Stem Cell Therapy for Myocardial Infarction Recovery: Advances, Challenges, and Future Directions
Abstract
1. Introduction
2. Literature Review Methodology
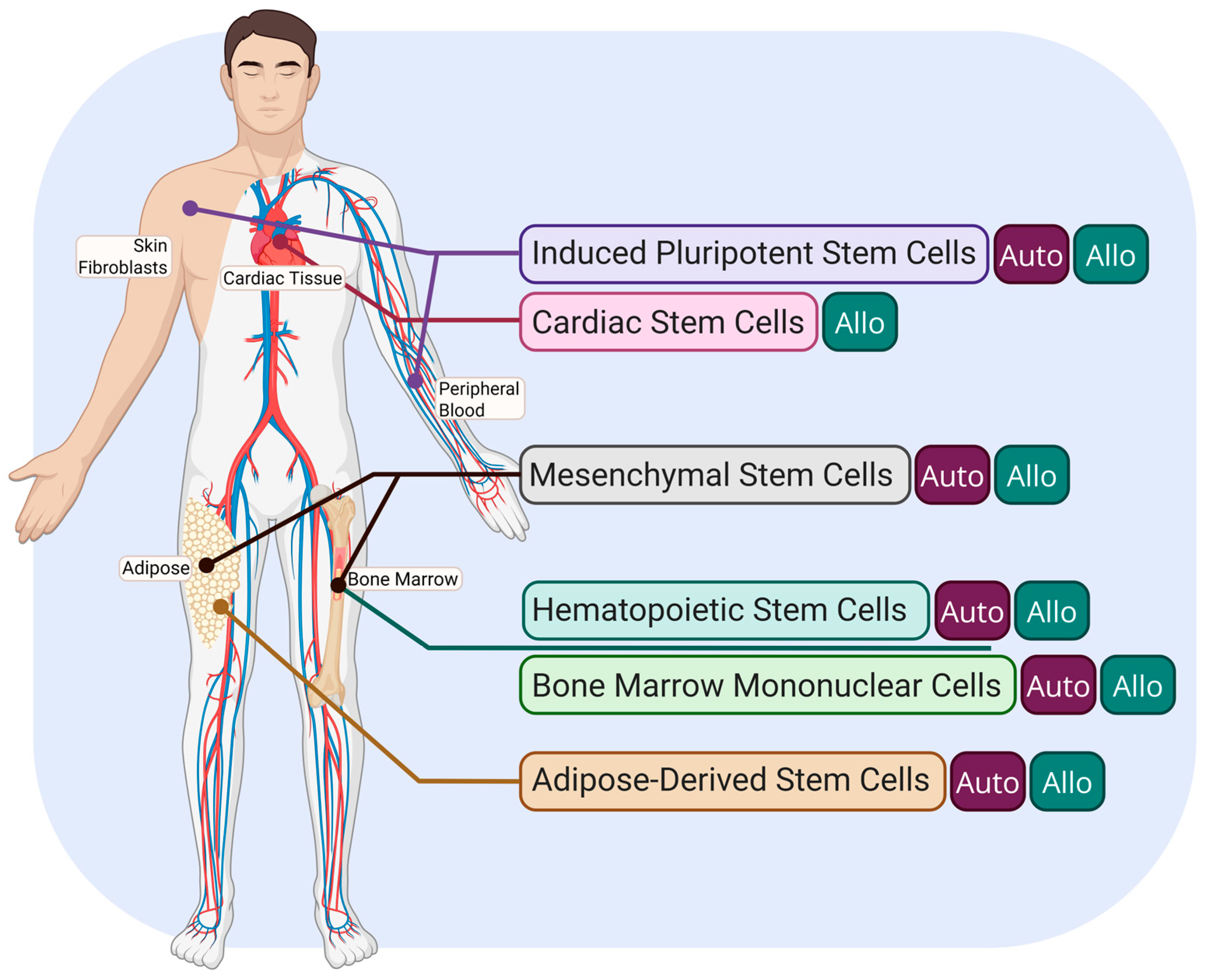
3. Types of Stem Cells Currently Researched for MI Recovery
3.1. Cardiac Stem Cells (CSCs)
3.2. Mesenchymal Stem Cells (MSCs)
3.3. Bone Marrow Mononuclear Cells (BMMCs)
3.4. Induced Pluripotent Stem Cells (iPSCs)
3.5. Hematopoietic Stem Cells (HSCs)
3.6. Adipose-Derived Stem Cells (ASCs)
3.7. Other Stem Cell Types
4. Role of Stem Cells in Cardiac Regeneration Post-Myocardial Infarction
4.1. Restoration and Angiogenesis
4.2. Extracellular and Paracrine Signaling
4.3. Extracellular Vesicles (EVs) and Their Role in Cardiac Regeneration
5. Methods and Timing of Delivery
5.1. Direct Intramyocardial Injection
5.2. Intravenous Infusion
5.3. Intracoronary Infusion
5.4. Intramyocardial Administration Using Catheters
5.4.1. Intramyocardial Trans Endocardial Injection
5.4.2. Intramyocardial Trans Coronary Venous Injection
5.5. Retrograde Coronary Venous Delivery System
6. Summary of Clinical and Preclinical Trials
7. Limitations in Cell Survival and Retention
7.1. Tumorigenicity Risks
7.2. Fibrosis and Adverse Remodeling
8. Future Stem Cell Technologies/Treatments
8.1. Gene Editing
8.2. Small Extracellular Vesicles
8.3. Bioinks and Bioprinting
8.4. Feasibility Studies
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MI | Myocardial Infarction |
| MACE | Major Adverse Cardiac Events |
| LVEDV | Left Ventricular End-Diastolic Volume |
| LVESV | Left Ventricular End-Systolic Volume |
| CABGs | Coronary Artery Bypass Grafts |
| CABG | Coronary Artery Bypass Grafting |
| AMI | Acute Myocardial Infarction |
| STEMI | ST-Elevation Myocardial Infarction |
| SPECT | Single Photon Emission Computed Tomography |
| iPSCs | Induced Pluripotent Stem Cells |
| ASCs | Adipose-Derived Stem Cells |
| MSC | Mesenchymal Stem Cell |
| BMMSC | Bone Marrow Mesenchymal Stem Cell |
| HSC | Hematopoietic Stem Cell |
| EPC | Endothelial Progenitor Cell |
| CSC | Cardiac Stem Cell |
| ESCs | Embryonic Stem Cells |
| WJ-MSCs | Wharton’s Jelly Mesenchymal Stromal Cells |
| CM | Cardiomyocyte |
| CPC | Cardiac Progenitor Cell |
| CDCs | Cardiosphere-Derived Cells |
| EVs | Extracellular Vesicles |
| MVs | Microvesicles |
| ESV | Embryonic Stem Cell-Derived Vesicles |
| sEV | Small Extracellular Vesicle |
| BMMCs | Bone Marrow-Derived Mononuclear Cells |
| BM-MNCs | Bone Marrow Mononuclear Cells |
| BMCs | Bone Marrow Cells |
| GVHD | Graft-versus-Host Disease |
| Muse Cells | Multilineage-Differentiation Stress-Enduring Cells |
| SVPs | Saphenous Vein-Derived Pericytes |
| ECM | Extracellular Matrix |
| HGF | Hepatocyte Growth Factor |
| hiPSC | Human Induced Pluripotent Stem Cell |
References
- Mathers, C.D.; Boerma, T.; Ma Fat, D. Global and Regional Causes of Death. Br. Med. Bull. 2009, 92, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Ojha, N.; Dhamoon, A.S. Myocardial Infarction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Le, N.T.; Dunleavy, M.W.; Kumar, R.D.; Zhou, W.; Bhatia, S.S.; El-Hashash, A.H. Cellular Therapies for Idiopathic Pulmonary Fibrosis: Current Progress and Future Prospects. Am. J. Stem Cells 2024, 13, 191–211. [Google Scholar] [CrossRef] [PubMed]
- du Pré, B.C.; Doevendans, P.A.; van Laake, L.W. Stem Cells for Cardiac Repair: An Introduction. J. Geriatr. Cardiol. JGC 2013, 10, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine Mechanisms of Stem Cell Reparative and Regenerative Actions in the Heart. J. Mol. Cell. Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef]
- Hou, L.; Kim, J.J.; Woo, Y.J.; Huang, N.F. Stem Cell-Based Therapies to Promote Angiogenesis in Ischemic Cardiovascular Disease. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H455–H465. [Google Scholar] [CrossRef]
- Botleroo, R.A.; Bhandari, R.; Ahmed, R.; Kareem, R.; Gyawali, M.; Venkatesan, N.; Ogeyingbo, O.D.; Elshaikh, A.O. Stem Cell Therapy for the Treatment of Myocardial Infarction: How Far Are We Now? Cureus 2021, 13, e17022. [Google Scholar] [CrossRef]
- Vasu, S.; Zhou, J.; Chen, J.; Johnston, P.V.; Kim, D.-H. Biomaterials-Based Approaches for Cardiac Regeneration. Korean Circ. J. 2021, 51, 943–960. [Google Scholar] [CrossRef]
- Hong, K.U.; Guo, Y.; Li, Q.-H.; Cao, P.; Al-Maqtari, T.; Vajravelu, B.N.; Du, J.; Book, M.J.; Zhu, X.; Nong, Y.; et al. C-Kit+ Cardiac Stem Cells Alleviate Post-Myocardial Infarction Left Ventricular Dysfunction despite Poor Engraftment and Negligible Retention in the Recipient Heart. PLoS ONE 2014, 9, e96725. [Google Scholar] [CrossRef]
- Fernández-Avilés, F.; Sanz-Ruiz, R.; Bogaert, J.; Casado Plasencia, A.; Gilaberte, I.; Belmans, A.; Fernández-Santos, M.E.; Charron, D.; Mulet, M.; Yotti, R.; et al. Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients with ST-Segment Elevation Myocardial Infarction and Left Ventricular Dysfunction. Circ. Res. 2018, 123, 579–589. [Google Scholar] [CrossRef]
