ML210 Antagonizes ABCB1- Not ABCG2-Mediated Multidrug Resistance in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Cytotoxicity Assay
2.3. Cell Cycle Assay
2.4. Drug Accumulation Assay
2.5. Western Blot Assay
2.6. Docking Analysis
2.7. Statistical Analysis
3. Results
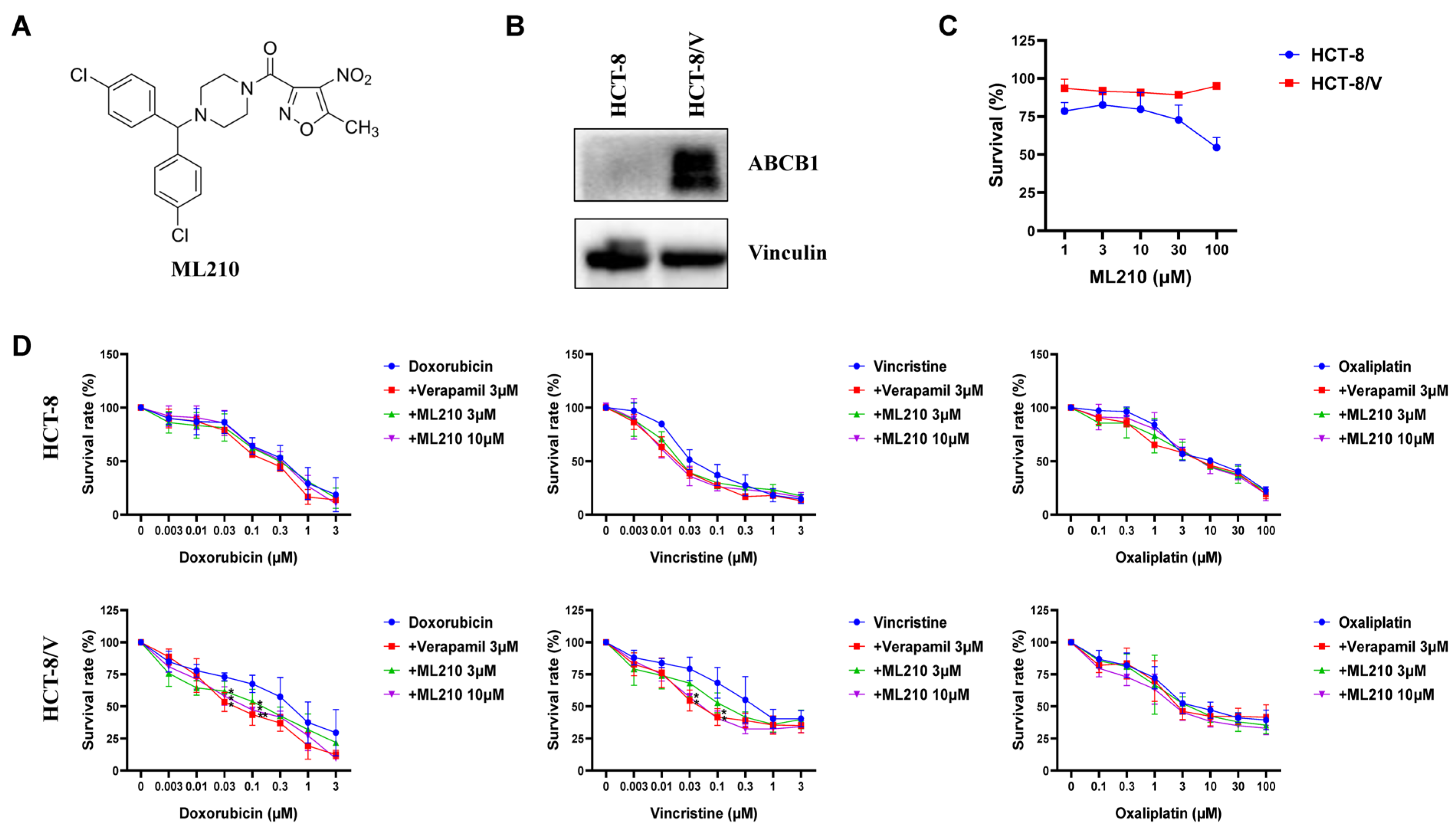
3.1. ML210 Antagonizes ABCB1-Mediated MDR in CRC Cells
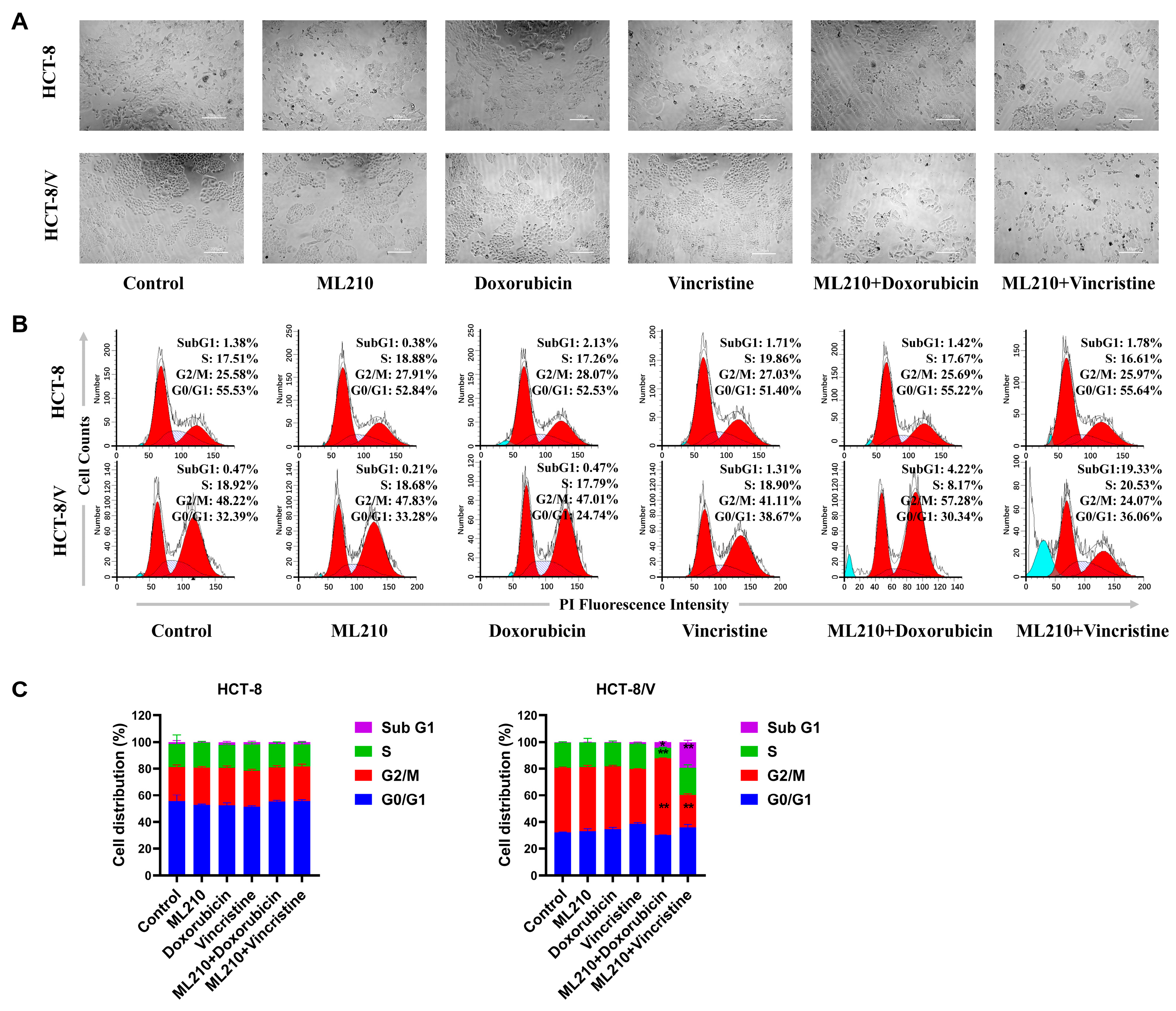
3.2. The Combination of ML210 with Doxorubicin or Vincristine Induces Cell Cycle Arrest in ABCB1-Overexpressing CRC Cells
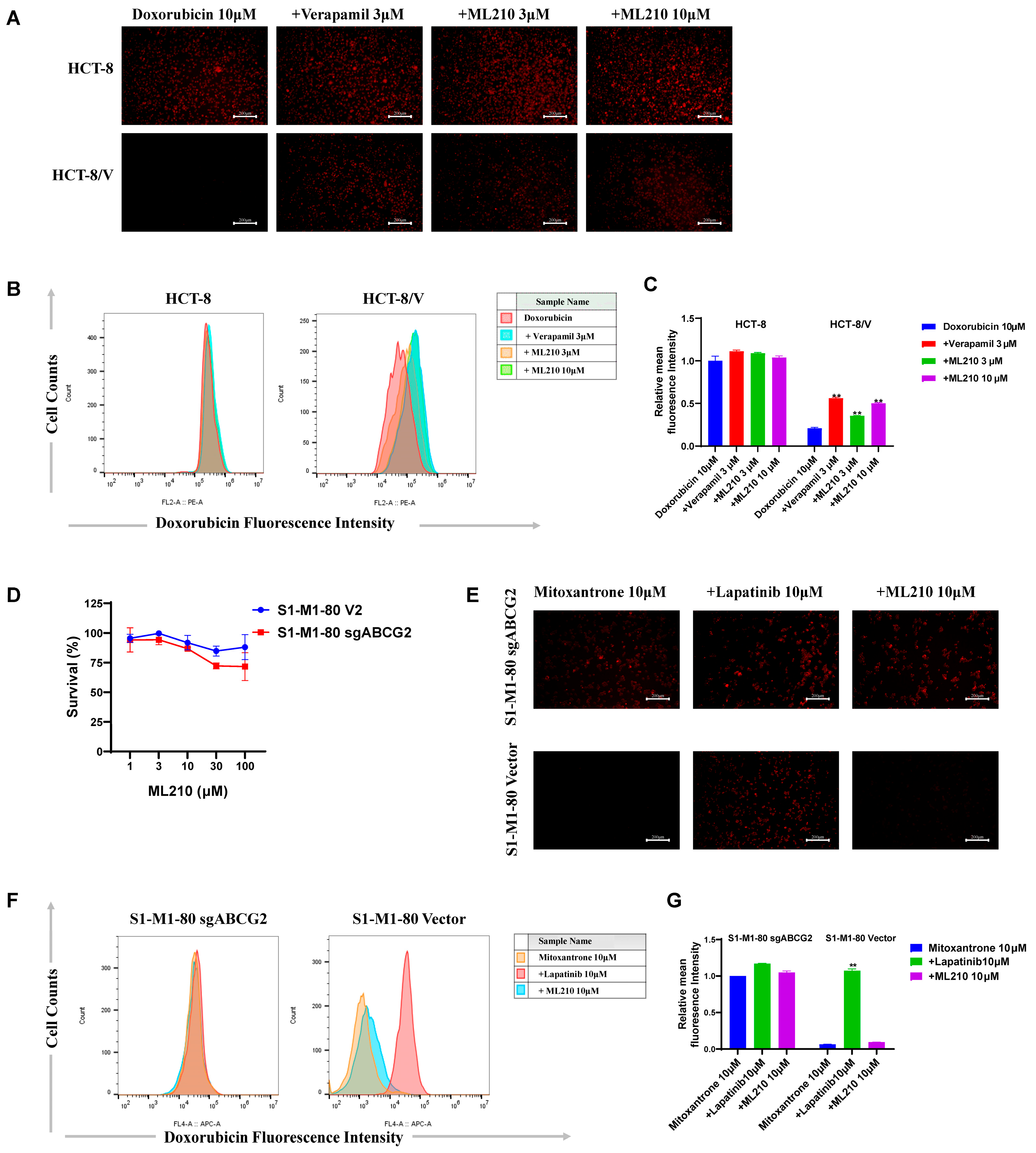
3.3. ML210 Enhances Doxorubicin Accumulation in ABCB1-Overexpressing CRC Cells and Does Not Enhance Mitoxantrane Accumulation in ABCG2-Overexpressing CRC Cells
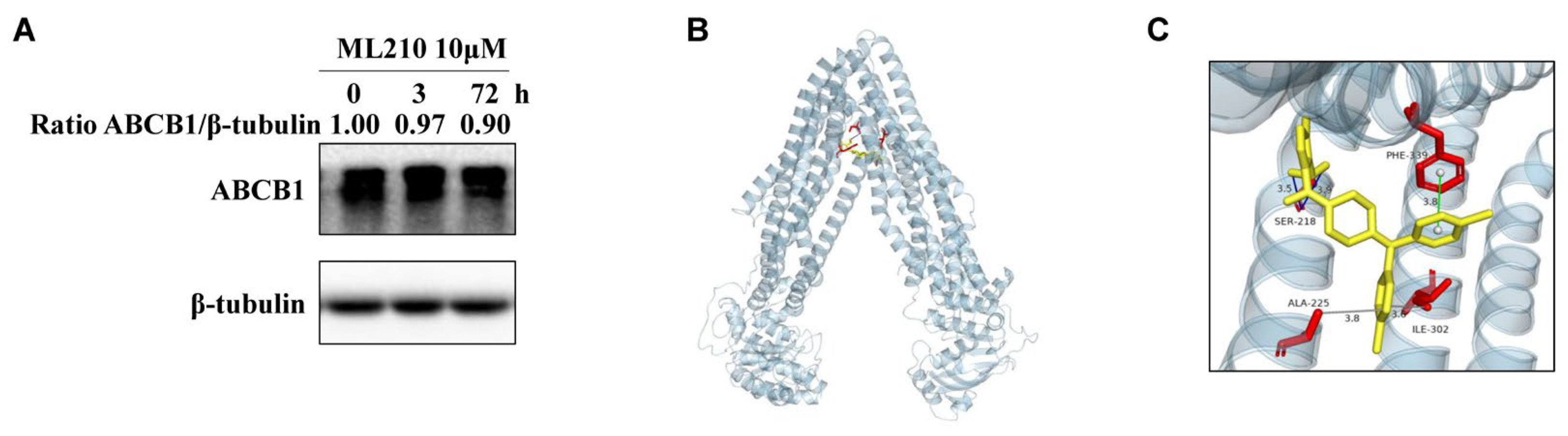
3.4. ML210 Does Not Affect ABCB1 Protein Levels and Its Binding Model with ABCB1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klimeck, L.; Heisser, T.; Hoffmeister, M.; Brenner, H. Colorectal cancer: A health and economic problem. Best Pract. Res. Clin. Gastroenterol. 2023, 66, 101839. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Chan, D.S.; Lau, R.; Aune, D.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE 2011, 6, e20456. [Google Scholar] [CrossRef]
- Krämer, H.U.; Schöttker, B.; Raum, E.; Brenner, H. Type 2 diabetes mellitus and colorectal cancer: Meta-analysis on sex-specific differences. Eur. J. Cancer 2012, 48, 1269–1282. [Google Scholar] [CrossRef]
- Miao, C.; Huang, Y.; Zhang, C.; Wang, X.; Wang, B.; Zhou, X.; Song, Y.; Wu, P.; Chen, Z.S.; Feng, Y. Post-translational modifications in drug resistance. Drug Resist. Updates 2025, 78, 101173. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, B.; Chen, Z.; Chen, Z.S. Progress in the studies on the molecular mechanisms associated with multidrug resistance in cancers. Acta Pharm. Sin. B 2023, 13, 982–997. [Google Scholar] [CrossRef]
