Resting-State Functional MRI in Dyslexia: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Selection Criteria
2.3. Exclusion Criteria
2.4. Selection Process and Data Compilation
2.5. Data Collection
2.6. Risk-of-Bias Assessment
2.7. Methodological Quality Assessment
2.8. Data Analysis
3. Results
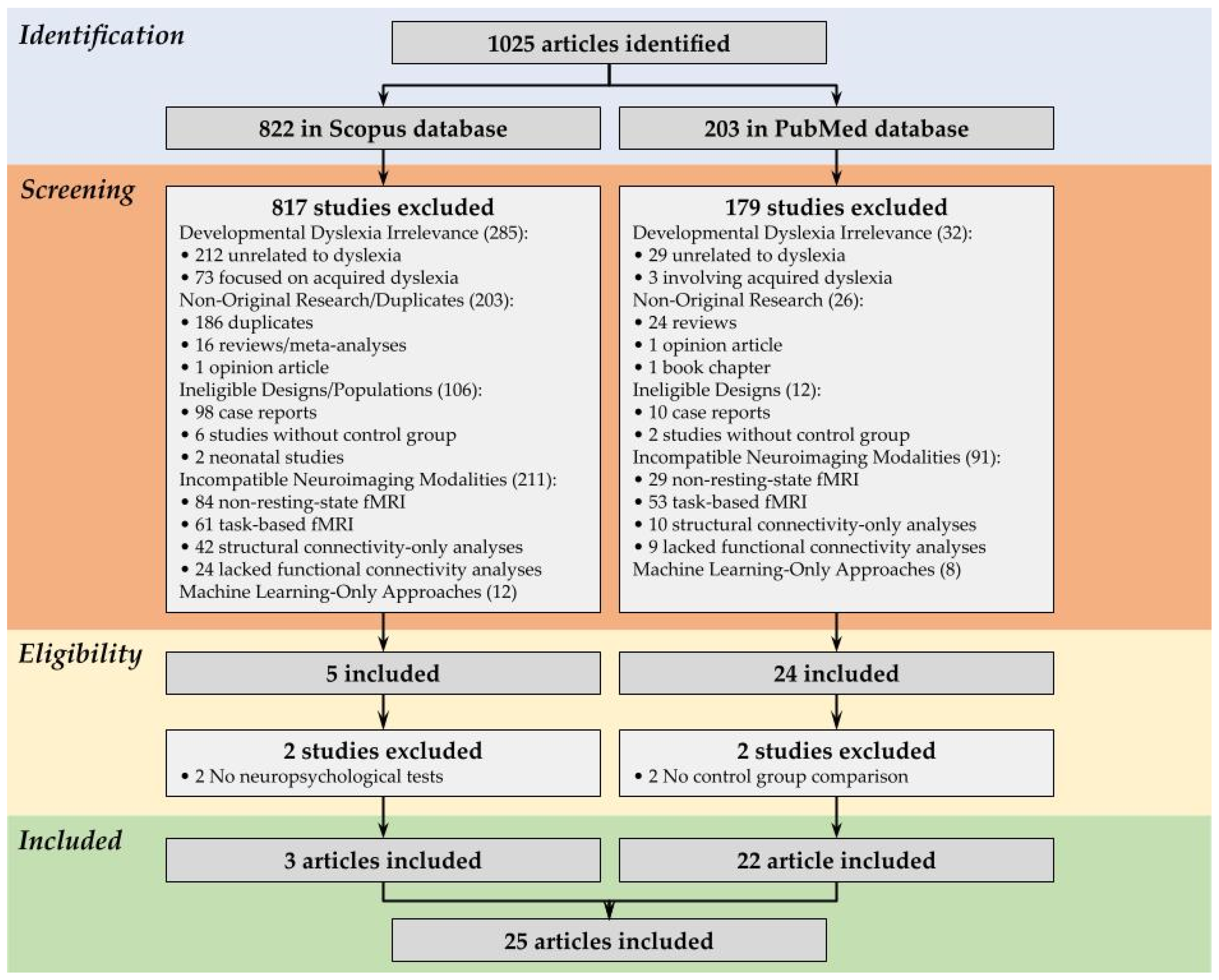
3.1. Study Selection
3.2. Methodological Quality Assessment Results
3.3. Study Characteristics
3.3.1. Demographic Data
3.3.2. Neuropsychological Tests for Dyslexia Evaluation
3.4. Brain Functional Connectivity (rs-fMRI)
3.4.1. rs-fMRI Acquisition
3.4.2. rs-fMRI Processing
3.4.3. rs-fMRI Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bigozzi, L.; Tarchi, C.; Vagnoli, L.; Valente, E.; Pinto, G. Reading Fluency As a Predictor of School Outcomes across Grades 4–9. Front. Psychol. 2017, 8, 200. [Google Scholar] [CrossRef]
- Huettig, F.; Pickering, M.J. Literacy Advantages Beyond Reading: Prediction of Spoken Language. Trends Cogn. Sci. 2019, 23, 464–475. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2022; Volume 5. [Google Scholar]
- Harrison, J.E.; Weber, S.; Jakob, R.; Chute, C.G. ICD-11: An international classification of diseases for the twenty-first century. BMC Med. Inform. Decis. Mak. 2021, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.L.; Pennington, B.F. Developmental dyslexia. Lancet 2012, 379, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Arnett, A.B.; Pennington, B.F.; Peterson, R.L.; Willcutt, E.G.; DeFries, J.C.; Olson, R.K. Explaining the sex difference in dyslexia. J. Child Psychol. Psychiatry 2017, 58, 719–727. [Google Scholar] [CrossRef]
- Turker, S.; Kuhnke, P.; Cheung, V.K.M.; Weise, K.; Hartwigsen, G. Neurostimulation improves reading and alters communication within reading networks in dyslexia. Ann. N. Y. Acad. Sci. 2025, 1544, 172–189. [Google Scholar] [CrossRef]
- Elnakib, A.; Soliman, A.; Nitzken, M.; Casanova, M.F.; Gimel’farb, G.; El-Baz, A. Magnetic resonance imaging findings for dyslexia: A review. J. Biomed. Nanotechnol. 2014, 10, 2778–2805. [Google Scholar] [CrossRef]
- Ramus, F.; Altarelli, I.; Jednoróg, K.; Zhao, J.; Scotto di Covella, L. Neuroanatomy of developmental dyslexia: Pitfalls and promise. Neurosci. Biobehav. Rev. 2018, 84, 434–452. [Google Scholar] [CrossRef]
- Mongerson, C.R.L.; Jennings, R.W.; Borsook, D.; Becerra, L.; Bajic, D. Resting-State Functional Connectivity in the Infant Brain: Methods, Pitfalls, and Potentiality. Front. Pediatr. 2017, 5, 159. [Google Scholar] [CrossRef]
- Martins, B.; Baba, M.Y.; Dimateo, E.M.; Costa, L.F.; Camara, A.S.; Lukasova, K.; Nucci, M.P. Investigating Dyslexia through Diffusion Tensor Imaging across Ages: A Systematic Review. Brain Sci. 2024, 14, 349. [Google Scholar] [CrossRef]
- Perdue, M.V.; Mahaffy, K.; Vlahcevic, K.; Wolfman, E.; Erbeli, F.; Richlan, F.; Landi, N. Reading intervention and neuroplasticity: A systematic review and meta-analysis of brain changes associated with reading intervention. Neurosci. Biobehav. Rev. 2022, 132, 465–494. [Google Scholar] [CrossRef]
- Turker, S.; Kuhnke, P.; Jiang, Z.; Hartwigsen, G. Disrupted network interactions serve as a neural marker of dyslexia. Commun. Biol. 2023, 6, 1114. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.E.; Welch, V. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Case-Control Studies in Meta-Analysis. Eur. J. Epidemiol. 2011, 25, 603–605. [Google Scholar]
- Carra, M.C.; Romandini, P.; Romandini, M. Risk of Bias Evaluation of Cross-Sectional Studies: Adaptation of the Newcastle-Ottawa Scale. J. Periodontal Res. 2025. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Gamer, M.; Lemon, J.; Singh, I. irr: Various Coefficients of Interrater Reliability and Agreement, R package version 0.84.1. 2010. Available online: https://CRAN.R-project.org/package=irr (accessed on 5 May 2025).
