Therapeutic Potential of Chick Early Amniotic Fluid in Mitigating Ionizing-Radiation-Induced Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of ceAF
2.3. IR-Induced Damage Procedure
2.4. ceAF Intervention and General Index Detection
2.5. Exercise Test
2.6. Hematoxylin and Eosin (H&E) Staining
2.7. Analysis of Peripheral Blood
2.8. Flow Cytometry Analysis
2.9. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Western Blot Analysis
2.11. Enzyme Linked Immunosorbent Assay (ELISA)
2.12. Statistical Analysis
3. Results
3.1. Supplementation with ceAF Reduces IR-Induced Mortality and Enhances the Exercise Capacity of Mice Following IR
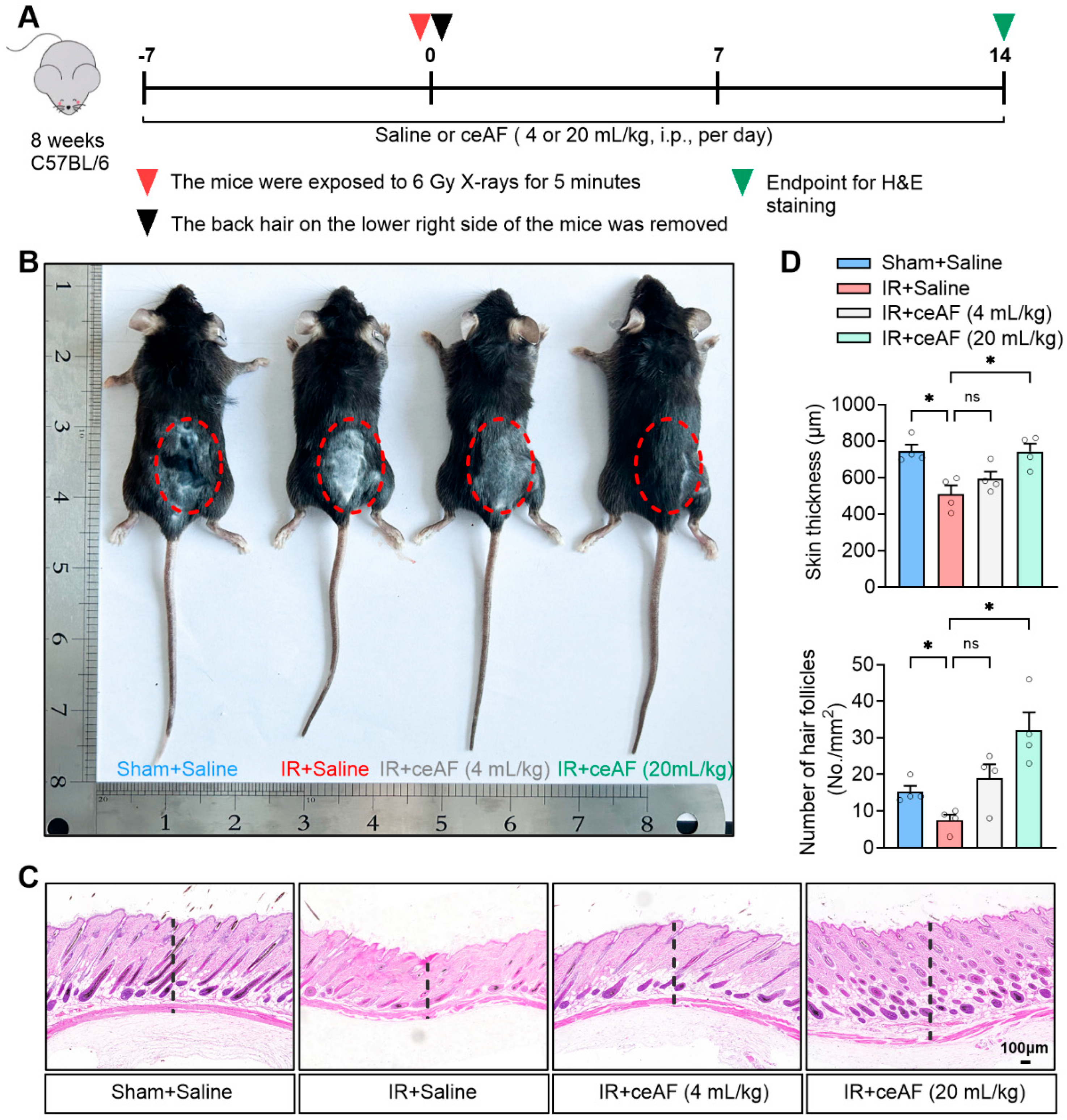
3.2. Supplementation with ceAF Reverses IR-Induced Skin Damage in Mice
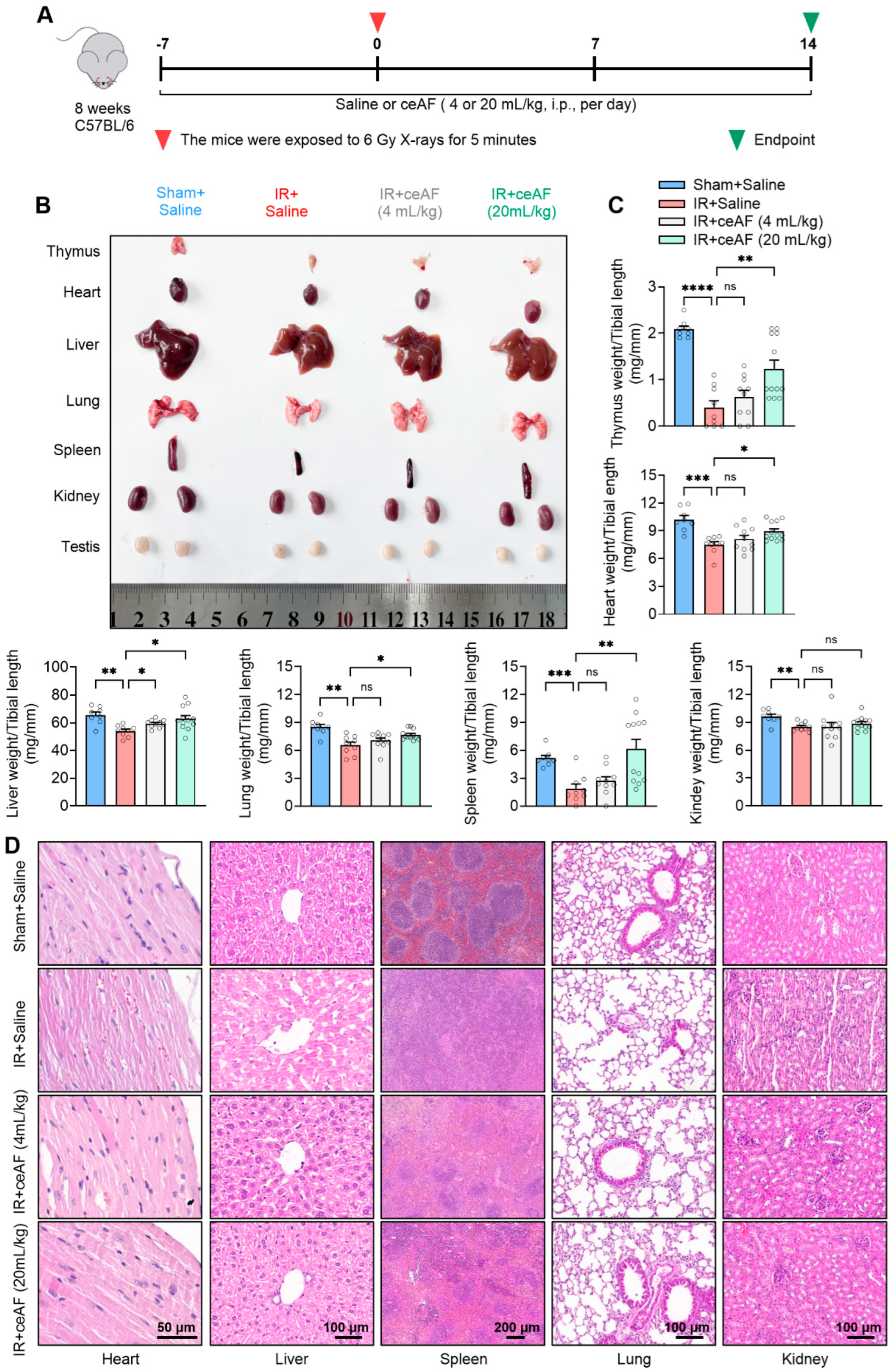
3.3. ceAF Ameliorates IR-Induced Organ Injury
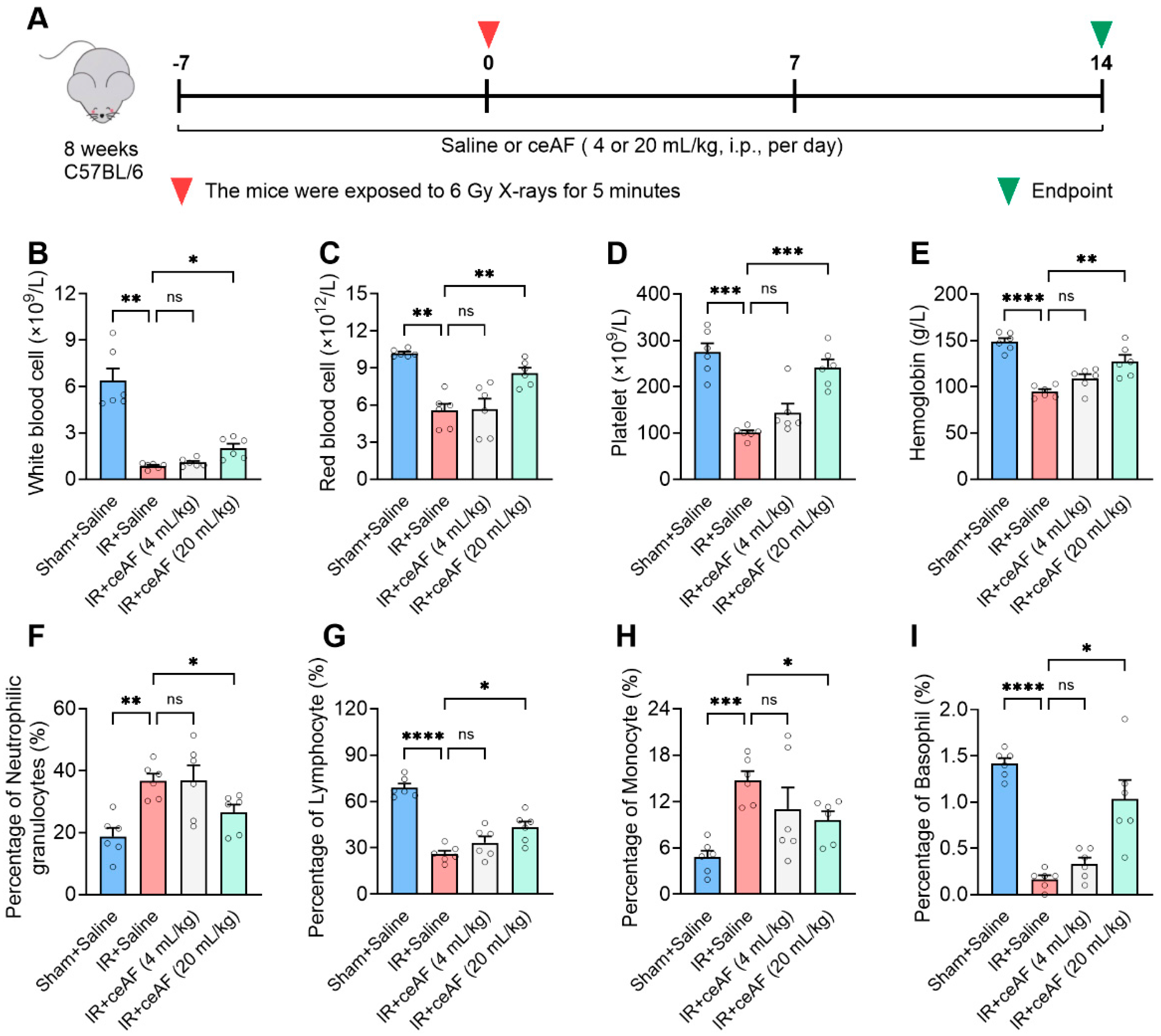
3.4. ceAF Relieves IR-Induced Injury of the Spleen Hematopoietic System
3.5. ceAF Improves Spleen Immune Function in IR-Treated Mice
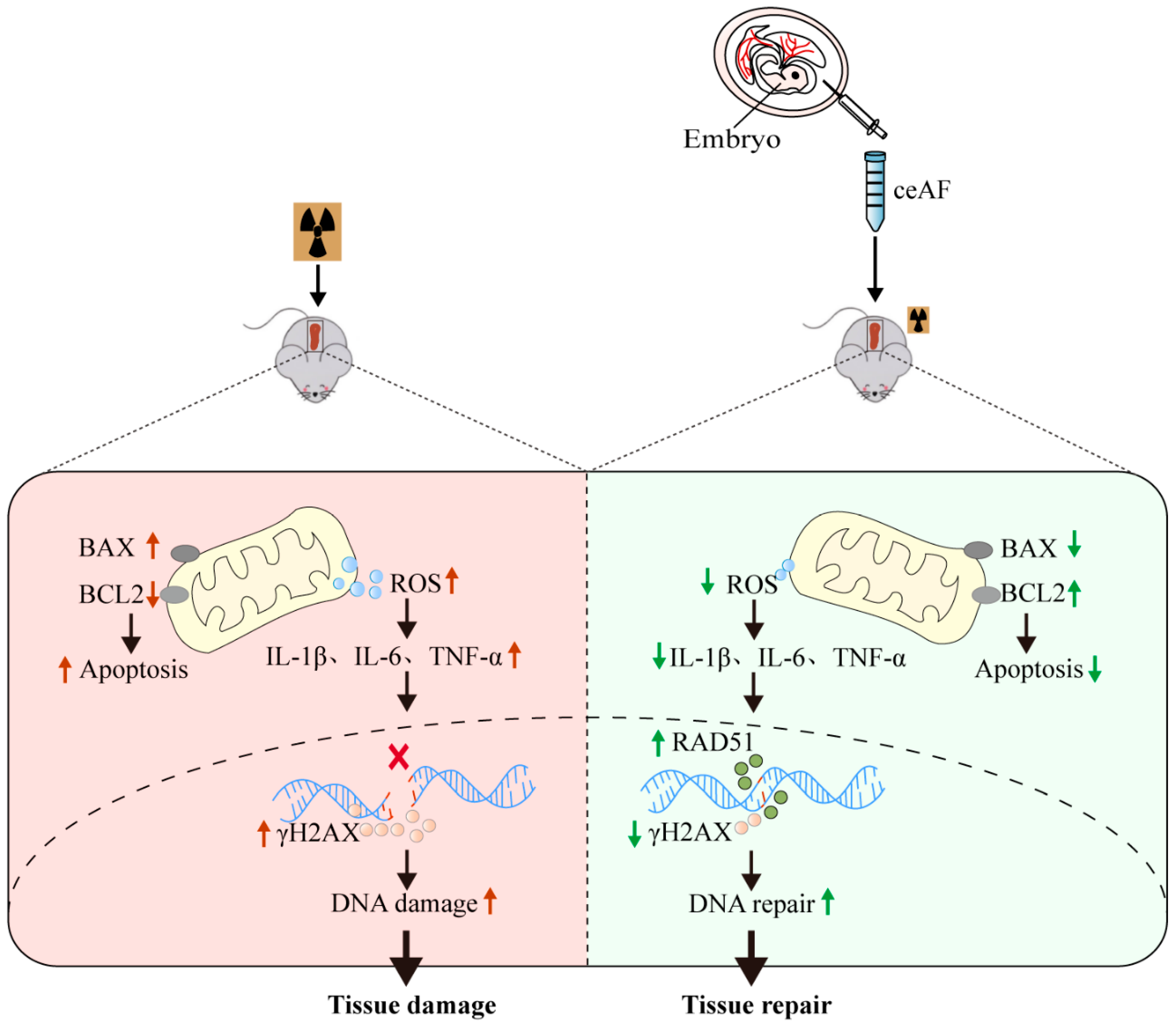
3.6. ceAF Suppresses IR-Induced Oxidative Stress and Inflammation and Effectively Enhances DNA Repair After IR-Induced Damage to the Spleen
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lumniczky, K.; Impens, N.; Armengol, G.; Candeias, S.; Georgakilas, A.G.; Hornhardt, S.; Martin, O.A.; Rodel, F.; Schaue, D. Low dose ionizing radiation effects on the immune system. Environ. Int. 2021, 149, 106212. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, H.; Bo, X.; Chen, H. Ionizing radiation damage and repair from 3D-genomic perspective. Trends Genet. 2023, 39, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, J.; Fan, X.; Liu, H.; Zhu, M.; Yang, M.; Zhang, X.; Zhang, H.; Yu, F. Ionizing Radiation-Induced Ferroptosis Based on Nanomaterials. Int. J. Nanomed. 2022, 17, 3497–3507. [Google Scholar] [CrossRef]