- Avolio, E.; Meloni, M.; Spencer, H.L.; Riu, F.; Katare, R.; Mangialardi, G.; Oikawa, A.; Rodriguez-Arabaolaza, I.; Dang, Z.; Mitchell, K.; et al. Combined Intramyocardial Delivery of Human Pericytes and Cardiac Stem Cells Additively Improves the Healing of Mouse Infarcted Hearts through Stimulation of Vascular and Muscular Repair. Circ. Res. 2015, 116, e81–e94. [Google Scholar] [CrossRef]
- Attar, A.; Nouri, F.; Yazdanshenas, A.; Hessami, K.; Vosough, M.; Abdi-Ardekani, A.; Izadpanah, P.; Ramzi, M.; Kojouri, J.; Pouladfar, G.; et al. Single vs. Double Intracoronary Injection of Mesenchymal Stromal Cell after Acute Myocardial Infarction: The Study Protocol from a Randomized Clinical Trial: BOOSTER-TAHA7 Trial. Trials 2022, 23, 293. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.M.; El-Shal, A.S.; Zidan, H.E.; Mazen, N.F.; Abd El-Haleem, M.R.; Abd El Motteleb, D.M. Comparing the Effects of MSCs and CD34+ Cell Therapy in a Rat Model of Myocardial Infarction. IUBMB Life 2016, 68, 343–354. [Google Scholar] [CrossRef] [PubMed]
- van der Spoel, T.I.G.; Gathier, W.A.; Koudstaal, S.; van Slochteren, F.; Of Lorkeers, S.J.; Sluijter, J.P.G.; Hoefer, I.E.; Steendijk, P.; Cramer, M.J.M.; Doevendans, P.A.; et al. Autologous Mesenchymal Stem Cells Show More Benefit on Systolic Function Compared to Bone Marrow Mononuclear Cells in a Porcine Model of Chronic Myocardial Infarction. J. Cardiovasc. Transl. Res. 2015, 8, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; et al. Intracoronary Infusion of Wharton’s Jelly-Derived Mesenchymal Stem Cells in Acute Myocardial Infarction: Double-Blind, Randomized Controlled Trial. BMC Med. 2015, 13, 162. [Google Scholar] [CrossRef]
- Rodrigo, S.F.; van Ramshorst, J.; Hoogslag, G.E.; Boden, H.; Velders, M.A.; Cannegieter, S.C.; Roelofs, H.; Al Younis, I.; Dibbets-Schneider, P.; Fibbe, W.E.; et al. Intramyocardial Injection of Autologous Bone Marrow-Derived Ex Vivo Expanded Mesenchymal Stem Cells in Acute Myocardial Infarction Patients Is Feasible and Safe up to 5 Years of Follow-Up. J. Cardiovasc. Transl. Res. 2013, 6, 816–825. [Google Scholar] [CrossRef]
- Attar, A.; Hosseinpour, A.; Hosseinpour, H.; Kazemi, A. Major Cardiovascular Events after Bone Marrow Mononuclear Cell Transplantation Following Acute Myocardial Infarction: An Updated Post-BAMI Meta-Analysis of Randomized Controlled Trials. BMC Cardiovasc. Disord. 2022, 22, 259. [Google Scholar] [CrossRef]
- Haddad, K.; Potter, B.J.; Matteau, A.; Reeves, F.; Leclerc, G.; Rivard, A.; Gobeil, F.; Roy, D.-C.; Noiseux, N.; Mansour, S. Analysis of the COMPARE-AMI Trial: First Report of Long-Term Safety of CD133+ Cells. Int. J. Cardiol. 2020, 319, 32–35. [Google Scholar] [CrossRef]
- Laguna, G.; DI Stefano, S.; Maroto, L.; Fulquet, E.; Echevarría, J.R.; Revilla, A.; Urueña, N.; Sevilla, T.; Arnold, R.; Ramos, B.; et al. Effect of Direct Intramyocardial Autologous Stem Cell Grafting in the Sub-Acute Phase after Myocardial Infarction. J. Cardiovasc. Surg. 2018, 59, 259–267. [Google Scholar] [CrossRef]
- Nair, V.; Madan, H.; Sofat, S.; Ganguli, P.; Jacob, M.J.; Datta, R.; Bharadwaj, P.; Sarkar, R.S.; Pandit, A.J.; Nityanand, S.; et al. Efficacy of Stem Cell in Improvement of Left Ventricular Function in Acute Myocardial Infarction--MI3 Trial. Indian J. Med. Res. 2015, 142, 165–174. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, S.; Lv, X.; Duan, F.; Wang, H.; Gao, Y.; Wang, J. Effects of Bone Marrow Mononuclear Cells Delivered through a Graft Vessel for Patients with Previous Myocardial Infarction and Chronic Heart Failure: An Echocardiographic Study of Left Atrium Function. Echocardiography 2016, 33, 1835–1843. [Google Scholar] [CrossRef]
- Yang, H.; Shao, N.; Holmström, A.; Zhao, X.; Chour, T.; Chen, H.; Itzhaki, I.; Wu, H.; Ameen, M.; Cunningham, N.J.; et al. Transcriptome Analysis of Non Human Primate-Induced Pluripotent Stem Cell-Derived Cardiomyocytes in 2D Monolayer Culture vs. 3D Engineered Heart Tissue. Cardiovasc. Res. 2021, 117, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Jun Hong, S.; Rogers, P.I.; Kihlken, J.; Warfel, J.; Bull, C.; Deuter-Reinhard, M.; Feng, D.; Xie, J.; Kyle, A.; Merfeld-Clauss, S.; et al. Intravenous Xenogeneic Transplantation of Human Adipose-Derived Stem Cells Improves Left Ventricular Function and Microvascular Integrity in Swine Myocardial Infarction Model. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2015, 86, E38–E48. [Google Scholar] [CrossRef]
- Sasaki, N.; Itakura, Y.; Mohsin, S.; Ishigami, T.; Kubo, H.; Chiba, Y. Cell Surface and Functional Features of Cortical Bone Stem Cells. Int. J. Mol. Sci. 2021, 22, 11849. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Minatoguchi, S.; Kanamori, H.; Mikami, A.; Okura, H.; Dezawa, M.; Minatoguchi, S. Stem Cell Therapy for Acute Myocardial Infarction-Focusing on the Comparison between Muse Cells and Mesenchymal Stem Cells. J. Cardiol. 2022, 80, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T. Overview of the Most Prominent Stem Cell Population Origins for Post-MI Therapies. Available online: https://app.biorender.com/illustrations/67d20536719d6d5bbe39b236 (accessed on 30 March 2025).
