Segmental Pulse Volume Recordings at the Forefoot Level as a Valuable Diagnostic Tool for Detection of Peripheral Arterial Disease in the Diabetic Foot Syndrome
Abstract
1. Introduction
2. Patients and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Clinical Characterization
2.3. Segmental Pulse Volume Recordings
2.4. Digital Subtraction Angiography
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics (Patient-Based Analysis)
3.2. Clinical and Hemodynamic Characteristics (Limb-Based Analysis)
3.3. Angiographic Characteristics (Limb-Based Analysis)
3.4. Pulse Volume Recordings (Limb-Based Analysis)
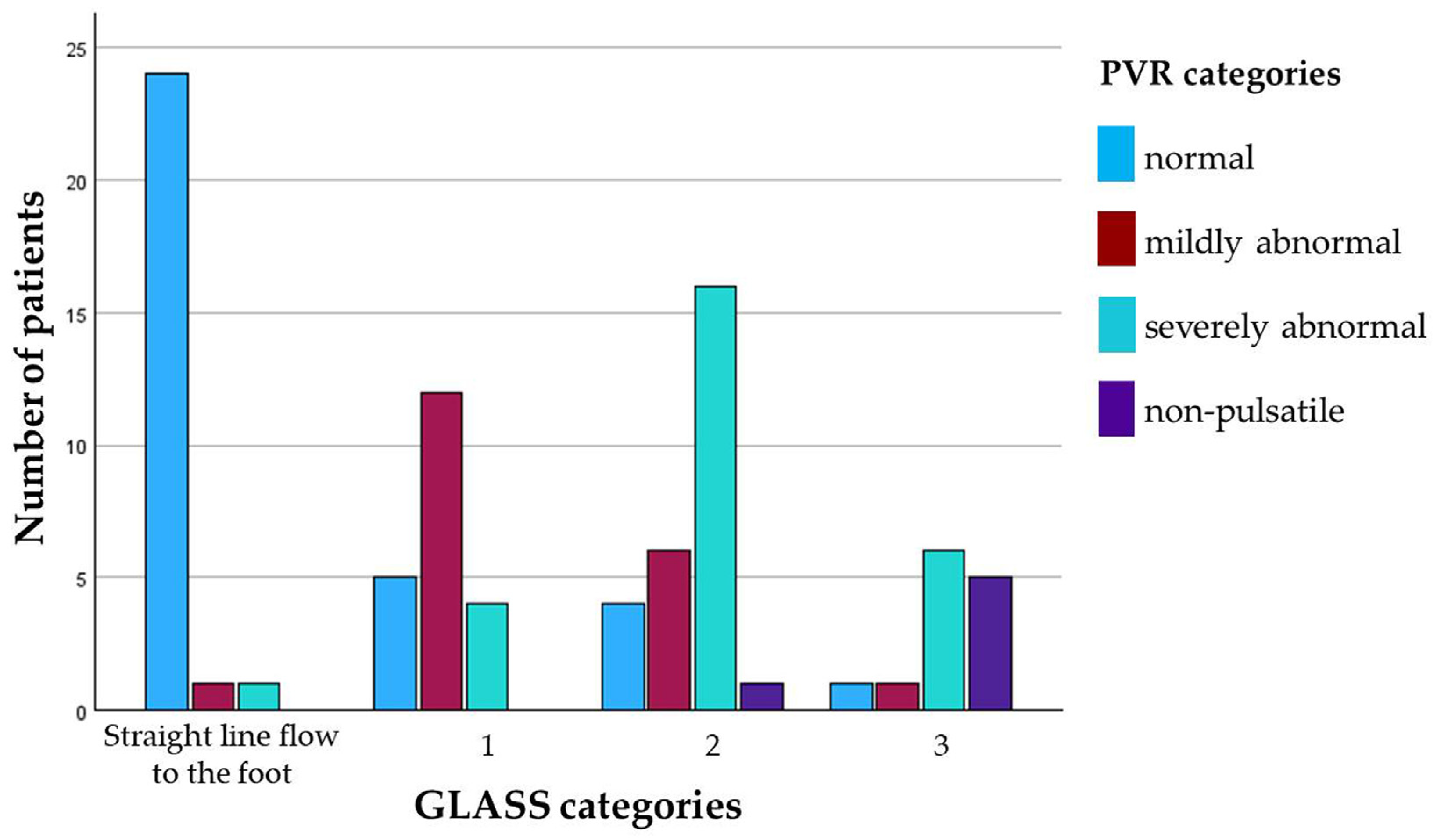
3.4.1. Visual Semiquantitative Analysis
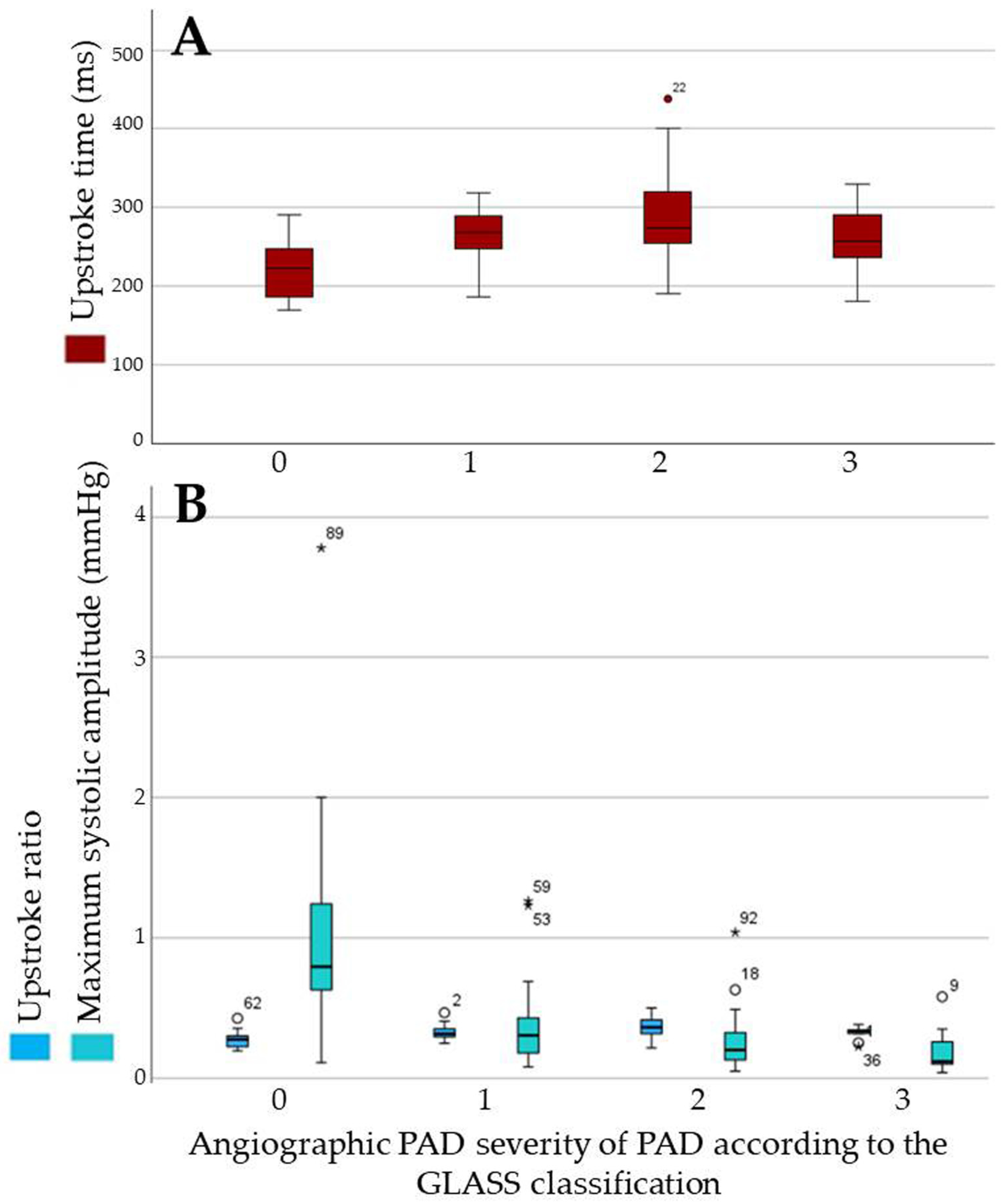
3.4.2. Quantitative Parameters
3.4.3. Subgroup Analysis: Quantitative Parameters in Legs with ABI Values ≥ 1.3
3.4.4. Subgroup Analysis: Quantitative Parameters in Limbs Affected by Foot Infection
3.4.5. Sensitivity to Change After Revascularization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; E Sheffer, K.; Carrillo-Larco, R.M.; E Bennett, J.; E Shaw, J.; Paciorek, C.J.; Singleton, R.K.; Pires, A.B.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.A.; Mills, J.L.; Armstrong, D.G.; Conte, M.S.; Kirsner, R.S.; Minc, S.D.; Plutzky, J.; Southerland, K.W.; Tomic-Canic, M. Current Status and Principles for the Treatment and Prevention of Diabetic Foot Ulcers in the Cardiovascular Patient Population: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e232–e253. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2019, 58, S1–S109.e33. [Google Scholar] [CrossRef]
- Fitridge, R.; Chuter, V.; Mills, J.; Hinchliffe, R.; Azuma, N.; Behrendt, C.A.; Boyko, E.J.; Conte, M.S.; Humphries, M.; Kirksey, L.; et al. The intersocietal IWGDF, ESVS, SVS guidelines on peripheral artery disease in people with diabetes and a foot ulcer. Diabetes Metab. Res. Rev. 2024, 40, e3686. [Google Scholar] [CrossRef]
- Chuter, V.; Schaper, N.; Hinchliffe, R.; Mills, J.; Azuma, N.; Behrendt, C.A.; Boyko, E.J.; Conte, M.S.; Humphries, M.; Kirksey, L.; et al. Performance of non-invasive bedside vascular testing in the prediction of wound healing or amputation among people with foot ulcers in diabetes: A systematic review. Diabetes Metab. Res. Rev. 2024, 40, e3701. [Google Scholar] [CrossRef]