- Mladenova, V.; Mladenov, E.; Stuschke, M.; Iliakis, G. DNA Damage Clustering after Ionizing Radiation and Consequences in the Processing of Chromatin Breaks. Molecules 2022, 27, 1540. [Google Scholar] [CrossRef] [PubMed]
- Einor, D.; Bonisoli-Alquati, A.; Costantini, D.; Mousseau, T.A.; Moller, A.P. Ionizing radiation, antioxidant response and oxidative damage: A meta-analysis. Sci. Total Environ. 2016, 548–549, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Koturbash, I.; Merrifield, M.; Kovalchuk, O. Fractionated exposure to low doses of ionizing radiation results in accumulation of DNA damage in mouse spleen tissue and activation of apoptosis in a p53/Atm-independent manner. Int. J. Radiat. Biol. 2017, 93, 148–155. [Google Scholar] [CrossRef]
- Das, U.; Biswas, S.; Sengupta, A.; Manna, K.; Chakraborty, A.; Dey, S. Ferulic acid (FA) abrogates ionizing radiation-induced oxidative damage in murine spleen. Int. J. Radiat. Biol. 2016, 92, 806–818. [Google Scholar] [CrossRef]
- Ali, E.N.; Mansour, S.Z. Boswellic acids extract attenuates pulmonary fibrosis induced by bleomycin and oxidative stress from gamma irradiation in rats. Chin. Med. 2011, 6, 36. [Google Scholar] [CrossRef]
- Yu, X.; Li, M.; Zhu, L.; Li, J.; Zhang, G.; Fang, R.; Wu, Z.; Jin, Y. Amifostine-loaded armored dissolving microneedles for long-term prevention of ionizing radiation-induced injury. Acta Biomater. 2020, 112, 87–100. [Google Scholar] [CrossRef]
- Ji, L.; Cui, P.; Zhou, S.; Qiu, L.; Huang, H.; Wang, C.; Wang, J. Advances of Amifostine in Radiation Protection: Administration and Delivery. Mol. Pharm. 2023, 20, 5383–5395. [Google Scholar] [CrossRef]