- Tan, S.; Deng, S.; Song, X.; Su, X.; Zhao, J.; Yang, K.; Li, H.; Wang, D.; Fu, J.; Gong, R.; et al. Altered effective connectivity on rapid automatized naming deficits in Chinese children with developmental dyslexia: An rs-fMRI study with Ganger causality analysis. J. Psychiatr. Res. 2024, 175, 235–242. [Google Scholar] [CrossRef]
- Gengeç Benli, Ş.; İçer, S.; Demirci, E.; Karaman, Z.F.; Ak, Z.; Acer, İ.; Sağır, G.R.; Aker, E.; Sertkaya, B. Data-driven exploratory method investigation on the effect of dyslexia education at brain connectivity in Turkish children: A preliminary study. Brain Struct. Funct. 2024, 229, 1697–1712. [Google Scholar] [CrossRef]
- Farah, R.; Dworetsky, A.; Coalson, R.S.; Petersen, S.E.; Schlaggar, B.L.; Rosch, K.S.; Horowitz-Kraus, T. An executive-functions-based reading training enhances sensory-motor systems integration during reading fluency in children with dyslexia. Cereb. Cortex 2024, 34, bhae166. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Yang, Y.; Bi, H.Y. Disruption of dynamic functional connectivity in children with developmental dyslexia. Lang. Cogn. Neurosci. 2023, 38, 621–635. [Google Scholar] [CrossRef]
- Gosse, C.; Dricot, L.; Van Reybroeck, M. Evidence of Altered Functional Connectivity at Rest in the Writing Network of Children with Dyslexia. Brain Sci. 2022, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, J.; Lv, Y.; Wang, F.; Ding, G.; Zhang, M.; Meng, X.; Song, Y. Failure of resting-state frontal-occipital connectivity in linking visual perception with reading fluency in Chinese children with developmental dyslexia. NeuroImage 2021, 233, 117911. [Google Scholar] [CrossRef] [PubMed]
- Sturm, V.E.; Roy, A.R.K.; Datta, S.; Wang, C.; Sible, I.J.; Holley, S.R.; Watson, C.; Palser, E.R.; Morris, N.A.; Battistella, G.; et al. Enhanced visceromotor emotional reactivity in dyslexia and its relation to salience network connectivity. Cortex 2021, 134, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Estivill, R.; Forne, S.; Lopez-Sala, A.; Falcon, C.; Caldu, X.; Sopena, J.M.; Sans, A.; Adan, A.; Grau, S.; Bargallo, N.; et al. Functional connectivity alterations associated with literacy difficulties in early readers. Brain Imaging Behav. 2021, 15, 2109–2120. [Google Scholar] [CrossRef]
- Horowitz-Kraus, T.; DiFrancesco, M.; Greenwood, P.; Scott, E.; Vannest, J.; Hutton, J.; Dudley, J.; Altaye, M.; Farah, R. Longer Screen Vs. Reading Time is Related to Greater Functional Connections Between the Salience Network and Executive Functions Regions in Children with Reading Difficulties vs. Typical Readers. Child Psychiatry Hum. Dev. 2021, 52, 681–692. [Google Scholar] [CrossRef]
- Greenwood, P.; Dudley, J.; Hutton, J.; DiFrancesco, M.; Farah, R.; Horowitz-Kraus, T. Higher maternal education is related to negative functional connectivity between attention system networks and reading-related regions in children with reading difficulties compared to typical readers. Brain Res. 2021, 1766, 147532. [Google Scholar] [CrossRef]
- Cross, A.M.; Ramdajal, R.; Peters, L.; Vandermeer, M.R.J.; Hayden, E.P.; Frijters, J.C.; Steinbach, K.A.; Lovett, M.W.; Archibald, L.M.D.; Joanisse, M.F. Resting-state functional connectivity and reading subskills in children. NeuroImage 2021, 243, 118529. [Google Scholar] [CrossRef]
- Nachshon, O.; Farah, R.; Horowitz-Kraus, T. Decreased Functional Connectivity Between the Left Amygdala and Frontal Regions Interferes With Reading, Emotional, and Executive Functions in Children With Reading Difficulties. Front. Hum. Neurosci. 2020, 14, 104. [Google Scholar] [CrossRef]
- Margolis, A.E.; Pagliaccio, D.; Davis, K.S.; Thomas, L.; Banker, S.M.; Cyr, M.; Marsh, R. Neural correlates of cognitive control deficits in children with reading disorder. Brain Imaging Behav. 2020, 14, 1531–1542. [Google Scholar] [CrossRef]
- Kuhl, U.; Neef, N.E.; Kraft, I.; Schaadt, G.; Dorr, L.; Brauer, J.; Czepezauer, I.; Muller, B.; Wilcke, A.; Kirsten, H.; et al. The emergence of dyslexia in the developing brain. NeuroImage 2020, 211, 116633. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Higuchi, H.; Uno, A.; Yokota, S.; Asano, K.; Taki, Y.; Kawashima, R. Association Between Resting-State Functional Connectivity and Reading in Two Writing Systems in Japanese Children With and Without Developmental Dyslexia. Brain Connect. 2020, 10, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.; Zivan, M.; Farah, R.; Horowitz-Kraus, T. Greater functional connectivity within the cingulo-opercular and ventral attention networks is related to better fluent reading: A resting-state functional connectivity study. NeuroImage Clin. 2020, 26, 102214. [Google Scholar] [CrossRef]
- Buchweitz, A.; Costa, A.C.; Toazza, R.; de Moraes, A.B.; Cara, V.M.; Esper, N.B.; Aguzzoli, C.; Gregolim, B.; Dresch, L.F.; Soldatelli, M.D.; et al. Decoupling of the Occipitotemporal Cortex and the Brain’s Default-Mode Network in Dyslexia and a Role for the Cingulate Cortex in Good Readers: A Brain Imaging Study of Brazilian Children. Dev. Neuropsychol. 2019, 44, 146–157. [Google Scholar] [CrossRef]
- Horowitz-Kraus, T.; Woodburn, M.; Rajagopal, A.; Versace, A.L.; Kowatch, R.A.; Bertocci, M.A.; Bebko, G.; Almeida, J.R.C.; Perlman, S.B.; Travis, M.J.; et al. Decreased functional connectivity in the fronto-parietal network in children with mood disorders compared to children with dyslexia during rest: An fMRI study. NeuroImage Clin. 2018, 18, 582–590. [Google Scholar] [CrossRef]
