Elevated Expression of TGFB1 in PBMCs Is Associated with Intracranial Aneurysm Formation, but TGFB3 Expression Implicated Rupture
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Sample Collection and Storage
2.3. Peripheral Blood Mononuclear Cell (PBMCs) Isolation Using Density Gradient Centrifugation with Ficoll Histopaque
- Measured 3 mL of Ficoll Histopaque into each of three 10 mL centrifuge tubes.
- Gently layered 3 mL of blood on top of the Ficoll Histopaque using a 1 mL automatic pipette, layering very slowly to ensure that the blood and Ficoll Histopaque remained as two distinct layers.
- Centrifuged the tubes (without delay) for 30 min at 400× g at 20 °C.
- Aspirated the whitish buffy coat (approximately 1.5 mL) of PBMCs that formed at the interface between the Ficoll Histopaque and medium.
- Washed the PBMCs three times by centrifuging at 250× g for 10 min each time with 10 mL of sterile PBS.
- Added 600 µL of RTL buffer with β-mercaptoethanol to the PBMCs, mixed, divided into two Eppendorf tubes, and frozen at −80 °C until RNA isolation.
2.4. Bio-Plex TGFβ Assays
2.5. RNA Isolation
2.6. Reverse Transcription
2.7. Real-Time RT-PCR
2.8. Statistical Analysis
3. Results
3.1. Demographic Data and Results of Routine Laboratory Tests for Each Group
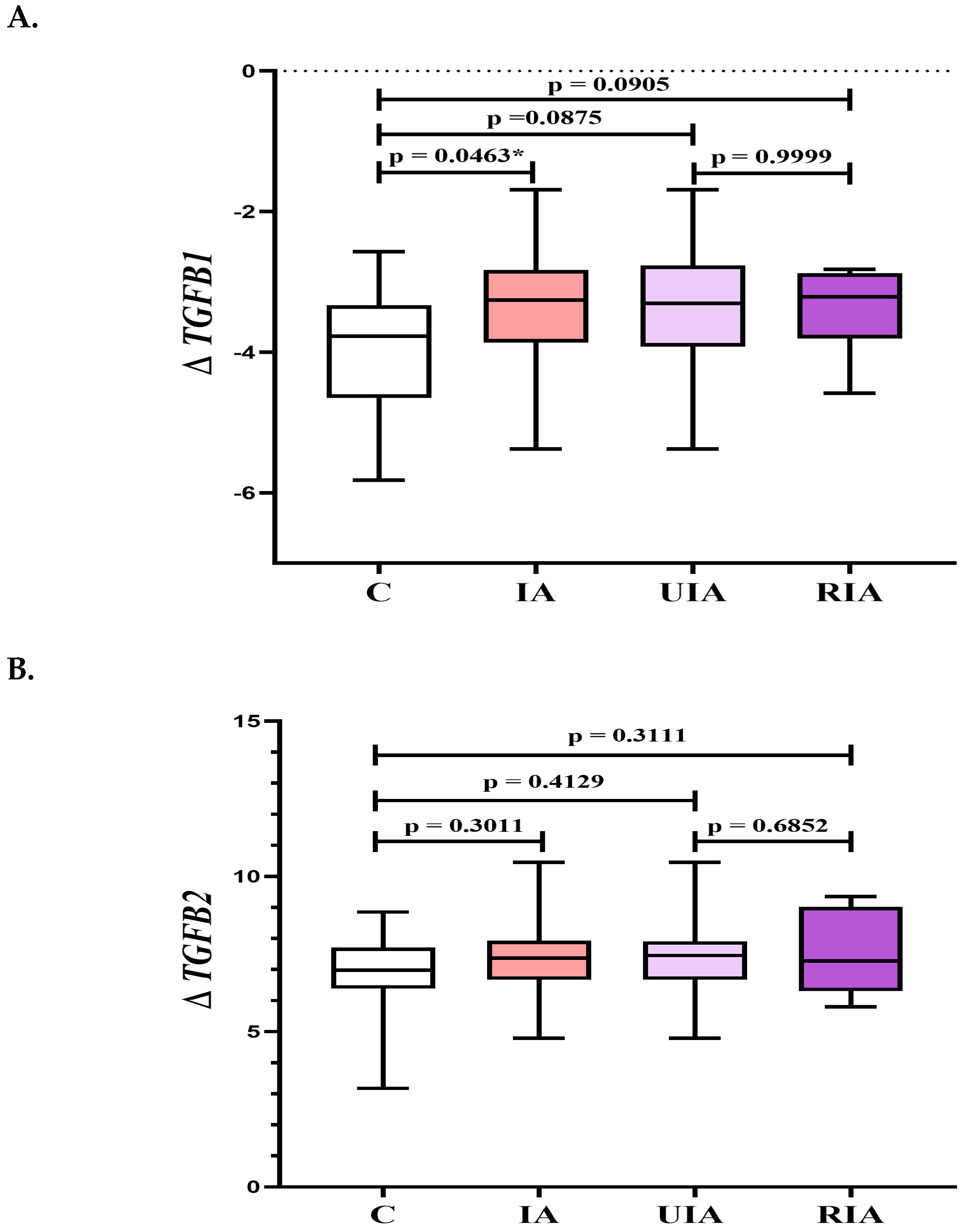
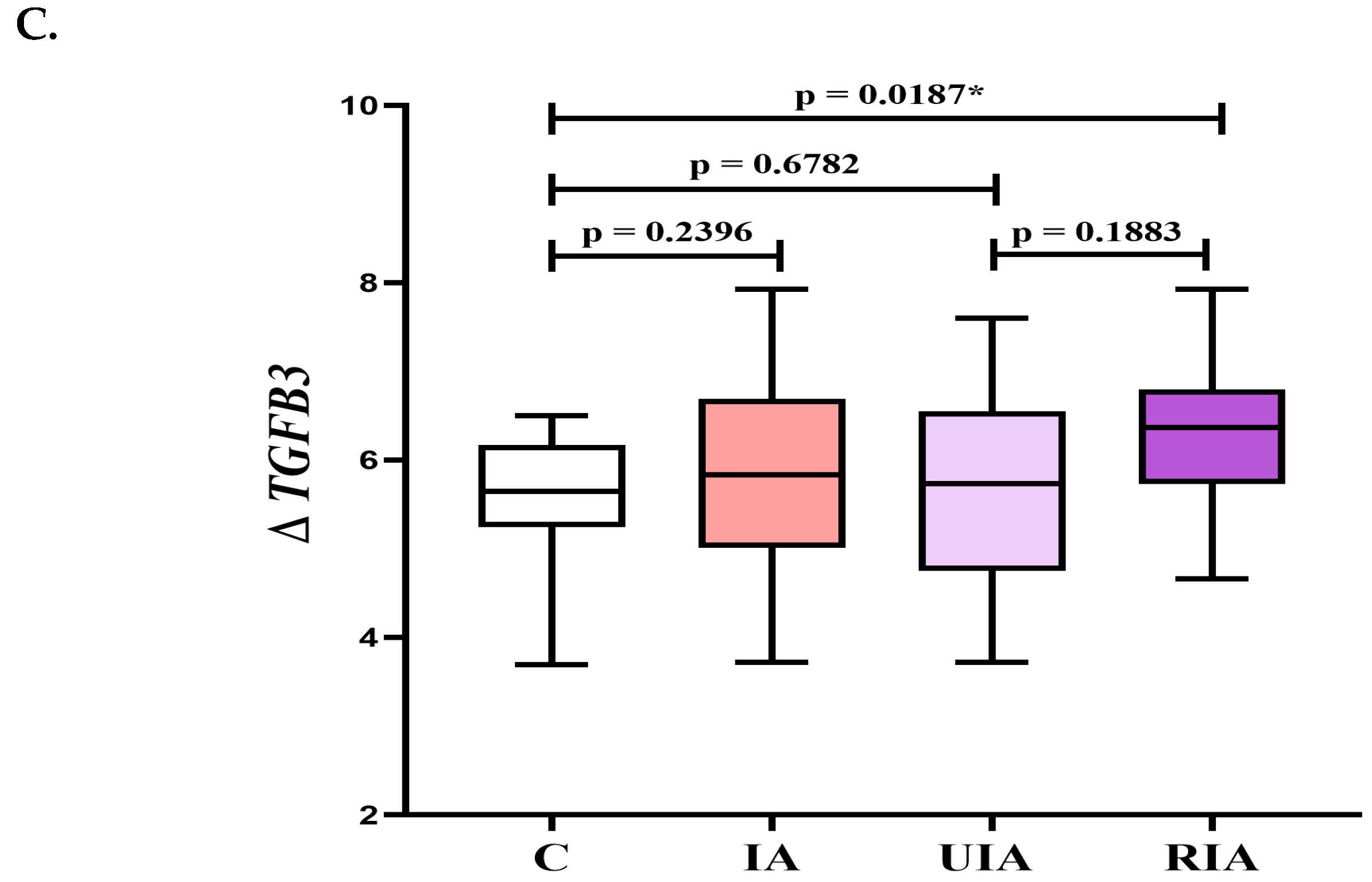
3.2. Gene Expression Profiles of TGFB Factors in PBMCs: TGFB1 and TGFB3 as Indicators of Aneurysm Formation and Rupture
3.3. Increased Serum TGF-β1 and TGF-β3 Concentrations as Promising Noninvasive Parameters for Assessing the Risk of Aneurysm Rupture
3.4. Serum TGF-β1 and TGF-β3 Levels Are Associated with the Risk Factors for Intracranial Aneurysms
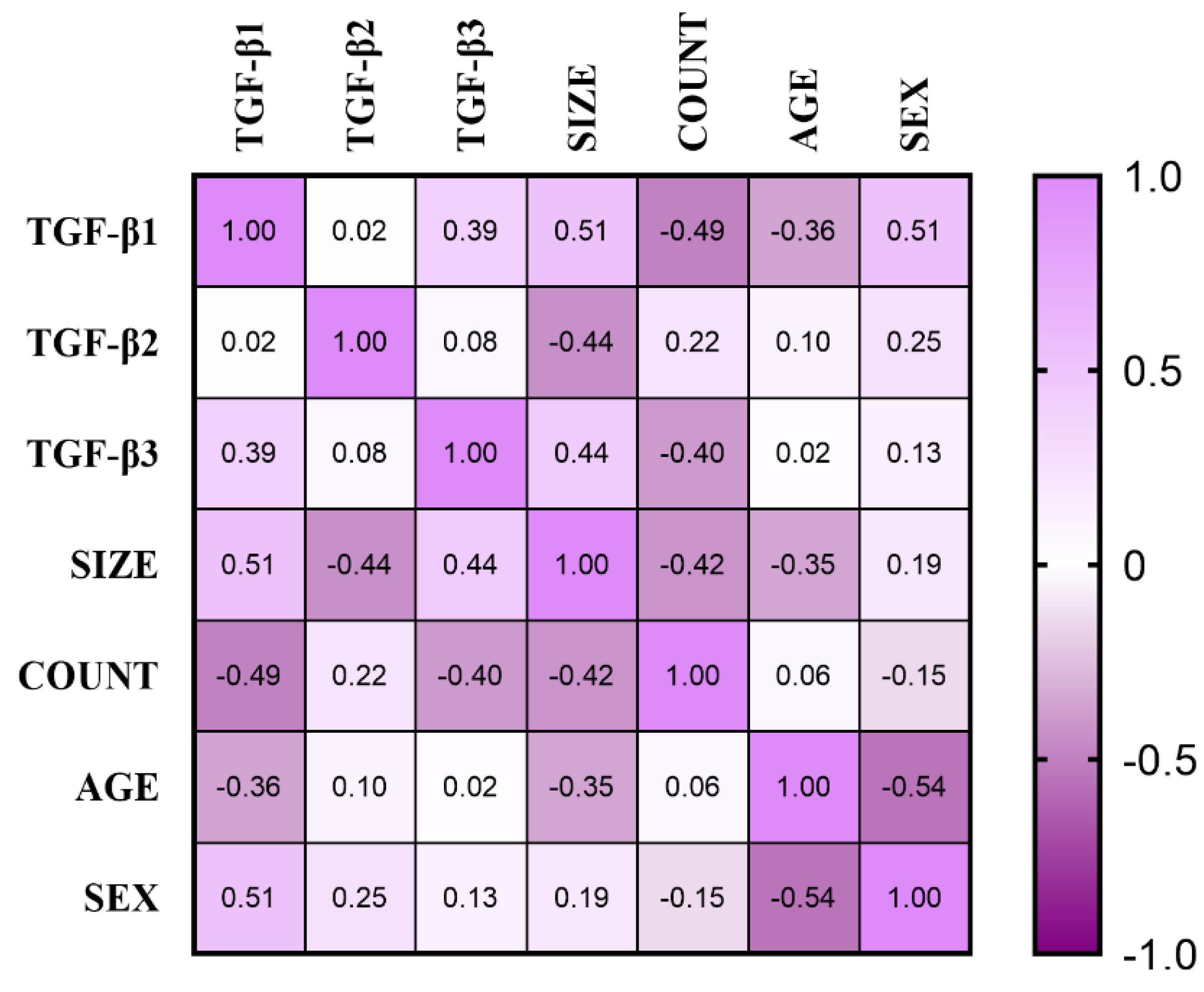
3.5. Correlation Coefficient Results in Unruptured Intracranial Aneurysm (UIA) Group
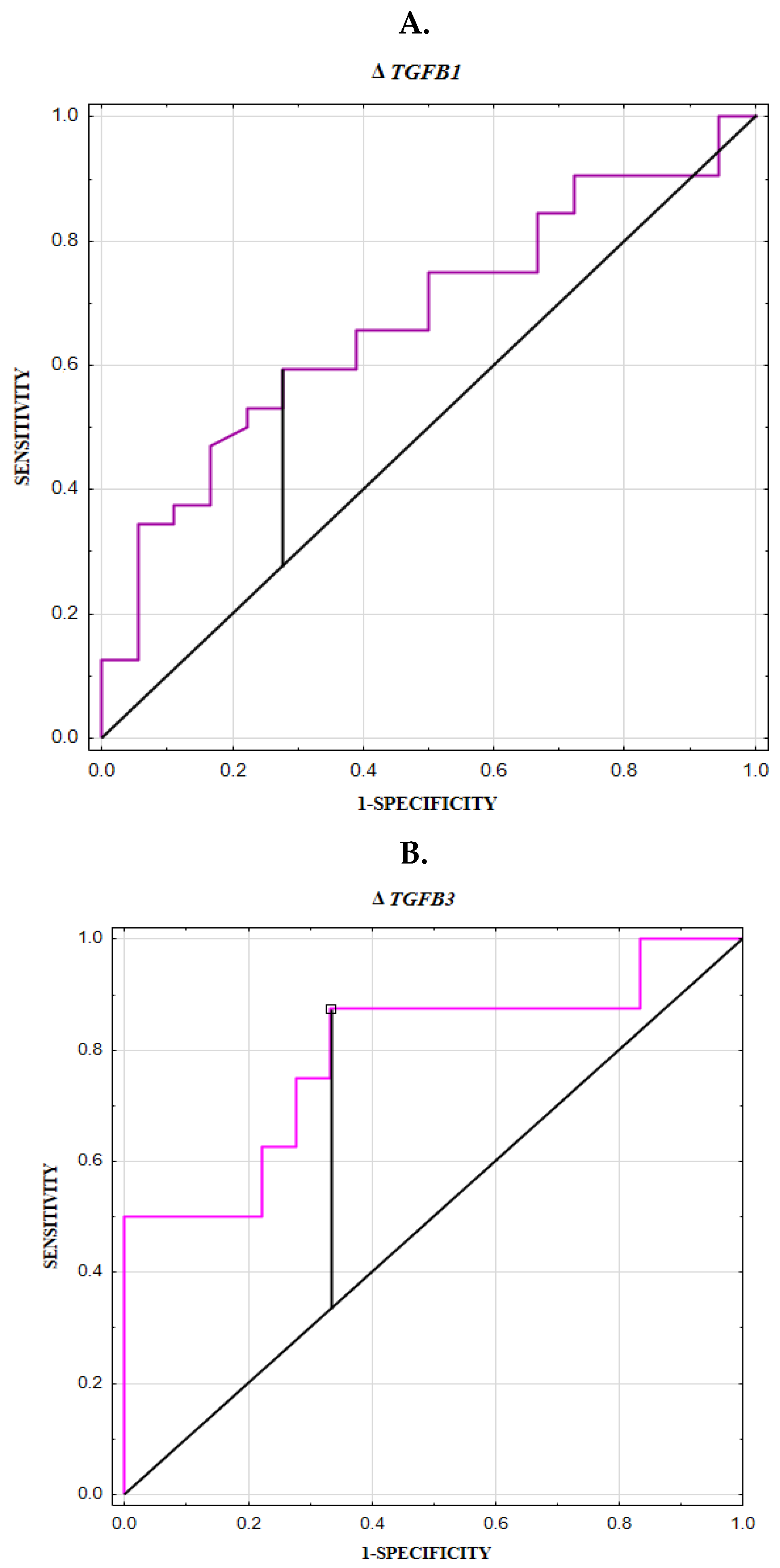
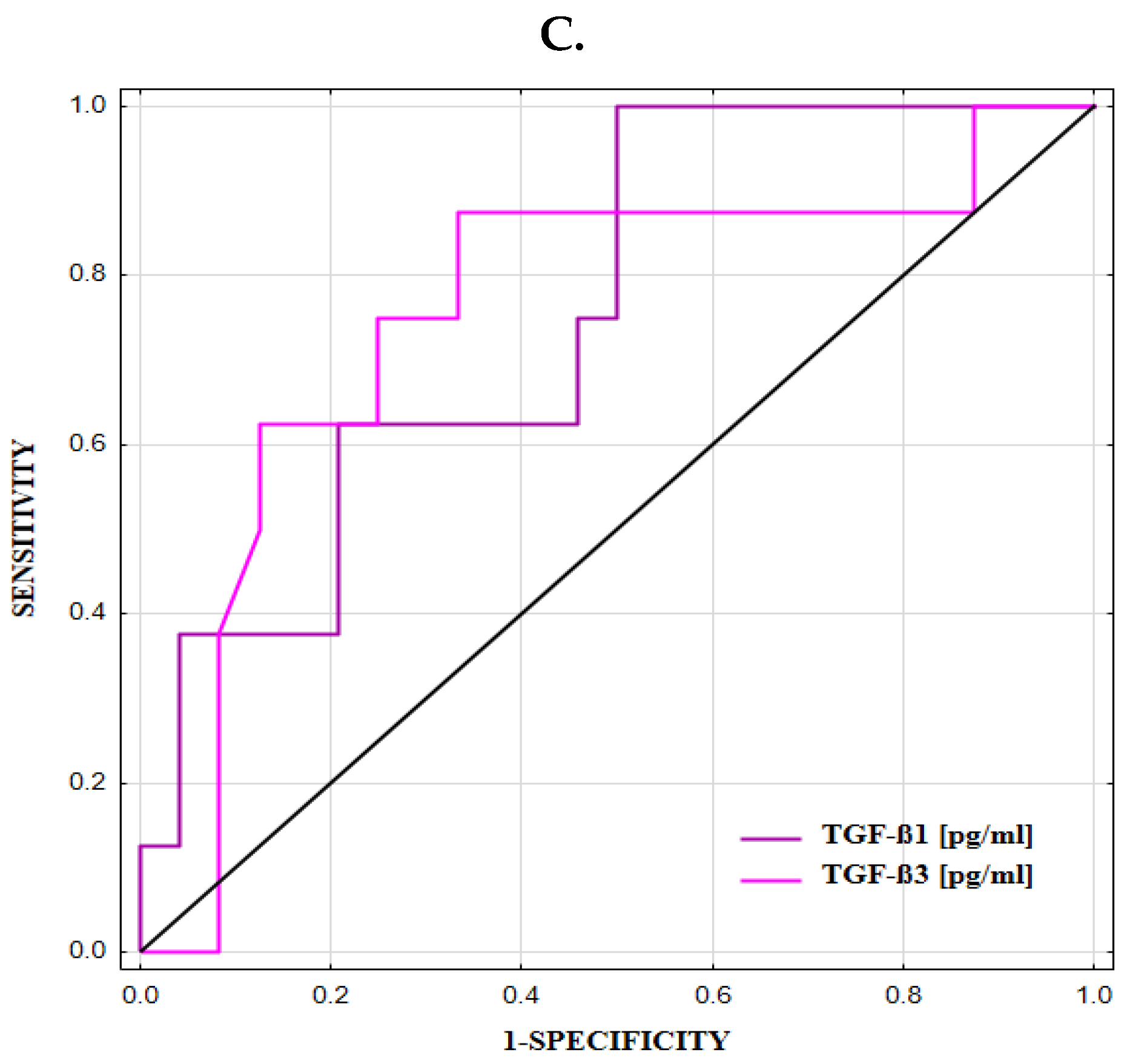
3.6. ROC Curve for TGFB1 and TGFB3 Expression and Concentrations
4. Discussion
Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | diagnostic accuracy |
| APTT | activated partial thromboplastin time |
| AUC | area under the ROC curve |
| cDNA | complementary DNA |
| Cut-off | (cut-off based on the highest Youden index) |
| ECs | endothelial cells |
| EEL | external elastic lamina |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HIF1A-AS1 | hypoxia-inducible factor 1α-antisense RNA |
| IA | intracranial aneurysm |
| IEL | internal elastic lamina |
| INR | international normalized ratio |
| K+ | potassium ion |
| MCV | mean corpuscular volume |
| mRNA | messenger RNA |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NOX4 | NADPH oxidase 4 |
| NPV | negative predictive value |
| PBMCs | peripheral blood mononuclear cells |
| PPV | positive predictive value |
| PT | prothrombin time |
| RBC | red blood cell count |
| RIA | ruptured intracranial aneurysm |
| RNA | ribonucleid acid |
| SAH | subarachnoid hemorrhage |
| Se | diagnostic sensitivity |
| SE | standard error |
| Smad | similar to mother against decapentaplegic |
| SMCs | smooth muscle cells |
| Sp | diagnostic specificity |
| SPARC | secreted protein acidic and rich in cysteine |
