Bioinformatics-Guided Experimental Validation Identifies NQO1 as a Senescence-Ferroptosis Hub in Liver Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Gene Identification
2.2. Gene Enrichment Analysis of Differential Genes in LF-Aging-Ferroptosis
2.3. Identification of hub DEGs
2.4. Immune Infiltration Analysis
2.5. Molecular Docking
2.6. Animal Models
2.7. Cell Culture
2.8. Antibodies and Reagents
2.9. RNA Interference and Transfection
2.10. Real-Time Fluorescence Quantitative PCR (RT-qPCR)
2.11. Western Blot (WB)
2.12. Histological Analysis
2.13. Immunofluorescence Staining
2.14. Cell Cycle Analysis
2.15. Senescence Associated β-Gal Staining (SA-β-Gal Staining)
2.16. Statistical Analysis
3. Results
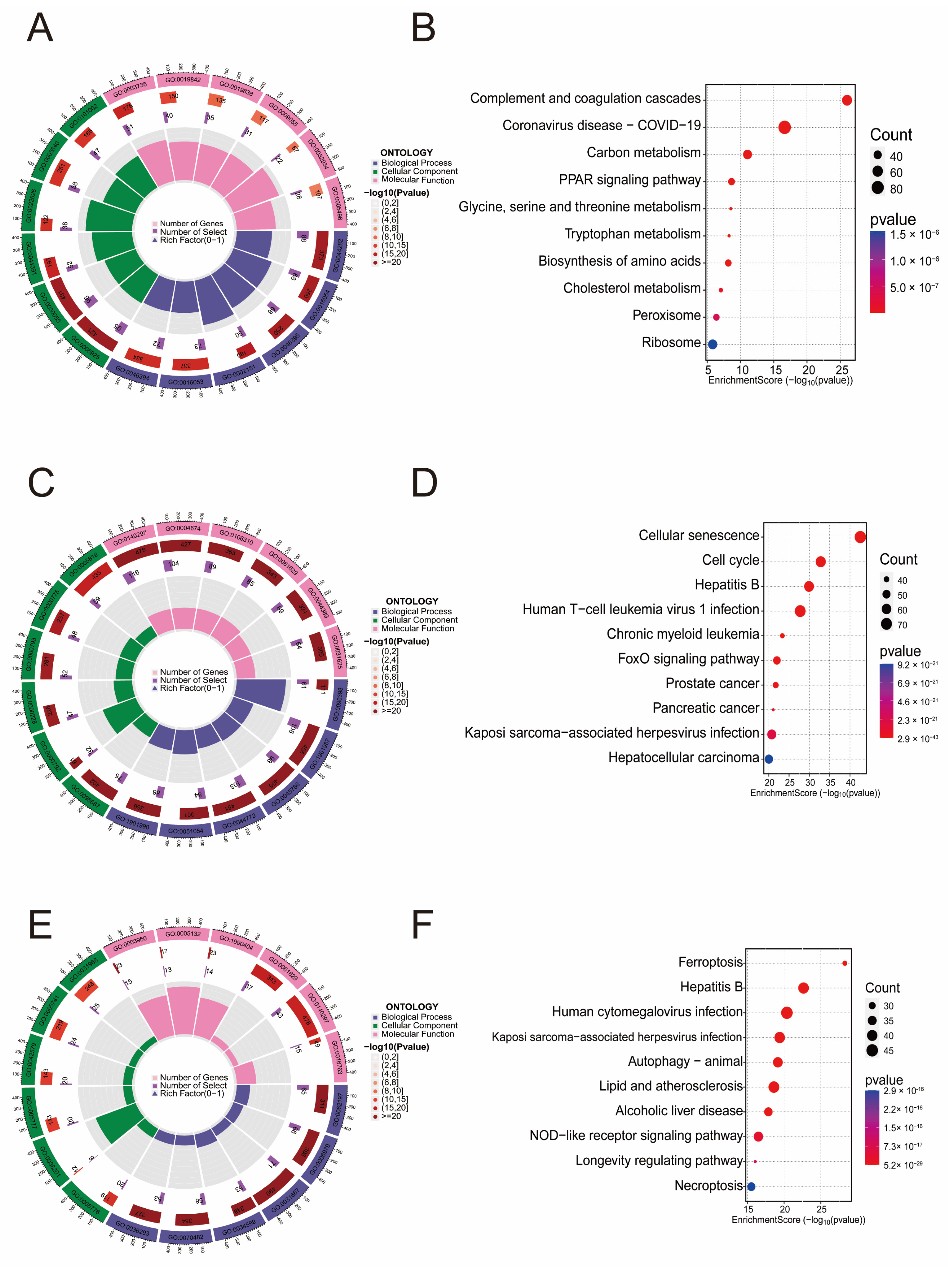
3.1. Enrichment Analysis of Genes Related to LF, Aging, and Ferroptosis
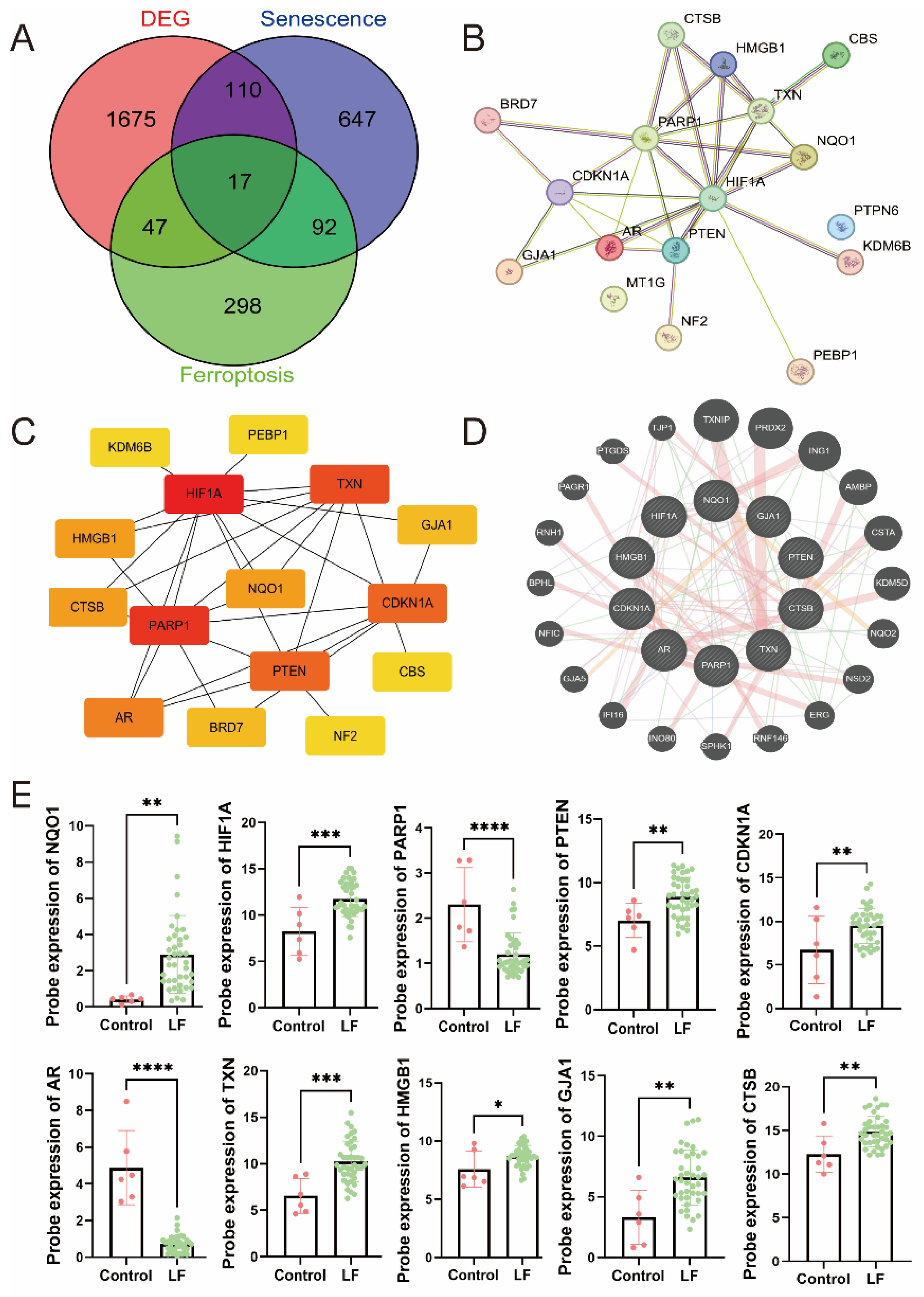
3.2. Identification of Hub DEGs Related to LF, Aging, and Ferroptosis
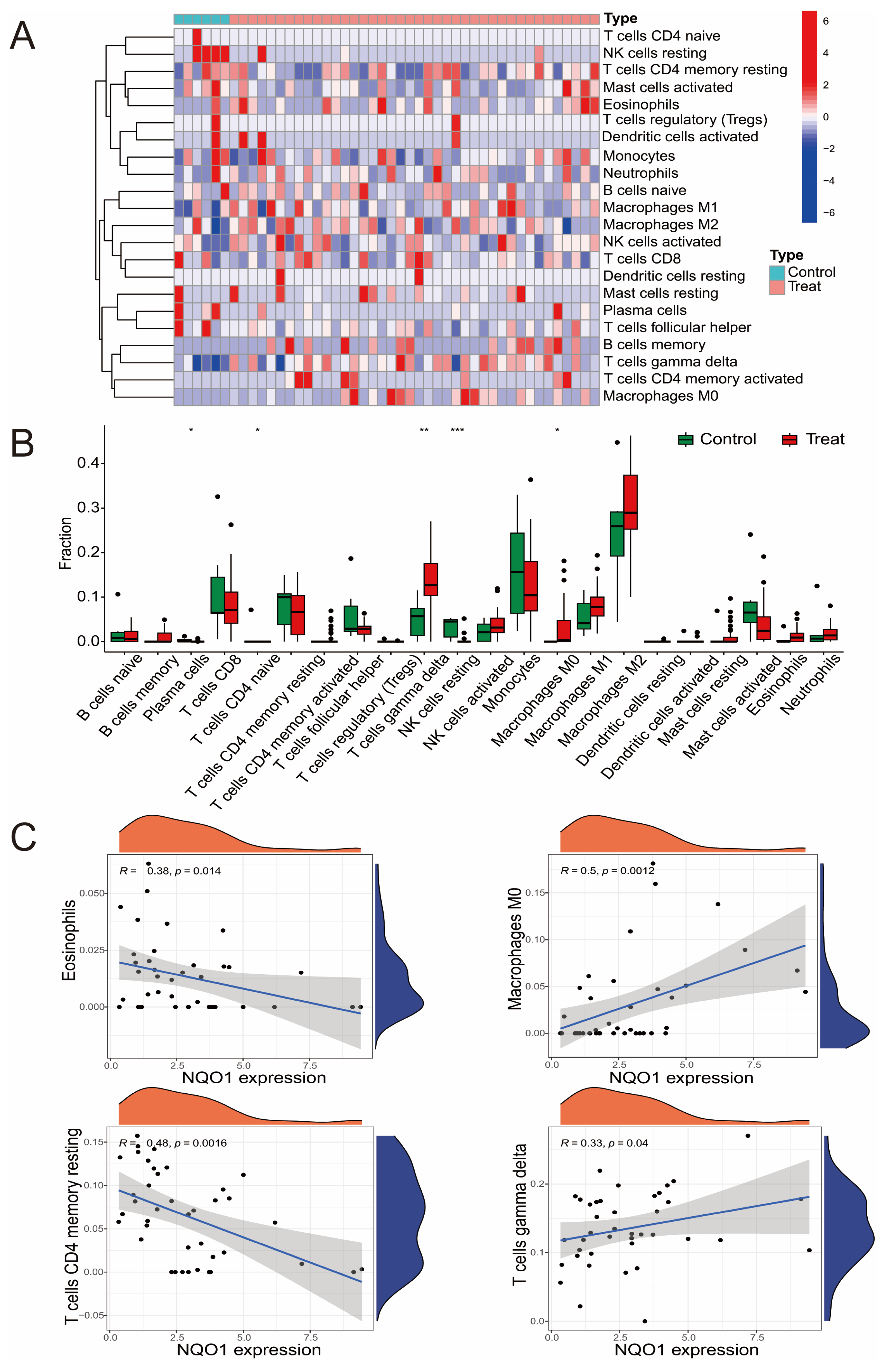
3.3. The Relationship Between Hub DEGs and Immune Cells
3.4. Clinical Relevance of NQO1
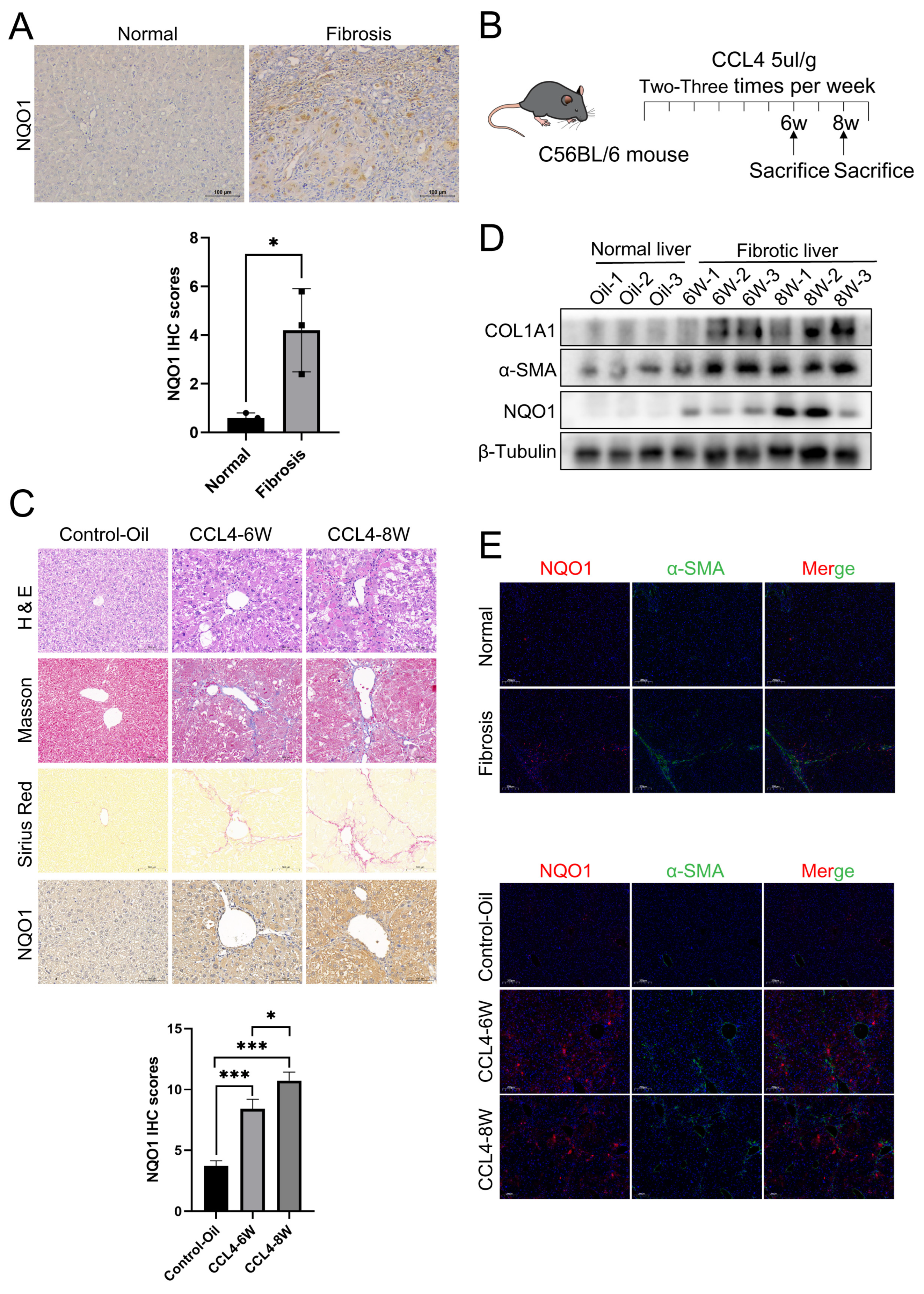
3.5. The Expression of NQO1 Is Elevated in LF
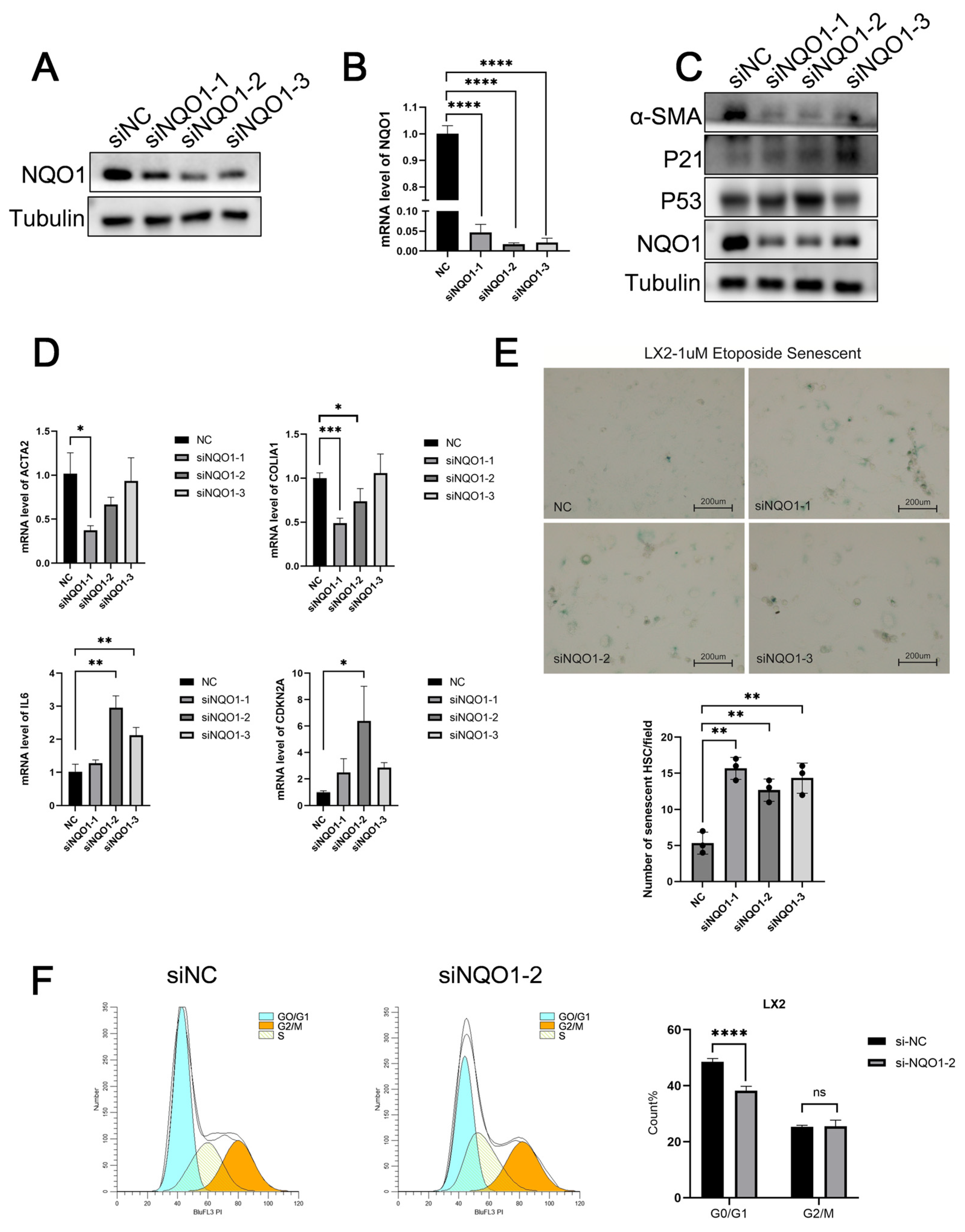
3.6. NQO1 Knockdown Induces Senescence and Suppresses LF
