Dapagliflozin’s Effects on Urinary Albumin and Non-Albumin Proteins in Diabetic and Non-Diabetic Kidney Transplant Recipients
Abstract
1. Introduction
2. Methods
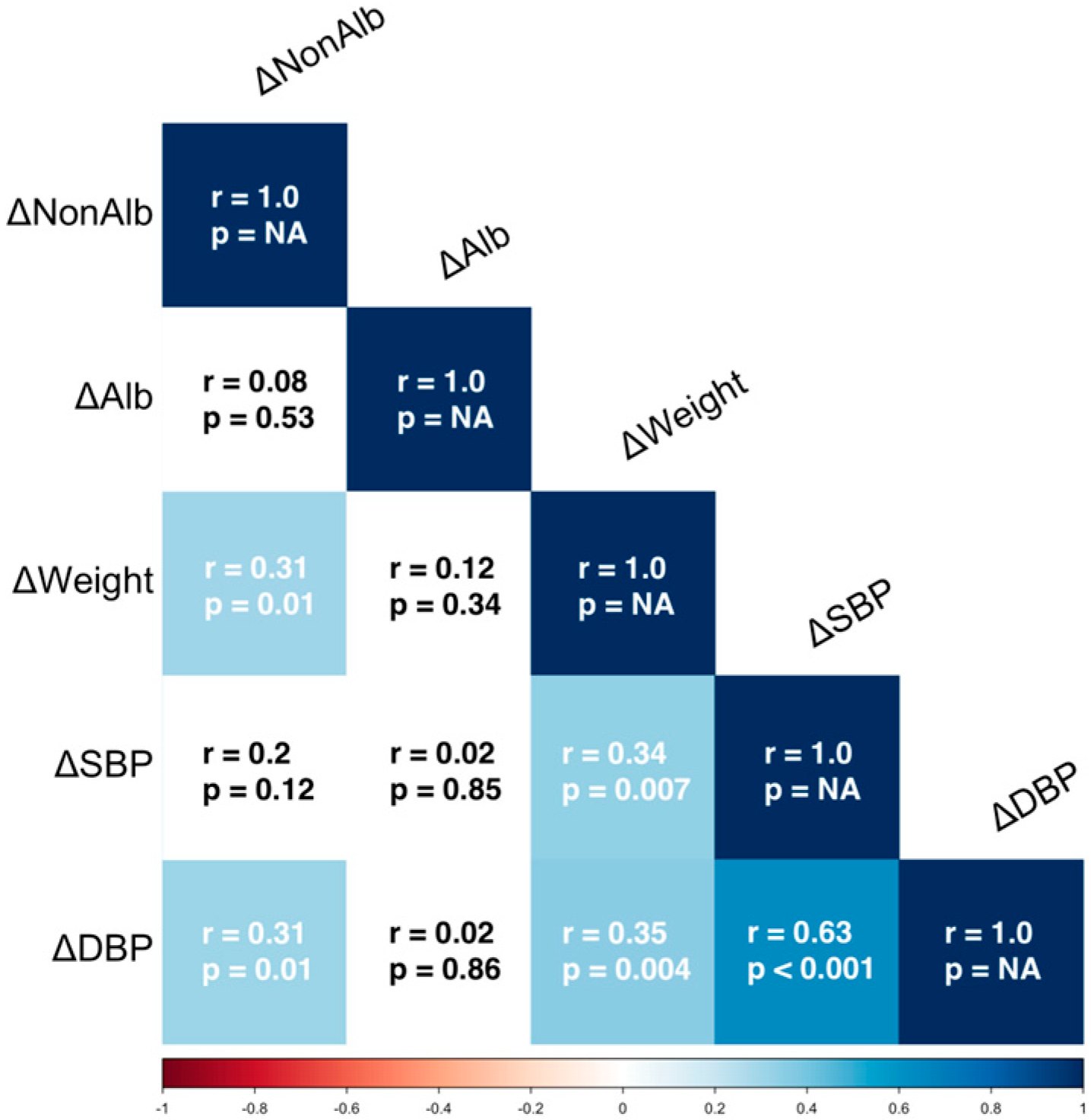
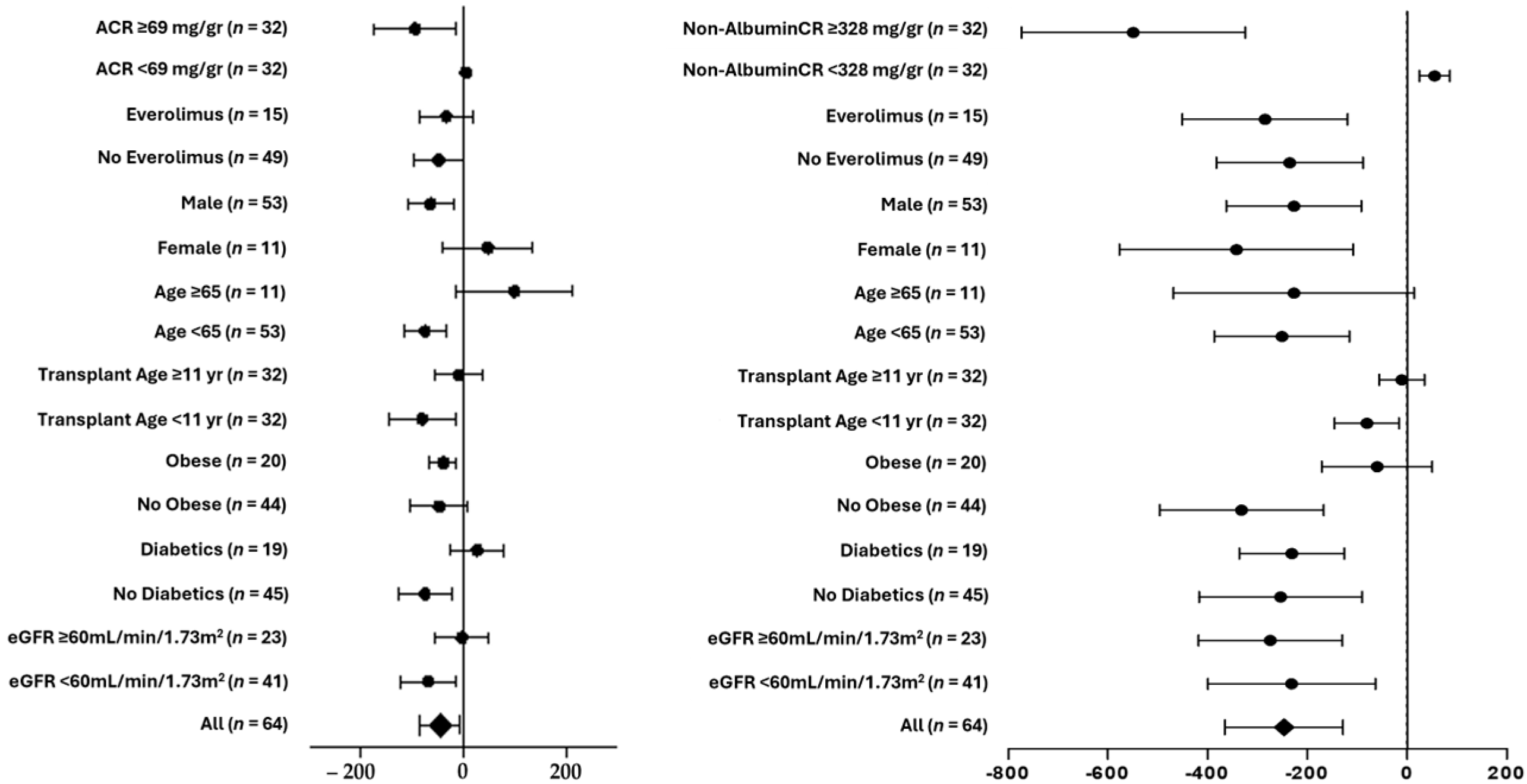
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Lee, T.; DeFronzo, R.A. Why Do SGLT2 Inhibitors Inhibit Only 30–50% of Renal Glucose Reabsorption in Humans? Diabetes 2012, 61, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Mariani, M.V.; Lavalle, C.; Palombi, M.; Pierucci, N.; Trivigno, S.; D’Amato, A.; Filomena, D.; Cipollone, P.; Laviola, D.; Piro, A.; et al. SGLT2i Reduce Arrhythmic Events in Heart Failure Patients with Cardiac Implantable Electronic Devices. ESC Heart Fail. 2025, 12, 2125–2133. [Google Scholar] [CrossRef]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. Summary of the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104, 708–714. [Google Scholar] [CrossRef]
- Poggio, E.D.; Augustine, J.J.; Arrigain, S.; Brennan, D.C.; Schold, J.D. Long-Term Kidney Transplant Graft Survival—Making Progress When Most Needed. Am. J. Transplant. 2021, 21, 2824–2832. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.; Wang, Z.; Benoit, S.; Burrows, N.R.; Imperatore, G.; Albright, A.L.; Patzer, R. Long-Term Mortality among Kidney Transplant Recipients with and without Diabetes: A Nationwide Cohort Study in the USA. BMJ Open Diabetes Res. Care 2021, 9, e001962. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.J.; Bujáki, B.; Bidiga, L.; Kardos, L.; Nemes, B.; Balla, J.; Szabó, R.P. Relevance of Proteinuria in Kidney Transplant Recipients and Allograft Outcomes. Transplant. Proc. 2024, 56, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Shlipak, M.G. The Promise of Tubule Biomarkers in Kidney Disease: A Review. Am. J. Kidney Dis. 2021, 78, 719–727. [Google Scholar] [CrossRef]
- Shamseddin, M.K.; Knoll, G.A. Posttransplantation Proteinuria: An Approach to Diagnosis and Management. Clin. J. Am. Soc. Nephrol. 2011, 6, 1786–1793. [Google Scholar] [CrossRef]