| TGF-β | transforming growth factor β |
| TβR | TGF-β receptor |
| UIA | unruptured intracranial aneurysm |
| WBC | white blood cell count |
References
- Ingall, T.; Asplund, K.; Mähönen, M.; Bonita, R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 2000, 31, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Østbye, T.; Levy, A.R.; Mayo, N.E. Hospitalization and Case-Fatality Rates for Subarachnoid Hemorrhage in Canada from 1982 Through 1991. Stroke 1997, 28, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Rui, Y.-N.; Hagan, J.P.; Kim, D.H. Intracranial Aneurysms: Pathology, Genetics, and Molecular Mechanisms. NeuroMolecular Med. 2019, 21, 325–343. [Google Scholar] [CrossRef]
- Etminan, N.; Rinkel, G.J. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat. Rev. Neurol. 2016, 12, 699–713. [Google Scholar] [CrossRef]
- Shih, S.-C.; Ju, M.; Liu, N.; Mo, J.-R.; Ney, J.J.; Smith, L.E.H. Transforming growth factor β1 induction of vascular endothelial growth factor receptor 1: Mechanism of pericyte-induced vascular survival in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 15859–15864. [Google Scholar] [CrossRef]
- Song, Y.; Liu, P.; Li, Z.; Shi, Y.; Huang, J.; Li, S.; Liu, Y.; Zhang, Z.; Wang, Y.; Zhu, W.; et al. The Effect of Myosin Light Chain Kinase on the Occurrence and Development of Intracranial Aneurysm. Front. Cell. Neurosci. 2018, 12, 416. [Google Scholar] [CrossRef]
- Kamińska, J.; Lyson, T.; Chrzanowski, R.; Sawicki, K.; Milewska, A.J.; Tylicka, M.; Zińczuk, J.; Matowicka-Karna, J.; Dymicka-Piekarska, V.; Mariak, Z.; et al. Ratio of IL-8 in CSF Versus Serum Is Elevated in Patients with Unruptured Brain Aneurysm. J. Clin. Med. 2020, 9, 1761. [Google Scholar] [CrossRef]
- Kamińska, J.; Dymicka-Piekarska, V.; Chrzanowski, R.; Sawicki, K.; Milewska, A.J.; Zińczuk, J.; Tylicka, M.; Jadeszko, M.; Mariak, Z.; Kratz, E.M.; et al. IL-6 Quotient (The Ratio of Cerebrospinal Fluid IL-6 to Serum IL-6) as a Biomarker of an Unruptured Intracranial Aneurysm. J. Inflamm. Res. 2021, 14, 6103–6114. [Google Scholar] [CrossRef]
- Kamińska, J.; Maciejczyk, M.; Ćwiklińska, A.; Matowicka-Karna, J.; Koper-Lenkiewicz, O.M. Pro-Inflammatory and Anti-Inflammatory Cytokines Levels are Significantly Altered in Cerebrospinal Fluid of Unruptured Intracranial Aneurysm (UIA) Patients. J. Inflamm. Res. 2022, 15, 6245–6261. [Google Scholar] [CrossRef]
- Rodrigues, V.J.; Elsayed, S.; Loeys, B.L.; Dietz, H.C.; Yousem, D.M. Neuroradiologic Manifestations of Loeys-Dietz Syndrome Type 1. Am. J. Neuroradiol. 2009, 30, 1614–1619. [Google Scholar] [CrossRef]
- Vanakker, O.M.; Hemelsoet, D.; De Paepe, A. Hereditary Connective Tissue Diseases in Young Adult Stroke: A Comprehensive Synthesis. Stroke Res. Treat. 2011, 2011, 712903. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Brinjikji, W.; Kallmes, D.F. Prevalence of Intracranial Aneurysms in Patients with Connective Tissue Diseases: A Retrospective Study. Am. J. Neuroradiol. 2016, 37, 1422–1426. [Google Scholar] [CrossRef]
- Mattson, M.P.; Barger, S.W.; Furukawa, K.; Bruce, A.J.; Wyss-Coray, T.; Mark, R.J.; Mucke, L. Cellular signaling roles of TGFβ, TNFα and βAPP in brain injury responses and Alzheimer’s disease. Brain Res. Rev. 1997, 23, 47–61. [Google Scholar] [CrossRef]