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | biological process |
| CC | cellular component |
| CCl4 | carbon tetrachloride |
| DAB | diaminobenzidine |
| DEGs | differential Expressed Genes |
| ECM | extensive extracellular matrix |
| GEO | Gene Expression Omnibus |
| GWAS | genome-wide association study |
| GO | Gene Ontology |
| H&E | hematoxylin and eosin |
| HSCs | hepatic stellate cells |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LF | liver fibrosis |
| MF | molecular function |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| PPI | protein-protein interaction |
| PSR | Picro Sirius Red |
| RT-qPCR | Real-time fluorescence quantitative PCR |
| SASPs | senescence-associated secretory phenotypes |
| Tregs | regulatory T cells |
| WB | Western blot |
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Sanfeliu-Redondo, D.; Gibert-Ramos, A.; Gracia-Sancho, J. Cell senescence in liver diseases: Pathological mechanism and theranostic opportunity. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 477–492. [Google Scholar] [CrossRef]
- López-Otín, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 2023, 35, 12–35. [Google Scholar] [CrossRef]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Chen, M.; Zhao, Y.; Zhang, B.; Chen, X.; Zhao, J.; Liang, H.; Chen, Q. Nano-encapsulation of drugs to target hepatic stellate cells: Toward precision treatments of liver fibrosis. J. Control. Release 2024, 376, 318–336. [Google Scholar] [CrossRef]
- Shojaie, L.; Iorga, A.; Dara, L. Cell Death in Liver Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef]
- Lu, J.L.; Yu, C.X.; Song, L.J. Programmed cell death in hepatic fibrosis: Current and perspectives. Cell Death Discov. 2023, 9, 449. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Boaru, D.L.; Minaya-Bravo, A.M.; De Castro-Martinez, P.; Fraile-Martinez, O.; Garcia-Montero, C.; Cobo-Prieto, D.; Barrena-Blázquez, S.; Lopez-Gonzalez, L.; Albillos, A.; et al. Improving understanding of ferroptosis: Molecular mechanisms, connection with cellular senescence and implications for aging. Heliyon 2024, 10, e39684. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Feng, B.; Yu, J.; Yan, L.; Che, L.; Zhuo, Y.; Luo, Y.; Yu, B.; Wu, D.; Chen, D. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol. 2021, 46, 102131. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wei, C.; Liu, G.; Zhang, L.; Li, J.; Li, L.; Cai, S.; Fang, L. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell Prolif. 2022, 55, e13158. [Google Scholar] [CrossRef]