- Ponticelli, C.; Graziani, G. Proteinuria after Kidney Transplantation. Transpl. Int. 2012, 25, 909–917. [Google Scholar] [CrossRef]
- Schwaiger, E.; Burghart, L.; Signorini, L.; Ristl, R.; Kopecky, C.; Tura, A.; Pacini, G.; Wrba, T.; Antlanger, M.; Schmaldienst, S.; et al. Empagliflozin in Posttransplantation Diabetes Mellitus: A Prospective, Interventional Pilot Study on Glucose Metabolism, Fluid Volume, and Patient Safety. Am. J. Transplant. 2019, 19, 907–919. [Google Scholar] [CrossRef]
- Patel, N.; Hindi, J.; Farouk, S.S. Sodium-Glucose Cotransporter 2 Inhibitors and Kidney Transplantation: What Are We Waiting For? Kidney360 2021, 2, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Halden, T.A.S.; Kvitne, K.E.; Midtvedt, K.; Rajakumar, L.; Robertsen, I.; Brox, J.; Bollerslev, J.; Hartmann, A.; Åsberg, A.; Jenssen, T. Efficacy and Safety of Empagliflozin in Renal Transplant Recipients With Posttransplant Diabetes Mellitus. Diabetes Care 2019, 42, 1067–1074. [Google Scholar] [CrossRef]
- Mahalwar, G.; Mathew, R.O.; Rangaswami, J. Sodium-Glucose Cotransporter 2 Inhibitors and Cardiorenal Outcomes in Kidney Transplantation. Curr. Opin. Nephrol. Hypertens. 2024, 33, 53–60. [Google Scholar] [CrossRef]
- Maigret, L.; Basle, L.; Chatelet, V.; Ecotiere, L.; Perrin, P.; Golbin, L.; Bertrand, D.; Anglicheau, D.; Poulain, C.; Garrouste, C.; et al. Sodium-Glucose Cotransporter-2 Inhibitor in Diabetic and Nondiabetic Renal Transplant Recipients. Kidney Int. Rep. 2025, 10, 816–827. [Google Scholar] [CrossRef]
- AlKindi, F.; Al-Omary, H.L.; Hussain, Q.; Al Hakim, M.; Chaaban, A.; Boobes, Y. Outcomes of SGLT2 Inhibitors Use in Diabetic Renal Transplant Patients. Transplant. Proc. 2020, 52, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Fructuoso, A.I.; Bedia Raba, A.; Banegas Deras, E.; Vigara Sánchez, L.A.; Valero San Cecilio, R.; Franco Esteve, A.; Cruzado Vega, L.; Gavela Martínez, E.; González Garcia, M.E.; Saurdy Coronado, P.; et al. Sodium-Glucose Cotransporter-2 Inhibitor Therapy in Kidney Transplant Patients with Type 2 or Post-Transplant Diabetes: An Observational Multicentre Study. Clin. Kidney J. 2023, 16, 1022–1034. [Google Scholar] [CrossRef]