- Agrawal, S.K. Aneurysm embolization with biologically active coils: An animal study. Neurol. Res. 2013, 35, 37–43. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.-K.L.; Flavell, R.A. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- de Caestecker, M. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev. 2004, 15, 1–11. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.G. TGF-β signaling from receptors to smads. Cold Spring Harb. Perspect. Biol. 2016, 8, 022061. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rodríguez-Vita, J.; Sanchez-Lopez, E.; Carvajal, G.; Egido, J. TGF-β signaling in vascular fibrosis. Cardiovasc. Res. 2007, 74, 196–206. [Google Scholar] [CrossRef]
- Rotzer, D.; Roth, M.; Lutz, M.; Lindemann, D.; Sebald, W.; Knaus, P. Type III TGF-β receptor-independent signalling of TGFβ2 via TβRII-B, an alternatively spliced TGF-β type II receptor. EMBO J. 2001, 20, 480–490. [Google Scholar] [CrossRef]
- Frösen, J.; Piippo, A.; Paetau, A.; Kangasniemi, M.; Niemelä, M.; Hernesniemi, J.; Jääskeläinen, J. Growth factor receptor expression and remodeling of saccular cerebral artery aneurysm walls: Implications for biological therapy preventing rupture. Neurosurgery 2006, 58, 534–541. [Google Scholar] [CrossRef]
- Tsai, S.; Hollenbeck, S.T.; Ryer, E.J.; Edlin, R.; Yamanouchi, D.; Kundi, R.; Wang, C.; Liu, B.; Kent, K.C. TGF-β through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am. J. Physiol. Circ. Physiol. 2009, 297, H540–H549. [Google Scholar] [CrossRef] [PubMed]
- Vincze, C.; Pál, G.; Wappler, E.A.; Szabó, É.R.; Nagy, Z.G.; Lovas, G.; Dobolyi, A. Distribution of mRNAs encoding transforming growth factors-β1, -2, and -3 in the intact rat brain and after experimentally induced focal ischemia. J. Comp. Neurol. 2010, 518, 3752–3770. [Google Scholar] [CrossRef] [PubMed]
- Supriya, M.; Christopher, R.; Devi, B.I.; Bhat, D.I.; Shukla, D.; Kalpana, S.R. Altered MicroRNA Expression in Intracranial Aneurysmal Tissues: Possible Role in TGF-β Signaling Pathway. Cell. Mol. Neurobiol. 2022, 42, 2393–2405. [Google Scholar] [CrossRef]
- Kataoka, K.; Taneda, M.; Asai, T.; Kinoshita, A.; Ito, M.; Kuroda, R. Structural Fragility and Inflammatory Response of Ruptured Cerebral Aneurysms. Stroke 1999, 30, 1396–1401. [Google Scholar] [CrossRef]
- Frösen, J.; Piippo, A.; Paetau, A.; Kangasniemi, M.; Niemelä, M.; Hernesniemi, J.; Jääskeläinen, J. Remodeling of Saccular Cerebral Artery Aneurysm Wall Is Associated with Rupture. Stroke 2004, 35, 2287–2293. [Google Scholar] [CrossRef]
- Travis, M.A.; Sheppard, D. TGF-β Activation and Function in Immunity. Annu. Rev. Immunol. 2014, 32, 51–82. [Google Scholar] [CrossRef]
- Liu, Z.; Ajimu, K.; Yalikun, N.; Zheng, Y.; Xu, F. Potential Therapeutic Strategies for Intracranial Aneurysms Targeting Aneurysm Pathogenesis. Front. Neurosci. 2019, 13, 1238. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The role of transforming growth factor (TGF)-β in the infarcted myocardium. J. Thorac. Dis. 2017, 9, S52–S63. [Google Scholar] [CrossRef]