- Cao, P.; Eckstein, H.H.; De Rango, P.; Setacci, C.; Ricco, J.B.; de Donato, G.; Becker, F.; Robert-Ebadi, H.; Diehm, N.; Schmidli, J.; et al. Chapter II: Diagnostic methods. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2011, 42 (Suppl. S2), S13–S32. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef]
- Chuter, V.; Schaper, N.; Mills, J.; Hinchliffe, R.; Russell, D.; Azuma, N.; Behrendt, C.A.; Boyko, E.J.; Conte, M.S.; Humphries, M.; et al. Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: A systematic review. Diabetes Metab. Res. Rev. 2024, 40, e3683. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Early, K.B.; Bruemmer, D.; Ebekozien, O.; Echouffo-Tcheugui, J.B.; et al. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.W., Jr. The dysvascular foot: A system for diagnosis and treatment. Foot Ankle 1981, 2, 64–122. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.; Gardin, J.M.; Jaff, M.; Mohler, E.; Roman, M.; Naqvi, T.Z. Guidelines for noninvasive vascular laboratory testing: A report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology. Vasc. Med. 2006, 11, 183–200. [Google Scholar] [CrossRef]
- Diehm, N.; Shang, A.; Silvestro, A.; Do, D.D.; Dick, F.; Schmidli, J.; Mahler, F.; Baumgartner, I. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2006, 31, 59–63. [Google Scholar] [CrossRef]
- Lanzer, P.; Hannan, F.M.; Lanzer, J.D.; Janzen, J.; Raggi, P.; Furniss, D.; Schuchardt, M.; Thakker, R.; Fok, P.W.; Saez-Rodriguez, J.; et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1145–1165. [Google Scholar] [CrossRef]
- Edmonds, M.E.; Morrison, N.; Laws, J.W.; Watkins, P.J. Medial arterial calcification and diabetic neuropathy. Br. Med. J. Clin. Res. Ed. 1982, 284, 928–930. [Google Scholar] [CrossRef]
- Bourron, O.; Aubert, C.E.; Liabeuf, S.; Cluzel, P.; Lajat-Kiss, F.; Dadon, M.; Komajda, M.; Mentaverri, R.; Brazier, M.; Pierucci, A.; et al. Below-knee arterial calcification in type 2 diabetes: Association with receptor activator of nuclear factor κB ligand, osteoprotegerin, and neuropathy. J. Clin. Endocrinol. Metab. 2014, 99, 4250–4258. [Google Scholar] [CrossRef]
- Normahani, P.; Poushpas, S.; Alaa, M.; Bravis, V.; Sounderajah, V.; Aslam, M.; Jaffer, U. Diagnostic Accuracy of Point-of-care Tests Used to Detect Arterial Disease in Diabetes: TEsting for Arterial Disease in Diabetes (TrEAD) Study. Ann. Surg. 2022, 276, e605–e612. [Google Scholar] [CrossRef]