- Selem, S.; Hatzistergos, K.E.; Hare, J.M. Chapter 19-Cardiac Stem Cells: Biology and Therapeutic Applications. In Principles of Regenerative Medicine, 2nd ed.; Atala, A., Lanza, R., Thomson, J.A., Nerem, R., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 327–346. ISBN 978-0-12-381422-7. [Google Scholar]
- Li, Q.; Guo, Y.; Ou, Q.; Chen, N.; Wu, W.-J.; Yuan, F.; O’Brien, E.; Wang, T.; Luo, L.; Hunt, G.N.; et al. Intracoronary Administration of Cardiac Stem Cells in Mice: A New, Improved Technique for Cell Therapy in Murine Models. Basic Res. Cardiol. 2011, 106, 849–864. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Szaraz, P.; Gratch, Y.S.; Iqbal, F.; Librach, C.L. In Vitro Differentiation of Human Mesenchymal Stem Cells into Functional Cardiomyocyte-like Cells. J. Vis. Exp. JoVE 2017, 55757. [Google Scholar] [CrossRef]
- Lee, H.; Hong, I. Double-edged Sword of Mesenchymal Stem Cells: Cancer-promoting versus Therapeutic Potential. Cancer Sci. 2017, 108, 1939–1946. [Google Scholar] [CrossRef]
- Bone Marrow Derived Mononuclear Cell-an Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/bone-marrow-derived-mononuclear-cell (accessed on 12 March 2025).
- Raab, S.; Klingenstein, M.; Liebau, S.; Linta, L. A Comparative View on Human Somatic Cell Sources for iPSC Generation. Stem Cells Int. 2014, 2014, 768391. [Google Scholar] [CrossRef]
- Lalit, P.A.; Hei, D.J.; Raval, A.N.; Kamp, T.J. iPS Cells for Post-Myocardial Infarction Repair: Remarkable Opportunities and Challenges. Circ. Res. 2014, 114, 1328–1345. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hong, S.-H. Hematopoietic Stem Cells and Their Roles in Tissue Regeneration. Int. J. Stem Cells 2019, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-Derived Stem Cells: Implications in Tissue Regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.A.; Krishna, K.S.; Berrocal, R.; Rao, K.S.; Sambasiva Rao, K.R.S. Myocardial Infarction and Stem Cells. J. Pharm. Bioallied Sci. 2011, 3, 182–188. [Google Scholar] [CrossRef]
- Mohsin, S.; Troupes, C.D.; Starosta, T.; Sharp, T.E.; Agra, E.J.; Smith, S.; Duran, J.M.; Zalavadia, N.; Zhou, Y.; Kubo, H.; et al. Unique Features of Cortical Bone Stem Cells Associated with Repair of the Injured Heart. Circ. Res. 2015, 117, 1024–1033. [Google Scholar] [CrossRef]
- Assmus, B.; Schächinger, V.; Teupe, C.; Britten, M.; Lehmann, R.; Döbert, N.; Grünwald, F.; Aicher, A.; Urbich, C.; Martin, H.; et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002, 106, 3009–3017. [Google Scholar] [CrossRef]
- Sanganalmath, S.K.; Bolli, R. Cell Therapy for Heart Failure: A Comprehensive Overview of Experimental and Clinical Studies, Current Challenges, and Future Directions. Circ. Res. 2013, 113, 810–834. [Google Scholar] [CrossRef]
- Roche, E.T.; Hastings, C.L.; Lewin, S.A.; Shvartsman, D.; Brudno, Y.; Vasilyev, N.V.; O’Brien, F.J.; Walsh, C.J.; Duffy, G.P.; Mooney, D.J. Comparison of Biomaterial Delivery Vehicles for Improving Acute Retention of Stem Cells in the Infarcted Heart. Biomaterials 2014, 35, 6850–6858. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, J.H.; Lee, Y.H.; Lee, J.H.; Kim, S.S.; Kim, M.Y.; Lee, M.G.; Kang, W.Y.; Lee, K.S.; Ahn, Y.K.; et al. Improvement in Left Ventricular Function with Intracoronary Mesenchymal Stem Cell Therapy in a Patient with Anterior Wall ST-Segment Elevation Myocardial Infarction. Cardiovasc. Drugs Ther. 2018, 32, 329–338. [Google Scholar] [CrossRef]
- González-King, H.; Rodrigues, P.G.; Albery, T.; Tangruksa, B.; Gurrapu, R.; Silva, A.M.; Musa, G.; Kardasz, D.; Liu, K.; Kull, B.; et al. Head-to-Head Comparison of Relevant Cell Sources of Small Extracellular Vesicles for Cardiac Repair: Superiority of Embryonic Stem Cells. J. Extracell. Vesicles 2024, 13, e12445. [Google Scholar] [CrossRef]
- Meng, W.-T.; Guo, H.-D. Small Extracellular Vesicles Derived from Induced Pluripotent Stem Cells in the Treatment of Myocardial Injury. Int. J. Mol. Sci. 2023, 24, 4577. [Google Scholar] [CrossRef]
- Terashvili, M.; Bosnjak, Z.J. Stem Cell Therapies in Cardiovascular Disease. J. Cardiothorac. Vasc. Anesth. 2019, 33, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Sürder, D.; Schwitter, J.; Moccetti, T.; Astori, G.; Rufibach, K.; Plein, S.; Lo Cicero, V.; Soncin, S.; Windecker, S.; Moschovitis, A.; et al. Cell-Based Therapy for Myocardial Repair in Patients with Acute Myocardial Infarction: Rationale and Study Design of the SWiss Multicenter Intracoronary Stem Cells Study in Acute Myocardial Infarction (SWISS-AMI). Am. Heart J. 2010, 160, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Duan, C.-Y.; Luo, C.-F.; Ou, C.-W.; Wu, Z.-Y.; Zhang, J.-W.; Ni, X.-B.; Chen, P.-Y.; Chen, M.