Cardiac Amyloidosis: A Narrative Review of Diagnostic Advances and Emerging Therapies
Abstract
1. Introduction
2. Materials and Methods
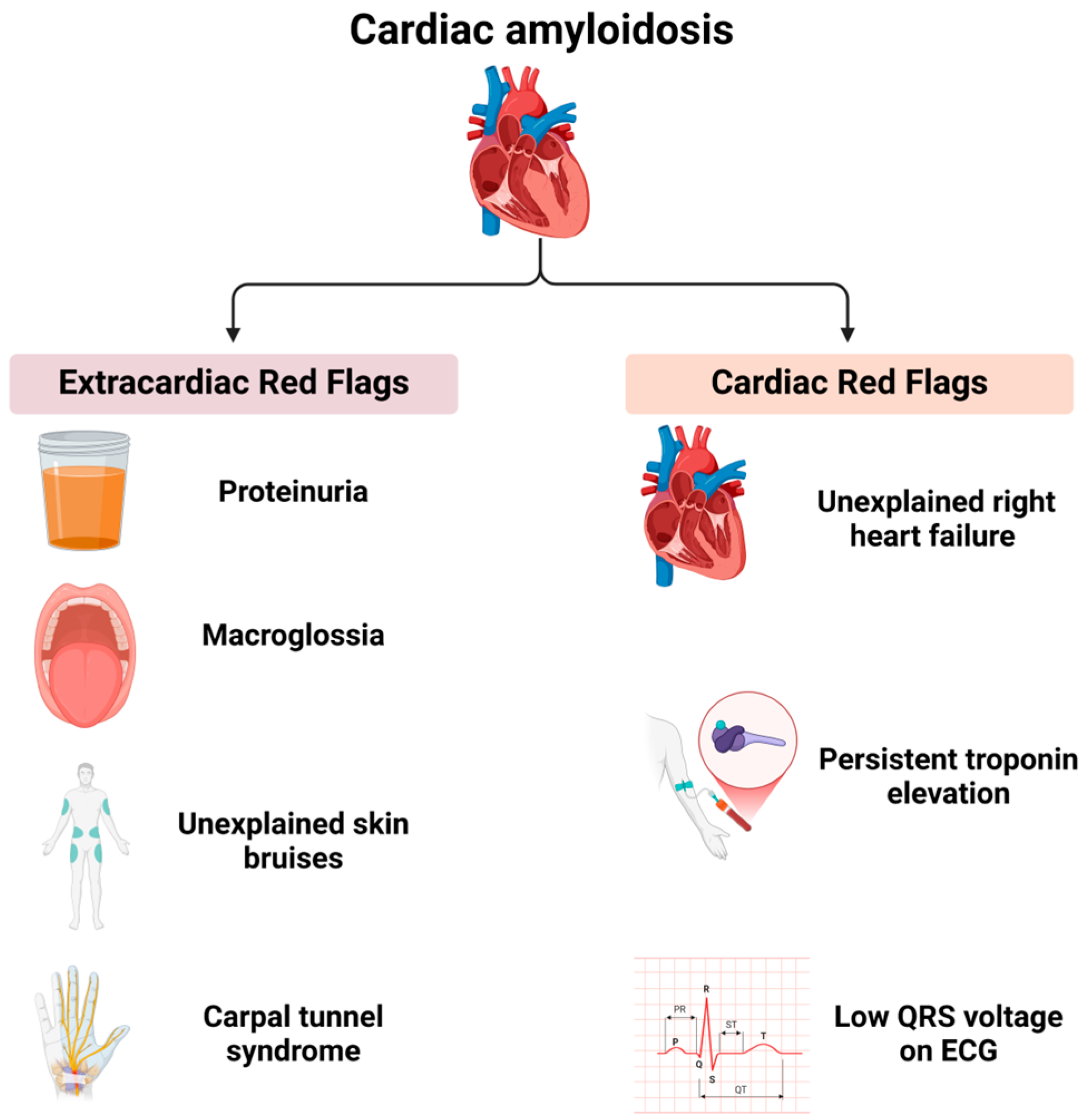
3. Clinical and Multimodal Imaging Features of Cardiac Amyloidosis
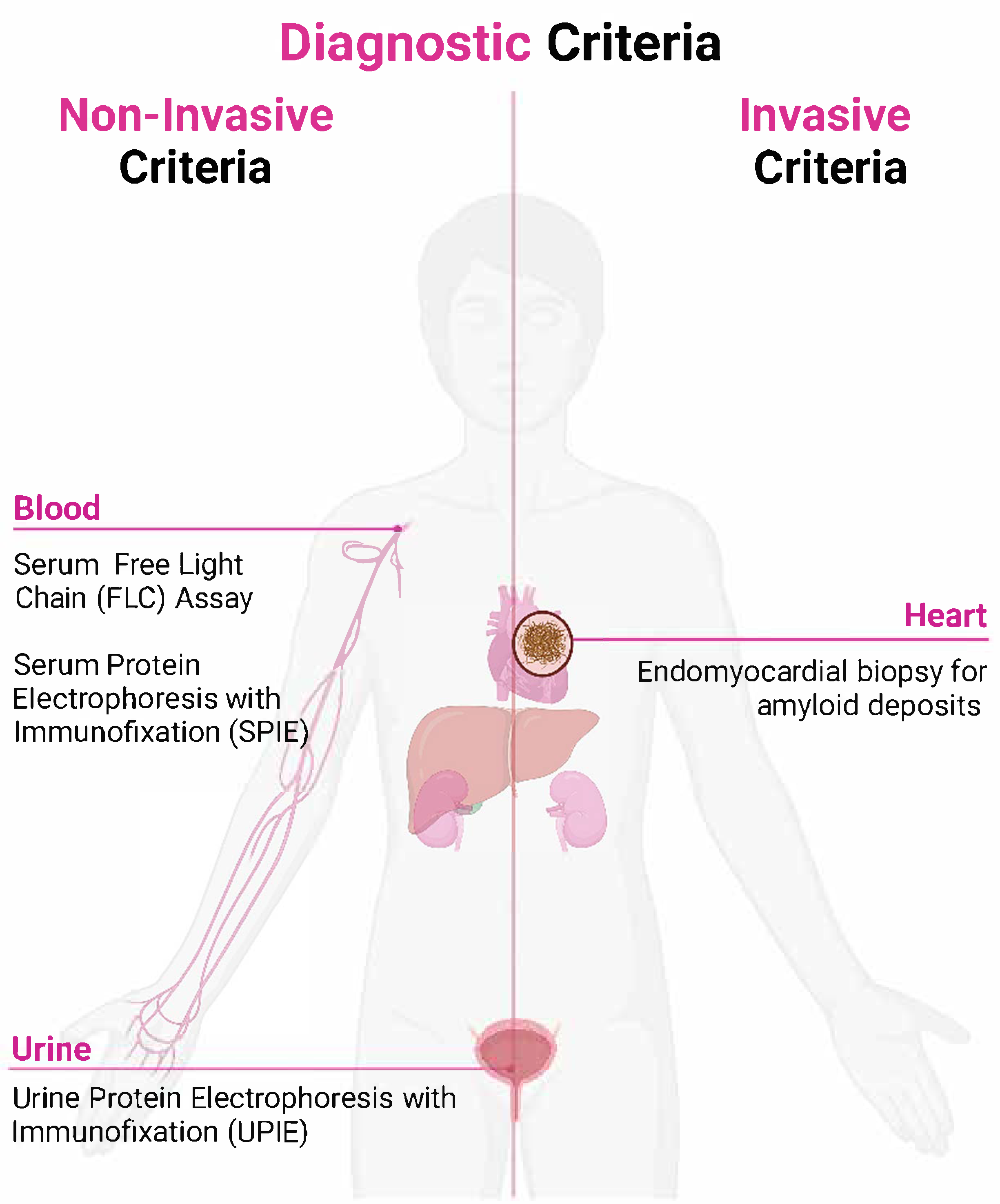
4. Diagnostic Criteria for Cardiac Amyloidosis
4.1. Non-Invasive Diagnostic Criteria
4.2. Invasive Diagnostic Criteria
4.3. Challenges in Interpretation
- Suspicion phase: identifying clinical and imaging features that raise suspicion for cardiac amyloidosis.