- Subhan, B.S.; Kwong, J.; Kuhn, J.F.; Monas, A.; Sharma, S.; Rabbani, P.S. Amniotic fluid-derived multipotent stromal cells drive diabetic wound healing through modulation of macrophages. J. Transl. Med. 2021, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Shorey-Kendrick, L.E.; Crosland, B.A.; Spindel, E.R.; McEvoy, C.T.; Wilmarth, P.A.; Reddy, A.P.; Zientek, K.D.; Roberts, V.H.J.; D’Mello, R.J.; Ryan, K.S.; et al. The amniotic fluid proteome changes across gestation in humans and rhesus macaques. Sci. Rep. 2023, 13, 17039. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Nagura, H.; Ueno, S.; Nakashima, A. Amino Acid Composition of Amniotic Fluid during the Perinatal Period Reflects Mother’s Fat and Carbohydrate Intake. Nutrients 2021, 13, 2136. [Google Scholar] [CrossRef]
- Moran, E.T., Jr. Nutrition of the developing embryo and hatchling. Poult. Sci. 2007, 86, 1043–1049. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, Y.; Gao, X.; Zhang, L.; Li, B.; Chen, J.; Zhou, X.; Cai, M.; Sun, W.; Zhang, Y.; et al. Therapeutic application of chick early amniotic fluid: Effective rescue of acute myocardial ischemic injury by intravenous administration. Cell Regen. 2022, 11, 9. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Zhang, L.; Zheng, Y.; Qian, J.; Sun, N.; Ding, X.; Cui, B. Chick early amniotic fluid component improves heart function and protects against inflammation after myocardial infarction in mice. Front. Cardiovasc. Med. 2022, 9, 1042852. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Yu, J.; Cheng, S.; Khan, Z.A.; Luo, Y.; Luo, H. Guanosine and Deoxyinosine Structural Analogs Extracted from Chick Early Amniotic Fluid Promote Cutaneous Wound Healing. Int. J. Mol. Sci. 2023, 24, 12817. [Google Scholar] [CrossRef]