-S. Impact of Timing Following Acute Myocardial Infarction on Efficacy and Safety of Bone Marrow Stem Cells Therapy: A Network Meta-Analysis. Stem Cells Int. 2016, 2016, 1031794. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Liu, D.; Zhong, Y.; Huang, R.-C. Effects of Timing on Intracoronary Autologous Bone Marrow-Derived Cell Transplantation in Acute Myocardial Infarction: A Meta-Analysis of Randomized Controlled Trials. Stem Cell Res. Ther. 2017, 8, 231. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Chugh, A.; Yang, P.C.; Zhao, D.X.M.; Ellis, S.G.; Forder, J.R.; Perin, E.C.; et al. TIME Trial: Effect of Timing of Stem Cell Delivery Following ST-Elevation Myocardial Infarction on the Recovery of Global and Regional Left Ventricular Function: Final 2-Year Analysis. Circ. Res. 2018, 122, 479–488. [Google Scholar] [CrossRef]
- Chan, J.L.; Miller, J.G.; Zhou, Y.; Robey, P.G.; Stroncek, D.F.; Arai, A.E.; Sachdev, V.; Horvath, K.A. Intramyocardial Bone Marrow Stem Cells in Patients Undergoing Cardiac Surgical Revascularization. Ann. Thorac. Surg. 2020, 109, 1142–1149. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Wolint, P.; Winklhofer, S.; Stolzmann, P.; Cesarovic, N.; Fleischmann, T.; Nguyen, T.D.L.; Frauenfelder, T.; Böni, R.; Scherman, J.; et al. Transcatheter Based Electromechanical Mapping Guided Intramyocardial Transplantation and in Vivo Tracking of Human Stem Cell Based Three Dimensional Microtissues in the Porcine Heart. Biomaterials 2013, 34, 2428–2441. [Google Scholar] [CrossRef]
- Patel, A.N.; Geffner, L.; Vina, R.F.; Saslavsky, J.; Urschel, H.C.; Kormos, R.; Benetti, F. Surgical Treatment for Congestive Heart Failure with Autologous Adult Stem Cell Transplantation: A Prospective Randomized Study. J. Thorac. Cardiovasc. Surg. 2005, 130, 1631–1638. [Google Scholar] [CrossRef]
- Pompilio, G.; Steinhoff, G.; Liebold, A.; Pesce, M.; Alamanni, F.; Capogrossi, M.C.; Biglioli, P. Direct Minimally Invasive Intramyocardial Injection of Bone Marrow-Derived AC133+ Stem Cells in Patients with Refractory Ischemia: Preliminary Results. Thorac. Cardiovasc. Surg. 2008, 56, 71–76. [Google Scholar] [CrossRef]
- Hamano, K.; Nishida, M.; Hirata, K.; Mikamo, A.; Li, T.S.; Harada, M.; Miura, T.; Matsuzaki, M.; Esato, K. Local Implantation of Autologous Bone Marrow Cells for Therapeutic Angiogenesis in Patients with Ischemic Heart Disease: Clinical Trial and Preliminary Results. Jpn. Circ. J. 2001, 65, 845–847. [Google Scholar] [CrossRef]
- Martens, A.; Rojas, S.V.; Baraki, H.; Rathert, C.; Schecker, N.; Zweigerdt, R.; Schwanke, K.; Rojas-Hernandez, S.; Martin, U.; Saito, S.; et al. Substantial Early Loss of Induced Pluripotent Stem Cells Following Transplantation in Myocardial Infarction. Artif. Organs 2014, 38, 978–984. [Google Scholar] [CrossRef]
- Zhang, H.; Song, P.; Tang, Y.; Zhang, X.; Zhao, S.; Wei, Y.; Hu, S. Injection of Bone Marrow Mesenchymal Stem Cells in the Borderline Area of Infarcted Myocardium: Heart Status and Cell Distribution. J. Thorac. Cardiovasc. Surg. 2007, 134, 1234–1240. [Google Scholar] [CrossRef]
- Hudson, W.; Collins, M.C.; deFreitas, D.; Sun, Y.S.; Muller-Borer, B.; Kypson, A.P. Beating and Arrested Intramyocardial Injections Are Associated with Significant Mechanical Loss: Implications for Cardiac Cell Transplantation. J. Surg. Res. 2007, 142, 263–267. [Google Scholar] [CrossRef]
- Liang, X.; Liu, J.; Li, M.; Lin, F.; Zhuang, R.; Meng, Q.; Ma, X.; Xin, Y.; Gong, X.; He, Z.; et al. Intravenously Administered Human Umbilical Cord-Derived Mesenchymal Stem Cell (HucMSC) Improves Cardiac Performance Following Infarction via Immune Modulation. Stem Cells Int. 2023, 2023, 6256115. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S. Pulmonary Passage Is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Freyman, T.; Polin, G.; Osman, H.; Crary, J.; Lu, M.; Cheng, L.; Palasis, M.; Wilensky, R.L. A Quantitative, Randomized Study Evaluating Three Methods of Mesenchymal Stem Cell Delivery Following Myocardial Infarction. Eur. Heart J. 2006, 27, 1114–1122. [Google Scholar] [CrossRef]
- Strauer, B.E.; Brehm, M.; Zeus, T.; Köstering, M.; Hernandez, A.; Sorg, R.V.; Kögler, G.; Wernet, P. Repair of Infarcted Myocardium by Autologous Intracoronary Mononuclear Bone Marrow Cell Transplantation in Humans. Circulation 2002, 106, 1913–1918. [Google Scholar] [CrossRef]
- Keith, M.C.L.; Tokita, Y.; Tang, X.-L.; Ghafghazi, S.; Moore, J.B.; Hong, K.U.; Elmore, J.B.; Amraotkar, A.R.; Guo, H.; Ganzel, B.L.; et al. Effect of the Stop-Flow Technique on Cardiac Retention of c-Kit Positive Human Cardiac Stem Cells after Intracoronary Infusion in a Porcine Model of Chronic Ischemic Cardiomyopathy. Basic Res. Cardiol. 2015, 110, 503. [Google Scholar] [CrossRef]
- Tseliou, E.; Kanazawa, H.; Dawkins, J.; Gallet, R.; Kreke, M.; Smith, R.; Middleton, R.; Valle, J.; Marbán, L.; Kar, S.; et al. Widespread Myocardial Delivery of Heart-Derived Stem Cells by Nonocclusive Triple-Vessel Intracoronary Infusion in Porcine Ischemic Cardiomyopathy: Superior Attenuation of Adverse Remodeling Documented by Magnetic Resonance Imaging and Histology. PLoS ONE 2016, 11, e0144523. [Google Scholar] [CrossRef]
