1. Introduction
Post-laser-assisted keratomileusis (LASIK) ectasia (PLE) is a rare adverse outcome following LASIK. Characteristic signs of this complication are decreased corneal biomechanical stability, stromal thinning, keratometric inferior steepening, progressive corneal irregularity and, ultimately, irregular corneal astigmatism [
1,
2,
3,
4]. This leads to deterioration in uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), and quality of vision (QoV), and can be accompanied by increased light sensitivity, glare, halos, and rarely monocular diplopia [
5]. Collagen cross-linking (CXL) has shown efficacy in halting the progression of PLE, although residual refractive errors may remain [
6,
7,
8,
9,
10]. The traditional epithelium-off procedure, however, is not free from complications, such as persistent epithelial defects, infection, corneal scarring and recurrent erosion, as well as an extended recovery period with pain and discomfort [
11]. Epithelium-on techniques minimize recovery time and complications but have not shown the same efficacy [
12,
13].
A less invasive technique, termed under-flap corneal crosslinking (ufCXL) was introduced to treat early PLE before a significant change in corneal irregularity or loss of CDVA occurs. This method involves re-lifting the flap, soaking the stromal bed with riboflavin, repositioning the flap, and applying ultraviolet (UV) light. CXL therefore occurs under the flap, but not in the flap itself [
14]. In a 36-month study, ufCXL was demonstrated to be safe to halt the progression of PLE with statistical maintenance of visual function, refractive astigmatism, and keratometry [
15]. ufCXL is performed without combined laser ablation, and as such, visual acuity and QoV are typically only stabilized but not improved [
15]. On rare occasions, ufCXL, like regular CXL, can also induce corneal changes, such as flattening, which may impact refractive and visual outcomes [
16,
17,
18].
In post-ufCXL patients with reduced UDVA, we hypothesize that a topography-guided photorefractive keratectomy (TGPRK) enhancement can be safely performed on the LASIK flap surface once topography and refraction show stability, with central corneal thickness back to baseline levels, several months after ufCXL. The concept of performing laser ablation along with CXL in ectatic corneas is not new and is reported to be safe, although somewhat controversial [
19,
20]. For example, PLE and keratoconus (KC) patients undergoing same-day combined TGPRK with CXL have both demonstrated comparable stability to conventional CXL [
19,
20]. The literature suggests that once a flap is made, it no longer contributes to the biomechanical stability of the cornea [
21]. We therefore hypothesized that the flap stroma, which did not receive crosslinking, could be safely ablated without biomechanical impact. A shallow, TGPRK ablation on the flap surface was performed to improve refraction and vision post-ufCXL. This small case series reports the visual and refractive outcomes and long-term stability of six PLE eyes that underwent TGPRK enhancement on the flap surface at least 10 months after ufCXL with stable refraction. We investigate the safety and effectiveness of this technique for improving UDVA with the aim of reducing spectacle dependence without compromising corneal stability.
2. Patients and Methods
This retrospective interventional case series included six eyes from five patients diagnosed with PLE after microkeratome LASIK. Staged TG PRK was performed after ectatic stabilization with ufCXL. This study was approved by the Ethics Review Board of the Canadian Ophthalmic Research Review and Ethics Compliance Team (CORRECT). All patients provided written consent for the use of anonymized data for research. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
2.1. Standardized Identification of Post-Lasik Ectasia
In contrast to previous CXL studies where corneal ectasia is commonly treated at an advanced stage with CDVA significantly reduced [
6], the current ufCXL technique aims to treat milder ectasia before a significant reduction in CDVA. Inclusion criteria were eyes that had myopic LASIK with early to moderate post-LASIK ectasia, defined as presenting with the new onset of progressive manifest refraction cylinder up to 2.50 diopters (D), a new decrease in uncorrected distance visual acuity (UDVA) not greater than 20/100, corrected distance visual acuity (CDVA) of 20/25 or better, and new topographic irregular astigmatism and/or inferior steepening consistent with post-LASIK ectasia, as previously reported [
14]. All cases were sent to a consultant group of five highly experienced surgeons who confirmed the diagnosis of PLE using previously published standardized criteria for requiring ufCXL intervention. These included topographical changes consistent with PLE with a decrease in UDVA and/or CDVA, and/or a decrease in quality of vision symptoms as subjectively described by the patient as causing new or worsening halos, glare, ghosting, or shadowing [
14].