- Dong, Y.; Li, S.; Sun, X.; Wang, Y.; Lu, T.; Wo, Y.; Leng, X.; Kong, D.; Jiao, W. Desmoglein 3 and Keratin 14 for Distinguishing Between Lung Adenocarcinoma and Lung Squamous Cell Carcinoma. Onco Targets Ther. 2020, 13, 11111–11124. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, Z.; Zhang, W.; Su, G.; Yang, P. Integrated Analysis of Key Pathways and Drug Targets Associated With Vogt-Koyanagi-Harada Disease. Front. Immunol. 2020, 11, 587443. [Google Scholar] [CrossRef]
- Gorochowski, T.E.; Grierson, C.S.; di Bernardo, M. Organization of feed-forward loop motifs reveals architectural principles in natural and engineered networks. Sci. Adv. 2018, 4, eaap9751. [Google Scholar] [CrossRef]
- Yang, C.; Wu, Q.; Huang, K.; Wang, X.; Yu, T.; Liao, X.; Huang, J.; Zhu, G.; Gong, Y.; Han, C.; et al. Genome-Wide Profiling Reveals the Landscape of Prognostic Alternative Splicing Signatures in Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2019, 9, 511. [Google Scholar] [CrossRef]
- Wisdom, A.J.; Mowery, Y.M.; Hong, C.S.; Himes, J.E.; Nabet, B.Y.; Qin, X.; Zhang, D.; Chen, L.; Fradin, H.; Patel, R.; et al. Single cell analysis reveals distinct immune landscapes in transplant and primary sarcomas that determine response or resistance to immunotherapy. Nat. Commun. 2020, 11, 6410. [Google Scholar] [CrossRef]