- Delewi, R.; Andriessen, A.; Tijssen, J.G.P.; Zijlstra, F.; Piek, J.J.; Hirsch, A. Impact of Intracoronary Cell Therapy on Left Ventricular Function in the Setting of Acute Myocardial Infarction: A Meta-Analysis of Randomised Controlled Clinical Trials. Heart Br. Card. Soc. 2013, 99, 225–232. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Biondi-Zoccai, G.G.L.; Abbate, A.; Khianey, R.; Sheiban, I.; Bartunek, J.; Vanderheyden, M.; Kim, H.-S.; Kang, H.-J.; Strauer, B.E.; et al. Impact of Intracoronary Cell Therapy on Left Ventricular Function in the Setting of Acute Myocardial Infarction: A Collaborative Systematic Review and Meta-Analysis of Controlled Clinical Trials. J. Am. Coll. Cardiol. 2007, 50, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.S.; Jones, D.A.; Davies, C.; Locca, D.; Veerapen, J.; Reid, A.; Godec, T.; Martin, J.; Mathur, A. Cell Administration Routes for Heart Failure: A Comparative Re-Evaluation of the REGENERATE-DCM and REGENERATE-IHD Trials. Regen. Med. 2022, 17, 891–903. [Google Scholar] [CrossRef]
- Fiarresga, A.; Mata, M.F.; Cavaco-Gonçalves, S.; Selas, M.; Simões, I.N.; Oliveira, E.; Carrapiço, B.; Cardim, N.; Cabral, J.M.S.; Ferreira, R.C.; et al. Intracoronary Delivery of Human Mesenchymal/Stromal Stem Cells: Insights from Coronary Microcirculation Invasive Assessment in a Swine Model. PLoS ONE 2015, 10, e0139870. [Google Scholar] [CrossRef]
- Bartunek, J.; Vanderheyden, M.; Vandekerckhove, B.; Mansour, S.; De Bruyne, B.; De Bondt, P.; Van Haute, I.; Lootens, N.; Heyndrickx, G.; Wijns, W. Intracoronary Injection of CD133-Positive Enriched Bone Marrow Progenitor Cells Promotes Cardiac Recovery after Recent Myocardial Infarction: Feasibility and Safety. Circulation 2005, 112, I178–I183. [Google Scholar] [CrossRef]
- Gao, L.R.; Pei, X.T.; Ding, Q.A.; Chen, Y.; Zhang, N.K.; Chen, H.Y.; Wang, Z.G.; Wang, Y.F.; Zhu, Z.M.; Li, T.C.; et al. A Critical Challenge: Dosage-Related Efficacy and Acute Complication Intracoronary Injection of Autologous Bone Marrow Mesenchymal Stem Cells in Acute Myocardial Infarction. Int. J. Cardiol. 2013, 168, 3191–3199. [Google Scholar] [CrossRef]
- Hong, S.J.; Hou, D.; Brinton, T.J.; Johnstone, B.; Feng, D.; Rogers, P.; Fearon, W.F.; Yock, P.; March, K.L. Intracoronary and Retrograde Coronary Venous Myocardial Delivery of Adipose-Derived Stem Cells in Swine Infarction Lead to Transient Myocardial Trapping with Predominant Pulmonary Redistribution. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2014, 83, E17–E25. [Google Scholar] [CrossRef]
- Perin, E.C.; Dohmann, H.F.R.; Borojevic, R.; Silva, S.A.; Sousa, A.L.S.; Mesquita, C.T.; Rossi, M.I.D.; Carvalho, A.C.; Dutra, H.S.; Dohmann, H.J.F.; et al. Transendocardial, Autologous Bone Marrow Cell Transplantation for Severe, Chronic Ischemic Heart Failure. Circulation 2003, 107, 2294–2302. [Google Scholar] [CrossRef]
- Losordo, D.W.; Schatz, R.A.; White, C.J.; Udelson, J.E.; Veereshwarayya, V.; Durgin, M.; Poh, K.K.; Weinstein, R.; Kearney, M.; Chaudhry, M.; et al. Intramyocardial Transplantation of Autologous CD34+ Stem Cells for Intractable Angina: A Phase I/IIa Double-Blind, Randomized Controlled Trial. Circulation 2007, 115, 3165–3172. [Google Scholar] [CrossRef]
- Fukushima, S.; Varela-Carver, A.; Coppen, S.R.; Yamahara, K.; Felkin, L.E.; Lee, J.; Barton, P.J.R.; Terracciano, C.M.N.; Yacoub, M.H.; Suzuki, K. Direct Intramyocardial but Not Intracoronary Injection of Bone Marrow Cells Induces Ventricular Arrhythmias in a Rat Chronic Ischemic Heart Failure Model. Circulation 2007, 115, 2254–2261. [Google Scholar] [CrossRef]
- Pätilä, T.; Miyagawa, S.; Imanishi, Y.; Fukushima, S.; Siltanen, A.; Mervaala, E.; Kankuri, E.; Harjula, A.; Sawa, Y. Comparison of Arrhythmogenicity and Proinflammatory Activity Induced by Intramyocardial or Epicardial Myoblast Sheet Delivery in a Rat Model of Ischemic Heart Failure. PLoS ONE 2015, 10, e0123963. [Google Scholar] [CrossRef]
- Thompson, C.A.; Nasseri, B.A.; Makower, J.; Houser, S.; McGarry, M.; Lamson, T.; Pomerantseva, I.; Chang, J.Y.; Gold, H.K.; Vacanti, J.P.; et al. Percutaneous Transvenous Cellular Cardiomyoplasty. A Novel Nonsurgical Approach for Myocardial Cell Transplantation. J. Am. Coll. Cardiol. 2003, 41, 1964–1971. [Google Scholar] [CrossRef]
- Raake, P.; von Degenfeld, G.; Hinkel, R.; Vachenauer, R.; Sandner, T.; Beller, S.; Andrees, M.; Kupatt, C.; Schuler, G.; Boekstegers, P. Myocardial Gene Transfer by Selective Pressure-Regulated Retroinfusion of Coronary Veins: Comparison with Surgical and Percutaneous Intramyocardial Gene Delivery. J. Am. Coll. Cardiol. 2004, 44, 1124–1129. [Google Scholar] [CrossRef]