- Lemke, A.; Brokmeier, H.M.; Leung, S.B.; Mara, K.C.; Mour, G.K.; Wadei, H.M.; Hill, J.M.; Stegall, M.; Kudva, Y.C.; Shah, P.; et al. Sodium-Glucose Cotransporter 2 Inhibitors for Treatment of Diabetes Mellitus after Kidney Transplantation. Clin. Transpl. 2022, 36, e14718. [Google Scholar] [CrossRef] [PubMed]
- Secondulfo, C.; Vecchione, N.; Russo, D.; Hamzeh, S.; Iacuzzo, C.; Apicella, L.; Di Pietro, R.A.; Pisani, A.; Amicone, M.; Cirillo, M.; et al. Impact of SGLT2 Inhibitors on Magnesium in Kidney Transplant Patients with and Without Diabetes. Int. J. Mol. Sci. 2025, 26, 2904. [Google Scholar] [CrossRef] [PubMed]
- Bilancio, G.; Celano, M.; Cozza, V.; Zingone, F.; Palladino, G.; Cirillo, M. Early Prediction of Cardiovascular Disease in Kidney Transplant Recipients. Transplant. Proc. 2017, 49, 2092–2098. [Google Scholar] [CrossRef]
- Agenzia Italiana Del Farmaco. Nota 100; Agenzia Italiana Del Farmaco: Roma, Italy, 2025.
- Levin, A.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. Executive Summary of the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: Known Knowns and Known Unknowns. Kidney Int. 2024, 105, 684–701. [Google Scholar] [CrossRef]
- Uitrakul, S.; Aksonnam, K.; Srivichai, P.; Wicheannarat, S.; Incomenoy, S. The Incidence and Risk Factors of Urinary Tract Infection in Patients with Type 2 Diabetes Mellitus Using SGLT2 Inhibitors: A Real-World Observational Study. Medicines 2022, 9, 59. [Google Scholar] [CrossRef]
- Aydin, A.; Ahmed, K.; Zaman, I.; Khan, M.S.; Dasgupta, P. Recurrent Urinary Tract Infections in Women. Int. Urogynecol. J. 2015, 26, 795–804. [Google Scholar] [CrossRef]
- Myers, G.L. Recommendations for Improving Serum Creatinine Measurement: A Report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin. Chem. 2006, 52, 5–18. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S20–S42. [Google Scholar] [CrossRef]