- Seedorf, K.; Weber, C.; Vinson, C.; Berger, S.; Vuillard, L.M.; Kiss, A.; Creusot, S.; Broux, O.; Geant, A.; Ilic, C.; et al. Selective disruption of NRF2-KEAP1 interaction leads to NASH resolution and reduction of liver fibrosis in mice. JHEP Rep. 2023, 5, 100651. [Google Scholar] [CrossRef]
- Xia, J.; Hu, J.N.; Wang, Z.; Cai, E.B.; Ren, S.; Wang, Y.P.; Lei, X.J.; Li, W. Based on network pharmacology and molecular docking to explore the protective effect of Epimedii Folium extract on cisplatin-induced intestinal injury in mice. Front. Pharmacol. 2022, 13, 1040504. [Google Scholar] [CrossRef]
- He, S.; Luo, Y.; Ma, W.; Wang, X.; Yan, C.; Hao, W.; Fang, Y.; Su, H.; Lai, B.; Liu, J.; et al. Endothelial POFUT1 controls injury-induced liver fibrosis by repressing fibrinogen synthesis. J. Hepatol. 2024, 81, 135–148. [Google Scholar] [CrossRef]
- Overstreet, J.M.; Wang, Y.; Wang, X.; Niu, A.; Gewin, L.S.; Yao, B.; Harris, R.C.; Zhang, M.Z. Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis. Faseb. J. 2017, 31, 4407–4421. [Google Scholar] [CrossRef]