- Majesky, M.W.; Lindner, V.; Twardzik, D.R.; Schwartz, S.M.; Reidy, M.A. Production of transforming growth factor β1 during repair of arterial injury. J. Clin. Investig. 1991, 88, 904–910. [Google Scholar] [CrossRef]
- Chen, C.-R.; Kang, Y.; Siegel, P.M.; Massagué, J. E2F4/5 and p107 as Smad Cofactors Linking the TGFβ Receptor to c-myc Repression. Cell 2002, 110, 19–32. [Google Scholar] [CrossRef]
- Siegel, P.M.; Massagué, J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Crist, A.M.; Lee, A.R.; Patel, N.R.; Westhoff, D.E.; Meadows, S.M. Vascular deficiency of Smad4 causes arteriovenous malformations: A mouse model of Hereditary Hemorrhagic Telangiectasia. Angiogenesis 2018, 21, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Sim, T.; Mathew-Joseph, S.; Pannu, H.; Milewicz, D.M.; Seidman, C.E.; Seidman, J.G.; Kim, D.H. Sequencing of TGF-β Pathway Genes in Familial Cases of Intracranial Aneurysm. Stroke 2009, 40, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Frösen, J. Smooth Muscle Cells and the Formation, Degeneration, and Rupture of Saccular Intracranial Aneurysm Wall—A Review of Current Pathophysiological Knowledge. Transl. Stroke Res. 2014, 5, 347–356. [Google Scholar] [CrossRef]
- Frösen, J.; Cebral, J.; Robertson, A.M.; Aoki, T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg. Focus 2019, 47, E21. [Google Scholar] [CrossRef]
- Nanjo, H.; Sho, E.; Komatsu, M.; Sho, M.; Zarins, C.K.; Masuda, H. Intermittent short-duration exposure to low wall shear stress induces intimal thickening in arteries exposed to chronic high shear stress. Exp. Mol. Pathol. 2006, 80, 38–45. [Google Scholar] [CrossRef]
- Darsaut, T.; Salazkin, I.; Ogoudikpe, C.; Gevry, G.; Bouzeghrane, F.; Raymond, J. Effects of stenting the parent artery on aneurysm filling and gene expression of various potential factors involved in healing of experimental aneurysms. Interv. Neuroradiol. 2006, 12, 289–302. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Chu, L.; Chen, W.; Du, Y.; Gu, J. Long non-coding RNA HIF1A-AS1 is upregulated in intracranial aneurysms and participates in the regulation of proliferation of vascular smooth muscle cells by upregulating TGF-β1. Exp. Ther. Med. 2018, 17, 1797–1801. [Google Scholar] [CrossRef]
- Plana, E.; Oto, J.; Medina, P.; Fernández-Pardo, Á.; Miralles, M. Novel contributions of neutrophils in the pathogenesis of abdominal aortic aneurysm, the role of neutrophil extracellular traps: A systematic review. Thromb. Res. 2020, 194, 200–208. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pietrzak, J.; Świechowski, R.; Wosiak, A.; Wcisło, S.; Balcerczak, E. ADAMTS Gene-Derived circRNA Molecules in Non-Small-Cell Lung Cancer: Expression Profiling, Clinical Correlations and Survival Analysis. Int. J. Mol. Sci. 2024, 25, 1897. [Google Scholar] [CrossRef] [PubMed]
- Bertoli-Avella, A.M.; Gillis, E.; Morisaki, H.; Verhagen, J.M.A.; De Graaf, B.M.; Van De Beek, G.; Gallo, E.; Kruithof, B.P.T.; Venselaar, H.; Myers, L.A.; et al. Mutations in a TGF-β ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J. Am. Coll. Cardiol. 2015, 65, 1324–1336. [Google Scholar] [CrossRef]
- Tingting, T.; Wenjing, F.; Qian, Z.; Hengquan, W.; Simin, Z.; Zhisheng, J.; Shunlin, Q. The TGF-β pathway plays a key role in aortic aneurysms. Clin. Chim. Acta 2020, 501, 222–228. [Google Scholar] [CrossRef]