- Ahmad, M.; Sun, Y.; Jia, X.; Li, J.; Zhang, L.; Yang, Z.; Lin, Y.; Zhang, X.; Khan, Z.A.; Qian, J.; et al. Therapeutic values of chick early amniotic fluid (ceAF) that facilitates wound healing via potentiating a SASP-mediated transient senescence. Genes. Dis. 2022, 9, 1345–1356. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Yan, X.; Qian, J.; Tan, Q. ceAF Ameliorates Diabetic Wound Healing by Alleviating Inflammation and Oxidative Stress via TLR4/NF-kappaB and Nrf2 Pathways. J. Diabetes Res. 2023, 2023, 2422303. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, Z.; Li, Y.; Huang, Q.; Xia, L.; Li, K. Delivery of manganese carbonyl to the tumor microenvironment using Tumor-Derived exosomes for cancer gas therapy and low dose radiotherapy. Biomaterials 2021, 274, 120894. [Google Scholar] [CrossRef]
- Ye, C.; Tong, Y.; Wu, N.; Wan, G.W.; Zheng, F.; Chen, J.Y.; Lei, J.Z.; Zhou, H.; Chen, A.D.; Wang, J.J.; et al. Inhibition of miR-135a-5p attenuates vascular smooth muscle cell proliferation and vascular remodeling in hypertensive rats. Acta Pharmacol. Sin. 2021, 42, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Geng, Z.; Zhang, L.L.; Zheng, F.; Zhou, Y.B.; Zhu, G.Q.; Xiong, X.Q. Chronic infusion of ELABELA alleviates vascular remodeling in spontaneously hypertensive rats via anti-inflammatory, anti-oxidative and anti-proliferative effects. Acta Pharmacol. Sin. 2022, 43, 2573–2584. [Google Scholar] [CrossRef]
- Ye, C.; Zheng, F.; Xu, T.; Wu, N.; Tong, Y.; Xiong, X.Q.; Zhou, Y.B.; Wang, J.J.; Chen, Q.; Li, Y.H.; et al. Norepinephrine acting on adventitial fibroblasts stimulates vascular smooth muscle cell proliferation via promoting small extracellular vesicle release. Theranostics 2022, 12, 4718–4733. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.N.; Kallenbach, J.G.; Orciuoli, H.M.; Paris, N.D.; Bachman, J.F.; Johnston, C.J.; Hernady, E.; Williams, J.P.; Dirksen, R.T.; Chakkalakal, J.V. Endurance exercise attenuates juvenile irradiation-induced skeletal muscle functional decline and mitochondrial stress. Skelet. Muscle 2022, 12, 8. [Google Scholar] [CrossRef]
- Tang, L.L.; Huang, C.L.; Zhang, N.; Jiang, W.; Wu, Y.S.; Huang, S.H.; Mao, Y.P.; Liu, Q.; Li, J.B.; Liang, S.Q.; et al. Elective upper-neck versus whole-neck irradiation of the uninvolved neck in patients with nasopharyngeal carcinoma: An open-label, non-inferiority, multicentre, randomised phase 3 trial. Lancet Oncol. 2022, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.W.; Wong, V.K.; Lo, H.H.; de Seabra Rodrigues Dias, I.R.; Leung, E.L.; Law, B.Y.; Liu, L. Natural products-based polypharmacological modulation of the peripheral immune system for the treatment of neuropsychiatric disorders. Pharmacol. Ther. 2020, 208, 107480. [Google Scholar] [CrossRef]
- Allen, H.L.; Deepe, G.S., Jr. B cells and CD4-CD8- T cells are key regulators of the severity of reactivation histoplasmosis. J. Immunol. 2006, 177, 1763–1771. [Google Scholar] [CrossRef]
- Verma, S.; Dutta, A.; Dahiya, A.; Kalra, N. Quercetin-3-Rutinoside alleviates radiation-induced lung inflammation and fibrosis via regulation of NF-kappaB/TGF-beta1 signaling. Phytomedicine 2022, 99, 154004. [Google Scholar] [CrossRef]
- Bang, E.; Kim, D.H.; Chung, H.Y. Protease-activated receptor 2 induces ROS-mediated inflammation through Akt-mediated NF-kappaB and FoxO6 modulation during skin photoaging. Redox Biol. 2021, 44, 102022. [Google Scholar] [CrossRef]
- Rump, A.; Becker, B.; Eder, S.; Lamkowski, A.; Abend, M.; Port, M. Medical management of victims contaminated with radionuclides after a “dirty bomb” attack. Mil. Med. Res. 2018, 5, 27. [Google Scholar] [CrossRef]