- Herity, N.A.; Lo, S.T.; Oei, F.; Lee, D.P.; Ward, M.R.; Filardo, S.D.; Hassan, A.; Suzuki, T.; Rezaee, M.; Carter, A.J.; et al. Selective Regional Myocardial Infiltration by the Percutaneous Coronary Venous Route: A Novel Technique for Local Drug Delivery. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2000, 51, 358–363. [Google Scholar] [CrossRef]
- Tuma, J.; Fernández-Viña, R.; Carrasco, A.; Castillo, J.; Cruz, C.; Carrillo, A.; Ercilla, J.; Yarleque, C.; Cunza, J.; Henry, T.D.; et al. Safety and Feasibility of Percutaneous Retrograde Coronary Sinus Delivery of Autologous Bone Marrow Mononuclear Cell Transplantation in Patients with Chronic Refractory Angina. J. Transl. Med. 2011, 9, 183. [Google Scholar] [CrossRef]
- Zakharova, L.; Nural-Guvener, H.; Feehery, L.; Popovic, S.; Nimlos, J.; Gaballa, M.A. Retrograde Coronary Vein Infusion of Cardiac Explant-Derived c-Kit+ Cells Improves Function in Ischemic Heart Failure. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Kesieme, E.B.; Buchan, K.G. Clinical Anatomy of the Coronary Venous System and Relevance to Retrograde Cardioplegia and Cardiac Electrophysiological Interventions. Clin. Anat. 2025, 38, 43–53. [Google Scholar] [CrossRef]
- Orihashi, K.; Miyashita, K.; Tashiro, M.; Kihara, K.; Kondo, N.; Yamamoto, M.; Hirose, N.; Fukutomi, T.; Nishimori, H. Avoidance of Coronary Sinus Injury During Retrograde Cardioplegia. Ann. Thorac. Surg. 2016, 102, e583–e586. [Google Scholar] [CrossRef]
- Dill, T.; Schächinger, V.; Rolf, A.; Möllmann, S.; Thiele, H.; Tillmanns, H.; Assmus, B.; Dimmeler, S.; Zeiher, A.M.; Hamm, C. Intracoronary Administration of Bone Marrow-Derived Progenitor Cells Improves Left Ventricular Function in Patients at Risk for Adverse Remodeling after Acute ST-Segment Elevation Myocardial Infarction: Results of the Reinfusion of Enriched Progenitor Cells And Infarct Remodeling in Acute Myocardial Infarction Study (REPAIR-AMI) Cardiac Magnetic Resonance Imaging Substudy. Am. Heart J. 2009, 157, 541–547. [Google Scholar] [CrossRef]
- Mathur, A.; Sim, D.S.; Choudry, F.; Veerapen, J.; Colicchia, M.; Turlejski, T.; Hussain, M.; Hamshere, S.; Locca, D.; Rakhit, R.; et al. Five-Year Follow-up of Intracoronary Autologous Cell Therapy in Acute Myocardial Infarction: The REGENERATE-AMI Trial. ESC Heart Fail. 2022, 9, 1152–1159. [Google Scholar] [CrossRef]
- Attar, A.; Farjoud Kouhanjani, M.; Hessami, K.; Vosough, M.; Kojuri, J.; Ramzi, M.; Hosseini, S.A.; Faghih, M.; Monabati, A. Effect of Once versus Twice Intracoronary Injection of Allogeneic-Derived Mesenchymal Stromal Cells after Acute Myocardial Infarction: BOOSTER-TAHA7 Randomized Clinical Trial. Stem Cell Res. Ther. 2023, 14, 264. [Google Scholar] [CrossRef]
- Attar, A.; Monabati, A.; Montaseri, M.; Vosough, M.; Hosseini, S.A.; Kojouri, J.; Abdi-Ardekani, A.; Izadpanah, P.; Azarpira, N.; Pouladfar, G.; et al. Transplantation of Mesenchymal Stem Cells for Prevention of Acute Myocardial Infarction Induced Heart Failure: Study Protocol of a Phase III Randomized Clinical Trial (Prevent-TAHA8). Trials 2022, 23, 632. [Google Scholar] [CrossRef]
- Chakravarty, T.; Makkar, R.R.; Ascheim, D.D.; Traverse, J.H.; Schatz, R.; DeMaria, A.; Francis, G.S.; Povsic, T.J.; Smith, R.R.; Lima, J.A.; et al. ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) Trial: Rationale and Design. Cell Transplant. 2017, 26, 205–214. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, J.; Zhang, N.; Li, W.; Wang, J.; Cai, G.; Chen, Y.; Yang, Y.; Liu, Z. Bone Marrow Mesenchymal Stem Cells Transfer in Patients with ST-Segment Elevation Myocardial Infarction: Single-Blind, Multicenter, Randomized Controlled Trial. Stem Cell Res. Ther. 2021, 12, 33. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, S.-H.; Youn, Y.-J.; Ahn, M.-S.; Kim, J.-Y.; Yoo, B.-S.; Yoon, J.; Kwon, W.; Hong, I.-S.; Lee, K.; et al. A Randomized, Open-Label, Multicenter Trial for the Safety and Efficacy of Adult Mesenchymal Stem Cells after Acute Myocardial Infarction. J. Korean Med. Sci. 2014, 29, 23–31. [Google Scholar] [CrossRef]
- Quijada, P.; Salunga, H.T.; Hariharan, N.; Cubillo, J.D.; El-Sayed, F.G.; Moshref, M.; Bala, K.M.; Emathinger, J.M.; De La Torre, A.; Ormachea, L.; et al. Cardiac Stem Cell Hybrids Enhance Myocardial Repair. Circ. Res. 2015, 117, 695–706. [Google Scholar] [CrossRef]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary Cardiosphere-Derived Cells after Myocardial Infarction: Evidence of Therapeutic Regeneration in the Final 1-Year Results of the CADUCEUS Trial (CArdiosphere-Derived aUtologous Stem CElls to Reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef]
- Tendera, M.; Wojakowski, W.; Ruzyłło, W.; Chojnowska, L.; Kepka, C.; Tracz, W.; Musiałek, P.; Piwowarska, W.; Nessler, J.; Buszman, P.; et al. Intracoronary Infusion of Bone Marrow-Derived Selected CD34+CXCR4+ Cells and Non-Selected Mononuclear Cells in Patients with Acute STEMI and Reduced Left Ventricular Ejection Fraction: Results of Randomized, Multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur. Heart J. 2009, 30, 1313–1321. [Google Scholar] [CrossRef]