- De Martin, E.; Fulgenzi, C.A.M.; Celsa, C.; Laurent-Bellue, A.; Torkpour, A.; Lombardi, P.; D’Alessio, A.; Pinato, D.J. Immune checkpoint inhibitors and the liver: Balancing therapeutic benefit and adverse events. Gut 2024, 6, 332125. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, D.; Ma, K.; Wu, X.; Hao, H.; Jiang, S. NAD(P)H:Quinone Oxidoreductase 1 (NQO1) as a Therapeutic and Diagnostic Target in Cancer. J. Med. Chem. 2018, 61, 6983–7003. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef]
- Ji, X.; Luo, H.; Li, X.; Wang, S.; Xia, L.; Ni, M.; Wang, J.; Peng, C.; Wu, X.; Tan, R.; et al. Structural characterization, anti-aging activity and mechanisms investigation in vivo of a polysaccharide from Anthriscus sylvestris. Int. J. Biol. Macromol. 2024, 279 Pt 3, 135256. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L. Navigating the complex role of senescence in liver disease. Chin. Med J. 2024, 137, 3061–3072. [Google Scholar] [CrossRef]
- Almalki, W.H.; Almujri, S.S. Aging, ROS, and cellular senescence: A trilogy in the progression of liver fibrosis. Biogerontology 2024, 26, 10. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Li, F.; Timchenko, N.; Tsai, R.Y.L. Transcriptional control of a stem cell factor nucleostemin in liver regeneration and aging. PLoS ONE 2024, 19, e0310219. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, X.; Qin, B.; Hu, R.; Miao, R.; Zhou, Y.; Wang, L.; Liu, T. Targeting NQO1 induces ferroptosis and triggers anti-tumor immunity in immunotherapy-resistant KEAP1-deficient cancers. Drug Resist. Updat. 2024, 77, 101160. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Lu, L.; Pan, S.S.; Wei, X.Q.; Miao, R.R.; Liu, X.H.; Xue, M.; Lin, X.K.; Xu, H.L. Targeting NQO1/GPX4-mediated ferroptosis by plumbagin suppresses in vitro and in vivo glioma growth. Br. J. Cancer 2022, 127, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Yang, W.; Sun, C.; Wang, X. Dissecting the potential role of ferroptosis in liver diseases: An updated review. Free. Radic. Res. 2023, 57, 282–293. [Google Scholar] [CrossRef]
- Aizawa, S.; Brar, G.; Tsukamoto, H. Cell Death and Liver Disease. Gut Liver 2020, 14, 20–29. [Google Scholar] [CrossRef]
- Xing, Z.Y.; Zhang, C.J.; Liu, L.J. Targeting both ferroptosis and pyroptosis may represent potential therapies for acute liver failure. World J. Gastroenterol. 2024, 30, 3791–3798. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Yuan, Y.M.; Zhao, Z.H.; Yao, Q.H.; Ye, X.Q.; Wang, Y.Y.; Liu, H.M.; Jha, R.; Balasubramanian, B.; Liu, W.C. Phlorotannin Alleviates Liver Injury by Regulating Redox Balance, Apoptosis, and Ferroptosis of Broilers under Heat Stress. Antioxidants 2024, 13, 1048. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fu, C.; Yang, Z.; Tan, Y.; Li, H.; Zhang, X.; Chen, M.; Peng, F.; Li, N. Bioinformatics-Guided Experimental Validation Identifies NQO1 as a Senescence-Ferroptosis Hub in Liver Fibrosis. Biomedicines 2025, 13, 1249. https://doi.org/10.3390/biomedicines13051249
Zhang X, Fu C, Yang Z, Tan Y, Li H, Zhang X, Chen M, Peng F, Li N. Bioinformatics-Guided Experimental Validation Identifies NQO1 as a Senescence-Ferroptosis Hub in Liver Fibrosis. Biomedicines. 2025; 13(5):1249. https://doi.org/10.3390/biomedicines13051249
Chicago/Turabian StyleZhang, Xinying, Chunmeng Fu, Ziyue Yang, Yue Tan, Huan Li, Xiangqian Zhang, Mengru Chen, Fang Peng, and Ning Li. 2025. "Bioinformatics-Guided Experimental Validation Identifies NQO1 as a Senescence-Ferroptosis Hub in Liver Fibrosis" Biomedicines 13, no. 5: 1249. https://doi.org/10.3390/biomedicines13051249
APA StyleZhang, X., Fu, C., Yang, Z., Tan, Y., Li, H., Zhang, X., Chen, M., Peng, F., & Li, N. (2025). Bioinformatics-Guided Experimental Validation Identifies NQO1 as a Senescence-Ferroptosis Hub in Liver Fibrosis. Biomedicines, 13(5), 1249. https://doi.org/10.3390/biomedicines13051249