- Elghazaly, H.; Howard, T.; Sanjay, S.; Mohamed, O.G.; Sounderajah, V.; Mehar, Z.; Davies, A.H.; Jaffer, U.; Normahani, P. Evaluating the prognostic performance of bedside tests used for peripheral arterial disease diagnosis in the prediction of diabetic foot ulcer healing. BMJ Open Diabetes Res. Care 2023, 11, e003110. [Google Scholar] [CrossRef]
- Darling, R.C.; Raines, J.K.; Brener, B.J.; Austen, W.G. Quantitative segmental pulse volume recorder: A clinical tool. Surgery 1972, 72, 873–877. [Google Scholar] [PubMed]
- Symes, J.F.; Graham, A.M.; Mousseau, M. Doppler waveform analysis versus segmental pressure and pulse-volume recording: Assessment of occlusive disease in the lower extremity. Can. J. Surgery. J. Can. Chir. 1984, 27, 345–347. [Google Scholar]
- Gibbons, G.W.; Wheelock, F.C., Jr.; Siembieda, C.; Hoar, C.S., Jr.; Rowbotham, J.L.; Persson, A.B. Noninvasive prediction of amputation level in diabetic patients. Arch. Surg. 1979, 114, 1253–1257. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Lowenstein, D.H.; Klein, M.F. Combining segmental systolic pressures and plethysmography to diagnose arterial occlusive disease of the legs. Am. J. Surg. 1979, 138, 211–218. [Google Scholar] [CrossRef]
- Randhawa, M.S.; Reed, G.W.; Grafmiller, K.; Gornik, H.L.; Shishehbor, M.H. Prevalence of Tibial Artery and Pedal Arch Patency by Angiography in Patients with Critical Limb Ischemia and Noncompressible Ankle Brachial Index. Circ. Cardiovasc. Interv. 2017, 10, e004605. [Google Scholar] [CrossRef]
- Czihal, M.; Hoffmann, U. Letter by Czihal and Hoffmann Regarding Article, “Prevalence of Tibial Artery and Pedal Arch Patency by Angiography in Patients with Critical Limb Ischemia and Noncompressible Ankle Brachial Index”. Circ. Cardiovasc. Interv. 2017, 10, e005702. [Google Scholar] [CrossRef]
- Babaei, M.R.; Malek, M.; Rostami, F.T.; Emami, Z.; Madani, N.H.; Khamseh, M.E. Non-invasive vascular assessment in people with type 2 diabetes: Diagnostic performance of Plethysmographic-and-Doppler derived ankle brachial index, toe brachial index, and pulse volume wave analysis for detection of peripheral arterial disease. Prim. Care Diabetes 2020, 14, 282–289. [Google Scholar] [CrossRef]