2.2. ufCXL Technique
As previously described [
15], the flap edge was identified at the slit lamp and a small 1- to 2-clock hour opening was created. Under the excimer laser microscope (EX500, Alcon Laboratories, Fort Worth, TX, USA), the entire flap was lifted. Under-flap stromal bed thickness needed to be 300 µm or greater prior to riboflavin administration. A circular sponge soaked in 0.25% isotonic riboflavin was placed on the stromal bed and rewetted at 60 s intervals for 5 min. Care was taken not to soak the flap with riboflavin. After 5 min, the bed was dried and excess riboflavin was removed with a surgical spear. The flap was replaced with minimal irrigation. Ultraviolet light (CCL-VARIO Cross-linking Radiation system; Peschke Trade GmbH, Hünenberg, Switzerland) was applied for 5 min at 18 mW/cm
2, resulting in a total irradiation dose of 5.4 J/cm
2 at the corneal surface. Lubricating or topical anesthetic drops were applied every 30 s during light exposure. The flap was assessed at the slit lamp prior to discharge. No bandage contact lens was applied.
2.3. Post-ufCXL Regimen
Gatifloxacin 0.3% (Zymar; Allergan, Inc., Irvine, CA, USA) four times a day for 5 days and prednisolone acetate 1% (Pred Forte; Allergan, Inc.) every hour for day 1, every 2 h for day 2, and four times a day for days 3, 4, and 5 were given postoperatively. Refresh tear drops were used every half hour for day 1, hourly for day 2, and then four times a day for 5 days. Strict instructions to avoid eye rubbing and squeezing of the eyelids were given to all patients. Patients wore protective sunglasses for 48 h postoperatively.
2.4. Topography-Guided PRK
Once a minimum stabilization period of at least 10 months post-ufCXL was confirmed, based on stable topography and refraction, patients with residual refractive errors were considered for TGPRK enhancement. Preoperatively, a minimum of 4 high-quality reproducible scans were obtained using Allegro Topolyzer Vario (Alcon Laboratories, Inc., Fort Worth, TX, USA) along with an additional 2 scans using Orbscan-IIz (Bausch & Lomb, Inc., Vaughan, ON, Canada). The corneal astigmatism magnitude and axis derived from these scans were averaged according to a previously published protocol [
22]. The calculated astigmatism magnitude and axis were then inputted into the Contoura surgical planning software v.1.76r76 (Alcon Laboratories, Inc., Fort Worth, TX, USA). Before proceeding with excimer ablation, the Orbscan elevation map was compared with the HOA ablation depth planning software map to ensure concordance, v.1.76r76. Excimer ablation of the anterior corneal HOAs and calculated refraction was undertaken. An optical zone size of 5.0 mm or 5.5 mm was selected depending on the topographic cone peak location within or outside of the central 3 mm ring, respectively.
Following the application of topical proparacaine hydrochloride 0.5% anesthetic drops (Alcaine, Alcon Laboratories, Inc.), a 6.5 mm zone of the epithelium was partially removed using a 40 μm phototherapeutic keratectomy (PTK). A small myopic ablation (approximately −0.75 D) was performed to offset any hyperopic treatment effect from PTK. TGPRK was then executed with the WaveLight EX500 excimer laser, ablating flap stroma (which had not been crosslinked during ufCXL). Mitomycin-C (0.02%) was applied to the stromal bed for 2 min to mitigate postoperative haze formation. The postoperative regimen included the administration of Zymar 4 times a day for 7 days, Acuvail once per day for 2 days, and Maxidex 4 times a day for 8 weeks. The bandage contact lens was removed on the fifth day.
