A Rare Coexistence of Klippel–Trenaunay Syndrome and Cardiac Sarcoidosis
Abstract
1. Background
2. Case Presentation
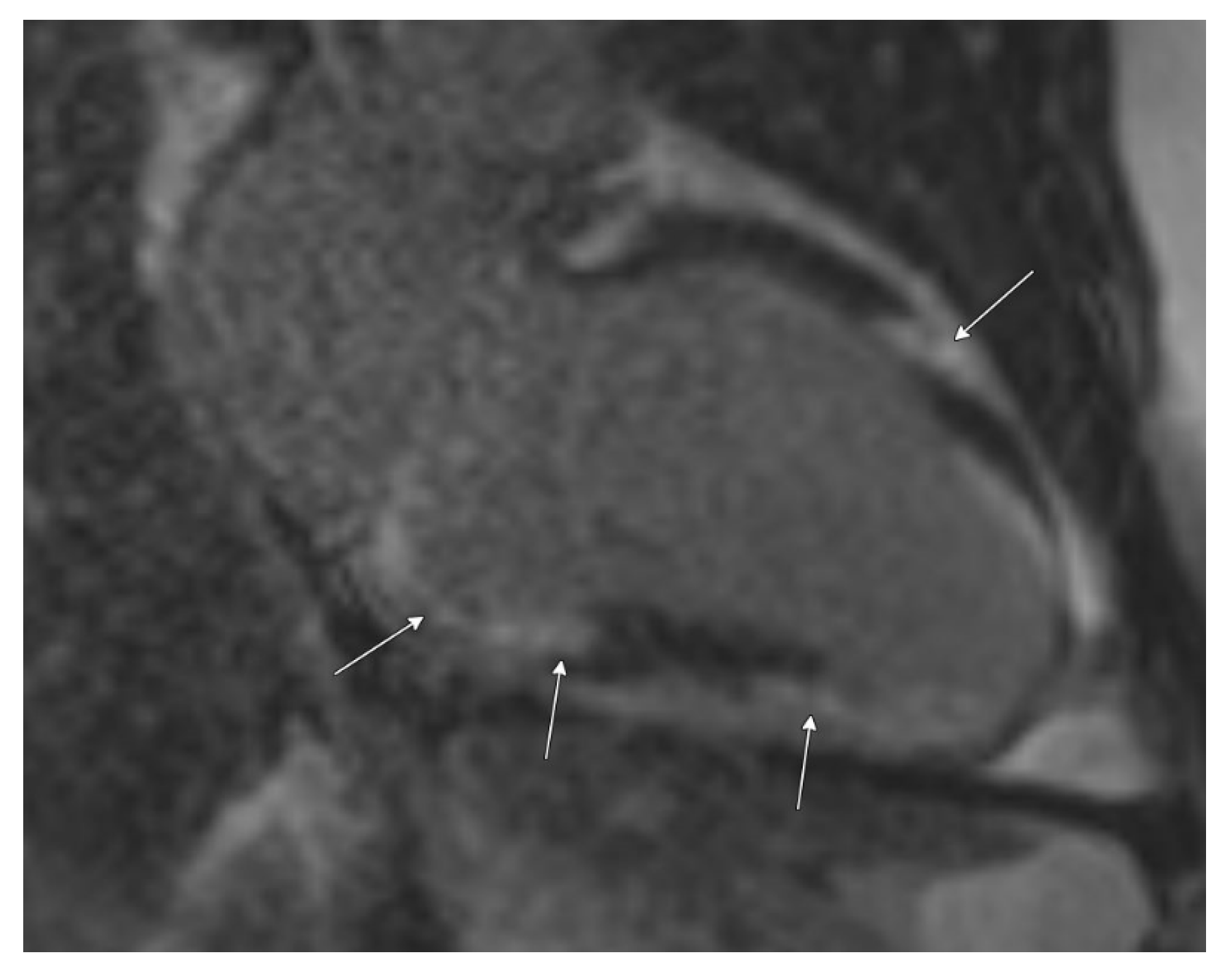
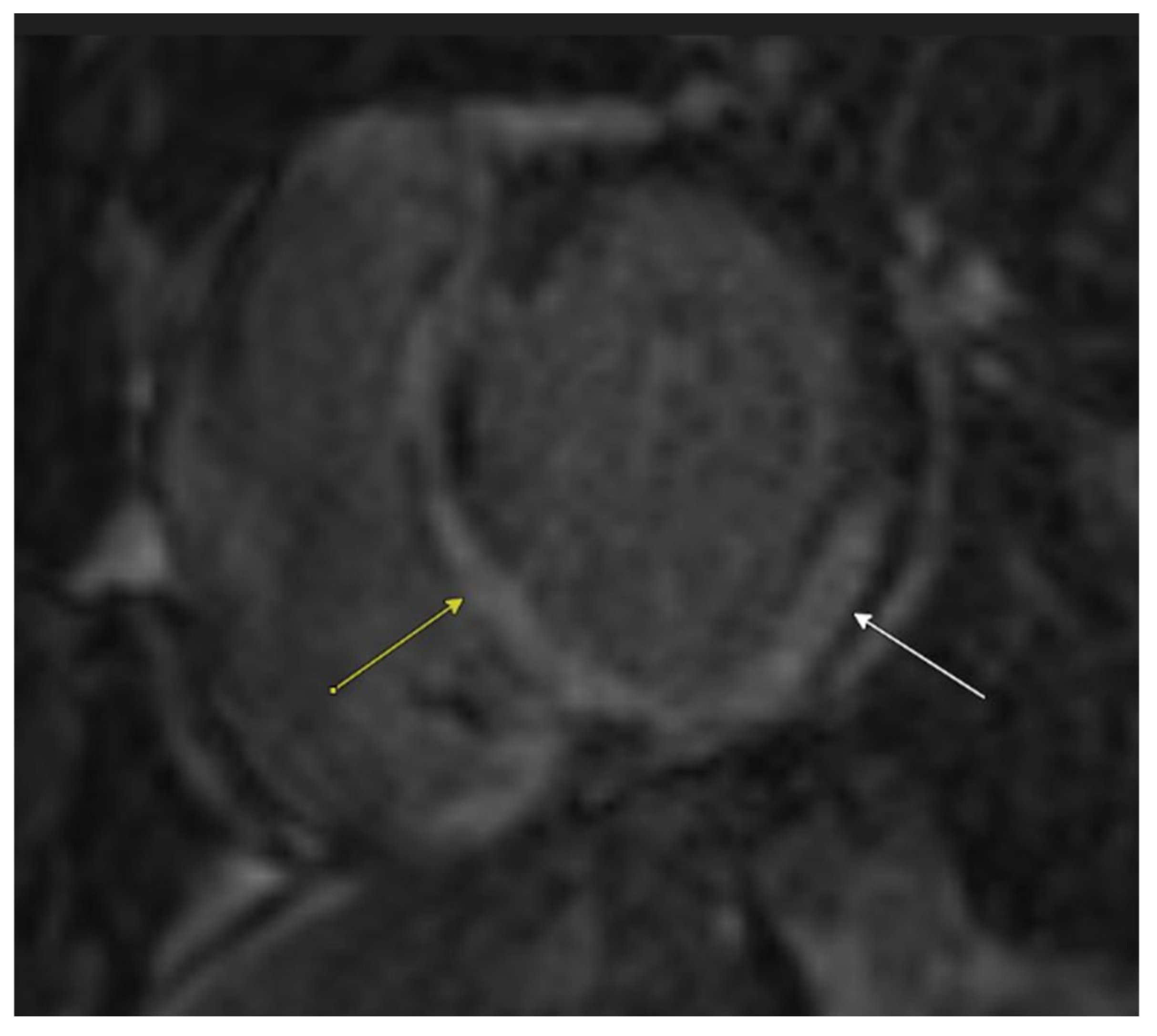
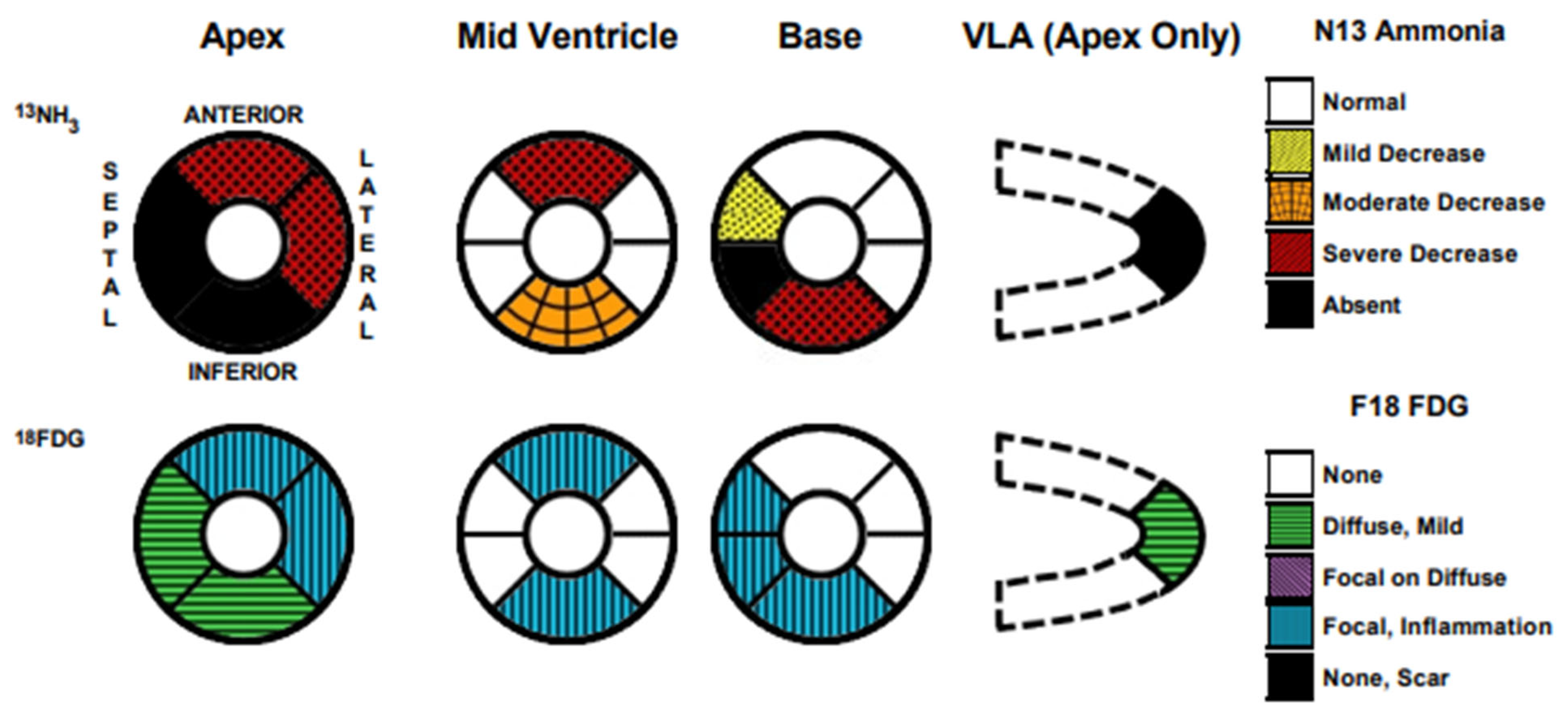
2.1. Initial Workup
2.2. Diagnosis and Management
2.3. Follow-Up
3. Discussion
3.1. Diagnosis and Management of KT Syndrome
3.2. Diagnosis and Management of CS
3.3. Complexities Associated with the Coexistence of KTS and CS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | Cardiac Sarcoidosis |
| CTEPH | Chronic thromboembolic pulmonary hypertension |
| DVT | Deep vein thrombosis |
| GDMT | Guideline-directed medical therapy |
| ICD | Implantable cardioverter defibrillator |
| KT Syndrome | Klippel–Trenaunay syndrome |
| PET | Positron Emission Tomography |
| PTE | Pulmonary thromboembolism |
| RWMA | Regional wall motion abnormalities |
References
- Berry, S.A.; Peterson, C.; Mize, W.; Bloom, K.; Zachary, C.; Blasco, P.; Hunter, D. Klippel-Trenaunay syndrome. Am. J. Med. Genet. 1998, 79, 319–326. [Google Scholar] [CrossRef]
- Asghar, F.; Aqeel, R.; Farooque, U.; Haq, A.; Taimur, M. Presentation and Management of Klippel-Trenaunay Syndrome: A Review of Available Data. Cureus 2020, 12, e8023. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.W.; Park, J.B. Cardiac sarcoidosis. Heart 2023, 109, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Gubala, A.; Venkatesh, K.; Akhter, M.; Meyer, T.E.; Fitzgibbons, T.P. High-Output Heart Failure in a Patient with Klippel-Trénaunay Syndrome: A Case Report. Cureus 2023, 15, e38963. [Google Scholar] [CrossRef]