- Definitive diagnosis phase: confirming the presence of amyloid deposits and accurately typing the amyloid fibrils to guide targeted treatments [40].
5. Discussion
6. Limitations of the Study
7. Future Directions
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Rubin, J.; Maurer, M.S. Cardiac Amyloidosis: Overlooked, Underappreciated, and Treatable. Annu. Rev. Med. 2020, 71, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, D.R.; Pastore, R.; Pool, S.; Malendowicz, S.; Kane, I.; Shivji, A.; Embury, S.H.; Ballas, S.K.; Buxbaum, J.N. Revised Transthyretin Ile 122 Allele Frequency in African-Americans. Hum. Genet. 1996, 98, 236–238. [Google Scholar] [CrossRef]
- Sipe, J.D.; Cohen, A.S. Review: History of the Amyloid Fibril. J. Struct. Biol. 2000, 130, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Buxbaum, J.N.; Eisenberg, D.S.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Sipe, J.D.; Westermark, P. Amyloid Nomenclature 2018: Recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2018, 25, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.H.; Alexander, K.M.; Liao, R.; Dorbala, S. AL (Light-Chain) Cardiac Amyloidosis. JACC 2016, 68, 1323–1341. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Mirzoyev, S.A.; Edwards, W.D.; Dogan, A.; Grogan, D.R.; Dunlay, S.M.; Roger, V.L.; Gertz, M.A.; Dispenzieri, A.; Zeldenrust, S.R.; et al. Left Ventricular Amyloid Deposition in Patients with Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2014, 2, 113–122. [Google Scholar] [CrossRef]
- Castaño, A.; Narotsky, D.L.; Hamid, N.; Khalique, O.K.; Morgenstern, R.; DeLuca, A.; Rubin, J.; Chiuzan, C.; Nazif, T.; Vahl, T.; et al. Unveiling Transthyretin Cardiac Amyloidosis and Its Predictors among Elderly Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Eur. Heart J. 2017, 38, 2879–2887. [Google Scholar] [CrossRef]
- Pollak, A.; Falk, R.H. Left Ventricular Systolic Dysfunction Precipitated by Verapamil in Cardiac Amyloidosis. Chest 1993, 104, 618–620. [Google Scholar] [CrossRef]
- Laptseva, N.; Rossi, V.A.; Sudano, I.; Schwotzer, R.; Ruschitzka, F.; Flammer, A.J.; Duru, F. Arrhythmic Manifestations of Cardiac Amyloidosis: Challenges in Risk Stratification and Clinical Management. J. Clin. Med. 2023, 12, 2581. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Elshazly, M.B.; Puri, R.; Saliba, W.; Kanj, M.; Vakamudi, S.; Patel, D.R.; Baranowski, B.; et al. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis: Predictors, Prevalence, and Efficacy of Rhythm Control Strategies. JACC Clin. Electrophysiol. 2020, 6, 1118–1127. [Google Scholar] [CrossRef]
- Mitrani, L.R.; De Los Santos, J.; Driggin, E.; Kogan, R.; Helmke, S.; Goldsmith, J.; Biviano, A.B.; Maurer, M.S. Anticoagulation with Warfarin Compared to Novel Oral Anticoagulants for Atrial Fibrillation in Adults with Transthyretin Cardiac Amyloidosis: Comparison of Thromboembolic Events and Major Bleeding. Amyloid 2021, 28, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, J.; Jaber, W.; Maurer, M.; Sperry, B.; Hanna, M.; Collier, P.; Patel, D.R.; Wazni, O.M.; Donnellan, E. Electrophysiological Manifestations of Cardiac Amyloidosis: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncology 2021, 3, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Liżewska-Springer, A.; Sławiński, G.; Lewicka, E. Arrhythmic Sudden Cardiac Death and the Role of Implantable Cardioverter-Defibrillator in Patients with Cardiac Amyloidosis—A Narrative Literature Review. J. Clin. Med. 2021, 10, 1858. [Google Scholar] [CrossRef] [PubMed]
- Belfeki, N.; Ghriss, N.; Monchi, M.; Moini, C. State of the Art of Cardiac Amyloidosis. Biomedicines 2023, 11, 1045. [Google Scholar] [CrossRef]
- Maurer, M.S.; Dunnmon, P.; Fontana, M.; Quarta, C.C.; Prasad, K.; Witteles, R.M.; Rapezzi, C.; Signorovitch, J.; Lousada, I.; Merlini, G. Proposed Cardiac End Points for Clinical Trials in Immunoglobulin Light Chain Amyloidosis: Report From the Amyloidosis Forum Cardiac Working Group. Circ. Heart Fail. 2022, 15, e009038. [Google Scholar] [CrossRef]
- aus dem Siepen, F.; Hansen, T. Diagnosing AL and ATTR Amyloid Cardiomyopathy: A Multidisciplinary Approach. J. Clin. Med. 2024, 13, 5873. [Google Scholar] [CrossRef]
- Selvanayagam, J.B.; Hawkins, P.N.; Paul, B.; Myerson, S.G.; Neubauer, S. Evaluation and Management of the Cardiac Amyloidosis. JACC 2007, 50, 2101–2110. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Hawkins, P.N.; Fontana, M. Cardiac Amyloidosis. Clin. Med. 2018, 18, s30–s35. [Google Scholar] [CrossRef]
- Pan, J.A.; Kerwin, M.J.; Salerno, M. Native T1 Mapping, Extracellular Volume Mapping, and Late Gadolinium Enhancement in Cardiac Amyloidosis: A Meta-Analysis. JACC Cardiovasc. Imaging 2020, 13, 1299–1310. [Google Scholar] [CrossRef]
- Rapezzi, C.; Elliott, P.; Damy, T.; Nativi-Nicolau, J.; Berk, J.L.; Velazquez, E.J.; Boman, K.; Gundapaneni, B.; Patterson, T.A.; Schwartz, J.H.; et al. Efficacy of Tafamidis in Patients with Hereditary and Wild-Type Transthyretin Amyloid Cardiomyopathy. JACC Heart Fail. 2021, 9, 115–123. [Google Scholar] [CrossRef]
- Gertz, M.A.; Dispenzieri, A.; Sher, T. Pathophysiology and Treatment of Cardiac Amyloidosis. Nat. Rev. Cardiol. 2015, 12, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Porcari, A.; Patel, R.K.; Razvi, Y.; Sinigiani, G.; Martinez-Naharro, A.; Venneri, L.; Moon, J.; Rauf, M.U.; Lachmann, H.; et al. Rare Forms of Cardiac Amyloidosis: Diagnostic Clues and Phenotype in Apo AI and AIV Amyloidosis. Circ. Cardiovasc. Imaging 2023, 16, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Bampatsias, D.; Papamichail, A.; Kuno, T.; Skoularigis, J.; Xanthopoulos, A.; Triposkiadis, F. Invasive and Non-Invasive Diagnostic Pathways in the Diagnosis of Cardiac Amyloidosis. J. Cardiovasc. Dev. Dis. 2023, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, G.; Aimo, A.; Barison, A.; Genovesi, D.; Buda, G.; Passino, C.; Emdin, M. Keys to Early Diagnosis of Cardiac Amyloidosis: Red Flags from Clinical, Laboratory and Imaging Findings. Eur. J. Prev. Cardiol. 2020, 27, 1806–1815. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and Treatment of Cardiac Amyloidosis: A Position Statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Moore, P.T.; Burrage, M.K.; Mackenzie, E.; Law, W.P.; Korczyk, D.; Mollee, P. The Utility of 99mTc-DPD Scintigraphy in the Diagnosis of Cardiac Amyloidosis: An Australian Experience. Heart Lung Circ. 2017, 26, 1183–1190. [Google Scholar] [CrossRef]