- Horowitz-Kraus, T.; DiCesare, C.; Kiefer, A.W. Longer Fixation Times During Reading Are Correlated With Decreased Connectivity in Cognitive-Control Brain Regions During Rest in Children. Mind Brain Educ. 2018, 12, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.; Margolis, A.E.; Thomas, L.; Huo, Z.; Marsh, R. Amygdala sub-regional functional connectivity predicts anxiety in children with reading disorder. Dev. Sci. 2018, 21, e12631. [Google Scholar] [CrossRef]
- Zhou, W.; Xia, Z.; Bi, Y.; Shu, H. Altered connectivity of the dorsal and ventral visual regions in dyslexic children: A resting-state fMRI study. Front. Hum. Neurosci. 2015, 9, 495. [Google Scholar] [CrossRef]
- Horowitz-Kraus, T.; Toro-Serey, C.; DiFrancesco, M. Increased Resting-State Functional Connectivity in the Cingulo-Opercular Cognitive-Control Network after Intervention in Children with Reading Difficulties. PLoS ONE 2015, 10, e0133762. [Google Scholar] [CrossRef]
- Horowitz-Kraus, T.; DiFrancesco, M.; Kay, B.; Wang, Y.; Holland, S.K. Increased resting-state functional connectivity of visual- and cognitive-control brain networks after training in children with reading difficulties. NeuroImage Clin. 2015, 8, 619–630. [Google Scholar] [CrossRef]
- Cheema, K.; Ostevik, A.V.; Westover, L.; Hodgetts, W.E.; Cummine, J. Resting-state networks and reading in adults with and without reading impairments. J. Neurolinguistics 2021, 60, 101016. [Google Scholar] [CrossRef]
- Schurz, M.; Wimmer, H.; Richlan, F.; Ludersdorfer, P.; Klackl, J.; Kronbichler, M. Resting-State and Task-Based Functional Brain Connectivity in Developmental Dyslexia. Cereb. Cortex 2015, 25, 3502–3514. [Google Scholar] [CrossRef] [PubMed]
- de León Rodríguez, D.; Buetler, K.A.; Eggenberger, N.; Laganaro, M.; Nyffeler, T.; Annoni, J.-M.; Müri, R.M. The Impact of Language Opacity and Proficiency on Reading Strategies in Bilinguals: An Eye Movement Study. Front. Psychol. 2016, 7, 649. [Google Scholar] [CrossRef]
- Borleffs, E.; Maassen, B.A.M.; Lyytinen, H.; Zwarts, F. Cracking the Code: The Impact of Orthographic Transparency and Morphological-Syllabic Complexity on Reading and Developmental Dyslexia. Front. Psychol. 2019, 9, 2534. [Google Scholar] [CrossRef] [PubMed]
- Patriat, R.; Molloy, E.K.; Meier, T.B.; Kirk, G.R.; Nair, V.A.; Meyerand, M.E.; Prabhakaran, V.; Birn, R.M. The effect of resting condition on resting-state fMRI reliability and consistency: A comparison between resting with eyes open, closed, and fixated. NeuroImage 2013, 78, 463–473. [Google Scholar] [CrossRef]
- Kumar, V.A.; Lee, J.; Liu, H.-L.; Allen, J.W.; Filippi, C.G.; Holodny, A.I.; Hsu, K.; Jain, R.; McAndrews, M.P.; Peck, K.K.; et al. Recommended Resting-State fMRI Acquisition and Preprocessing Steps for Preoperative Mapping of Language and Motor and Visual Areas in Adult and Pediatric Patients with Brain Tumors and Epilepsy. Am. J. Neuroradiol. 2024, 45, 139–148. [Google Scholar] [CrossRef]
- Paz-Alonso, P.M.; Oliver, M.; Lerma-Usabiaga, G.; Caballero-Gaudes, C.; Quiñones, I.; Suárez-Coalla, P.; Duñabeitia, J.A.; Cuetos, F.; Carreiras, M. Neural correlates of phonological, orthographic and semantic reading processing in dyslexia. NeuroImage Clin. 2018, 20, 433–447. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, Y.; Yang, X.; Yu, W.; Wan, Y. Dyslexia: A Bibliometric and Visualization Analysis. Front. Public Health 2022, 10, 915053. [Google Scholar] [CrossRef]
- Cao, F.; Sussman, B.L.; Rios, V.; Yan, X.; Wang, Z.; Spray, G.J.; Mack, R.M. Different mechanisms in learning different second languages: Evidence from English speakers learning Chinese and Spanish. NeuroImage 2017, 148, 284–295. [Google Scholar] [CrossRef]
- Miles, E. Dyslexia may show a different face in different languages. Dyslexia 2000, 6, 193–201. [Google Scholar] [CrossRef]
- Macdonald, S.; Deacon, L. Twice upon a time: Examining the effect socio-economic status has on the experience of dyslexia in the United Kingdom. Dyslexia 2019, 25, 3–19. [Google Scholar] [CrossRef]
- Wolf, M.; Gotlieb, R.J.M.; Kim, S.A.; Pedroza, V.; Rhinehart, L.V.; Tempini, M.L.G.; Sears, S. Towards a dynamic, comprehensive conceptualization of dyslexia. Ann. Dyslexia 2024, 74, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lee, H.L.; Zhang, Q.; Liu, T.; Geng, L.B.; Seghier, M.L.; Shakeshaft, C.; Twomey, T.; Green, D.W.; Yang, Y.M.; et al. Developmental dyslexia in Chinese and English populations: Dissociating the effect of dyslexia from language differences. Brain 2010, 133, 1694–1706. [Google Scholar] [CrossRef]
- Landerl, K.; Harald, F.H.; Moritz, H.; De Jong, P.F.; Alain, D.; George, M.; Rauno, P.; Georgiou, G.K. Phonological Awareness and Rapid Automatized Naming as Longitudinal Predictors of Reading in Five Alphabetic Orthographies with Varying Degrees of Consistency. Sci. Stud. Read. 2019, 23, 220–234. [Google Scholar] [CrossRef]
- Raimondo, L.; Oliveira, ĺ.A.F.; Heij, J.; Priovoulos, N.; Kundu, P.; Leoni, R.F.; van der Zwaag, W. Advances in resting state fMRI acquisitions for functional connectomics. NeuroImage 2021, 243, 118503. [Google Scholar] [CrossRef] [PubMed]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 2014, 84, 320–341. [Google Scholar] [CrossRef]