- Chingale, M.; Zhu, D.; Cheng, K.; Huang, K. Bioengineering Technologies for Cardiac Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 9, 681705. [Google Scholar] [CrossRef]
- Saleem, A.; Abbas, M.K.; Wang, Y.; Lan, F. hPSC Gene Editing for Cardiac Disease Therapy. Pflugers Arch. 2022, 474, 1123–1132. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence Supporting Paracrine Hypothesis for Akt-Modified Mesenchymal Stem Cell-Mediated Cardiac Protection and Functional Improvement. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 661–669. [Google Scholar] [CrossRef]
- Perin, E.C.; López, J. Methods of Stem Cell Delivery in Cardiac Diseases. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, S110–S113. [Google Scholar] [CrossRef]
- Caplice, N.M. The Future of Cell Therapy for Acute Myocardial Infarction. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, S129–S132. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Sikkel, M.B.; Hayward, C.; MacLeod, K.T.; Harding, S.E.; Lyon, A.R. SERCA2a Gene Therapy in Heart Failure: An Anti-Arrhythmic Positive Inotrope. Br. J. Pharmacol. 2014, 171, 38–54. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S. Gene Therapy for Heart Failure: Back to the Bench. Mol. Ther. 2015, 23, 1551–1552. [Google Scholar] [CrossRef]
- Tompkins, B.A.; Balkan, W.; Winkler, J.; Gyöngyösi, M.; Goliasch, G.; Fernández-Avilés, F.; Hare, J.M. IMPACT: Preclinical Studies of Cell Therapy for Human Disease. Circ. Res. 2018, 122, 1006–1020. [Google Scholar] [CrossRef]
- Choudry, F.; Hamshere, S.; Saunders, N.; Veerapen, J.; Bavnbek, K.; Knight, C.; Pellerin, D.; Locca, D.; Westwood, M.; Rakhit, R.; et al. A Randomized Double-Blind Control Study of Early Intra-Coronary Autologous Bone Marrow Cell Infusion in Acute Myocardial Infarction: The REGENERATE-AMI Clinical Trial. Eur. Heart J. 2016, 37, 256–263. [Google Scholar] [CrossRef]
| Stem Cell Type | Common Cell Origin | Possible Lineages | Therapeutic Concerns | Reference(s) |
|---|---|---|---|---|
| Cardiac Stem Cells (CSCs) | Heart tissue | Cardiomyogenic, Endothelial, Smooth Muscle | Potential for GVHD (allogeneic use), low engraftment | [9,10,11,27,28] |
| Mesenchymal Stem Cells (MSCs) | Bone marrow, adipose tissue, umbilical cord | Osteogenic, Chondrogenic, Adipogenic, Myogenic, Tenogenic, Neurogenic | Tumorigenic risk | [12,13,16,28,30,31] |
| Bone Marrow Mononuclear Cells (BMMCs) | Bone marrow | Hematopoietic, Endothelial, Mesenchymal | Limited efficacy alone | [14,17,19,20,21,32] |
| Induced Pluripotent Stem Cells (iPSCs) | Skin-derived fibroblasts, peripheral blood | Ectodermal, Mesodermal, Endodermal | Teratoma formation risk | [22,33,34] |
| Hematopoietic Stem Cells (HSCs) | Bone marrow | Myeloid, Lymphoid | Low engraftment | [13,35] |
| Adipose-Derived Stem Cells (ASCs) | Adipose tissue | Osteogenic, Chondrogenic, Adipogenic, Myogenic, Neurogenic, Angiogenic. | Tumorigenic risk | [23,36] |
| Muse Cells | Various tissues (bone marrow, etc.) | Mesodermal, Endodermal, Ectodermal | Limited clinical relevance, long isolation time | [25] |
| Cortical Bone-Derived Stem Cells (CBSCs) | Cortical bone | Osteogenic, Chondrogenic, Adipogenic, Angiogenics | Limited established research | [24,38] |
| Embryonic Stem Cells (ESCs) | Embryos | Ectodermal, Mesodermal, Endodermal | Ethical concerns | [37] |
| Type of Stem Cell | Study Type | Delivery Route/Method | Dosage | Population Size | Results | Reference |
|---|---|---|---|---|---|---|
| Wharton’s Jelly Mesenchymal Stromal Cell (WJ-MSC) | Clinical, Single-blind, Randomized, Multicenter Trial | Intracoronary injection (once vs. twice) | Single dose 107 WJ-MSCs, Single (107) + Booster dose (107) = 207 cells | N = 65 | Baseline LVEF: 40% in all groups. Single MSC increased LVEF by 4.54 ± 2% (one dose), Booster dose increased by 7.45 ± 2% (p < 0.001). Echocardiography: 6.71 ± 2.4% (one dose), 10.71 ± 2.5% (booster). | [84] |
| Bone Marrow Mononuclear Cell (BM-MNC) | Clinical, Phase 3 Randomized Trial (3-Year Follow-Up) | Intracoronary infusion | Single dose of 107 cells | N = 390 | Trial incomplete; expected outcome: evaluate prevention of HF post-AMI. | [85] |
| Autologous Cardiosphere-Derived Cells (CDCs) | Clinical, Double-Blind, Placebo-Controlled Trial | Intracoronary (IC) injection | 2.5 × 107 cells | N = 134 | IC infusion of CDCs (CAP-1002) was safe and feasible. No significant reduction in scar size at 6 or 12 months. LVEDV and LVESV reduced at 6 months, NT-proBNP decreased. | [86] |
| Bone Marrow-Derived Cells (BMCs) Autologous | Clinical, Randomized Controlled Trial (85 Patients, AMI) | Intracoronary infusion into infarct-related artery | 19.8 × 107 cells | N = 85 | MACE incidence: BMCs (26.1%), Placebo (18%). No mortality, no significant difference in MI or revascularization between groups. | [83] |