- Luo, M.; Yang, X.; Chen, H.N.; Nice, E.C.; Huang, C. Drug resistance in colorectal cancer: An epigenetic overview. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188623. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Deng, Z.F.; Wu, X.T.; Lu, Y.; Zhu, Z.Y.; Wen, Q.; Zhang, W.; Zhang, H.Y.; Chen, X.Z.; Wu, Y.S.; et al. Low miR-224-5p in exosomes confers colorectal cancer 5-FU resistance by upregulating S100A4. Drug Resist. Updates 2025, 79, 101211. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Li, W.; Wang, Y.; Zhang, Z.; Zhang, L.; Zhang, W.; Xing, Y.; Zhou, C. Inhibition of CDK1 Overcomes Oxaliplatin Resistance by Regulating ACSL4-mediated Ferroptosis in Colorectal Cancer. Adv. Sci. 2023, 10, e2301088. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Dhillon, S. Regorafenib: A Review in Metastatic Colorectal Cancer. Drugs 2018, 78, 1133–1144. [Google Scholar] [CrossRef]
- Alkharsan, A.; Safaralizadeh, R.; Khalaj-Kondori, M.; HosseinpourFeizi, M. Examination of the effects of capecitabine treatment on the HT-29 colorectal cancer cell line and HCG 11, HCG 15, and HCG 18 lncRNAs in CRC patients before and after chemotherapy. Naunyn Schmiedebergs Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Magadmi, R.; Alyoubi, R.; Moshrif, T.; Bakhshwin, D.; Suliman, B.A.; Kamel, F.; Jamal, M.; Burzangi, A.S.; Basit, S. Polymorphisms in the Drug Transporter Gene ABCB1 Are Associated with Drug Response in Saudi Epileptic Pediatric Patients. Biomedicines 2023, 11, 2505. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Xu, B.Q.; Wang, Y.Y.; Zhang, P.W.; Shu, Y.B.; Shi, Z. GSK2606414 Sensitizes ABCG2-Overexpressing Multidrug-Resistant Colorectal Cancer Cells to Chemotherapeutic Drugs. Biomedicines 2023, 11, 3103. [Google Scholar] [CrossRef]
- Moinul, M.; Amin, S.A.; Jha, T.; Gayen, S. Updated chemical scaffolds of ABCG2 inhibitors and their structure-inhibition relationships for future development. Eur. J. Med. Chem. 2022, 241, 114628. [Google Scholar] [CrossRef]
- Mora Lagares, L.; Pérez-Castillo, Y.; Minovski, N.; Novič, M. Structure-Function Relationships in the Human P-Glycoprotein (ABCB1): Insights from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2021, 23, 362. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef]