- McNorgan, C.; Smith, G.J.; Edwards, E.S. Integrating functional connectivity and MVPA through a multiple constraint network analysis. NeuroImage 2020, 208, 116412. [Google Scholar] [CrossRef]
- Bahrami, M.; Laurienti, P.J.; Shappell, H.M.; Simpson, S.L. Brain Network Analysis: A Review on Multivariate Analytical Methods. Brain Connect. 2023, 13, 64–79. [Google Scholar] [CrossRef]
- Seitzman, B.A.; Snyder, A.Z.; Leuthardt, E.C.; Shimony, J.S. The State of Resting State Networks. Top. Magn. Reson. Imaging 2019, 28, 189–196. [Google Scholar] [CrossRef]
- Gordon, E.M.; Lynch, C.J.; Gratton, C.; Laumann, T.O.; Gilmore, A.W.; Greene, D.J.; Ortega, M.; Nguyen, A.L.; Schlaggar, B.L.; Petersen, S.E.; et al. Three Distinct Sets of Connector Hubs Integrate Human Brain Function. Cell Rep. 2018, 24, 1687–1695.e1684. [Google Scholar] [CrossRef]
- Taran, N.; Farah, R.; DiFrancesco, M.; Altaye, M.; Vannest, J.; Holland, S.; Rosch, K.; Schlaggar, B.L.; Horowitz-Kraus, T. The role of visual attention in dyslexia: Behavioral and neurobiological evidence. Hum. Brain Mapp. 2022, 43, 1720–1737. [Google Scholar] [CrossRef]
- Raschle, N.M.; Zuk, J.; Gaab, N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc. Natl. Acad. Sci. USA 2012, 109, 2156–2161. [Google Scholar] [CrossRef]
- Maurer, U.; Brem, S.; Bucher, K.; Kranz, F.; Benz, R.; Steinhausen, H.C.; Brandeis, D. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain 2007, 130, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Taran, N.; Farah, R.; Gashri, C.; Gitman, E.; Rosch, K.; Schlaggar, B.L.; Horowitz-Kraus, T. Executive functions–based reading training engages the cingulo-opercular and dorsal attention networks. Netw. Neurosci. 2023, 7, 1452–1482. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Wu, M.H.; Liederman, J.; Fisher, J.M. Greater Pre-Stimulus Effective Connectivity from the Left Inferior Frontal Area to other Areas is Associated with Better Phonological Decoding in Dyslexic Readers. Front. Syst. Neurosci. 2010, 4, 156. [Google Scholar] [CrossRef]
- Haft, S.; Duong, P.; Ho, T.; Hendren, R.; Hoeft, F. Anxiety and Attentional Bias in Children with Specific Learning Disorders. J. Abnorm. Child. Psychol. 2018, 47, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Zuk, J.; Perdue, M.V.; Becker, B.; Yu, X.; Chang, M.; Raschle, N.M.; Gaab, N. Neural correlates of phonological processing: Disrupted in children with dyslexia and enhanced in musically trained children. Dev. Cogn. Neurosci. 2018, 34, 82–91. [Google Scholar] [CrossRef]
- Koyama, M.S.; Kelly, C.; Shehzad, Z.; Penesetti, D.; Castellanos, F.X.; Milham, M.P. Reading networks at rest. Cereb. Cortex 2010, 20, 2549–2559. [Google Scholar] [CrossRef]

| Ref. | Year | Demographic Data | Neuropsychological Tests for Dyslexia Evaluation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Language | Group | N | Sex W:M | Age (Years) | Years of Education (or Level) | IQ | Word Reading Accuracy | Pseudoword Reading Accuracy | Text Reading Accuracy | RAN | Phonological Task | Attention | Other | ||
| rs-fMRI in Children | ||||||||||||||||
| Tan S et al. [20] | 2024 | China | Chinese | Control DYS | 36 64 | 8:28 25:39 | 9.4 ± 1.1 9.8 ± 1.1 | 2nd to 5th grade | WISC-IV (FSIQ ≥ 80) | CRTB | NR | NR | Digits, Letters, Colors and Shapes | NR | Conners Teacher Rating Scale (CBRS) | PRS |
| Gengeç B et al. [21] | 2024 | Turkey | Turkish | Control DYS DYSe | 15 13 15 | 10:5 3:10 6:9 | 10.1 ± 1.5 8.9 ± 1.7 9.4 ± 1.5 | E-DYS: DYS with special education for 12–24 months | WISC-IV (≥80) | Number of words read per minute | NR | NR | NR | NR | NR | Motor coordination assessment |
| Farah R et al. [22] | 2024 | USA | English | Control DYS | 65 55 | 29:36 24:31 | 10.1 ± 1.7 9.9 ± 1.3 | NR | Non-verbal Intelligence Test (TONI) | Letter–Word and Word Attack subtests (WJ-III); Sight Word Efficiency subtest (TOWRE) | Timed pseudoword decoding efficiency (TOWRE) | NR | NR | Elision (CTOPP) | NR | Stroop, Stories listening |
| Li Y et al. [23] | 2022 | China | Chinese | Control DYS | 38 22 | 17:21 17:5 | 10.9 ± 0.8 10.7 ± 0.9 | NR | Non-verbal (Raven) | NR | NR | Reading Fluency Test | NR | Oddity detection task | NR | Vocabulary (CRM) |
| Gosse C et al. [24] | 2022 | Belgium | French | Control DYS | 16 16 | 9:5 5:9 | 9.3 ± 1.0 9.4 ± 1.2 | NR | Non-verbal (WISC-IV) | Analytic Battery of Written Language (Regular and Irregular Words) | Analytic Battery of Written Language (Nonwords) | NR | NR | NR | NR | Spelling (BALE), Concise Evaluation Scale for Children’s Handwriting |
| Yang X et al. [25] | 2021 | China | Chinese | Control DYS | 25 18 | 13:12 6:12 | 10.4 ± 1.0 10.2 ± 1.0 | 10:12 (4°:6°) | Non-verbal (Raven) | CRTB | NR | Reading Fluency Test | NR | NR | NR | NR |
| Sturm VE et al. [26] | 2021 | USA | English | Control DYS | 22 32 | 11:11 15:17 | 10.5 ± 1.5 10.3 ± 1.4 | NR | Non-verbal (WASI) | Sight Word Efficiency (TOWRE-2) and Letter–Word (WJ-IV) | Phonemic Decoding Efficiency (TOWRE-2) and Word Attack (WJ-IV) | Paragraph Reading (GORT-5) | NR | NR | NR | Social Skills, Anxiety and Depression (BASC-2) |
| Mateu-Estivill R et al. [27] | 2021 | Spain | Catalan | Control DYS | 30 34 | NR | 7.4 ± 0.5 7.1 ± 0.7 | 1st or 2nd grade | VIQ and PIQ (WISC-IV) | Words subtest (TALE-C) | Pseudowords subtest (PROLEC-R) | Text subtests (TALE-C) | Letters, Objects and Colors | NR | NR | Executive Function and Verbal Fluency (FAS, Animals), Spelling (TALE-C), Digit span (WISC-IV) |