- Hara, H.; Takeda, N.; Fujiwara, T.; Yagi, H.; Maemura, S.; Kanaya, T.; Nawata, K.; Morita, H.; Komuro, I. Activation of TGF-β signaling in an aortic aneurysm in a patient with Loeys-Dietz syndrome caused by a novel loss-of-function variant of TGFBR1. Hum. Genome Var. 2019, 6, 6–9. [Google Scholar] [CrossRef]
- Takeda, N.; Hara, H.; Fujiwara, T.; Kanaya, T.; Maemura, S.; Komuro, I. TGF-β signaling-related genes and thoracic aortic aneurysms and dissections. Int. J. Mol. Sci. 2018, 19, 2125. [Google Scholar] [CrossRef]
- Chen, J.; Chang, R. Association of TGF-β Canonical Signaling-Related Core Genes with Aortic Aneurysms and Aortic Dissections. Front. Pharmacol. 2022, 13, 888563. [Google Scholar] [CrossRef]
- Dai, J.; Losy, F.; Guinault, A.-M.; Pages, C.; Anegon, I.; Desgranges, P.; Becquemin, J.-P.; Allaire, E. Overexpression of Transforming Growth Factor-β1 Stabilizes Already-Formed Aortic Aneurysms. Circulation 2005, 112, 1008–1015. [Google Scholar] [CrossRef]
- Soto, M.E.; Rodríguez-Brito, M.; Pérez-Torres, I.; Herrera-Alarcon, V.; Martínez-Hernández, H.; Hernández, I.; Castrejón-Téllez, V.; Peña-Ocaña, B.A.; Alvarez-Leon, E.; Manzano-Pech, L.; et al. Analysis of FBN1, TGFβ2, TGFβR1 and TGFβR2 mRNA as Key Molecular Mechanisms in the Damage of Aortic Aneurysm and Dissection in Marfan Syndrome. Int. J. Mol. Sci. 2025, 26, 3067. [Google Scholar] [CrossRef]
- Itoh, F.; Itoh, S.; Adachi, T.; Ichikawa, K.; Matsumura, Y.; Takagi, T.; Festing, M.; Watanabe, T.; Weinstein, M.; Karlsson, S.; et al. Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. Blood 2012, 119, 5320–5328. [Google Scholar] [CrossRef]
- DiRenzo, D.M.; Chaudhary, M.A.; Shi, X.; Franco, S.R.; Zent, J.; Wang, K.; Guo, L.-W.; Kent, K.C. A crosstalk between TGF-β/Smad3 and Wnt/β-catenin pathways promotes vascular smooth muscle cell proliferation. Cell. Signal. 2016, 28, 498–505. [Google Scholar] [CrossRef]
- Ryan, S.T.; Koteliansky, V.E.; Gotwals, P.J.; Lindner, V. Transforming Growth Factor-Beta-Dependent Events in Vascular Remodeling following Arterial Injury. J. Vasc. Res. 2003, 40, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Flood, C.; Akinwunmi, J.; Lagord, C.; Daniel, M.; Berry, M.; Jackowski, A.; Logan, A. Transforming Growth Factor-β1 in the Cerebrospinal Fluid of Patients with Subarachnoid Hemorrhage: Titers Derived from Exogenous and Endogenous Sources. J. Cereb. Blood Flow Metab. 2001, 21, 157–162. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, N.K.; Linn, F.H.H.; van der Plas, J.A.; Algra, A.; Rinkel, G.J.E. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef]
- Recouvreux, M.V.; Lapyckyj, L.; Camilletti, M.A.; Guida, M.C.; Ornstein, A.; Rifkin, D.B.; Becu-Villalobos, D.; Díaz-Torga, G. Sex Differences in the Pituitary Transforming Growth Factor-β1 System: Studies in a Model of Resistant Prolactinomas. Endocrinology 2013, 154, 4192–4205. [Google Scholar] [CrossRef]
- Sathyan, S.; Koshy, L.V.; Srinivas, L.; Easwer, H.V.; Premkumar, S.; Nair, S.; Bhattacharya, R.N.; Alapatt, J.P.; Banerjee, M. Pathogenesis of intracranial aneurysm is mediated by proinflammatory cytokine TNFA and IFNG and through stochastic regulation of IL10 and TGFB1 by comorbid factors. J. Neuroinflammation 2015, 12, 135. [Google Scholar] [CrossRef]