2.5. Data and Statistical Analysis
Data from ophthalmic examinations pre-LASIK, pre-ufCXL, post-ufCXL, pre-TGPRK and at 1, 3, 6, 12, 24, and 36 months and later time points post-TGPRK were collected for analysis, including slit-lamp examinations, manifest refraction sphere, cylinder, and spherical equivalent (SEQ), uncorrected and corrected distance visual acuity (UDVA and CDVA, respectively), maximum keratometry (Kmax), central pachymetry, corneal irregularity indices, and presence of haze and/or other complications at the slit lamp. Keratometry and corneal irregularity indices were obtained with the Orbscan IIz system (Bausch & Lomb) on each eye and time point. Failure of ufCXL or PLE progression post-TGPRK was defined as an increase in Kmax of 1.00 D or greater over the pre-ufCXL value, similar to previous CXL studies [
15,
22]. Data were analyzed using MATLAB R2024B (MathWorks, Inc., Natick, MA, USA). For normally distributed variables, paired
t-tests were used to compare pre- and post-TGPRK outcomes, while the two-sample Wilcoxon signed-rank test was applied for non-normally distributed data. The Kolmogorov–Smirnov test was employed to assess the normality of data distributions. Statistical significance was defined as a
p-value less than 0.05.
4. Discussion
While PLE is rare, with incidence ranging from 0.04 to 0.6%, its progression can result in biomechanical corneal decompensation, stromal thinning, keratometric inferior steepening, progressive corneal irregularity with irregular corneal astigmatism, and ultimately, significant quality of vision and quality of life deterioration [
1,
2,
3,
4]. CXL has shown excellent efficacy and safety in halting the progression of PLE as well as keratoconus [
6,
7,
8,
9,
10]. However, the traditional epithelium-off procedure is not free from complications, such as persistent epithelial defects, infection, corneal scarring and recurrent erosion, as well as an extended recovery with pain and discomfort [
11].
Under-flap CXL has been recently introduced as a less invasive technique to halt and stabilize early PLE. It is performed in early cases of PLE, when cornea biomechanics and vision are only minimally affected, with a small amount of irregular astigmatism and before any significant CDVA loss occurs [
14]. More advanced cases of ectasia, in the author’s practice, are treated with same-day sequential TGPRK with CXL [
22]. In ufCXL, the LASIK flap is lifted, riboflavin is applied to the stromal bed, the flap is repositioned, and UV light is administered to the corneal surface, thereby achieving crosslinking exclusively within the stromal bed beneath the flap [
14]. The epithelium remains intact and the CXL effect is achieved deeper in the corneal stroma below the flap since the epithelium stays on. ufCXL has fewer complications than standard epithelium-off CXL, minimizing dry eye, pain, and haze, with minimal quicker recovery [
14].
In a published series of 20 eyes treated with ufCXL, a 36-month follow-up demonstrated stable safety, visual outcomes, refractive astigmatism and maximum keratometry, with preservation of normal endothelial cell density, and a consistently deep stromal demarcation line [
15]. No eye showed progression of more than 1.00 diopter in maximum keratometry 3 years after treatment, highlighting ufCXL potential to halt early PLE progression [
15].
Although ufCXL effectively stabilizes early PLE, it does not correct the residual refractive errors already induced by the ectasia [
15]. Furthermore, the unpredictable corneal flattening associated with CXL, and the variable epi remodeling coupled with regression over time, can worsen these residual errors after ufCXL [
23,
24]. Further interventions to improve UDVA and overall quality of vision once ufCXL has biomechanically strengthened the corneal stroma below the flap and stabilized PLE remain warranted for certain patients. The current preliminary case series explored the efficacy, safety, accuracy, and stability of performing topography-guided PRK once PLE stabilization was confirmed 18 months (median) after ufCXL. The aim was to reduce refractive error and to regularize the corneal surface to improve UDVA and QoV without compromising the long-term stability achieved by ufCXL.