| Horowitz-Kraus T et al. [28] | 2021 | USA | English | Control DYS | 28 29 | NR | 10 ± 1.3 9.8 ± 1.3 | NR | Non-verbal Intelligence test (TONI-3) and VIQ (PPVT-4) | TOSREC, Sight Word Efficiency (TOWRE) and Letter–Word (WJ) | Pseudoword Efficiency (TOWRE) and Word Attack (WJ) | Passage Comprehension (WJ) | Numbers, Objects, Colors and Letters (CTOPP) | Elision (CTOPP) | Conners Comprehensive Behavior Rating Scales (CBRS), Sky Search (TEA-Ch), Sustained (CPT) | Working memory (Digit Span—WISC), Coding and Symbol Search (WISC), Switching (Stroop), Fluency (DKEFS), EF (BRIEF) |
| Greenwood P et al. [29] | 2021 | USA | English | Control DYS | 21 24 | 6:15 12:12 | 10.0 ± 1.5 9.8 ± 1.4 | NR | Non-verbal Intelligence test (TONI-IV) | Sight Word Efficiency (TOWRE) and Letter–Word (WJ) | Pseudoword Efficiency (TOWRE) and Word Attack (WJ) | TOSREC, Passage Comprehension (WJ) and GORT | NR | Elision and Blending Words (CTOPP-2) | Conners Self-report Test (CBRS) | NR |
| Cross AM et al. [30] | 2021 | Canada | English | Control DYS | 65 18 | 45:38 (Total) | 10.9 ± 1.0 | NR | Non-verbal (WASI-II) | Sight Word Efficiency (TOWRE-2) | Phonemic Decoding Efficiency (TOWRE-2) | Passage Comprehension (WJ-III) | Letters | NR | NR | NR |
| Nachshon O et al. [31] | 2020 | USA | English | Control DYS | 37 27 | 15:22 15:12 | 9.9 ± 1.0 10.7 ± 1.1 | NR | Non-verbal Intelligence test (TONI-3) and VIQ (PPVT-4) | Sight Word Efficiency (TOWRE) and Letter–Word (WJ) | Pseudowords Reading Efficiency (TOWRE) and Pseudoword Reading (WJ) | Passage Comprehension (WJ) | Numbers and Letters (CTOPP) | Elision (CTOPP) | Sky Search (TEA-Ch) | Inhibition (STROOP), Working memory (Digit Span—WISC), Symbol Search (WISC), Switching (Wisconsin, EF (BRIEF), Coding (WISC) |
| Margolis AE et al. [32] | 2020 | USA | English | Control DYS | 18 20 | 10:8 8:12 | 10.4 ± 1.7 9.8 ± 1.3 | NR | FIQ, VIQ and PIQ (WASI) | TOWRE-2 and WJ-III | TOWRE-2 | GORT-5 | NR | NR | NR | Rapid Simon Task |
| Kuhl U et al. [33] | 2020 (pub) 2012–2013 (initial data) 2015–2015 (final data) | Germany | German | Control DYS | 16 16 | 7:9 5:11 | BL: 5.6 ± 4 5.8 ± 4 AL: 8.8 ± 3 8.4 ± 3 | BL: Kindergarten (before literacy) AL: Second grade | BL: Non-verbal (WPPSI-III) AL: Non-verbal (WISC-IV) | BL: - AL: SLRT-II | NR | NR | BL: Colored Objects (BISC) AL: - | BL: BISC AL: Base competence for school years (BAKO) | NR | BL: Short-term memory (K-ABC) AL: German spelling test (DERET 1-2) and Heidelberg math’s test (HRT1-4) |
| Hashimoto T et al. [34] | 2020 | Japan | Japanese (syllabic Hiragana and logographic Kanji) | Control DYS | 46 22 | 6:40 3:19 | 10.5 10.7 | NR | FSIQ, VIQ and PIQ (WISC-III or IV) | Standard comprehension test of abstract words | NR | NR | Type not specified | Backward word repetition (3 mola and 4 mola word) | NR | Rey–Osterrieth Complex Figure (copy, immediate recall and delayed recall) |
| Freedman L et al. [35] | 2020 | USA | English | Control DYS | 30 26 | 16:14 10:16 | 10.0 ± 1.4 10.0 ± 1.3 | NR | Non-verbal Intelligence test (TONI-3) | Letter–Word (WJ-III) and Sight Word Efficiency (TOWRE) | Word Attack (WJ-III) | NR | NR | NR | Visual attention (TEA-Ch) | Digit span (WISC-III), Switching (D-KEFS), Wisconsin Card Sorting Task, Inhibition (BRIEF) |
| Davis K et al. [39] | 2018 | USA | English | Control DYS | 21 24 | NR | 10.2 ± 1.8 9.8 ± 1.2 | NR | VIQ and non-verbal (WASI) and WISC-IV | Letter–Word and spelling (WJ-III) | Word Attack (WJ-III) and Phonemic Decoding Efficiency (TOWRE-2) | Reading Fluency (WJ-III) and GORT-5 | Letters and Digits (CTOPP-2) | NR | NR | Children’s memory scale (CMS) story memory, word pairs and numbers; RCMAS, CDRS-R |
| Buchweitz A et al. [36] | 2018 (pub) 2014–15 (tests) | Brazil | Brazilian Portuguese | Control DYS | 16 16 | 7:9 5:11 | 8.4 ± 0.5 9.6 ± 0.8 | NR | WASI | Word Reading Task (LPI) | Pseudoword Reading Task (LPI) | Reading Comprehension test | NR | NR | NR | NR |
| Horowitz-Kraus T, Wood et al. [37] | 2018 | USA | English | Control DYS MD BD | 17 15 14 11 | 9:8 7:8 11:3 3:9 | 9.8 ± 1.4 10.3 ± 1.5 15.5 ± 1.8 16.7 ± 1.8 | NR | NR | Letter–Word (WJ-III) | Word Attack (WJ-III) | NR | NR | Elision (CTOPP) | NR | NR |
| Horowitz-Kraus T, DiCesare et al. [38] | 2018 | USA | English | Control DYS | 9 10 | 4:5 2:8 | 10.2 ± 0.7 9.9 ± 0.9 | NR | Non-verbal IQ (TONI-3) and VIQ (PPVT-4) | Letter–Word (WJ-III) | Word Attack (WJ-III) | TOSREC | NR | Elision (CTOPP) | Conners Comprehensive Behavior Rating Scales (CBRS) | Shifting (Color-word Stroop), Visual attention (Sky Search), Working memory (Digit-span), and Learning (Wisconsin) |
| Zhou W et al. [40] | 2015 | China | Chinese | Control DYS | 26 21 | 16:10 9:12 | 12.0 ± 1.2 12.0 ± 1.6 | NR | Non-verbal (C-WISC and Raven) | NR | NR | Reading Fluency Test | RAN | Phoneme deletion and Lexical decision | NR | Morphological production |
| Horowitz-Kraus T, Toro-Serey C et al. [41] | 2015 | USA | English | Control DYS | 17 15 | 8:9 8:7 | 9.8 ± 1.4 10.3 ± 1.5 | NR | Non-verbal Intelligence test (TONI-3) | TOWRE | TOWRE | NR | Objects (CTOPP) | Elision (CTOPP) | Conners Comprehensive Behavior Rating Scales (CBRS) and Speed and accuracy (TEA-Ch) | Fluency abilities (D-KEFS), Switching (WCST), Inhibition abilities (Stroop—D-KEFS) |
