 2025. "Preliminary Evaluation of 3D-Printed Alginate/Gelatin Scaffolds for Protein Fast Release as Suitable Devices for Personalized Medicine" Biomedicines 13, no. 6: 1365. https://doi.org/10.3390/biomedicines13061365
APA StyleGhezzi, B., Foresti, R., Scialoia, L. P., Botti, M., Mersanne, A., Ratto, F., Rossi, F., Martini, C., Perini, P., Favari, E., & Freyrie, A. (2025). Preliminary Evaluation of 3D-Printed Alginate/Gelatin Scaffolds for Protein Fast Release as Suitable Devices for Personalized Medicine. Biomedicines, 13(6), 1365. https://doi.org/10.3390/biomedicines13061365