- Sho, E.; Sho, M.; Nanjo, H.; Kawamura, K.; Masuda, H.; Dalman, R.L. Comparison of cell-type-specific vs transmural aortic gene expression in experimental aneurysms. J. Vasc. Surg. 2005, 41, 844–852. [Google Scholar] [CrossRef]
- Henderson, E.L.; Geng, Y.-J.; Sukhova, G.K.; Whittemore, A.D.; Knox, J.; Libby, P. Death of Smooth Muscle Cells and Expression of Mediators of Apoptosis by T Lymphocytes in Human Abdominal Aortic Aneurysms. Circulation 1999, 99, 96–104. [Google Scholar] [CrossRef]
- Fukui, D.; Miyagawa, S.; Soeda, J.; Tanaka, K.; Urayama, H.; Kawasaki, S. Overexpression of transforming growth factor β1 in smooth muscle cells of human abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 540–545. [Google Scholar] [CrossRef]
| Aneurysm Geometry Characteristics | ||||||||
| IA Group No. (%) | UIA Group No. (%) | RIA Group No. (%) | ||||||
| Aneurysm Location | ||||||||
| MCA | 3 (9%) | 2 (8%) | 1 (12.5%) | |||||
| ACA | 1 (3%) | 1 (4%) | - | |||||
| ICA | 13 (41%) | 12 (50%) | 1 (12.5%) | |||||
| AComA | 11 (34%) | 5 (21%) | 6 (75%) | |||||
| BA | 4 (13%) | 4 (17%) | - | |||||
| Aneurysm size [mm] | ||||||||
| <5 mm | 9 (28%) | 7 (29%) | 2 (25%) | |||||
| 5–10 mm | 17 (53%) | 12 (50%) | 5 (62.5%) | |||||
| >10 mm | 6 (19%) | 5 (21%) | 1 (12.5%) | |||||
| Number of aneurysms | ||||||||
| Single | 22 (69%) | 17 (71%) | 5 (62.5%) | |||||
| Multiple | 10 (31%) | 7 (29%) | 3 (37.5%) | |||||
| Shape of aneurysms | ||||||||
| Saccular | 19 (59%) | 14 (58%) | 5 (62.5%) | |||||
| Polycyclic | 13 (41%) | 10 (42%) | 3 (37.5%) | |||||
| Demographic Data and Basic Laboratory Parameters | ||||||||
| Median (25th and 75th percentile) | 2-tailed p-Value | |||||||
| IA Group (n = 32) | UIA Group (n = 24) | RIA Group (n = 8) | Control Group (n = 20) | IA vs.C | UIA vs. C | RIA vs.C | UIA vs. RIA | |
| Sex female/male | 22/10 | 18/6 | 4/4 | 14/6 | 0.9244 | 0.7102 | 0.5746 | 0.3784 |
| Age mean (range) | 53 (24–72) | 54 (24–71) | 47 (26–72) | 51 (24–71) | 0.5822 | 0.3086 | 0.5002 | 0.2927 |
| WBC [×103/µL] | 7.19 (5.86–8.877) | 6.48 (5.46–7.96) | 9.79 (7.23–15.33) | 5.73 (5.19–6.76) | 0.0118 | 0.1018 | 0.0007 | 0.0065 |
| RBC [×106/µL] | 4.54 (4.20–4.77) | 4.53 (4.20–4.77) | 4.63 (4.04–4.79) | 4.71 (4.36–5.18) | 0.0369 | 0.0483 | 0.1816 | 0.8814 |
| HGB [g/dL] | 13.5 (12.8–14.4) | 13.5 (12.8–14.4) | 13.7 (12.2–14.7) | 14.0 (13.1–14.6) | 0.3551 | 0.3555 | 0.6358 | 0.9490 |
| HCT [%] | 38.9 (37.4–42.4) | 38.8 (37.6–42.6) | 39.5 (34.8–41.8) | 40.8 (38.9–43.9) | 0.0487 | 0.0832 | 0.1226 | 0.5641 |
| MCV [fl] | 87.7 (85.60–91.30) | 89.6 (86.5–91.9) | 86.0 (83.7–86.4) | 86.6 (85.8–88.3) | 0.2347 | 0.0339 | 0.1991 | 0.0328 |
| PLT [×103/µL] | 227 (193–252) | 217 (191–248) | 242 (222–279) | 239 (218–290) | 0.1214 | 0.0605 | 0.9009 | 0.2197 |
| PCT [%] | 0.23 (0.21–0.26) | 0.23 (0.21–0.25) | 0.24 (0.22–0.27) | 0.25 (0.23–0.30) | 0.1625 | 0.1355 | 0.5661 | 0.5935 |
| MPV [fl] | 10.3 (9.9–12.0) | 10.4 (10.1–11.2) | 9.9 (9.4–10.5) | 10.2 (9.8–10.7) | 0.3173 | 0.1177 | 0.5327 | 0.1035 |