- Mansour, S.Z.; El-Kabany, H. Effects of Fructus Piperis Longi extract on fibrotic liver of gamma-irradiated rats. Chin. Med. 2009, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, M.E.; Vanpouille-Box, C.; Melero, I.; Formenti, S.C.; Demaria, S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018, 39, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, H.; Wu, S.; Gao, L.; Gao, Y.; Peng, R.; Cui, X.; Xiong, C.; Hu, W.; Wang, D. Molecular mechanism of damage and repair of mouse thymus lymphocytes induced by radiation. Chin. Med. J. 2002, 115, 1070–1073. [Google Scholar]
- Yang, H.; Huang, F.; Tao, Y.; Zhao, X.; Liao, L.; Tao, X. Simvastatin ameliorates ionizing radiation-induced apoptosis in the thymus by activating the AKT/sirtuin 1 pathway in mice. Int. J. Mol. Med. 2017, 40, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, M.; Deng, X.; Da, Q.; Li, M.; Huang, H.; Zhao, L.; Jing, L.; Wang, H. Anti irradiation nanoparticles shelter immune organ from radio-damage via preventing the IKK/IkappaB/NF-kappaB activation. Mol. Cancer 2024, 23, 234. [Google Scholar] [CrossRef]
- Sheng, H.; Huang, Y.; Xiao, Y.; Zhu, Z.; Shen, M.; Zhou, P.; Guo, Z.; Wang, J.; Wang, H.; Dai, W.; et al. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J. Immunother. Cancer 2020, 8, e000340. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Z.; Qi, F.; Wang, D. Applications of human amniotic fluid stem cells in wound healing. Chin. Med. J. 2022, 135, 2272–2281. [Google Scholar] [CrossRef]