The concept of same-day sequential CXL and TGPRK for PLE and keratoconus is well-established, with numerous short- and long-term studies demonstrating its safety and efficacy [
19,
20,
22,
24]. Protocols have shown improvements in UDVA and QoV, and studies on ectatic eyes treated with simultaneous CXL and TGPRK report stability comparable to conventional CXL alone [
19,
20,
22]. The current approach differs in several ways. TGPRK is deferred until ufCXL corneal stabilization is confirmed. The ufCXL technique also allows the flap to remain untouched by cross-linking and preserved for future ablation. This contrasts with traditional staged CXL and TGPRK in separate sessions where cross-linked tissue gets ablated. Simultaneous same-day and traditional separate session ablation protocols also do not aim for full visual rehabilitation but rather regularize the cornea and minimize tissue removal. Because with ufCXL, PLE is detected early, prior to significant corneal distortion or induced astigmatism, the required correction is minimal, resulting in less tissue ablation.
The current study’s post-ufCXL TGPRK takes advantage of the LASIK flap stroma for excimer ablation. Because ufCXL does not cross-link the flap, this stromal flap tissue remains available for refractive correction and corneal regularization. Ablating non-crosslinked tissue reduces variability in laser ablation compared to ablating crosslinked tissue [
25]. Furthermore, once a LASIK flap is created, it contributes minimally to corneal biomechanics [
21]. Performing a shallow surface ablative procedure of the non-crosslinked flap tissue in these stabilized ectatic eyes is therefore inherently safer. By confining the ablation to the flap stroma, biomechanical risk is minimized. Moreover, performing the TGPRK several months after ufCXL allows clinicians to confirm corneal stability before correcting PLE-induced and CXL-induced changes, with the intent of improving UDVA and overall visual quality.
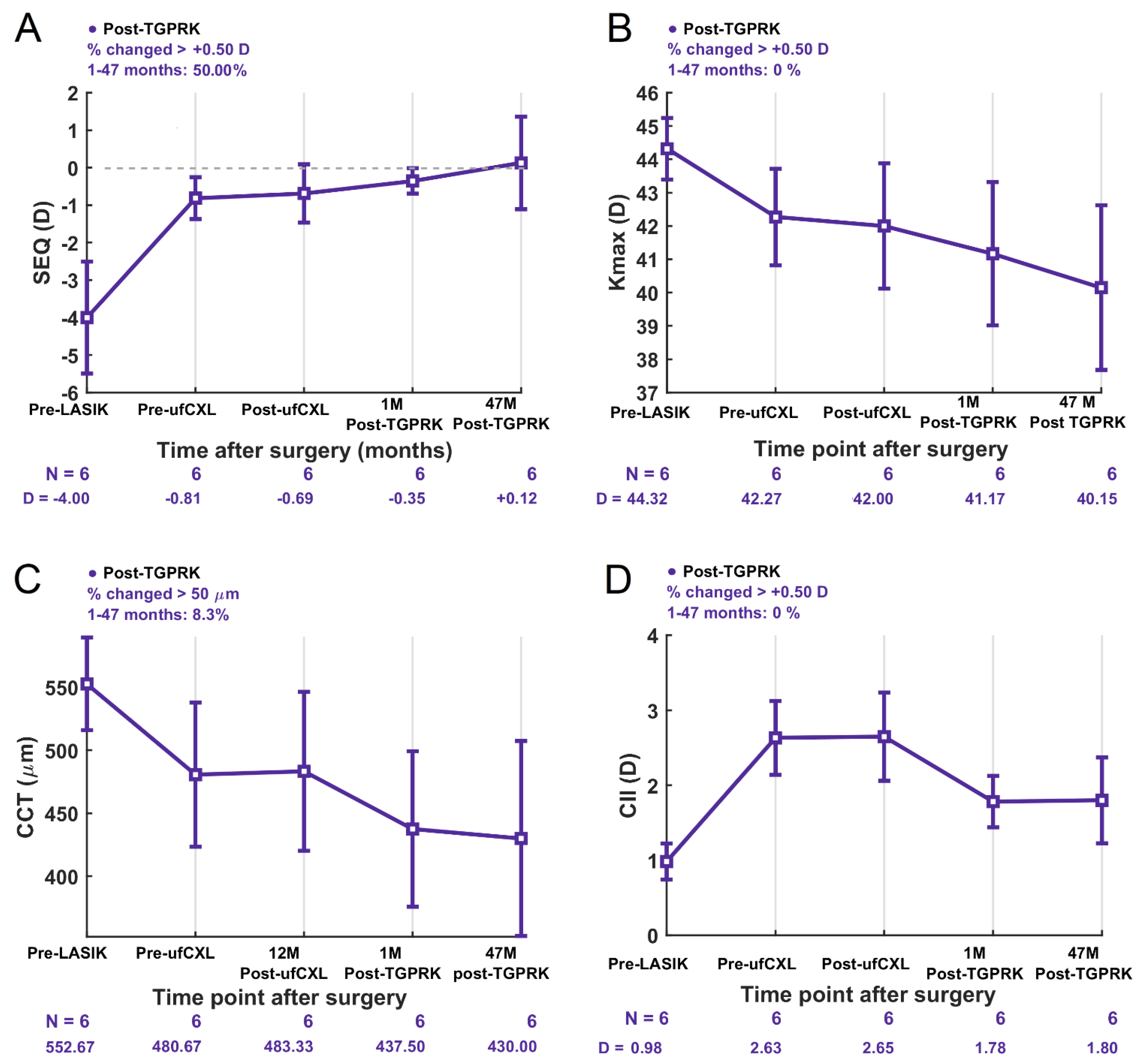
We report long-term, 4-year stability and no progression of ectasia following TGPRK treatment in six post-ufCXL eyes with mild to moderate PLE. At 1 month post-TGPRK, there was already a statistically significant increase in the percentage of eyes achieving monocular cumulative Snellen UDVA of 20/20 and 20/25 or better compared to pre-TGPRK with patients gaining an average 0.28 LogMAR of UDVA. Still four years after TGPRK, patients gained an average of 2 lines of functional UDVA