| PDW [fl] | 11.8 (10.90–13.40) | 12.1 (11.2–13.7) | 11.0 (9.6–12.4) | 11.4 (10.9–12.2) | 0.2499 | 0.0832 | 0.5327 | 0.1603 |
| P-LCR [%] | 26.9 (23.7–32.9) | 27.8 (25.0–34.6) | 23.4 (20.0–29.1) | 25.2 (22.7–31.2) | 0.2347 | 0.0790 | 0.5661 | 0.1241 |
| PT [s] | 14.3 (13.6–14.8) | 14.3 (13.8–14.7) | 13.9 (13.3–15.2) | 13.0 (12.8–13.9) | 0.0003 | 0.0003 | 0.0428 | 0.6540 |
| INR | 1.06 (1.03–1.11) | 1.07 (1.04–1.10) | 1.03 (1.02–1.14) | 0.98 (0.98–1.01) | <0.0001 | <0.0001 | 0.0047 | 0.7816 |
| APTT [s] | 30.0 (27.1–31.6) | 30.7 (28.0–32.1) | 27.5 (25.6–29.2) | 26.8 (25.8–28.6) | 0.0074 | 0.0006 | 0.9801 | 0.0462 |
| Fibrinogen [mg/dL] | 305 (268–361) | 302 (265–361) | 309 (284–360) | 262 (250–300) | 0.0118 | 0.0339 | 0.0285 | 0.5355 |
| Na+ [mmol/L] | 140 (139–142) | 140 (139–142) | 141 (139–142) | 140 (139–141) | 0.3173 | 0.4617 | 0.2806 | 0.7169 |
| K+ [mmol/L] | 4.1 (3.9–4.5) | 4.2 (4.0–4.6) | 3.6 (3.1–4.3) | 4.4 (4.2–4.6) | 0.0510 | 0.2011 | 0.0114 | 0.0368 |
| Glucose [mg/dL] | 102 (91–130) | 98 (91–125) | 122 (94–155) | 94 (89–100) | 0.0351 | 0.1069 | 0.0247 | 0.3345 |
| Urea [mg/dL] | 29.96 (21.40–36.38) | 27.82 (21.40–36.38) | 29.96 (29.64–34.24) | 25.03 (21.90–28.03) | 0.0753 | 0.2183 | 0.0328 | 0.6540 |
| Creatinine [mg/dL] | 0.71 (0.62–0.83) | 0.71 (0.64–0.82) | 0.70 (0.54–0.87) | 0.73 (0.68–0.81) | 0.4841 | 0.5360 | 0.5661 | 0.9830 |
| eGFR [mL/min] | 104 (84–123) | 101 (84–121) | 108 (89–137) | 101 (93–112) | 0.8595 | 0.9164 | 0.4688 | 0.4284 |
| Parameter [pg/mL] | Median (25th and 75th Percentile) | 2-Tailed p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| IA Group (n = 32) | UIA Group (n = 24) | RIA Group (n = 8) | C Group (n = 20) | IA vs. C | UIA vs. C | RIA vs. C | UIA vs. RIA | |
| TGF-β1 | 48,466 (38,597–60,862) | 45,206 (37,359–56,918) | 60,183 (46,060–65,440) | 52,784 (38,485–61,275) | 0.7024 | 0.3199 | 0.2806 | 0.0328 * |
| TGF-β2 | 1108 (964–1318) | 1048 (960–1288) | 1274 (1111–1343) | 1017 (942–1222) | 0.3575 | 0.7751 | 0.0625 | 0.1241 |
| TGF-β3 | 441 (403–490) | 418 (393–466) | 491 (449–502) | 453 (399–517) | 0.3196 | 0.1220 | 0.5410 | 0.0301 * |
| Parameter [pg/mL] | Sex | 2-Tailed p-Value | ||
| Female n = 18 | Male n = 6 | |||
| TGF-β1 | 43,448 (35,530–50,411) | 57,870 (55,473–63,605) | 0.0118 * | |
| TGF-β2 | 991 (942–1250) | 1174 (1047–1300) | 0.2509 | |
| TGF-β3 | 418 (389–454) | 416 (408–470) | 0.5805 | |
| Age | 2-Tailed p-Value | |||
| < 60 years n = 12 | ≥ 60 years n = 12 | |||
| TGF-β1 | 56,846 (43,177–62,751) | 40,887 (37,461–45,206) | 0.0205 * | |
| TGF-β2 | 1005 (937–1276) | 1052 (983–11,288) | 0.4776 | |
| TGF-β3 | 418 (386–462) | 424 (396–461) | 0.9778 | |
| Size of Aneurysms | 2-Tailed p-Value | |||
<5 mm n = 7 | 5–10 mm n = 12 | >10 mm n = 5 | <5 mm vs. 5–10 mm <5 mm vs. >10 mm 5–10 mm vs. >10 mm | |
| TGF-β1 | 37,257 (34,543–48,961) | 45,206 (38,778–53,149) | 61,027 (56,991–64,225) | 0.2268 0.1490 0.0818 |
| TGF-β2 | 1250 (993–1359) | 1019 (967–1133) | 942 (932–1252) | 0.1956 0.1490 0.5743 |
| TGF-β3 | 408 (364–419) | 426 (396–449) | 470 (420–508) | 0.4320 0.0303 * 0.1037 |
| Count of Aneurysms | 2-Tailed p-Value | |||
| Single n = 17 | Multiple n = 7 | |||
| TGF-β1 | 50,411 (42,635–56,991) | 35,530 (29,885–42,969) | 0.0337 * | |