- Attallah, N.; Yassine, L. Use of Empagliflozin in Recipients of Kidney Transplant: A Report of 8 Cases. Transpl. Proc. 2019, 51, 3275–3280. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-Y.; Chang, L.-Y.; Chen, J.-Y.; Pan, H.-C.; Tseng, C.-S.; Chueh, J.S.; Wu, V.-C. The Outcomes of SGLT-2 Inhibitor Utilization in Diabetic Kidney Transplant Recipients. Nat. Commun. 2024, 15, 10043. [Google Scholar] [CrossRef]
- Quilis, A.; Gavela, E.; Kánter, J.; Castro-Alonso, C.; Calatayud, E.; Vivó, E.; Parra, M.; Gandia, P.; Sancho, A. Use of SGLT2i in Non-Diabetic Kidney Transplant Recipients. Transpl. Proc. 2025, 57, 24–26. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kwon, S.; Jeon, Y.; Kim, Y.H.; Kwon, H.; Kim, Y.S.; Lee, H.; Kim, Y.-L.; Kim, C.-D.; Park, S.-H.; et al. The Efficacy and Safety of SGLT2 Inhibitor in Diabetic Kidney Transplant Recipients. Transplantation 2022, 106, e404–e412. [Google Scholar] [CrossRef] [PubMed]
- Mahling, M.; Schork, A.; Nadalin, S.; Fritsche, A.; Heyne, N.; Guthoff, M. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibition in Kidney Transplant Recipients with Diabetes Mellitus. Kidney Blood Press. Res. 2019, 44, 984–992. [Google Scholar] [CrossRef]
- Knoll, G.A. Proteinuria in Kidney Transplant Recipients: Prevalence, Prognosis, and Evidence-Based Management. Am. J. Kidney Dis. 2009, 54, 1131–1144. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Filippidis, G.; Efthymiadi, M.; Liakopoulos, V.; Stefanidis, I. Dapagliflozin Prevents High-Glucose-Induced Cellular Senescence in Renal Tubular Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 16107. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Golfinopoulos, S.; Efthymiadi, M.; Poulianiti, C.; Polyzou Konsta, M.A.; Liakopoulos, V.; Stefanidis, I. Routes of Albumin Overload Toxicity in Renal Tubular Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 9640. [Google Scholar] [CrossRef]