- Ioannou, A.; Patel, R.K.; Razvi, Y.; Porcari, A.; Knight, D.; Martinez-Naharro, A.; Kotecha, T.; Venneri, L.; Chacko, L.; Brown, J.; et al. Multi-Imaging Characterization of Cardiac Phenotype in Different Types of Amyloidosis. Cardiovasc. Imaging 2023, 16, 464–477. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, E.; McPhail, E.D.; Salas-Anton, C.; Dominguez, F.; Gertz, M.A.; Dispenzieri, A.; Dasari, S.; Milani, P.; Verga, L.; Grogan, M.; et al. Histological Typing in Patients with Cardiac Amyloidosis. JACC 2024, 83, 1085–1099. [Google Scholar] [CrossRef]
- Leung, N.; Nasr, S.H.; Sethi, S. How I Treat Amyloidosis: The Importance of Accurate Diagnosis and Amyloid Typing. Blood 2012, 120, 3206–3213. [Google Scholar] [CrossRef]
- Morfino, P.; Aimo, A.; Castiglione, V.; Chianca, M.; Vergaro, G.; Cipolla, C.M.; Fedele, A.; Emdin, M.; Fabiani, I.; Cardinale, D. Cardiovascular Toxicity from Therapies for Light Chain Amyloidosis. Front. Cardiovasc. Med. 2023, 10, 1212983. [Google Scholar] [CrossRef]
- Hill, M.M.; Dasari, S.; Mollee, P.; Merlini, G.; Costello, C.E.; Hazenberg, B.P.C.; Grogan, M.; Dispenzieri, A.; Gertz, M.A.; Kourelis, T.; et al. The Clinical Impact of Proteomics in Amyloid Typing. Mayo Clin. Proc. 2021, 96, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Brownrigg, J.; Lorenzini, M.; Lumley, M.; Elliott, P. Diagnostic Performance of Imaging Investigations in Detecting and Differentiating Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. ESC Heart Fail. 2019, 6, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Dicorato, M.M.; Basile, P.; Muscogiuri, G.; Carella, M.C.; Naccarati, M.L.; Dentamaro, I.; Guglielmo, M.; Baggiano, A.; Mushtaq, S.; Fusini, L.; et al. Novel Insights into Non-Invasive Diagnostic Techniques for Cardiac Amyloidosis: A Critical Review. Diagnostics 2024, 14, 2249. [Google Scholar] [CrossRef]
- Cotella, J.; Randazzo, M.; Maurer, M.S.; Helmke, S.; Scherrer-Crosbie, M.; Soltani, M.; Goyal, A.; Zareba, K.; Cheng, R.; Kirkpatrick, J.N.; et al. Limitations of Apical Sparing Pattern in Cardiac Amyloidosis: A Multicentre Echocardiographic Study. Eur. Heart J.—Cardiovasc. Imaging 2024, 25, 754–761. [Google Scholar] [CrossRef]
- Paeng, J.C.; Choi, J.Y. Nuclear Imaging for Cardiac Amyloidosis: Bone Scan, SPECT/CT, and Amyloid-Targeting PET. Nucl. Med. Mol. Imaging 2021, 55, 61–70. [Google Scholar] [CrossRef]
- Auer, B.; Kijewski, M.F.; Dorbala, S. Quantitative ATTR-Cardiac Amyloidosis SPECT/CT Imaging: The Time Is Now! J. Nucl. Cardiol. 2023, 30, 1246–1249. [Google Scholar] [CrossRef]
- Oerlemans, M.I.F.J.; Rutten, K.H.G.; Minnema, M.C.; Raymakers, R.A.P.; Asselbergs, F.W.; de Jonge, N. Cardiac Amyloidosis: The Need for Early Diagnosis. Neth. Heart J. 2019, 27, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Berk, J.L. Transthyretin (TTR) Cardiac Amyloidosis. Circulation 2012, 126, 1286–1300. [Google Scholar] [CrossRef]
- Siddiqi, O.K.; Ruberg, F.L. Cardiac Amyloidosis: An Update on Pathophysiology, Diagnosis, and Treatment. Trends Cardiovasc. Med. 2018, 28, 10–21. [Google Scholar] [CrossRef]
- Kyriakou, P.; Mouselimis, D.; Tsarouchas, A.; Rigopoulos, A.; Bakogiannis, C.; Noutsias, M.; Vassilikos, V. Diagnosis of Cardiac Amyloidosis: A Systematic Review on the Role of Imaging and Biomarkers. BMC Cardiovasc. Disord 2018, 18, 221. [Google Scholar] [CrossRef]
- Maloberti, A.; Ciampi, C.; Politi, F.; Fabbri, S.; Musca, F.; Giannattasio, C. Cardiac Amyloidosis Red Flags: What All the Cardiologist Have to Know. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 21, 200271. [Google Scholar] [CrossRef] [PubMed]
- Debonnaire, P.; Claeys, M.; Smet, M.D.; Trenson, S.; Lycke, M.; Demeester, C.; Droogenbroeck, J.V.; Vriese, A.S.D.; Verhoeven, K.; Vantomme, N.; et al. Trends in Diagnosis, Referral, Red Flag Onset, Patient Profiles and Natural Outcome of de Novo Cardiac Amyloidosis and Their Multidisciplinary Implications. Acta Cardiol. 2022, 77, 791–804. [Google Scholar] [CrossRef]
- Blanco-López, E.; Martínez-del Río, J.; López-Calles, A.; Negreira-Caamaño, M.; Águila-Gordo, D.; Soto-Martín, P.; Soto-Pérez, M.M.; Cubides-Novoa, A.F.; Gonzalez-Barderas, M.; Sánchez-Pérez, I.; et al. Cardiac Amyloidosis and Red Flags: Natural History and Its Impact in Morbimortality. Med. Clínica (Engl. Ed.) 2025, 164, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Farzaneh-Far, A.; Mamas, M.A. Red Flags in Cardiac Amyloidosis. Eur. J. Prev. Cardiol. 2020, 27, 1804–1805. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, M.; Carpinteiro, A.; Rischpler, C.; Hagenacker, T.; Rassaf, T.; Luedike, P. Diagnosing Cardiac Amyloidosis in Every-Day Practice: A Practical Guide for the Cardiologist. IJC Heart Vasc. 2020, 28, 100519. [Google Scholar] [CrossRef]
- Westin, O.; Fosbøl, E.L.; Maurer, M.S.; Leicht, B.P.; Hasbak, P.; Mylin, A.K.; Rørvig, S.; Lindkær, T.H.; Johannesen, H.H.; Gustafsson, F. Screening for Cardiac Amyloidosis 5 to 15 Years After Surgery for Bilateral Carpal Tunnel Syndrome. J. Am. Coll. Cardiol. 2022, 80, 967–977. [Google Scholar] [CrossRef]
- Yamamoto, H.; Yokochi, T. Transthyretin Cardiac Amyloidosis: An Update on Diagnosis and Treatment. ESC Heart Fail. 2019, 6, 1128–1139. [Google Scholar] [CrossRef]
- Driggin, E.; Maurer, M.S. The Quintessential Form of Diastolic Heart Failure in Older Adults: Wild Type Transthyretin Cardiac Amyloidosis. Clin. Cardiol. 2020, 43, 171–178. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Panagiotopoulos, I.; Kouroutzoglou, A.; Koutsis, G.; Toskas, P.; Lazaros, G.; Toutouzas, K.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C. Prevalence and Clinical Outcomes of Transthyretin Amyloidosis: A Systematic Review and Meta-Analysis. Eur. J. Heart Fail. 2022, 24, 1677–1696. [Google Scholar] [CrossRef]
- Novosad, O.; Rudiuk, T.; Shevchuk, L.; Kundina, V.; Schmidt, A.G. Secondary Systemic AL-Amyloidosis Associated with Multiple Myeloma: Clinical Case and Literature Review. Res. Res. Square 2022. [Google Scholar] [CrossRef]