| TGF-β2 | 1049 (961–1250) | 1047 (960–1351) | 0.5761 | |
| TGF-β3 | 438 (408–477) | 408 (364–417) | 0.0645 | |
| Shape of Aneurysms | ||||
| Saccular n = 14 | Polycyclic n = 10 | 2-Tailed p-Value | ||
| TGF-β1 | 45,206 (37,257–56,991) | 46,954 (38,416–50,825) | 0.9771 | |
| TGF-β2 | 1097 (961–1252) | 1012 (960–1300) | 0.7521 | |
| TGF-β3 | 422 (391–454) | 430 (408–470) | 0.7521 | |
| Smoking | 2-Tailed p-Value | |||
| No n = 20 | Yes n = 4 | |||
| TGF-β1 | 45,206 (38,041–56,846) | 43,834 (33,571–58,930) | 0.8519 | |
| TGF-β2 | 1018 (951–1199) | 1289 (1123–1346) | 0.0969 | |
| TGF-β3 | 418 (405–449) | 419 (338–504) | 0.9701 | |
| Hypertension | 2-Tailed p-Value | |||
| No n = 10 | Yes n = 14 | |||
| TGF-β1 | 53,149 (37,666–61,027) | 42,802 (37,257–50,411) | 0.3407 | |
| TGF-β2 | 1083 (961–1253) | 1020 (942–1326) | 0.2591 | |
| TGF-β3 | 411 (364–470) | 419 (408–445) | 0.4031 | |
| Cut-Off | Youden Index | AUC ± SE | Se [%] | Sp [%] | PPV [%] | NPV [%] | ACC [%] | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| ΔTGFB1 | −3.412 | 0.32 | 0.671 ±0.077 | 60 | 72 | 79 | 50 | 64 | 0.0267 * |
| Cut-Off | Youden Index | AUC ± SE | Se [%] | Sp [%] | PPV [%] | NPV [%] | ACC [%] | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| ΔTGFB3 | 5.683 | 0.54 | 0.792 ± 0.106 | 88 | 67 | 54 | 54 | 73 | 0.0061 * |
| Cut-Off | Youden Index | AUC ± SE | Se [%] | Sp [%] | PPV [%] | NPV [%] | ACC [%] | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| TGF-β1 [pg/mL] | 44,973 | 0.50 | 0.755 ± 0.092 | 100 | 50 | 40 | 100 | 63 | 0.0056 * |
| TGF-β3 [pg/mL] | 444 | 0.54 | 0.758 ± 0.106 | 86 | 67 | 47 | 94 | 72 | 0.0150 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutkowska, K.; Koper-Lenkiewicz, O.M.; Żebrowska-Nawrocka, M.; Jakoniuk, M.; Łysoń, T.; Tylicka, M.; Balcerczak, E.; Matowicka-Karna, J.; Kamińska, J. Elevated Expression of TGFB1 in PBMCs Is Associated with Intracranial Aneurysm Formation, but TGFB3 Expression Implicated Rupture. Biomedicines 2025, 13, 1273. https://doi.org/10.3390/biomedicines13061273
Sutkowska K, Koper-Lenkiewicz OM, Żebrowska-Nawrocka M, Jakoniuk M, Łysoń T, Tylicka M, Balcerczak E, Matowicka-Karna J, Kamińska J. Elevated Expression of TGFB1 in PBMCs Is Associated with Intracranial Aneurysm Formation, but TGFB3 Expression Implicated Rupture. Biomedicines. 2025; 13(6):1273. https://doi.org/10.3390/biomedicines13061273
Chicago/Turabian StyleSutkowska, Kinga, Olga Martyna Koper-Lenkiewicz, Marta Żebrowska-Nawrocka, Marta Jakoniuk, Tomasz Łysoń, Marzena Tylicka, Ewa Balcerczak, Joanna Matowicka-Karna, and Joanna Kamińska. 2025. "Elevated Expression of TGFB1 in PBMCs Is Associated with Intracranial Aneurysm Formation, but TGFB3 Expression Implicated Rupture" Biomedicines 13, no. 6: 1273. https://doi.org/10.3390/biomedicines13061273
APA StyleSutkowska, K., Koper-Lenkiewicz, O. M., Żebrowska-Nawrocka, M., Jakoniuk, M., Łysoń, T., Tylicka, M., Balcerczak, E., Matowicka-Karna, J., & Kamińska, J. (2025). Elevated Expression of TGFB1 in PBMCs Is Associated with Intracranial Aneurysm Formation, but TGFB3 Expression Implicated Rupture. Biomedicines, 13(6), 1273. https://doi.org/10.3390/biomedicines13061273