- Lu, D.-F.; Zheng, R.; Li, A.; Zhang, J.-Q. Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists on Proteinuria and Weight in a Diabetes Cohort. World J. Diabetes 2025, 16, 98552. [Google Scholar] [CrossRef]
- Praga, M.; Morales, E. Weight Loss and Proteinuria. Contrib. Nephrol. 2006, 151, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Gong, Y.; Zhen, Q.; Gu, L.; Yang, J.; Kang, M.; Zhang, A.; Shen, T.; Wang, Y.; Liu, F.; et al. Effect of Weight Loss on Proteinuria in Adults with Type 2 Diabetes: A Real-World Study. Diabetes Res. Clin. Pract. 2023, 206, 111021. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, E.; Bruschi, M.; Candiano, G.; Boccardi, C.; Citti, L.; Mangraviti, S.; Rosso, G.; Larti, A.; Rosati, A.; Ghiggeri, G.M.; et al. Posttransplant Proteinuria Associated with Everolimus. Transpl. Proc. 2009, 41, 1216–1217. [Google Scholar] [CrossRef] [PubMed]
| Number of patients | 64 |
| Men, % | 82.8% |
| Age, years | 55.1 (41.8–62.1) |
| Primary Kidney Disease | |
| Unknown, % | 40.6% |
| Glomerular, % | 35.9% |
| Genetic, % | 10.9% |
| Interstitial, % | 1.6% |
| Metabolic, % | 1.6% |
| Other, % | 9.4% |
| Immunosuppressive Therapy | |
| Cyclosporine, % | 31.3% |
| Tacrolimus, % | 64.1% |
| Everolimus, % | 23.5% |
| Mycophenolate, % | 70.3% |
| Steroid, % | 95.3% |
| Transplant Age, years | 11.1 ± 8.4 |
| Obesity, % | 31.3% |
| Diabetes, % | 29.7% |
| PTDM, % (of diabetics) | 52.6% |
| Impaired Fasting Glucose | 12.5% |
| T0 (n = 64) | T1 (n = 64) | T2 (n = 64) | |
|---|---|---|---|
| Urinary protein/creatinine, mg/g | 383 (188–1461) | † 376 (148–940) ** | ‡ 418 (211–778) ns |
| Urinary albumin/creatinine, mg/g | 69 (13–385) | † 45 (12–252) * | ‡ 64 (14–214) ns |
| Urinary non-albumin/creatinine, mg/g | 328 (145–945) | † 260 (127–615) * | ‡ 308 (160–627) ns |
| 24 h urinary proteins, mg | 601 (243–1860) | † 472 (200–1209) *** | ‡ 492 (216–1078) ns |
| 24 h urinary albumin, mg | 97 (15–449) | † 59 (12–290) * | ‡ 60 (14–316) ns |
| 24 h urinary non-albumin proteins, mg | 520 (178–1200) | † 344 (176–888) *** | ‡ 389 (164–842) ns |