- Chu, S.T.; Han, Y.H.; Koh, J.A.; Kim, S.J.; Lee, H.C.; Kim, S.E.; Shin, Y.C.; Sir, J.J.; Choi, S.M.; Joo, S.B. A Case of Klippel-Trenaunay Syndrome with Acute Submassive Pulmonary Thromboembolism Treated with Thrombolytic Therapy. J. Cardiovasc. Ultrasound 2015, 23, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Zureigat, H.; Frank, R.; Shah, V.S.; Makarenko, V.; Hucker, W.; Ho, J.E.; Wood, M.J.; Osborne, M.T. Cardiac Sarcoidosis Initially Diagnosed as Spontaneous Coronary Artery Dissection. JACC Case Rep. 2021, 3, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, F.; Azuma, A.; Anzai, T.; Ishizaka, N.; Ishida, Y.; Isobe, M.; Inomata, T.; Ishibashi-Ueda, H.; Eishi, Y.; Kitakaze, M.; et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis—Digest Version. Circ. J. 2019, 83, 2329–2388. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A.; Costabel, U.; Drent, M.; Wells, A.; Maier, L.; Koth, L.; Shigemitsu, H.; Culver, D.A.; Gelfand, J.; Valeyre, D.; et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc. Diffuse Lung Dis. 2014, 31, 19–27. [Google Scholar] [PubMed]
- Cheng, R.K.; Kittleson, M.M.; Beavers, C.J.; Birnie, D.H.; Blankstein, R.; Bravo, P.E.; Gilotra, N.A.; Judson, M.A.; Patton, K.K.; Rose-Bovino, L. Diagnosis and Management of Cardiac Sarcoidosis: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e1197–e1216. [Google Scholar] [CrossRef] [PubMed]