- Chufan, E.E.; Kapoor, K.; Sim, H.M.; Singh, S.; Talele, T.T.; Durell, S.R.; Ambudkar, S.V. Multiple transport-active binding sites are available for a single substrate on human P-glycoprotein (ABCB1). PLoS ONE 2013, 8, e82463. [Google Scholar] [CrossRef] [PubMed]
- Skinner, K.T.; Palkar, A.M.; Hong, A.L. Genetics of ABCB1 in Cancer. Cancers 2023, 15, 4236. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.R.; Jeddi, F.; Soozangar, N.; Somi, M.H.; Shirmohamadi, M.; Khaze, V.; Samadi, N. Nrf2/P-glycoprotein axis is associated with clinicopathological characteristics in colorectal cancer. Biomed. Pharmacother. 2018, 104, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Badic, B.; Durand, S.; El Khoury, F.; De La Grange, P.; Gentien, D.; Simon, B.; Le Jossic-Corcos, C.; Corcos, L. Prognostic impact of cancer stem cell markers ABCB1, NEO1 and HIST1H2AE in colorectal cancer. Am. J. Transl. Res. 2020, 12, 5797–5807. [Google Scholar]
- Lei, Z.N.; Teng, Q.X.; Wu, Z.X.; Ping, F.F.; Song, P.; Wurpel, J.N.D.; Chen, Z.S. Overcoming multidrug resistance by knockout of ABCB1 gene using CRISPR/Cas9 system in SW620/Ad300 colorectal cancer cells. MedComm 2021, 2, 765–777. [Google Scholar] [CrossRef]
- Dahlmann, M.; Werner, R.; Kortüm, B.; Kobelt, D.; Walther, W.; Stein, U. Restoring Treatment Response in Colorectal Cancer Cells by Targeting MACC1-Dependent ABCB1 Expression in Combination Therapy. Front. Oncol. 2020, 10, 599. [Google Scholar] [CrossRef]
- Besse, A.; Stolze, S.C.; Rasche, L.; Weinhold, N.; Morgan, G.J.; Kraus, M.; Bader, J.; Overkleeft, H.S.; Besse, L.; Driessen, C. Carfilzomib resistance due to ABCB1/MDR1 overexpression is overcome by nelfinavir and lopinavir in multiple myeloma. Leukemia 2018, 32, 391–401. [Google Scholar] [CrossRef]
- Ye, P.; Xing, H.; Lou, F.; Wang, K.; Pan, Q.; Zhou, X.; Gong, L.; Li, D. Histone deacetylase 2 regulates doxorubicin (Dox) sensitivity of colorectal cancer cells by targeting ABCB1 transcription. Cancer Chemother. Pharmacol. 2016, 77, 613–621. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Wang, F.; Moyer, M.P.; Wei, Q.; Zhang, P.; Yang, Z.; Liu, W.; Zhang, H.; Chen, N.; et al. Long non-coding RNA CCAL regulates colorectal cancer progression by activating Wnt/β-catenin signalling pathway via suppression of activator protein 2α. Gut 2016, 65, 1494–1504. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, F.; Gu, X.; Feng, L.; Xu, M.; Li, T.; Liu, X.; Zhang, X. Binding of RNA m6A by IGF2BP3 triggers chemoresistance of HCT8 cells via upregulation of ABCB1. Am. J. Cancer Res. 2021, 11, 1428–1445. [Google Scholar]
- Schulz, J.A.; Hartz, A.M.S.; Bauer, B. ABCB1 and ABCG2 Regulation at the Blood-Brain Barrier: Potential New Targets to Improve Brain Drug Delivery. Pharmacol. Rev. 2023, 75, 815–853. [Google Scholar] [CrossRef] [PubMed]
- Myllynen, P.; Kummu, M.; Sieppi, E. ABCB1 and ABCG2 expression in the placenta and fetus: An interspecies comparison. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Walther, W.; Kobelt, D.; Bauer, L.; Aumann, J.; Stein, U. Chemosensitization by diverging modulation by short-term and long-term TNF-α action on ABCB1 expression and NF-κB signaling in colon cancer. Int. J. Oncol. 2015, 47, 2276–2285. [Google Scholar] [CrossRef]
- Wang, L.; Ji, H.; Ni, S.; Xu, J.; Zhang, Y.; Zhao, X.; Wu, X.; Tian, J.; Chen, J. Antimalarial activity and sensitization of chrysosplenetin against artemisinin-resistant genotype Plasmodium berghei K173 potentially via dual-mechanism of maintaining host P-glycoprotein homeostasis mediated by NF-κB p52 or PXR/CAR signaling pathways and regulating heme/haemozoin metabolism. Phytother. Res. 2023, 37, 2939–2956. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Wei, W.; Shi, M.; Xin, B.; Zhang, T.; Shen, X. Hypoxia regulates ABCG2 activity through the activivation of ERK1/2/HIF-1α and contributes to chemoresistance in pancreatic cancer cells. Cancer Biol. Ther. 2016, 17, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.W.; Fan, H.C.; Liu, J.M.; Fan, T.P.; Jing, J.; Yang, C.L.; Hsu, R.J. Estrogen Enhances the Expression of the Multidrug Transporter Gene ABCG2-Increasing Drug Resistance of Breast Cancer Cells through Estrogen Receptors. Int. J. Mol. Sci. 2017, 18, 163. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.C.; Chen, Y.; Shi, X.B.; Xing, Z.H.; He, Z.J.; Wang, S.T.; Liu, W.J.; Zhang, P.W.; Yu, Z.Z.; et al. AZ32 Reverses ABCG2-Mediated Multidrug Resistance in Colorectal Cancer. Front. Oncol. 2021, 11, 680663. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Shi, X.B.; Xing, Z.H.; He, Z.J.; Wang, S.T.; Li, Y.C.; Liu, W.J.; Zhang, P.W.; Yu, Z.Z.; et al. Inhibiting the Activity of ABCG2 by KU55933 in Colorectal Cancer. Recent Pat. Anticancer Drug Discov. 2022, 17, 387–395. [Google Scholar] [CrossRef]
- Fan, J.; To, K.K.W.; Chen, Z.S.; Fu, L. ABC transporters affects tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist. Updates 2023, 66, 100905. [Google Scholar] [CrossRef]
- Ni, C.; Hong, M. Oligomerization of drug transporters: Forms, functions, and mechanisms. Acta Pharm. Sin. B 2024, 14, 1924–1938. [Google Scholar] [CrossRef] [PubMed]
- Alketbi, L.; Al-Ali, A.; Talaat, I.M.; Hamid, Q.; Bajbouj, K. The Role of ATP-Binding Cassette Subfamily A in Colorectal Cancer Progression and Resistance. Int. J. Mol. Sci. 2023, 24, 1344. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Wang, J.Q.; Yang, Y.; Li, J.; Chen, Z.S. Natural Product as Substrates of ABC Transporters: A Review. Recent Pat. Anticancer Drug Discov. 2021, 16, 222–238. [Google Scholar] [CrossRef]
- Yan, X.J.; Gong, L.H.; Zheng, F.Y.; Cheng, K.J.; Chen, Z.S.; Shi, Z. Triterpenoids as reversal agents for anticancer drug resistance treatment. Drug Discov. Today 2014, 19, 482–488. [Google Scholar] [CrossRef]
- Shi, Z.; Tiwari, A.K.; Shukla, S.; Robey, R.W.; Kim, I.W.; Parmar, S.; Bates, S.E.; Si, Q.S.; Goldblatt, C.S.; Abraham, I.; et al. Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478. Biochem. Pharmacol. 2009, 77, 781–793. [Google Scholar] [CrossRef]
- Shi, Z.; Peng, X.X.; Kim, I.W.; Shukla, S.; Si, Q.S.; Robey, R.W.; Bates, S.E.; Shen, T.; Ashby, C.R., Jr.; Fu, L.W.; et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007, 67, 11012–11020. [Google Scholar] [CrossRef]