compared to pre-TGPRK, with no patients losing lines of UDVA nor CDVA. This demonstrates improved and sustained functional uncorrected vision maintained at an average of 4 years after the second intervention. Although visual improvements of an average gain of 2 lines in UDVA were seen long-term, not all patients reached 20/20 UDVA. Refractively, there was a clinically meaningful trend of reduced average defocus equivalent post-TGPRK, with significantly more eyes achieving a defocus equivalent of 0.75 D or less post-TGPRK compared to pre-TGPRK (67% vs. 0%). Overall, 4 years after TGPRK, none of the eyes showed an increase in Kmax (no steepening) with stable SEQ, cylinder, corneal thickness and corneal irregularity index. Although, introducing a TGPRK 12 months or more after ufCXL might introduce variability due to prior or still ongoing long-term ufCXL effects, as well as the difference in biomechanics in PLE corneas [
16,
25,
26], the attempt to achieved refractive astigmatism predictability of TGPRK enhancement post-ufCXL was reliable with R
2 or 0.99. The TIA to SIA graphs, however, showed a slight overcorrection of astigmatism (CI = 1.20) suggesting the astigmatism should be slightly undercorrected by 20% upon treating those eyes with TGPRK post-ufCXL.
With routine, annual post-LASIK follow-up, PLE can be identified before significant irreversible changes occur and ectatic progression is halted with ufCXL. Once corneal stability is confirmed, a surface TGPRK is performed to correct residual refractive error and regularize the cornea. This novel technique introduces a paradigm shift in the traditional view of PLE from a debilitating complication to a manageable treatable condition. In select cases, visual outcomes may reach a level where corrective eyewear is no longer required.
Limitations
The small sample size reflects the low incidence of PLE and may limit the statistical power of this study. This preliminary study should therefore be interpreted as proof-of-concept rather than definitive evidence of safety. Statistically significant improvements were found from pre- to post-TGPRK. Since only eyes with relatively mild to moderate PLE were included, the effectiveness of long-term sequential ufCXL and TGPRK in more advanced PLE cases remains unknown. Larger prospective studies are needed to confirm these findings and report more variables such as higher order aberration, QoV, corneal epithelium mapping looking at epithelial irregularity, and contrast sensitivity.