- Fukutake, M.; Ochiai, D.; Masuda, H.; Abe, Y.; Sato, Y.; Otani, T.; Sakai, S.; Aramaki-Hattori, N.; Shimoda, M.; Matsumoto, T.; et al. Human amniotic fluid stem cells have a unique potential to accelerate cutaneous wound healing with reduced fibrotic scarring like a fetus. Hum. Cell 2019, 32, 51–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Liu, Y.; Chen, Z.; Li, X.; Tang, L.; Li, J.; Duan, M.; Zhang, G. Human Amniotic Fluid Stem Cell-Derived Exosomes as a Novel Cell-Free Therapy for Cutaneous Regeneration. Front. Cell Dev. Biol. 2021, 9, 685873. [Google Scholar] [CrossRef]
- Karcher, D.M.; McMurtry, J.P.; Applegate, T.J. Developmental changes in amniotic and allantoic fluid insulin-like growth factor (IGF)-I and -II concentrations of avian embryos. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 404–409. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, W.; Wang, J.; Zhang, M.; Yang, S.; Tian, Y.; Vidyasagar, S.; Pena, L.A.; Zhang, K.; Cao, Y.; et al. Mitigation effect of an FGF-2 peptide on acute gastrointestinal syndrome after high-dose ionizing radiation. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Dos Santos, H.T.; Maslow, F.; Small, T.; Samuel, R.Z.; Lei, P.; Andreadis, S.T.; Baker, O.J. Fibrin hydrogels fortified with FGF-7/10 and laminin-1 peptides promote regeneration of irradiated salivary glands. Acta Biomater. 2023, 172, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, J.; Li, J.; Bwalya, C.; Xu, Y.; Niu, Y.; Zhang, Y.; Wu, J.; Xu, Y.; Chen, J.; et al. RhFGF21 Protects Epidermal Cells against UVB-Induced Apoptosis through Activating AMPK-Mediated Autophagy. Int. J. Mol. Sci. 2022, 23, 12466. [Google Scholar] [CrossRef] [PubMed]
- Bussink, J.; Kaanders, J.H.; van der Kogel, A.J. Microenvironmental transformations by VEGF- and EGF-receptor inhibition and potential implications for responsiveness to radiotherapy. Radiother. Oncol. 2007, 82, 10–17. [Google Scholar] [CrossRef]