| Horowitz-Kraus T, Difrancesco M et al. [42] | 2015 | USA | English | Control DYS | 18 18 | 9:9 9:9 | 9.8 ± 1.7 9.9 ± 1.3 | NR | Non-verbal Intelligence test (TONI-3) | TOWRE | Decoding subtest for nonwords reading (TOWRE) | Rate, Accuracy (GORT-IV) | NR | NR | Conners Comprehensive Behavior Rating Scales (CBRS) | NR |
| rs-fMRI in adults | ||||||||||||||||
| Cheema K et al. [43] | 2021 | Canada | English | Control DYS | 19 14 | 14:5 10:4 | 21.6 ± 2.0 24.4 ± 5.4 | NR | Non-verbal (WASI) | Sight Word Efficiency (TOWRE-1) and Word Identification | Pseudoword Decoding Efficiency (TOWRE-1) and Word Attack (WJ) | NR | NR | NR | NR | Spelling |
| Schurz M et al. [44] | 2015 | Germany | German | Control DYS | 14 15 | 0:14 0:15 | 17.8 ± 1.2 18.3 ± 1.1 | NR | VIQ, PIQ (WAIS-R) | Word Reading | Non-word Reading | Sentence Reading | Objects | Lexical decision task | NR | NR |
| Ref. | rs-fMRI Acquisition | rs-fMRI Process | rs-fMRI Outcomes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equipment Field | Sequence | TR/TE (ms) | Slice Number | Slice Thickness (mm) | FOV | Volumes (Time) | Condition | Software Version | Denoising Process | Atlas | Type of Analyze | ROIs | Difference in FC of Groups | Clinical-Connectivity Correlation | |||
| Outlier Parameters | Temporal Filter (Hz) | Spatial Smoothed * (mm) | |||||||||||||||
| rs-fMRI in Children | |||||||||||||||||
| Tan S et al. [20] | Siemens 3 T | EPI | 2000/30 | 32 | 3.5 | 224 | NR | Awake with eyes closed | DPABI 5.1 | >3 mm 3° rotation | 0.01–0.08 | 6 | MNI | ROI-to-ROI (Granger causality analysis) | Left SOG, PreCG, IFG tri, MFG, SMG, SMA, PG, right PG, bilaterally MOG, and STG | DYS showed decreased EC from left PreCG to right PreCu and AG, and left ACC and calcarine, as well as from left MFG to left calcarine, compared to controls. Enhanced EC from right Cu to left IFG tri (DYS > controls). | Letter-RAN scores positively correlated with EC from left PG to left ACC. |
| Gengeç B et al. [21] | Siemens 1.5 T | T2* | 2800/53 | 25 | 5 | 192 | 133 | Awake eyes open | FSL MELODIC 6.0.4 | >2 mm or mean of 0.5 mm | 0.01 | 5 | MNI | ICA AROMA-dual regression | VIS: right occipital lobe sub-gyral; DMN: bilateral MTG; FPN: l-STG, l-MTG, l-IPL, right cerebellum cerebellar tonsil, right PreCu; VAN: bilateral insula; OFN: r-ACC; LMN: l-IPL | VIS: DYS decreased FC in the right compared to EDYS and in the left MOG in EDYS compared to controls Compared to controls: DMN: DYS decreases FC in the left MTG; FPN: DYS increases FC in the STG; VAN: DYS increases FC in the left IA OFN: DYS decreases FC in the right dACC; LMN: DYS decreases FC in the left IPL | NR |
| Farah R et al. [22] | Philips 3 T (dataset 1 and 2)/Philips 3T (dataset 3) | EPI (dataset 1 and 2)/EPI multi-band (dataset 3) | 2000/30 (dataset 1 and 2)/700/30 (dataset 3) | 37 (dataset 1 and 2)/48 (dataset 3) | 4 (dataset 1)/3 (dataset 2 and 3) | NR | 180/300/857 | Eyes closed | NR | NR | 0.009–0.08 | 6 | Talairach | ROI-to-ROI | SMd, SMl, CON, auditory, DMN, PMN, VIS, FPN, salience, VAN, DAN, MTL, BG, THA and cerebellum | No group differences | Reading fluency shows positive correlation with the FC in DYS within the SMd, FPN, and between the SMd and auditory networks for both groups, as well as between pseudoword reading fluency and FC in DYS within the SMl and VIS |
| Li Y et al. [23] | Siemens 3T | EPI | 2000/31.8|2000/30 | 54|32 | 2|3 | 192 | 160 | 240 | Awake with eyes closed, not thinking about anything | DPABI 4.5 and SPM 8 Dynamic BC toolbox | >3 mm or 3° rotation | 0.01–0.1 | 4 | MNI | Dynamic FC by ROI-to-ROI and ALFF and ReHo | Reading network: PreCG, PostCG, MFG, IFG, AG, IPL, STG, MTG, ITG, FG, MOG, and Cu bilaterally | DYS showed a greater temporal variability of FC at left IFG with the left MTG, and the left MOG with right than controls. DYS showed reduced ALFF and ReHo compared to controls. | Temporal variability in the left IFG negatively correlated with reading fluency and phonological awareness for both groups. |
| Gosse C et al. [24] | GE 3T | EPI | 1500/30 | 64 | 2 | 220 | 250 | Awake with eyes closed | Brain Voyager 20.4 | NR | 0.005 | 5 | MNI | Seed-to-voxel | Left GMFA located in BA6 in the posterior part of the MFG. | DYS reduction in FC at bilateral frontoparietal, limbic, and cerebellum, as well as the left temporolimbic and temporal areas compared to controls | Reading and handwriting scores correlated positively in almost all clusters in both groups |
| Yang X et al. [25] | Siemens 3T | EPI | 2400/30 | 40 | 3 | 192 | 180 | Awake with eyes closed | SPM 12 | FD > 2 mm; 2° rotation | 0.01–0.08 | 6 | MNI | ROI-to-ROI | V1, BA17 | NR | Positive correlation between FC (V1-MFG) and reading fluency in controls |
| Sturm VE et al. [26] | Siemens 3T | EPI | 2000/31| 1290/32.4 | 31|68 | 3.5|2.2 | 220|211 | 180|279 | Awake with eyes closed | SPM 12|CONN 17f | <2 mm | 0.01–0.1 | 8 | MNI | AAL | Seed-to-voxel | SAL network: right ventral AI, bilateral ACC | DYS showed stronger FC in right vAI with the pACC, the anterior MCC, amygdala, THA, hypothalamus, and brainstem | Positive correlation between FC of the right vAI—pACC, anterior MCC, and the right frontal pole with emotional facial behavior. |
| Mateu-Estivill R et al. [27] | Siemens 3T | EPI | 2500/29 | 40 | 3 | 240 | 242 | NR | SPM12|CONN | FD > 0.5 mm or SIC > 3 SD | 0.008–0.1 | 8 | MNI|Harvard–Oxford Atlas | MVPA | Seed-to-voxel | PCA —Cluster 1: PCC and right MFG, —Cluster 2: the left FFG | DYS higher FC right MFG with DMN and lower with bilateral AI and SMG | No significant correlations were found |