- Giblin, G.T.; Murphy, L.; Stewart, G.C.; Desai, A.S.; Di Carli, M.F.; Blankstein, R.; Givertz, M.M.; Tedrow, U.B.; Sauer, W.H.; Hunninghake, G.M.; et al. Cardiac Sarcoidosis: When and How to Treat Inflammation. Card. Fail. Rev. 2021, 7, e17. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.; Uusitalo, V.; Pöyhönen, P.; Mäyränpää, M.I.; Kupari, M. Cardiac sarcoidosis: Phenotypes, diagnosis, treatment, and prognosis. Eur. Heart J. 2023, 44, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Gilotra, N.; Okada, D.; Sharma, A.; Chrispin, J. Management of Cardiac Sarcoidosis in 2020. Arrhythm. Electrophysiol. Rev. 2020, 9, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Nordenswan, H.K.; Lehtonen, J.; Ekström, K.; Kandolin, R.; Simonen, P.; Mäyränpää, M.; Vihinen, T.; Miettinen, H.; Kaikkonen, K.; Haataja, P.; et al. Outcome of Cardiac Sarcoidosis Presenting with High-Grade Atrioventricular Block. Circ. Arrhythm. Electrophysiol. 2018, 11, e006145. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014, 11, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.F.; Lu, Q.; Yan, Z.X. Lymphatic malformation is a common component of Klippel-Trenaunay syndrome. J. Vasc. Surg. 2010, 52, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Gianlupi, A.; Harper, R.W.; Dwyre, D.M.; Marelich, G.P. Recurrent