- Toulany, M.; Minjgee, M.; Kehlbach, R.; Chen, J.; Baumann, M.; Rodemann, H.P. ErbB2 expression through heterodimerization with erbB1 is necessary for ionizing radiation- but not EGF-induced activation of Akt survival pathway. Radiother. Oncol. 2010, 97, 338–345. [Google Scholar] [CrossRef]
- Abdollahi, A.; Li, M.; Ping, G.; Plathow, C.; Domhan, S.; Kiessling, F.; Lee, L.B.; McMahon, G.; Grone, H.J.; Lipson, K.E.; et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J. Exp. Med. 2005, 201, 925–935. [Google Scholar] [CrossRef]
- Alexandru, O.; Sevastre, A.S.; Castro, J.; Artene, S.A.; Tache, D.E.; Purcaru, O.S.; Sfredel, V.; Tataranu, L.G.; Dricu, A. Platelet-Derived Growth Factor Receptor and Ionizing Radiation in High Grade Glioma Cell Lines. Int. J. Mol. Sci. 2019, 20, 4663. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Yang, H.; Wu, Y.; Zhao, Y.; Xin, W.; Han, D.; Sun, N.; Ye, C. Therapeutic Potential of Chick Early Amniotic Fluid in Mitigating Ionizing-Radiation-Induced Damage. Biomedicines 2025, 13, 1253. https://doi.org/10.3390/biomedicines13051253
Zhang K, Yang H, Wu Y, Zhao Y, Xin W, Han D, Sun N, Ye C. Therapeutic Potential of Chick Early Amniotic Fluid in Mitigating Ionizing-Radiation-Induced Damage. Biomedicines. 2025; 13(5):1253. https://doi.org/10.3390/biomedicines13051253
Chicago/Turabian StyleZhang, Ke, Hai Yang, Yueyue Wu, Yining Zhao, Wenxu Xin, Deshen Han, Ning Sun, and Chao Ye. 2025. "Therapeutic Potential of Chick Early Amniotic Fluid in Mitigating Ionizing-Radiation-Induced Damage" Biomedicines 13, no. 5: 1253. https://doi.org/10.3390/biomedicines13051253
APA StyleZhang, K., Yang, H., Wu, Y., Zhao, Y., Xin, W., Han, D., Sun, N., & Ye, C. (2025). Therapeutic Potential of Chick Early Amniotic Fluid in Mitigating Ionizing-Radiation-Induced Damage. Biomedicines, 13(5), 1253. https://doi.org/10.3390/biomedicines13051253