- Wu, Z.X.; Yang, Y.; Wang, J.Q.; Zhou, W.M.; Chen, J.; Fu, Y.G.; Patel, K.; Chen, Z.S.; Zhang, J.Y. Elevated ABCB1 Expression Confers Acquired Resistance to Aurora Kinase Inhibitor GSK-1070916 in Cancer Cells. Front. Pharmacol. 2020, 11, 615824. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.K.; Zhang, G.N.; Al Rihani, S.B.; Wei, M.N.; Gupta, P.; Zhang, X.Y.; Shukla, S.; Ambudkar, S.V.; Kaddoumi, A.; et al. Regorafenib overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter in colorectal cancer: In vitro and in vivo study. Cancer Lett. 2017, 396, 145–154. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, B.; Lin, Y.; Li, Y.D.; Wang, J.Q.; Chen, Z.; Ping, F.F.; Chen, Z.S. Ribociclib Inhibits P-gp-Mediated Multidrug Resistance in Human Epidermoid Carcinoma Cells. Front. Pharmacol. 2022, 13, 867128. [Google Scholar] [CrossRef]
- Shi, Z.; Jain, S.; Kim, I.W.; Peng, X.X.; Abraham, I.; Youssef, D.T.; Fu, L.W.; El Sayed, K.; Ambudkar, S.V.; Chen, Z.S. Sipholenol A, a marine-derived sipholane triterpene, potently reverses P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Cancer Sci. 2007, 98, 1373–1380. [Google Scholar] [CrossRef]
- Shi, Z.; Tiwari, A.K.; Shukla, S.; Robey, R.W.; Singh, S.; Kim, I.W.; Bates, S.E.; Peng, X.; Abraham, I.; Ambudkar, S.V.; et al. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011, 71, 3029–3041. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.G.; Zhang, Y.J.; Li, Y.; Zhao, J.M.; Zhang, W.J.; Jiang, Q.W.; Mei, X.L.; Xue, Y.Q.; Qin, W.M.; Yang, Y.; et al. Trametinib modulates cancer multidrug resistance by targeting ABCB1 transporter. Oncotarget 2015, 6, 15494–15509. [Google Scholar] [CrossRef]
- Lv, M.; Qiu, J.G.; Zhang, W.J.; Jiang, Q.W.; Qin, W.M.; Yang, Y.; Zheng, D.W.; Chen, Y.; Huang, J.R.; Wang, K.; et al. Wallichinine reverses ABCB1-mediated cancer multidrug resistance. Am. J. Trans. Res. 2016, 8, 2969. [Google Scholar]
- Fowers, K.D.; Kopecek, J. Targeting of multidrug-resistant human ovarian carcinoma cells with anti-P-glycoprotein antibody conjugates. Macromol. Biosci. 2012, 12, 502–514. [Google Scholar] [CrossRef]