| Horowitz-Kraus T et al. [28] | Philips 3T | EPI | 700/30 | NR | 3 | NR | 857 | NR | SPM 12|CONN 17f | NR | 0.008–0.2 | NR | MNI | ICA | SAL network: ACC, bilateral AI, bilateral rostral PFC; bilateral SMG | NR | DYS showed positive correlations between screen vs. reading time ratio and FC of SAL network and frontal EF regions, as well as between SAL network and bilateral cognitive control regions, associative visual processing regions, and negative correlation with FC subcortical cognitive control, language, and V1 region |
| Greenwood P et al. [29] | Philips 3T | T2* | 700/30 | 48 | 3 | 200 | 857 | Awake, fixated on a cross | SPM 12|CONN | NR | 0.008–0.09 | 8 | MNI|WFU PickAtlas | Seed-to-voxel | CON: bilateral aPFC, lateral AI/FOp, medial AI/FOp, AI/FOp, dACC/medial SFC; FPN: bilateral DLPFC, IPL, IPS, PreCu, MCC; DAN: FEF, pIPS, aIPS, MT+, and left SMA/pre-SMA, right IFG, MFG, and AI; VAN: bilateral AI, SFG, and MTG, right SMG (TPJ), IFG/MFG, left STG, PreCu, MedFG, and sulcus callosomarginalis | No group differences | DYS shows a negative correlation between maternal education and FC between FPN and left central opercular cortex and occipital FFG |
| Cross AM et al. [30] | Siemens 3T | T2* | 1000/30 | 48 | 3 | 210 | 360 | Awake, fixated on a cross | SPM 12|CONN | FD < 0.5 mm or BSC > 3 SD | 0.008–0.09 | 5 | MNI | Seed-to-voxel | MFG, IFG opercularis, IFG triangularis, PreCG, STG posterior, AG, SPL, SMA, FFG, OP, THA | NR | Positive correlation between FC in left dorsal and anterior regions with decoding proficiency, and between left THA, and right FFG with sight word reading, RAN, and reading comprehension abilities. A negative association between reading comprehension and RSFC from SPL to SFG and MFG, in left hemisphere. |
| Nachshon O et al. [31] | Philips 3T | EPI | 2000/38 | NR | 5 | NR | 300 | Awake, fixated on a cross | SPM/CONN | NR | 0.008–0.2 | NR | FSL Harvard–Oxford | Seed-to-voxel and graph theory | Amygdala: BLA and CAN bilateral | DYS showed lower global efficiency values within the amygdala network, a greater FC of the bilateral amygdala with the right TP and the right MFG, and a lower FC between the left amygdala and the left FP. DYS decreases FC between the amygdala (left > right) with right FP, the left amygdala with left FP, and the right amygdala with left THA | Positive correlation between reading/EF measures and FC of left amygdala-bilateral FP, and negative correlation between emotional abilities and FC of left Amygdala-right FP |
| Margolis AE et al. [32] | GE 3T | EPI | 2000/30 | NR | 3 | 240 | 292 | NR | SPM 12|CONN 16b | FD > 0.5 mm or BSC > 3 | 0.01–0.1 | 8 | MNI | Seed-to-voxel | CON: AI/FP, aPFC, THA bilateral and dorsal ACC FPN: DLPFC, IPL, IPS, PreCu bilaterally | DYS reduced FC of CON with left FPN and temporal-parietal regions and increased FC with cerebellum than controls. DYS showed decreases FC from FPN to DMN in controls | Both groups have a negative correlation FC of FPN (left IPL and right SFG) with Simon’s performance. DYS showed a positive correlation between FC left IPL to PCC with word-reading accuracy. |
| Kuhl U et al. [33] | Siemens 3T | T2* | 2000/30 | NR | 3.99 | 192 | 75|118 | NR | FSL 5.0 and AFNI 17.2.17 | FD > 0.5 mm | 0.01–0.1 | 6 | MNI|Glasse atlas | ROI-to-ROI | Cortical: V1, and left MT, FG, A1, PT, BA6, BA44, and BA45/47 Subcortical areas: MGB, LGN, and iC | DYS showed lower FC in the left A1 and PT compared to controls, mainly in boys before literacy | NR |
| Hashimoto T et al. [34] | Philips 3T | EPI | 2000/30 | 34 | 3.75 | 240 | 160 | Awake eyes open | DPARSF|SPM8 | FD > 0.5 mm and BSC > 1.5% | 0.01–0.1 | 6 | MNI | Seed-to-voxel | The left lFG and IPL; bilateral FG and the right IOG | No group differences. | Control: fluent reading (Hiragana) positively correlated with FC between left FG and left ventral fronto-temporal area and fewer correlations in DYS. Control > DYS showed a positive correlation between the FC of left IPL-right MFG with Kanji accuracy |
| Freedman L et al. [35] | Philips 3T | T2* | 2000/30 | NR | 3 | NR | 300 | Awake, fixated on a cross | CONN 18 | NR | 0.008–0.2 | 8 | MNI|WFU PickAtlas | ROI-to-ROI | CON: bilateral aPFC and AI; FPN: bilateral DLPFC, IPL, IPS, PreCu; DAN: bilateral FEF and IPS; VAN: bilateral IFG, STG, MFG, IPL | CON and VAN FC differences between high and low fluency separation groups | NR |
| Davis K et al. [39] | GE 3T | EPI | 2200/30 | 140 | 3.5 | 240 | 273 | Minds wander, awake, looking at a cross | SPM 12 and CONN 16b | FD > 0.5 mm | 0.01–0.1 | 6 | MNI and Juelich Histological Atlas | Seed-to-voxel | Amygdala: BLA and CAN bilateral | DYS showed an increase in FC from left BLA to an MPFC compared to controls, as well as in bilateral CMA to MPFC (left SMFG and MFG) | Both groups showed FC from left BLA and bilateral CMA to mPFC positively predicted reading impairment and negatively predicted reading fluency. |
| Buchweitz A et al. [36] | GE 3T | EPI | 2000/30 | 29 | 3.5 | 220 | 210 | Awake looking at a cross | AFNI | >0.9 mm | 0.01–0.1 | 6 | Haskins Ped | MNI | Seed-to-voxel | Left FFG (VWFA), left AG, left IFG, left MTG, left STG | DYS showed lower FC between VWFA and PCC compared to controls. | NR |