pulmonary embolism associated with Klippel-Trenaunay-Weber syndrome. Chest 1999, 115, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Douma, R.A.; Oduber, C.E.; Gerdes, V.E.; van Delden, O.M.; van Eck-Smit, B.L.; Meijers, J.C.; van Beers, E.J.; Bouma, B.J.; van der Horst, C.M.; Bresser, P. Chronic pulmonary embolism in Klippel-Trenaunay syndrome. J. Am. Acad. Dermatol. 2012, 66, 71–77. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Desai, A.; Mautong, H.; Mergo, P.; Leoni, J.; Ruiz, J.; Goswami, R. A Rare Coexistence of Klippel–Trenaunay Syndrome and Cardiac Sarcoidosis. Biomedicines 2025, 13, 1326. https://doi.org/10.3390/biomedicines13061326
Sharma S, Desai A, Mautong H, Mergo P, Leoni J, Ruiz J, Goswami R. A Rare Coexistence of Klippel–Trenaunay Syndrome and Cardiac Sarcoidosis. Biomedicines. 2025; 13(6):1326. https://doi.org/10.3390/biomedicines13061326
Chicago/Turabian StyleSharma, Shriya, Aarti Desai, Hans Mautong, Patricia Mergo, Juan Leoni, Jose Ruiz, and Rohan Goswami. 2025. "A Rare Coexistence of Klippel–Trenaunay Syndrome and Cardiac Sarcoidosis" Biomedicines 13, no. 6: 1326. https://doi.org/10.3390/biomedicines13061326
APA StyleSharma, S., Desai, A., Mautong, H., Mergo, P., Leoni, J., Ruiz, J., & Goswami, R. (2025). A Rare Coexistence of Klippel–Trenaunay Syndrome and Cardiac Sarcoidosis. Biomedicines, 13(6), 1326. https://doi.org/10.3390/biomedicines13061326