- Nadali, F.; Pourfathollah, A.A.; Alimoghaddam, K.; Nikougoftar, M.; Rostami, S.; Dizaji, A.; Azizi, E.; Zomorodipour, A.; Ghavamzadeh, A. Multidrug resistance inhibition by antisense oligonucleotide against MDR1/mRNA in P-glycoprotein expressing leukemic cells. Hematology 2007, 12, 393–401. [Google Scholar] [CrossRef]
- Shi, Z.; Liang, Y.J.; Chen, Z.S.; Wang, X.W.; Wang, X.H.; Ding, Y.; Chen, L.M.; Yang, X.P.; Fu, L.W. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol. Ther. 2006, 5, 39–47. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, J.G.; Li, Y.; Di, J.M.; Zhang, W.J.; Jiang, Q.W.; Zheng, D.W.; Chen, Y.; Wei, M.N.; Huang, J.R.; et al. Targeting ABCB1-mediated tumor multidrug resistance by CRISPR/Cas9-based genome editing. Am. J. Transl. Res. 2016, 8, 3986–3994. [Google Scholar]
- Weïwer, M.; Bittker, J.A.; Lewis, T.A.; Shimada, K.; Yang, W.S.; MacPherson, L.; Dandapani, S.; Palmer, M.; Stockwell, B.R.; Schreiber, S.L.; et al. Development of small-molecule probes that selectively kill cells induced to express mutant RAS. Bioorg. Med. Chem. Lett. 2012, 22, 1822–1826. [Google Scholar] [CrossRef]
- Takemura, K.; Ikeda, K.; Miyake, H.; Sogame, Y.; Yasuda, H.; Okada, N.; Iwata, K.; Sakagami, J.; Yamaguchi, K.; Itoh, Y.; et al. Epithelial-Mesenchymal Transition Suppression by ML210 Enhances Gemcitabine Anti-Tumor Effects on PDAC Cells. Biomolecules 2025, 15, 70. [Google Scholar] [CrossRef]
- Azumi, M.; Kusama, K.; Yoshie, M.; Nakano, S.; Tsuru, A.; Kato, T.; Tamura, K. Involvement of ferroptosis in eribulin-induced cytotoxicity in ovarian clear cell carcinoma. Eur. J. Pharmacol. 2024, 971, 176544. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Li, H.; Wang, T.; Lu, F.; Liu, R.; Xie, G.; Song, L.; Huang, B.; Li, X.; et al. Enzalutamide inhibits PEX10 function and sensitizes prostate cancer cells to ROS activators. Cell Death Dis. 2024, 15, 559. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.; Barbosa, D.J.; Benfeito, S.; Silva, V.; Chavarria, D.; Borges, F.; Remião, F.; Silva, R. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol. Ther. 2023, 244, 108373. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, S. A Multifunctional γ-Polyglutamic Acid Hydrogel for Combined Tumor Photothermal and Chemotherapy. Gels 2025, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Z.; Li, K.; Xie, G.; Feng, Y.; Wang, Z.; Li, N.; Liu, R.; Ding, Y.; Wang, J.; et al. TrkA promotes MDM2-mediated AGPS ubiquitination and degradation to trigger prostate cancer progression. J. Exp. Clin. Cancer Res. 2024, 43, 16. [Google Scholar] [CrossRef]
- Cheff, D.M.; Huang, C.; Scholzen, K.C.; Gencheva, R.; Ronzetti, M.H.; Cheng, Q.; Hall, M.D.; Arnér, E.S.J. The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1. Redox Biol. 2023, 62, 102703. [Google Scholar] [CrossRef]
- Hu, Q.; Zhu, W.; Du, J.; Ge, H.; Zheng, J.; Long, S.; Fan, J.; Peng, X. A GPX4-targeted photosensitizer to reverse hypoxia-induced inhibition of ferroptosis for non-small cell lung cancer therapy. Chem. Sci. 2023, 14, 9095–9100. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Lin, S.; Liu, Y.; Li, W. Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging 2021, 13, 13515–13534. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-C.; Xiong, Y.-M.; Long, Z.-P.; Huang, Y.-P.; Shu, Y.-B.; He, K.; Sun, H.-Y.; Shi, Z. ML210 Antagonizes ABCB1- Not ABCG2-Mediated Multidrug Resistance in Colorectal Cancer. Biomedicines 2025, 13, 1245. https://doi.org/10.3390/biomedicines13051245
Li Y-C, Xiong Y-M, Long Z-P, Huang Y-P, Shu Y-B, He K, Sun H-Y, Shi Z. ML210 Antagonizes ABCB1- Not ABCG2-Mediated Multidrug Resistance in Colorectal Cancer. Biomedicines. 2025; 13(5):1245. https://doi.org/10.3390/biomedicines13051245
Chicago/Turabian StyleLi, Yan-Chi, Yu-Meng Xiong, Ze-Ping Long, Yi-Ping Huang, Yu-Bin Shu, Ke He, Hong-Yan Sun, and Zhi Shi. 2025. "ML210 Antagonizes ABCB1- Not ABCG2-Mediated Multidrug Resistance in Colorectal Cancer" Biomedicines 13, no. 5: 1245. https://doi.org/10.3390/biomedicines13051245
APA StyleLi, Y.-C., Xiong, Y.-M., Long, Z.-P., Huang, Y.-P., Shu, Y.-B., He, K., Sun, H.-Y., & Shi, Z. (2025). ML210 Antagonizes ABCB1- Not ABCG2-Mediated Multidrug Resistance in Colorectal Cancer. Biomedicines, 13(5), 1245. https://doi.org/10.3390/biomedicines13051245