- Meyr, A.J.; Mateen, S.; Skolnik, J.; Choi, E.T. Approximation of the Ankle-Brachial Index in the Setting of Medial Arterial Calcific Sclerosis. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2022, 61, 314–317. [Google Scholar] [CrossRef]
- Mateen, S.; Skolnik, J.; Oresanya, L.; Choi, E.T.; Meyr, A.J. Responsiveness and Inter-Rater Reliability of the Pulse Volume Recording Upstroke Ratio (PVRr). J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2022, 61, 486–489. [Google Scholar] [CrossRef]




| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patients with diabetes mellitus suffering from nontraumatic foot wounds | Patients with diabetic foot syndrome, Wagner stage 5 (necrosis of the complete foot) |
| Invasive angiography with complete depiction of the femoral, popliteal, below-the-knee, and below-the-ankle arteries | Patients with peripheral arterial disease without foot wounds, Rutherford categories I–IV |
| Pulse volume recordings of the forefoot within 4 weeks prior to invasive angiography | Patients with peripheral arterial disease affecting the iliac artery inflow |
| Patients with nonatherosclerotic peripheral arterial disease | |
| Patients with acute limb ischemia | |
| Patients with nontraumatic foot wounds not suffering from diabetes mellitus | |
| Patients suffering from traumatic foot wounds | |
| Patients with prior amputation proximal to the toe level | |
| Patients with non-analyzable PVRs due to motion artifacts |
| Variable | Limb-Based Analysis n = 90 |
|---|---|
| Known peripheral arterial disease, n (%) | 56 (62.2) |
| Prior lower extremity artery revascularization, n (%) | 40 (44.4) |
| Known peripheral neuropathy, n (%) | 49 (54.4) |
| Righ/left leg affected by diabetic foot ulcer, n (%) | 44 (48.9)/46 (51.1) |
| Diabetic foot ulcer duration, weeks (mean ± SD) | 18.9 ± 39.3 |
| Foot pulses absent in the affected leg, n (%) | 63 (70) |
| Clinical suspicion of ischemia, n (%) | 47 (52.2) |
| ABI < 0.9/≥1.3, n (%) * | 19 (21.1)/49 (54.4) |
| Clinical signs and symptoms of infection, n (%) | 59 (65.6%) |
| Wound swab positive, n (%) | 48 (53.3) |
| Radiographic suspicion of osteomyelitis, n (%) | 30 (66.7) |
| IMD | Straight-Line Inflow to the Foot | GLASS I | GLASS II | GLASS III |
|---|---|---|---|---|
| P0 | n = 18 | n = 13 | n = 16 * | n = 3 |