- Jaiswal, V.; Ang, S.P.; Chia, J.E.; Abdelazem, E.M.; Jaiswal, A.; Biswas, M.; Gimelli, A.; Parwani, P.; Siller-Matula, J.M.; Mamas, M.A. Echocardiographic Predictors of Presence of Cardiac Amyloidosis in Aortic Stenosis. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Llerena-Velastegui, J.; Zumbana-Podaneva, K. Advances in the Diagnosis and Management of Cardiac Amyloidosis: A Literature Review. Cardiol. Res. 2024, 15, 211–222. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.S.; Kim, S.-J. Diagnostic Performance of PET for Detection of Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. J. Cardiol. 2020, 76, 618–625. [Google Scholar] [CrossRef]
- Sattar, Y.; Maya, T.R.; Zafrullah, F.; Patel, N.; Latchana, S. Diagnosis and Management of a Cardiac Amyloidosis Case Mimicking Hypertrophic Cardiomyopathy. Cureus 2018, 10, e3749. [Google Scholar] [CrossRef] [PubMed]
- Herzog, E.; Leibowitz, D.; Gatt, M.E.; Koren, T.; Pollak, A. Pathway for the Diagnosis and Management of Cardiac Amyloidosis. Crit. Pathw. Cardiol. 2023, 22, 114–119. [Google Scholar] [CrossRef]
- Musetti, V.; Greco, F.; Castiglione, V.; Aimo, A.; Palmieri, C.; Genovesi, D.; Giorgetti, A.; Emdin, M.; Vergaro, G.; McDonnell, L.A.; et al. Tissue Characterization in Cardiac Amyloidosis. Biomedicines 2022, 10, 3054. [Google Scholar] [CrossRef] [PubMed]
- Garcia, Y.; Collins, A.B.; Stone, J.R. Abdominal Fat Pad Excisional Biopsy for the Diagnosis and Typing of Systemic Amyloidosis. Hum. Pathol. 2018, 72, 71–79. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, W.; Chen, X.; Hu, J.; Sun, Y.; Zhao, Y. Prognostic Impact of Light-Chain and Transthyretin-Related Categories in Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. Hell. J. Cardiol. 2019, 60, 375–383. [Google Scholar] [CrossRef]
- Meier-Ewert, H.K.; Sanchorawala, V.; Berk, J.L.; Ruberg, F.L. Cardiac Amyloidosis: Evolving Approach to Diagnosis and Management. Curr. Treat. Options Cardiovasc. Med. 2011, 13, 528–542. [Google Scholar] [CrossRef]
- Fanta, L.E.; Ewer, S.M.; Gimelli, G.; Reilly, N. Alcohol Septal Ablation for Left Ventricular Outflow Tract Obstruction in Cardiac Amyloidosis: New Indication for an Established Therapy. Catheter. Cardiovasc. Interv. 2022, 100, 910–914. [Google Scholar] [CrossRef]
- Tan, N.Y.; MOHSIN, Y.; Hodge, D.O.; Lacy, M.Q.; Packer, D.L.; Dispenzieri, A.; Grogan, M.; Asirvatham, S.J.; Madhavan, M.; McLeod, C.J. Catheter Ablation for Atrial Arrhythmias in Patients with Cardiac Amyloidosis. J. Cardiovasc. Electrophysiol. 2016, 27, 1167–1173. [Google Scholar] [CrossRef]
- Quinn, J.H.; Sviggum, E.B.; La Nou, A.T.; Burkhamer, K.J. Point-of-Care Ultrasound Leads to a Rare Incidental Diagnosis in a Patient with Acute Kidney Injury. Jaapa 2022, 35, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Uddin, Q.S.; Tharwani, Z.H.; Kashif, M.A.B.; Javaid, S.S.; Kumar, P.; Zia, M.T.; Javed, M.; Butt, M.S.; Asim, Z. Concomitant Transthyretin Cardiac Amyloidosis in Patients Undergoing TAVR for Aortic Stenosis: A Systemic Review and Meta-Analysis. Int. J. Cardiol. 2024, 402, 131854. [Google Scholar] [CrossRef]
- Aimo, A.; Tomasoni, D.; Porcari, A.; Vergaro, G.; Castiglione, V.; Passino, C.; Adamo, M.; Bellicini, M.G.; Lombardi, C.M.; Nardi, M.; et al. Left Ventricular Wall Thickness and Severity of Cardiac Disease in Women and Men with Transthyretin Amyloidosis. Eur. J. Heart Fail. 2023, 25, 510–514. [Google Scholar] [CrossRef]
- Melero Polo, J.; Roteta Unceta-Barrenechea, A.; Revilla Martí, P.; Pérez-Palacios, R.; Gracia Gutiérrez, A.; Bueno Juana, E.; Andrés Gracia, A.; Atienza Ayala, S.; Aibar Arregui, M.Á. Echocardiographic Markers of Cardiac Amyloidosis in Patients with Heart Failure and Left Ventricular Hypertrophy. Cardiol. J. 2023, 30, 266–275. [Google Scholar] [CrossRef]
- Aimo, A.; Merlo, M.; Porcari, A.; Georgiopoulos, G.; Pagura, L.; Vergaro, G.; Sinagra, G.; Emdin, M.; Rapezzi, C. Redefining the Epidemiology of Cardiac Amyloidosis. A Systematic Review and Meta-Analysis of Screening Studies. Eur. J. Heart Fail. 2022, 24, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ha, S.; Kim, Y. Cardiac Amyloidosis Imaging with Amyloid Positron Emission Tomography: A Systematic Review and Meta-Analysis. J. Nucl. Cardiol. 2020, 27, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Ceriello, L.; Khanji, M.Y.; Dangas, G.; Bucciarelli-Ducci, C.; Di Mauro, M.; Fedorowski, A.; Zimarino, M.; Gallina, S. Prognostic Significance of Cardiac Amyloidosis in Patients with Aortic Stenosis. JACC Cardiovasc. Imaging 2021, 14, 293–295. [Google Scholar] [CrossRef]
- Cai, S.; Haghbayan, H.; Chan, K.K.W.; Deva, D.P.; Jimenez-Juan, L.; Connelly, K.A.; Ng, M.-Y.; Yan, R.T.; Yan, A.T. Tissue Mapping by Cardiac Magnetic Resonance Imaging for the Prognostication of Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2024, 403, 131892. [Google Scholar] [CrossRef]
- See, A.S.Y.; Ho, J.S.-Y.; Chan, M.Y.; Lim, Y.C.; Yeo, T.-C.; Chai, P.; Wong, R.C.C.; Lin, W.; Sia, C.-H. Prevalence and Risk Factors of Cardiac Amyloidosis in Heart Failure: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2022, 31, 1450–1462. [Google Scholar] [CrossRef]
- Ho, J.S.-Y.; Kor, Q.; Kong, W.K.; Lim, Y.C.; Chan, M.Y.-Y.; Syn, N.L.; Ngiam, J.N.; Chew, N.W.; Yeo, T.-C.; Chai, P.; et al. Prevalence and Outcomes of Concomitant Cardiac Amyloidosis and Aortic Stenosis: A Systematic Review and Meta-Analysis. Hell. J. Cardiol. 2022, 64, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Roshankar, G.; Draycott, L.; Jimenez-Zepeda, V.; Fine, N.; Chan, D.; Han, D.; Miller, R.J.H. Diagnostic Accuracy of Bone Scintigraphy Imaging for Transthyretin Cardiac Amyloidosis: Systematic Review and Meta-Analysis. J. Nucl. Cardiol. 2023, 30, 2464–2476. [Google Scholar] [CrossRef]
- Kang, Y.; Qu, N.; Zhang, Z.; Zhang, Q.; Chen, X.; Fu, M. Tolerability and Effectiveness of Beta-Blockers in Patients with Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2024, 402, 131813. [Google Scholar] [CrossRef] [PubMed]
- de Campos, D.; Saleiro, C.; Botelho, A.; Costa, M.; Gonçalves, L.; Teixeira, R. Aortic Valve Intervention for Aortic Stenosis and Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. Future Cardiol. 2022, 18, 477–486. [Google Scholar] [CrossRef]
- Kwok, C.S.; Choy, C.H.; Pinney, J.; Townend, J.N.; Whelan, C.; Fontana, M.; Gillmore, J.D.; Steeds, R.P.; Moody, W.E. Effect of Beta-Blockade on Mortality in Patients with Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. ESC Heart Fail. 2024, 11, 3901–3910. [Google Scholar] [CrossRef]