| Horowitz-Kraus T, Wood et al. [37] | Philips 3T | EPI | 2000/38 | NR | 5 | NR | 165 | Awake, fixated on a cross | SPM 8|CONN 13p | FD > 2 mm | 0.008–0.2 | 8 | MNI|WFU PickAtlas | ROI-to-ROI and graph theory | CON: dACC and bilaterally aPFC, lateral AI/FOp, medial AI/FOp, and AI/FOp; FPN: bilateral DLPFC, bilateral IPL, bilateral, IPS, bilateral PreCu, mCC | No group differences in the FPN and CON | DYS had a lower reading score and higher FC FPN compared to the mood disorders group. DYS had a negative correlation between phonological awareness and FC of CON |
| Horowitz-Kraus T, DiCe et al. [38] | Philips 3T | EPI | 2000/38 | NR | 5 | NR | 300 | Awake, fixated on a cross | SPM 8|CONN | FD > 0.02 mm | 0.008–0.2 | 8 | MNI | ROI-to-ROI | Bilateral ACC/BA24 | DYS showed lower FC in ACC/BA24 with left IFG/BA45 | Lower negative FC between ACC and left IFG tri regions was correlated with longer fixation times during reading in DYS. |
| Zhou W et al. [40] | Siemens 3T | EPI | 2000/30 | 33 | 3 | 200 | 240 | Motionless and no thinking | DPARSF | NR | 0.01–0.08 | 4 | MNI | Seed-to-voxel | Left IPS, VWFA | DYS decreases FC between left IPS to the left MFG and VWFA to the anterior part of the left MFG compared to controls | Both groups showed a positive correlation in score of reading fluency with FC for IPS-MFG and VWFA-MFG |
| Horowitz-Kraus T, Toro-Serey C et al. [41] | Philips 3T | EPI | 2000/38 | NR | 5 | NR | 165 | Awake, fixated on a cross | SPM 8|CONN13p | FD > 2 mm | 0.008–0.2 | 8 | MNI | ROI-to-ROI | CON: bilateral aPFC, bilateral lateral AI, bilateral medial AI, bilateral AI, and dACC; FPN: mCC and bilateral DLPFC, IPL, IPS, and PreCu | No group differences in the FPN and CON | DYS showed a negative correlation between reading scores and FC of CON |
| Horowitz-Kraus T, Difrancesco M et al. [42] | Philips 3T | EPI | 2000/38 | NR | 5 | NR | 165 | Awake, fixated on a cross | SPM8 and GIFT | IC noise | NR | 8 | MNI | ICA/PCA | ICs— Visual: bilateral FFG, Cu, LG, ITG; Executive functions: bilateral SFG and MFG; Attention: bilateral ACC and SFG; SM: bilateral Pre and Pos CG; language: bilateral IFG, MFG, ACC, left SPL, SMG; Occipito-temporal: bilateral LG, MTG, FFG; DAN: bilateral PreCu, SFG, LG, MTG, AG; and Memory: parahippocampal gyrus | DYS showed greater FC between visual and SM IC compared with controls. DYS showed decreased FC between visual, EF, and Attention ICs compared to controls | Both groups had a negative correlation between visual and SM FC with word and nonword reading scores. |
| rs-fMRI in adults | |||||||||||||||||
| Cheema K et al. [43] | Siemens 3T | EPI | 1980/30 | 64 | 2.2 | NR | 182 | Awake, fixated on a cross | SPM12 CONN | FD > 0.9 mm; BSC > 5 z-value; | 0.01–0.1 | 8 | MNI | Seed-to-ROI | DMN: MPFC, bilateral LP, PCC; DAN: bilateral FEF and IPS; SAL: bilateral AI and RPFC, ACC, SMG | No group differences in seeds IFG, SMG, and FFG or DMN, DAN, and SAL | The between-group difference in left SMG-left AG FC showed a positive correlation with TOWRE SWE fluency behavior, and negative correlation for DYS |
| Schurz M et al. [44] | Philips 1.5T | EPI | 2200/45 | 25 | 5 | 220 | 110 | Awake eyes open | SPM 8|CONN 13i | FD > 0.5 mm; BSC > 1.5% or | 0.008–0.09 | 8 | MNI | Seed-to-voxel | Occipito-Temporal seeds: left FFG and left ITG | DYS showed stronger FC in left FFG with right PreCu, but lower with left IFG tri, as well as between the left ITG with bilateral IFG (op, orb and tri) and stronger with left PreCu. | Positive correlation for both groups: Reading and Verbal IQ with FC of FFG with left IFG (op and tri), and ITG and STG with left IFG tri;, only reading with FC of MTG with left IFG (ob and op) and between IFG with left MTG; reading and naming was correlated with ITG and STG with left IFG op. The negative correlation was found between IFG and left IPL/AG |
| Left temporo-parietal seeds: left STG and left IPL | DYS showed weaker FC in the left STG with left IFG op, and the left IPL with right hippocampus and left PreCu. | ||||||||||||||||
| Left temporal and frontal seeds: left MTG and left IFG | DYS showed stronger FC in left MTG with left IFG oper, right FP and left calcarine sulcus; and lower FC in left IFG with left MTG and SMA and stronger with right rolandic operculum. | ||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, B.; Verrone, I.A.B.; Sakamoto, M.M.I.; Baba, M.Y.; Yvata, M.E.; Lukasova, K.; Nucci, M.P. Resting-State Functional MRI in Dyslexia: A Systematic Review. Biomedicines 2025, 13, 1210. https://doi.org/10.3390/biomedicines13051210
Martins B, Verrone IAB, Sakamoto MMI, Baba MY, Yvata ME, Lukasova K, Nucci MP. Resting-State Functional MRI in Dyslexia: A Systematic Review. Biomedicines. 2025; 13(5):1210. https://doi.org/10.3390/biomedicines13051210
Chicago/Turabian StyleMartins, Bruce, Isabel A. B. Verrone, Mariana M. I. Sakamoto, Mariana Y. Baba, Melissa E. Yvata, Katerina Lukasova, and Mariana P. Nucci. 2025. "Resting-State Functional MRI in Dyslexia: A Systematic Review" Biomedicines 13, no. 5: 1210. https://doi.org/10.3390/biomedicines13051210
APA StyleMartins, B., Verrone, I. A. B., Sakamoto, M. M. I., Baba, M. Y., Yvata, M. E., Lukasova, K., & Nucci, M. P. (2025). Resting-State Functional MRI in Dyslexia: A Systematic Review. Biomedicines, 13(5), 1210. https://doi.org/10.3390/biomedicines13051210