| Bone Marrow Mesenchymal Stem Cells (BM-MSCs) | Clinical, Randomized, Single-Blind, Multicenter, Controlled Trial | Intracoronary infusion 15 days after PCI | 3.31 × 106 cells | N = 43 | IC BM-MSCs did not improve LV function or myocardial viability at 12-month follow-up. Safe, no toxic events. | [87] |
| Bone Marrow Mesenchymal Stem Cells (BM-MSCs) | Clinical, Randomized, Open-Label, Multicenter Trial | Intracoronary administration into infarct-related artery (IRA) at one month | 7.2 × 107 cells | N = 69 | LVEF by SPECT at 6 months: BM-MSC group (5.9% ± 8.5%) vs. Control (1.6% ± 7.0%, p = 0.037). No significant LVEDV or LVESV differences. | [88] |
| Bone Marrow Mesenchymal Stem Cells (BM-MSCs) | Clinical, Randomized, Single-Blind Trial | Intracoronary infusion 1 month post-PCI | 7.2 × 107 cells | N = 30 | LVEF increased by 8.8 ± 2.9% (BM-MSC) vs. 4.8 ± 1.9% (control) at 4 months (p = 0.031). No increased adverse events. Echocardiography showed sustained improvement at 12 months. | [42] |
| Allogeneic Human Cardiac Stem Cells (AlloCSC-01) | Clinical, Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial | Intracoronary infusion at days 5–7 post-STEMI | 3.5 × 107 cells | N = 49 | No deaths or MACE at 12 months. Infarct size reduction: −2.3% (95% CI, −6.5% to 1.9%). No differences in ventricular remodeling. Low immunogenicity. | [10] |
| Bone Marrow-Derived Progenitor Cells (BMCs) | Clinical, Randomized, Multicenter, Placebo-Controlled Trial | Intracoronary infusion post-STEMI | 2.0 × 107–2.5 × 107 cells | N = 54 | At 12 months, BMC treatment effect on EF was 2.8% (p = 0.26). In patients with EF ≤ 48.9%, BMCs improved EF (+6.6%, p = 0.01), reduced EDV increase (p = 0.02), and prevented ESV increase (p = 0.01). | [82] |
| Mesenchymal Stem Cells (MSCs) vs. CD34+ Cells | Preclinical, Rat Model | Intravenous injection | 2 × 106 cells | N = 48 | CD34+ cells showed superior efficacy over MSCs in infarct size reduction and angiogenesis markers (VEGF, VEGFR-2, Ang-1, Tie-2 upregulation). Both cell types improved myocardial tissue structure, but CD34+ had significantly better outcomes. | [13] |
| Mesenchymal Stem Cells (MSCs) vs. Bone Marrow Mononuclear Cells (BMMNC) | Preclinical, Porcine Model | Trans endocardial injection | 107 autologous MSCs or BMMNCs | N = 15 | MSCs improved LVEF significantly over BMMNCs at 4 weeks post-injection. BMMNCs showed no significant LVEF improvement. | [14] |
| Mesenchymal Stem Cells (MSCs) | Preclinical, mouse model | Intracoronary infusion, | 1.0 × 105 cells | N = 30 | Cardiac Stem Cell Hybrids Enhance Myocardial Repair. CCs improved left ventricular anterior wall thickness and increased capillary density. | [89] |
| Autologous Cardiosphere-Derived Cells (CDCs) | Clinical, Randomized, Controlled Trial | Intracoronary infusion | 12.5 to 25 × 106 cells | N = 17 | CDC therapy led to reduced scar size, increased viable myocardium, and improved regional function at 1-year follow-up. No significant safety concerns observed. | [90] |
| Bone Marrow Mononuclear Cells (BMMNC) | Clinical, Randomized, Controlled Trial | Delivery through graft vessel during CABG | 13.28 × 107 cells | N = 42 | CABG + BM MNC improved LA function more than CABG alone. LAGS, LVEF, and LAV significantly improved postoperatively. Two-dimensional strain imaging was a sensitive tool for LA function evaluation. | [21] |
| Mesenchymal Stem Cells (MSCs) (Autologous Bone Marrow-Derived Ex Vivo Expanded Mesenchymal Stem Cells) | Clinical, Randomized, Controlled Trial | Intramyocardial Injection | 31 × 106 cells | N = 54 | Intramyocardial MSC injection in AMI patients was feasible and safe for up to 5-year follow-up. LV function improved, no significant differences compared to controls in event-free survival. | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, N.T.; Dunleavy, M.W.; Zhou, W.; Bhatia, S.S.; Kumar, R.D.; Woo, S.T.; Ramirez-Pulido, G.; Ramakrishnan, K.S.; El-Hashash, A.H. Stem Cell Therapy for Myocardial Infarction Recovery: Advances, Challenges, and Future Directions. Biomedicines 2025, 13, 1209. https://doi.org/10.3390/biomedicines13051209
Le NT, Dunleavy MW, Zhou W, Bhatia SS, Kumar RD, Woo ST, Ramirez-Pulido G, Ramakrishnan KS, El-Hashash AH. Stem Cell Therapy for Myocardial Infarction Recovery: Advances, Challenges, and Future Directions. Biomedicines. 2025; 13(5):1209. https://doi.org/10.3390/biomedicines13051209
Chicago/Turabian StyleLe, Nicholas T., Matthew W. Dunleavy, William Zhou, Sumrithbir S. Bhatia, Rebecca D. Kumar, Suyin T. Woo, Gonzalo Ramirez-Pulido, Kaushik S. Ramakrishnan, and Ahmed H. El-Hashash. 2025. "Stem Cell Therapy for Myocardial Infarction Recovery: Advances, Challenges, and Future Directions" Biomedicines 13, no. 5: 1209. https://doi.org/10.3390/biomedicines13051209
APA StyleLe, N. T., Dunleavy, M. W., Zhou, W., Bhatia, S. S., Kumar, R. D., Woo, S. T., Ramirez-Pulido, G., Ramakrishnan, K. S., & El-Hashash, A. H. (2025). Stem Cell Therapy for Myocardial Infarction Recovery: Advances, Challenges, and Future Directions. Biomedicines, 13(5), 1209. https://doi.org/10.3390/biomedicines13051209