- Khan, L.A.; Shaikh, F.H.; Khan, M.S.; Zafar, B.; Farooqi, M.; Bold, B.; Aslam, H.M.; Essam, N.; Noor, I.; Siddique, A.; et al. Artificial Intelligence-Enhanced Electrocardiogram for the Diagnosis of Cardiac Amyloidosis: A Systemic Review and Meta-Analysis. Curr. Probl. Cardiol. 2024, 49, 102860. [Google Scholar] [CrossRef]
- Cannata, F.; Chiarito, M.; Pinto, G.; Villaschi, A.; Sanz-Sánchez, J.; Fazzari, F.; Regazzoli, D.; Mangieri, A.; Bragato, R.M.; Colombo, A.; et al. Transcatheter Aortic Valve Replacement in Aortic Stenosis and Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. ESC Heart Fail. 2022, 9, 3188–3197. [Google Scholar] [CrossRef]
- Sun, H.; Shi, Z.; Liu, W. The Value of the Electrocardiogram in the Recognition of Cardiac Amyloidosis: A Systematic Meta-Analysis. BMC Cardiovasc. Disord. 2024, 24, 488. [Google Scholar] [CrossRef]
- Halawa, A.; Woldu, H.G.; Kacey, K.G.; Alpert, M.A. Effect of ICD Implantation on Cardiovascular Outcomes in Patients with Cardiac Amyloidosis: A Systematic Review and Meta-Anaylsis. J. Cardiovasc. Electrophysiol. 2020, 31, 1749–1758. [Google Scholar] [CrossRef]
- Kato, S.; Azuma, M.; Horita, N.; Utsunomiya, D. Monitoring the Efficacy of Tafamidis in ATTR Cardiac Amyloidosis by MRI-ECV: A Systematic Review and Meta-Analysis. Tomography 2024, 10, 1303–1311. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Dong, F.; Chi, H. Diagnostic Sensitivity of Abdominal Fat Aspiration Biopsy for Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. Int. J. Surg. Pathol. 2024, 32, 286–293. [Google Scholar] [CrossRef]
- Albulushi, A.; Buraiki, J.A.; Aly, G.; Al-Wahshi, Y.; Jahangirifard, A. Role of Biomarkers in Early Diagnosis and Prognosis of Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2025, 50, 102883. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Glaudemans, A.W.J.M.; Bertagna, F.; Hazenberg, B.P.C.; Erba, P.A.; Giubbini, R.; Ceriani, L.; Prior, J.O.; Giovanella, L.; Slart, R.H.J.A. Diagnostic Accuracy of Bone Scintigraphy in the Assessment of Cardiac Transthyretin-Related Amyloidosis: A Bivariate Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1945–1955. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, C. Diagnostic Performance of CMR, SPECT, and PET Imaging for the Detection of Cardiac Amyloidosis: A Meta-Analysis. BMC Cardiovasc. Disord. 2021, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Leng, Z. An Analysis Regarding the Article “Artificial Intelligence-Enhanced Electrocardiogram for the Diagnosis of Cardiac Amyloidosis: A Systemic Review and Meta-Analysis”. Curr. Probl. Cardiol. 2024, 49, 102866. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, H.; Cui, W. Performance of Bone Tracer for Diagnosis and Differentiation of Transthyretin Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. Diagn. Interv. Radiol. 2021, 27, 802–810. [Google Scholar] [CrossRef]
- Riley, J.M.; Junarta, J.; Ullah, W.; Siddiqui, M.U.; Anzelmi, A.; Ruge, M.; Vishnevsky, A.; Alvarez, R.J.; Ruggiero, N.J.; Rajapreyar, I.N.; et al. Transcatheter Aortic Valve Implantation in Cardiac Amyloidosis and Aortic Stenosis. Am. J. Cardiol. 2023, 198, 101–107. [Google Scholar] [CrossRef]
- Cho, S.-G.; Han, S. Prognostic Value of Bone Scintigraphy in Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. Clin. Nucl. Med. 2025, 50, e34–e40. [Google Scholar] [CrossRef]
- Zhang, Y.; Chaolu, H. Diagnostic Role of NT-proBNP in Patients with Cardiac Amyloidosis Involvement: A Meta-Analysis. Arq. Bras. Cardiologia 2022, 119, 212–222. [Google Scholar] [CrossRef]
- Zhao, L.; Tian, Z.; Fang, Q. Diagnostic Accuracy of Cardiovascular Magnetic Resonance for Patients with Suspected Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2016, 16, 129. [Google Scholar] [CrossRef]
- Jaiswal, V.; Joshi, A.; Ishak, A.; Nataraj, M.; Ang, S.P.; Khan, N.; Daneshvar, F.; Aguilera-Alvarez, V.H.; Verma, D.; Shrestha, A.B.; et al. Meta-Analysis of Post-Transcatheter Aortic Valve Replacement Outcomes in Patients with Cardiac Amyloidosis and Aortic Stenosis. Int. J. Surg. 2023, 109, 2872–2874. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Nabeshima, Y.; Kitano, T.; Yang, L.-T.; Takeuchi, M. Diagnostic Accuracy and Prognostic Value of Relative Apical Sparing in Cardiac Amyloidosis—Systematic Review and Meta-Analysis. Circ. J. 2024, 89, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.M.; Brizneda, M.V.; Kwon, D.H.; Popovic, Z.B.; Flamm, S.D.; Hanna, M.; Griffin, B.P.; Xu, B. Reference Ranges, Diagnostic and Prognostic Utility of Native T1 Mapping and Extracellular Volume for Cardiac Amyloidosis: A Meta-Analysis. J. Magn. Reson. Imaging 2021, 53, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- American College of Cardiology. A Study to Evaluate Patisiran in Participants with Transthyretin Amyloidosis with Cardiomyopathy. 2023. Available online: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2023/10/30/15/55/apollo-b (accessed on 8 May 2025).
- European Society of Cardiology: Vutrisiran Offers a New Lifeline to Patients with Progressive Heart Condition. Available online: https://www.escardio.org/The-ESC/Press-Office/Press-releases/Vutrisiran-offers-a-new-lifeline-to-patients-with-progressive-heart-condition (accessed on 8 May 2025).
- Roberts, J.R.; Lan, M.L.; Mank, V.M.F.; Talavera, F.; Brent, L.H.; Besa, E.C.; Wolf, R.E.; Pumerantz, A.; Middleman, C.F.; Sprowl, G.M. Strategies for Shortening Time to Diagnosis of Transthyretin Amyloidosis. Medscape. Available online: https://emedicine.medscape.com/article/335301-treatment?form=fpf (accessed on 16 May 2025).
- Attruby: Uses, Dosage, Side Effects, Warnings. Available online: https://www.drugs.com/attruby.html (accessed on 8 May 2025).
- Gillmore, J.D.; Taubel, J.; Gane, E. First-in-Human in vivo CRISPR/Cas9 Editing of the TTR Gene by NTLA-2001 in Patients with Transthyretin (ATTR) Amyloidosis with Cardiomyopathy. In Proceedings of the American Heart Association Scientific Sessions, Chicago, IL, USA, 5–7 November 2022; Available online: https://www.acc.org/-/media/Clinical/PDF-Files/Approved-PDFs/2022/11/01/AHA22/Nov05/Nov05-5pm-First-in-Human-in-vivo-Crispr-Cas9-Editing-aha-2022.pdf (accessed on 11 April 2025).
- Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; Maurer, M.S.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient with Cardiac Amyloidosis. JACC 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.D.; Baljevic, M.; Campagnaro, E.; Castillo, J.J.; Costello, C.; D’Angelo, C.; Devarakonda, S.; et al. Systemic Light Chain Amyloidosis, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 67–81. [Google Scholar]
- Palladini, G.; Milani, P.; Merlini, G. Management of AL Amyloidosis in 2020. Blood 2020, 136, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Mallus, M.T.; Rizzello, V. Treatment of Amyloidosis: Present and Future. Eur. Heart J. Suppl. 2023, 25, B99–B103. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
| Amyloidosis Subtype | Clinical Manifestations |
|---|---|
| AL amyloidosis |
|
| ATTR amyloidosis |
|
| Type of Assessment | Main Findings |
|---|---|
| Clinical |
|
| Electrocardiography |
|
| Echocardiography |
|
| Biomarkers |
|
| Cardiac magnetic resonance |
|
| Scenario | Findings | Interpretation | Next Steps |
|---|---|---|---|
| Scenario 1 | Scintigraphy: no cardiac uptake. Monoclonal protein tests: negative. | Very low probability of cardiac amyloidosis; ATTR and AL amyloidosis are unlikely. | Consider alternative diagnoses. If suspicion persists, perform cardiac MRI (CMR) followed by cardiac or extracardiac biopsy, as scintigraphy may be negative in some ATTRv mutations (depending on TTR fibril composition) or rare subtypes of cardiac amyloidosis. |
| Scenario 2 | Scintigraphy: cardiac uptake present. Monoclonal protein tests: negative. | Grade 2 or 3 cardiac uptake: ATTR cardiac amyloidosis confirmed; proceed with genetic testing to distinguish between ATTRv and ATTRwt. Grade 1 cardiac uptake: non-invasive diagnosis is not possible. | Histological confirmation of amyloid deposits (cardiac or extracardiac biopsy) is required. |
| Scenario 3 | Scintigraphy: no cardiac uptake. Monoclonal protein tests: at least one abnormal. | AL amyloidosis must be ruled out. | Use CMR to confirm or exclude cardiac involvement. If CMR findings are supportive or inconclusive, perform cardiac or extracardiac biopsy for amyloid confirmation. If CMR findings do not support cardiac amyloidosis, diagnosis is unlikely. If CMR is unavailable, proceed directly to biopsy to avoid delays. Consultation with a hematologist is recommended. |
| Scenario 4 | Scintigraphy: cardiac uptake present. Monoclonal protein tests: at least one abnormal. | Possible scenarios: ATTR with concomitant monoclonal gammopathy of undetermined significance (MGUS) or other hematological disorders producing FLC, AL amyloidosis, or coexistence of ATTR and AL amyloidosis. | Diagnosis requires histological confirmation and amyloid typing, typically through an endomyocardial biopsy. |
| Title | Authors | Year | Study Type | Conclusion | Ref |
|---|---|---|---|---|---|
| Redefining the epidemiology of cardiac amyloidosis. A systematic review and meta-analysis of screening studies | Aimo et al. | 2022 | A systematic review and meta-analysis | Screening in certain clinical groups can identify a large number of patients who could benefit from treatment. ATTR-CA is the most common form of CA, but light-chain amyloidosis (AL-CA) is not rare, accounting for up to 18% of cases. The average age at diagnosis is between 74 and 90 years, and most patients are men (50–100%). | [66] |
| Cardiac amyloidosis imaging with amyloid positron emission tomography: A systematic review and meta-analysis | Kim et al. | 2020 | A systematic review and meta-analysis | Amyloid PET is a promising method for the diagnosis of cardiac amyloidosis, with high sensitivity and specificity. Semi-quantitative PET analysis helps differentiate between AL and ATTR, which is essential for selecting the appropriate treatment. The results support the use of PET in combination with other imaging techniques (MRI, bone scintigraphy) for the accurate diagnosis of CA. | [67] |
| Prevalence and clinical outcomes of ATTR: a systematic review and meta-analysis | Antonopoulos et al. | 2022 | A systematic review and meta-analysis | ATTR-CA is more common than previously thought, especially in populations with HFpEF, severe aortic stenosis, and hypertrophic cardiomyopathy. Survival depends on the ATTR subtype and genetic mutations. Patients with Val30Met have the worst prognosis. Tafamidis/patisiran therapy significantly improves survival, highlighting the importance of early diagnosis and appropriate treatment. Research gaps: additional epidemiological studies are needed to better understand the global distribution of ATTR. | [49] |
| Echocardiographic predictors of presence of cardiac amyloidosis in aortic stenosis | Jaiswal et al. | 2022 | A systematic review and meta-analysis | Echocardiography can help detect cardiac amyloidosis in patients with aortic stenosis, based on the parameters described above. Differences in wall thickness, LVMI, and diastolic parameters are useful for screening CA in patients with AS. Additional studies are needed to determine the optimal values of these parameters for more accurate differential diagnosis. | [51] |
| Concomitant transthyretin cardiac amyloidosis in patients undergoing TAVR for aortic stenosis: A systemic review and meta-analysis. | Fatima et al. | 2024 | A systematic review and meta-analysis | TTRCA is common in patients with severe aortic stenosis who require TAVR (13.3%). Patients with TTRCA have higher mortality and cardiovascular hospitalization rates compared to those without TTRCA, suggesting a more reserved prognosis. Larger studies are needed to determine the safety and efficacy of TAVR in patients with TTRCA, as current data come from small cohorts. | [63] |
| Prognostic Significance of Cardiac Amyloidosis in Patients With Aortic Stenosis: A Systematic Review and Meta-Analysis | Ricci et al. | 2021 | A systematic review and meta-analysis | In summary, transthyretin cardiac amyloidosis (CA) is associated with a significantly increased risk of all-cause mortality in elderly patients with aortic stenosis (AS). Additionally, maximum left ventricular wall thickness (LVWT) appears to be a key prognostic factor in individuals with both conditions, independent of age, left ventricular ejection fraction, and aortic valve replacement. Until further evidence from randomized controlled trials clarifies the effectiveness of amyloid-targeted and valve-focused therapies across varying disease severity phenotypes, treatment decisions for patients with dual pathology should be carefully assessed by the local heart team, with a personalized discussion of the benefit–risk balance for each patient. | [68] |
| Tissue mapping by cardiac magnetic resonance imaging for the prognostication of cardiac amyloidosis: A systematic review and meta-analysis | Cai et al. | 2024 | A systematic review and meta-analysis | The study concludes that cardiac magnetic resonance (CMR) tissue mapping is a valuable prognostic tool for assessing disease severity and predicting mortality in patients with cardiac amyloidosis. Higher native T1 times, increased extracellular volume (ECV), and lower myocardial-to-skeletal T2 ratios are significantly associated with worse outcomes. These findings support the clinical utility of CMR-based tissue characterization in risk stratification and guiding treatment decisions for patients with cardiac amyloidosis. | [69] |
| Prognostic impact of light-chain and transthyretin-related categories in cardiac amyloidosis: A systematic review and meta-analysis | Xin et al. | 2019 | A systematic review and meta-analysis | The study underscores the importance of distinguishing between amyloidosis subtypes, as they have distinct prognostic implications. | [58] |
| Prevalence and Risk Factors of Cardiac Amyloidosis in Heart Failure: A Systematic Review and Meta-Analysis | See et al. | 2022 | A systematic review and meta-analysis | The findings underscore the importance of maintaining a high index of suspicion for CA in HF patients, especially those presenting with the identified risk factors. Early recognition and appropriate diagnostic evaluations are crucial, as timely diagnosis of CA can significantly influence management strategies and improve patient outcomes. | [70] |
| Prevalence and outcomes of concomitant cardiac amyloidosis and aortic stenosis: A systematic review and meta-analysis | Ho et al. | 2021 | A systematic review and meta-analysis | The findings underscore the importance of considering the coexistence of CA in patients with AS, as this dual pathology is linked to poorer prognoses. Early detection and tailored management strategies are crucial to improve outcomes in this patient population. | [71] |