| T0 (n = 64) | T1 (n = 64) | T2 (n = 64) | |
|---|---|---|---|
| Body weight, kg | 81 ± 16 | † 80 ± 16 * | ‡ 80 ± 15 * |
| Systolic blood pressure, mmHg | 139 ± 15 | † 133 ± 13 ** | ‡ 134 ± 16 ns |
| Diastolic blood pressure, mmHg | 83 ± 10 | † 80 ± 9 ** | ‡ 78 ± 9 ns |
| Serum creatinine, mg/dL | 1.47 ± 0.45 | † 1.54 ± 0.52 * | ‡ 1.6 ± 0.60 * |
| eGFR, mL/min/1.73 m2 | 56 ± 18 | † 53 ± 19 ns | ‡ 52 ± 20 ns |
| Serum glucose, mg/dL | 108 ± 18 | † 103 ± 25 ns | ‡ 106 ± 28 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilancio, G.; Hamzeh, S.; Vecchione, N.; Russo, D.; Iacuzzo, C.; Apicella, L.; Di Pietro, R.A.; Vitale, P.; Amicone, M.; Pisani, A.; et al. Dapagliflozin’s Effects on Urinary Albumin and Non-Albumin Proteins in Diabetic and Non-Diabetic Kidney Transplant Recipients. Biomedicines 2025, 13, 1303. https://doi.org/10.3390/biomedicines13061303
Bilancio G, Hamzeh S, Vecchione N, Russo D, Iacuzzo C, Apicella L, Di Pietro RA, Vitale P, Amicone M, Pisani A, et al. Dapagliflozin’s Effects on Urinary Albumin and Non-Albumin Proteins in Diabetic and Non-Diabetic Kidney Transplant Recipients. Biomedicines. 2025; 13(6):1303. https://doi.org/10.3390/biomedicines13061303
Chicago/Turabian StyleBilancio, Giancarlo, Sarah Hamzeh, Nicoletta Vecchione, Dora Russo, Candida Iacuzzo, Luca Apicella, Renata Angela Di Pietro, Piercarla Vitale, Maria Amicone, Antonio Pisani, and et al. 2025. "Dapagliflozin’s Effects on Urinary Albumin and Non-Albumin Proteins in Diabetic and Non-Diabetic Kidney Transplant Recipients" Biomedicines 13, no. 6: 1303. https://doi.org/10.3390/biomedicines13061303
APA StyleBilancio, G., Hamzeh, S., Vecchione, N., Russo, D., Iacuzzo, C., Apicella, L., Di Pietro, R. A., Vitale, P., Amicone, M., Pisani, A., Cirillo, M., & Secondulfo, C. (2025). Dapagliflozin’s Effects on Urinary Albumin and Non-Albumin Proteins in Diabetic and Non-Diabetic Kidney Transplant Recipients. Biomedicines, 13(6), 1303. https://doi.org/10.3390/biomedicines13061303