| UST 221 ± 30 ms | UST 271 ± 38 ms | UST 291 ± 51 ms | UST 236 ± 22 ms | |
| USR 0.28 ± 0.05 | USR 0.32 ± 0.03 | USR 0.37 ± 0.08 | USR 0.29 ± 0.06 | |
| MSA 1.10 ± 0.78 mmHg | MSA 0.47 ± 0.38 mmHg | MSA 0.31 ± 0.26 mmHg | MSA 0.16 ± 0.16 mmHg | |
| P1 | n = 7 | n = 7 | n = 9 | n = 11 # |
| UST 215 ± 41 ms | UST 264 ± 38 ms | UST 296 ± 68 ms | UST 276 ± 53 ms | |
| USR 0.25 ± 0.03 | USR 0.33 ± 0.08 | USR 0.37 ± 0.06 | USR 0.33 ± 0.04 | |
| MSA 0.66 ± 0.48 mmHg | MSA 0.30 ± 0.14 mmHg | MSA 0.29 ± 0.05 mmHg | MSA 0.21 ± 0.19 mmHg | |
| P2 | n = 1 | n = 2 | n = 3 | n = 0 |
| UST 291 ms | UST 231 ± 46 ms | UST 277 ± 97 ms | ||
| USR 0.43 | USR 0.34 ± 0.11 | USR 0.33 ± 0.03 | ||
| MSA 0.65 mmHg | MSA 0.17 ± 0.01 mmHg | MSA 0.29 ± 0.05 mmHg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nützel, A.; Reik, L.J.U.; Hamberger, M.; Lottspeich, C.; Deniz, S.; Löw, A.; Schneider, H.; Polzer, H.; Baumbach, S.; Czihal, M. Segmental Pulse Volume Recordings at the Forefoot Level as a Valuable Diagnostic Tool for Detection of Peripheral Arterial Disease in the Diabetic Foot Syndrome. Biomedicines 2025, 13, 1281. https://doi.org/10.3390/biomedicines13061281
Nützel A, Reik LJU, Hamberger M, Lottspeich C, Deniz S, Löw A, Schneider H, Polzer H, Baumbach S, Czihal M. Segmental Pulse Volume Recordings at the Forefoot Level as a Valuable Diagnostic Tool for Detection of Peripheral Arterial Disease in the Diabetic Foot Syndrome. Biomedicines. 2025; 13(6):1281. https://doi.org/10.3390/biomedicines13061281
Chicago/Turabian StyleNützel, Andreas, Lilly Juliane Undine Reik, Maximilian Hamberger, Christian Lottspeich, Sinan Deniz, Anja Löw, Holger Schneider, Hans Polzer, Sebastian Baumbach, and Michael Czihal. 2025. "Segmental Pulse Volume Recordings at the Forefoot Level as a Valuable Diagnostic Tool for Detection of Peripheral Arterial Disease in the Diabetic Foot Syndrome" Biomedicines 13, no. 6: 1281. https://doi.org/10.3390/biomedicines13061281
APA StyleNützel, A., Reik, L. J. U., Hamberger, M., Lottspeich, C., Deniz, S., Löw, A., Schneider, H., Polzer, H., Baumbach, S., & Czihal, M. (2025). Segmental Pulse Volume Recordings at the Forefoot Level as a Valuable Diagnostic Tool for Detection of Peripheral Arterial Disease in the Diabetic Foot Syndrome. Biomedicines, 13(6), 1281. https://doi.org/10.3390/biomedicines13061281