| Diagnostic accuracy of bone scintigraphy imaging for transthyretin cardiac amyloidosis: systematic review and meta-analysis | Ahluwalia et al. | 2023 | A systematic review and meta-analysis | Bone scintigraphy imaging is highly accurate for identifying patients with ATTR-CM. The slight differences in specificity among the diagnostic approaches may have clinical implications, especially when applied to low-risk screening populations. The study highlights the importance of considering disease prevalence when interpreting diagnostic accuracy and suggests that quantitative analysis of SPECT imaging may offer the highest specificity among the evaluated methods. | [72] |
| Diagnostic performance of imaging investigations in detecting and differentiating cardiac amyloidosis: a systematic review and meta-analysis | Brownrigg et al. | 2019 | A systematic review and meta-analysis | The study concludes that while both CMR and nuclear scintigraphy are valuable tools for detecting cardiac amyloidosis, nuclear scintigraphy, especially when paired with monoclonal protein screening, offers superior performance in differentiating ATTR from AL amyloidosis. These findings support the integration of both imaging modalities into a non-invasive diagnostic algorithm for CA. | [32] |
| Tolerability and effectiveness of beta-blockers in patients with cardiac amyloidosis: A systematic review and meta-analysis | Kang et al. | 2024 | A systematic review and meta-analysis | The findings suggest that BB therapy may have limited effectiveness and tolerability in patients with cardiac amyloidosis. Clinicians are advised to exercise caution when prescribing BBs to this patient population, considering potential adverse effects and closely monitoring for signs of intolerance. Alternative therapeutic strategies should be explored to manage heart failure symptoms in CA patients. These conclusions align with previous research indicating challenges in BB tolerability among CA patients. For instance, a study published in Frontiers in Cardiovascular Medicine reported that over half of the patients prescribed BBs discontinued therapy due to adverse effects such as hypotension and bradycardia. | [73] |
| Aortic valve intervention for aortic stenosis and cardiac amyloidosis: a systematic review and meta-analysis | de Campos et al. | 2022 | A systematic review and meta-analysis | The findings suggest that aortic valve interventions can be beneficial for patients with both AS and CA, offering symptomatic improvement and potential survival benefits. However, the associated mortality rates underscore the importance of careful patient selection and comprehensive preoperative evaluation. Decisions regarding such interventions should involve a multidisciplinary heart team approach, considering the individual patient’s clinical condition and comorbidities. These conclusions align with previous research indicating that while aortic valve interventions in patients with AS and CA carry certain risks, they can lead to meaningful improvements in patient outcomes when appropriately applied. | [74] |
| Effect of beta-blockade on mortality in patients with cardiac amyloidosis: A systematic review and meta-analysis | Kwok et al. | 2024 | A systematic review and meta-analysis | The study concludes that beta-blocker therapy does not confer a mortality benefit in patients with cardiac amyloidosis and may be associated with potential harm, particularly in those with AL amyloidosis. These findings suggest that the routine use of beta-blockers in CA patients should be carefully reconsidered, and alternative therapeutic strategies may be warranted. Clinicians are advised to exercise caution when prescribing beta-blockers to this patient population and to monitor for adverse effects diligently. | [75] |
| Artificial intelligence-enhanced electrocardiogram for the diagnosis of cardiac amyloidosis: A systemic review and meta-analysis. | Khan et al. | 2024 | A systematic review and meta-analysis | The findings suggest that AI-enhanced ECG models are effective tools for detecting CA and its subtypes. These models may facilitate early diagnosis and intervention, potentially improving patient outcomes. The study highlights the promise of integrating AI with ECG analysis to enhance the non-invasive detection of cardiac amyloidosis. | [76] |
| Transcatheter aortic valve replacement in aortic stenosis and cardiac amyloidosis: a systematic review and meta-analysis | Cannata et al. | 2022 | A systematic review and meta-analysis | The study concludes that TAVR is an effective and safe treatment option for patients with concomitant AS and CA, offering a significant survival advantage over medical therapy alone. The safety profile of TAVR in these patients is comparable to those with AS alone, except for a non-significant trend toward a higher risk of permanent pacemaker implantation. These findings support the consideration of TAVR as a viable therapeutic option in this patient population. | [77] |
| The value of the electrocardiogram in the recognition of cardiac amyloidosis: a systematic meta-analysis. | Sun et al. | 2024 | A systematic review and meta-analysis | The study concludes that while ECGs exhibit high specificity in detecting cardiac amyloidosis, their sensitivity is relatively low. This implies that a positive ECG finding is quite reliable for diagnosing CA; however, a negative ECG does not definitively rule out the disease. Therefore, ECGs can be a valuable initial screening tool, but they should be complemented with other diagnostic methods, such as echocardiography, cardiac magnetic resonance imaging, or biopsy, to ensure accurate detection and diagnosis of cardiac amyloidosis. | [78] |
| Effect of ICD implantation on cardiovascular outcomes in patients with cardiac amyloidosis: A systematic review and meta-anaylsis | Halawa et al. | 2020 | A systematic review and meta-analysis | The study suggests that while ICDs can effectively manage ventricular arrhythmias in CA patients, the overall mortality remains high, and the benefit of ICD implantation may be limited to specific subgroups. These findings underscore the importance of individualized patient assessment when considering ICD therapy in the context of cardiac amyloidosis. These conclusions align with previous research indicating that ICDs may not significantly improve survival in CA patients, despite their efficacy in terminating arrhythmias. For instance, a study published in Europace in 2020 reported that ICD implantation in CA patients was not associated with longer survival, despite comparable event rates to non-CA patients. Given the complex nature of cardiac amyloidosis and the limited survival benefit observed with ICD therapy, a comprehensive, multidisciplinary approach is essential to optimize patient outcomes. | [79] |
| Monitoring the Efficacy of Tafamidis in ATTR Cardiac Amyloidosis by MRI-ECV: A Systematic Review and Meta-Analysis | Kato et al. | 2024 | A systematic review and meta-analysis | The findings suggest that MRI-ECV is a valuable non-invasive imaging biomarker for monitoring the efficacy of tafamidis in patients with ATTR-CM. The stability of MRI-ECV in tafamidis-treated patients indicates that the therapy effectively halts disease progression, whereas increases in MRI-ECV in untreated patients reflect ongoing disease advancement. This underscores the potential of MRI-ECV measurements in guiding clinical decisions and assessing therapeutic responses in ATTR-CM management. | [80] |
| Diagnostic performance of PET for detection of cardiac amyloidosis: A systematic review and meta-analysis | Kim et al. | 2020 | A systematic review and meta-analysis | The findings suggest that PET imaging is a highly sensitive and specific modality for the detection of cardiac amyloidosis. The significant difference in tracer uptake between CA patients and controls underscores the potential of PET as a valuable tool in the non-invasive diagnosis of this condition. These results support the integration of PET into clinical practice for the evaluation of suspected cardiac amyloidosis. | [53] |
| Diagnostic Sensitivity of Abdominal Fat Aspiration Biopsy for Cardiac Amyloidosis: A Systematic Review and Meta-Analysis | Wang et al. | 2024 | A systematic review and meta-analysis | The findings suggest that while abdominal fat aspiration biopsy is a valuable diagnostic tool for light-chain amyloidosis cardiomyopathy, its effectiveness is limited in diagnosing ATTR cardiomyopathy. Therefore, clinicians should consider alternative or additional diagnostic approaches when ATTR-CM is suspected. | [81] |
| Role of biomarkers in early diagnosis and prognosis of cardiac amyloidosis: A systematic review and meta-analysis | Albulushi et al. | 2025 | A systematic review and meta-analysis | The findings underscore the critical role of biomarkers in the early diagnosis and prognosis of cardiac amyloidosis. NT-proBNP and troponins are well-established markers for early detection, while novel biomarkers offer additional insights into disease progression and subtype differentiation. Integrating biomarker analysis with imaging studies may enhance diagnostic accuracy and inform treatment strategies for CA patients. | [82] |
| Native T1 Mapping, Extracellular Volume Mapping, and Late Gadolinium Enhancement in Cardiac Amyloidosis: A Meta-Analysis | Pan et al. | 2020 | A systematic review and meta-analysis | The findings suggest that ECV mapping offers superior diagnostic and prognostic utility in the assessment of cardiac amyloidosis compared to LGE and native T1 mapping. While native T1 mapping provides similar sensitivity and specificity to LGE without the need for contrast agents, ECV mapping stands out as the most effective technique for both diagnosing CA and predicting adverse outcomes. These results support the integration of ECV mapping into clinical practice for comprehensive evaluation of patients with suspected or confirmed cardiac amyloidosis. | [19] |
| Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: a bivariate meta-analysis | Treglia et al. | 2018 | A systematic review and meta-analysis | The findings suggest that bone scintigraphy with technetium-labeled radiotracers is a highly accurate non-invasive method for diagnosing cardiac transthyretin amyloidosis. The high sensitivity and specificity support its use as a reliable diagnostic tool in clinical practice. | [83] |
| Diagnostic performance of CMR, SPECT, and PET imaging for the detection of cardiac amyloidosis: a meta-analysis. | Wu and Yu | 2021 | A systematic review and meta-analysis | The findings suggest that SPECT imaging exhibits superior diagnostic performance in detecting cardiac amyloidosis compared to CMR and PET. However, all three imaging techniques are valuable tools in the non-invasive diagnosis of CA. The study highlights the importance of selecting the appropriate imaging modality based on clinical context, availability, and patient-specific factors. | [84] |
| An analysis regarding the article “Artificial intelligence-enhanced electrocardiogram for the diagnosis of cardiac amyloidosis: A systemic review and meta-analysis | Su and Leng | 2024 | A systematic review and meta-analysis | This analysis contributes to the ongoing discourse on the role of AI in cardiology by highlighting the strengths and limitations of current research on AI-enhanced ECGs for CA diagnosis. It underscores the importance of rigorous methodological standards and the need for continued investigation to ensure the safe and effective integration of AI tools into clinical practice. | [85] |
| Performance of bone tracer for diagnosis and differentiation of transthyretin cardiac amyloidosis: a systematic review and meta-analysis | Zhao et al. | 2021 | A systematic review and meta-analysis | The findings suggest that bone scintigraphy is a valuable non-invasive tool with high accuracy for diagnosing transthyretin cardiac amyloidosis. While it plays a modest role in differentiating ATTR-CA from AL-CA, certain tracers like 18F-NaF may offer enhanced differentiation capabilities. These results support the use of bone scintigraphy in clinical practice for the assessment of suspected cardiac amyloidosis. | [86] |
| Transcatheter Aortic Valve Implantation in Cardiac Amyloidosis and Aortic Stenosis | Riley et al. | 2023 | A systematic review and meta-analysis | The findings suggest that TAVI may offer a survival advantage over conservative medical therapy in patients with concomitant AS and CA. This supports the consideration of TAVI as a viable treatment option in this patient population. However, the authors note that the evidence is derived from a limited number of observational studies, underscoring the need for further research to confirm these results and to establish comprehensive management guidelines for patients with both AS and CA. | [87] |
| Prognostic Value of Bone Scintigraphy in Cardiac Amyloidosis: A Systematic Review and Meta-analysis | Cho and Han | 2025 | A systematic review and meta-analysis | The findings suggest that bone scintigraphy not only serves as a diagnostic tool for cardiac amyloidosis but also provides valuable prognostic information. Specific imaging biomarkers derived from bone scintigraphy can stratify patients based on risk, thereby guiding clinical decision-making and management strategies. The study underscores the importance of incorporating bone scintigraphy into the routine evaluation of patients with suspected or confirmed cardiac amyloidosis to inform prognosis and tailor therapeutic approaches. | [88] |
| Diagnostic Role of NT-proBNP in Patients with Cardiac Amyloidosis Involvement: A Meta-Analysis | Zhang and Chaolu | 2022 | A systematic review and meta-analysis | The findings suggest that NT-proBNP is a valuable biomarker for the early diagnosis of cardiac involvement in amyloidosis patients, demonstrating high sensitivity and specificity. Its use can aid clinicians in identifying cardiac amyloidosis, potentially leading to earlier interventions and improved patient outcomes. | [89] |
| Diagnostic accuracy of cardiovascular magnetic resonance for patients with suspected cardiac amyloidosis: a systematic review and meta-analysis. | Zhao et al. | 2016 | A systematic review and meta-analysis | The findings suggest that CMR is a valuable non-invasive tool for diagnosing cardiac amyloidosis, offering high sensitivity and specificity. The use of advanced CMR techniques, such as LGE and T1 mapping, enhances the detection of myocardial amyloid infiltration, thereby aiding in the early diagnosis and management of CA. | [90] |
| Meta-analysis of post-transcatheter aortic valve replacement outcomes in patients with cardiac amyloidosis and aortic stenosis | Jaiswal et al. | 2023 | A systematic review and meta-analysis | The findings suggest that patients with concomitant aortic stenosis and cardiac amyloidosis undergoing TAVR may face higher risks of adverse outcomes, including increased mortality within 30 days, stroke, acute kidney injury, and major bleeding events, compared to patients with aortic stenosis alone. These results underscore the importance of careful patient selection and risk stratification when considering TAVR for individuals with both AS and CA. Further research is warranted to develop strategies to mitigate these risks and improve outcomes in this patient population. | [91] |
| Diagnostic Accuracy and Prognostic Value of Relative Apical Sparing in Cardiac Amyloidosis—Systematic Review and Meta-Analysis | Lee et al. | 2024 | A systematic review and meta-analysis | The findings suggest that the relative apical sparing pattern in echocardiographic strain imaging is a valuable tool for both the diagnosis and prognosis of cardiac amyloidosis. Its application in clinical practice can aid in the early detection and management of CA, potentially leading to improved patient outcomes. | [92] |
| Diagnostic and Prognostic Utility of Native T1 Mapping and Extracellular Volume for Cardiac Amyloidosis: A Meta-Analysis | Wang et al. | 2021 | A systematic review and meta-analysis | The findings suggest that native T1 mapping and ECV are valuable tools in the diagnosis and prognosis of cardiac amyloidosis. Establishing specific reference ranges enhances their clinical applicability, aiding in early detection and risk stratification of patients with CA. | [93] |
| Intervention | Indication | Class/Level | Refference |
|---|---|---|---|
| Tafamidis | Symptomatic ATTR-CA (NYHA I–II) | I B | [25,104] |
| Patisiran/Inotersen | ATTRv with polyneuropathy (±cardiac involvement) | IIa B | [99] |
| Diflunisal | ATTR-CA when tafamidis unavailable or intolerant | IIb C | [25] |
| Bortezomib-based chemo (±daratumumab) | AL-CA with hematologic involvement | I B | [100] |
| Autologous stem-cell transplant | Selected low-risk AL-CA patients | IIa B | [101] |
| Diuretics | Volume overload | I C | [102] |
| Avoid β-blockers/ACE-I | Hypotension in restrictive physiology | IIb C | [73] |
| Anticoagulation | AF or atrial dysfunction in CA | I C | [103] |
| TAVR | Severe AS + CA (NYHA II–III) | IIa B | [105] |
| ICD | Secondary prevention in AL-CA with sustained VT/VF | IIa C | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Movila, D.E.; Motofelea, A.C.; Cozma, D.; Albai, O.; Sima, A.C.; Andor, M.; Ciocarlie, T.; Dragan, S.R. Cardiac Amyloidosis: A Narrative Review of Diagnostic Advances and Emerging Therapies. Biomedicines 2025, 13, 1230. https://doi.org/10.3390/biomedicines13051230
Movila DE, Motofelea AC, Cozma D, Albai O, Sima AC, Andor M, Ciocarlie T, Dragan SR. Cardiac Amyloidosis: A Narrative Review of Diagnostic Advances and Emerging Therapies. Biomedicines. 2025; 13(5):1230. https://doi.org/10.3390/biomedicines13051230
Chicago/Turabian StyleMovila, Dana Emilia, Alexandru Catalin Motofelea, Dragos Cozma, Oana Albai, Alexandra Christa Sima, Minodora Andor, Tudor Ciocarlie, and Simona Ruxanda Dragan. 2025. "Cardiac Amyloidosis: A Narrative Review of Diagnostic Advances and Emerging Therapies" Biomedicines 13, no. 5: 1230. https://doi.org/10.3390/biomedicines13051230
APA StyleMovila, D. E., Motofelea, A. C., Cozma, D., Albai, O., Sima, A. C., Andor, M., Ciocarlie, T., & Dragan, S. R. (2025). Cardiac Amyloidosis: A Narrative Review of Diagnostic Advances and Emerging Therapies. Biomedicines, 13(5), 1230. https://doi.org/10.3390/biomedicines13051230








