Patients with Higher Pulse Wave Velocity Are More Likely to Develop a More Severe Form of Knee Osteoarthritis: Implications for Cardiovascular Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- ▪
- Postmenopausal women with knee pain;
- ▪
- Provided written informed consent;
- ▪
- Were diagnosed with KOA (X-rays, ultrasound);
- ▪
- Were able to complete study procedures.
- ▪
- Reproductive age;
- ▪
- Systemic and local autoimmune diseases;
- ▪
- Hypothyroidism or hyperthyroidism;
- ▪
- Hormonal and anti-inflammatory therapy [including non-steroidal and steroidal anti-inflammatory drugs, Disease-Modifying Antirheumatic Drugs (DMARDs), or biological therapy];
- ▪
- Cardiac arrhythmias (atrial flutter/fibrillation, atrioventricular conduction disturbances, paroxysmal supraventricular tachycardia);
- ▪
- Acute or chronic myelo- and limfo-prolipherative diseases;
- ▪
- Chronic end-stage kidney, liver or heart failure (eGFR < 15 mL/min/1.75 m2; Child Pugh C and D; NT-proBNP > 1000 pg/mL);
- ▪
- Malignant diseases within the last 5 years or their therapy;
- ▪
- Life expectancy less than 6 months;
- ▪
- Mental illness or dementia;
- ▪
- Recent infection (last 4 weeks);
- ▪
- Intra-articular injections within the last 3 months;
- ▪
- Intensive physical therapy within the past 10 days;
- ▪
- Congenital or developmental disorders;
- ▪
- Knee arthroplasty.
2.2. Clinical Assessment and Measured Parameters
2.2.1. Medical Examinations
2.2.2. Radiographic and Ultrasound Imaging
2.3. Laboratory Analysis
Evaluation of Cardiovascular Risk, Atherosclerosis and Renal Function
2.4. Assessment of Pain and Functional Capacity
2.5. Pulse Wave Velocity Measurements
2.6. Statistical Analysis
3. Results
3.1. Recruitment and Allocation of Patients to the Assessment Group
3.1.1. Characteristics of Patients with Knee Osteoarthritis
3.1.2. Correlation of Cardiovascular Risk Factors with Radiological Grade of Knee Osteoarthritis
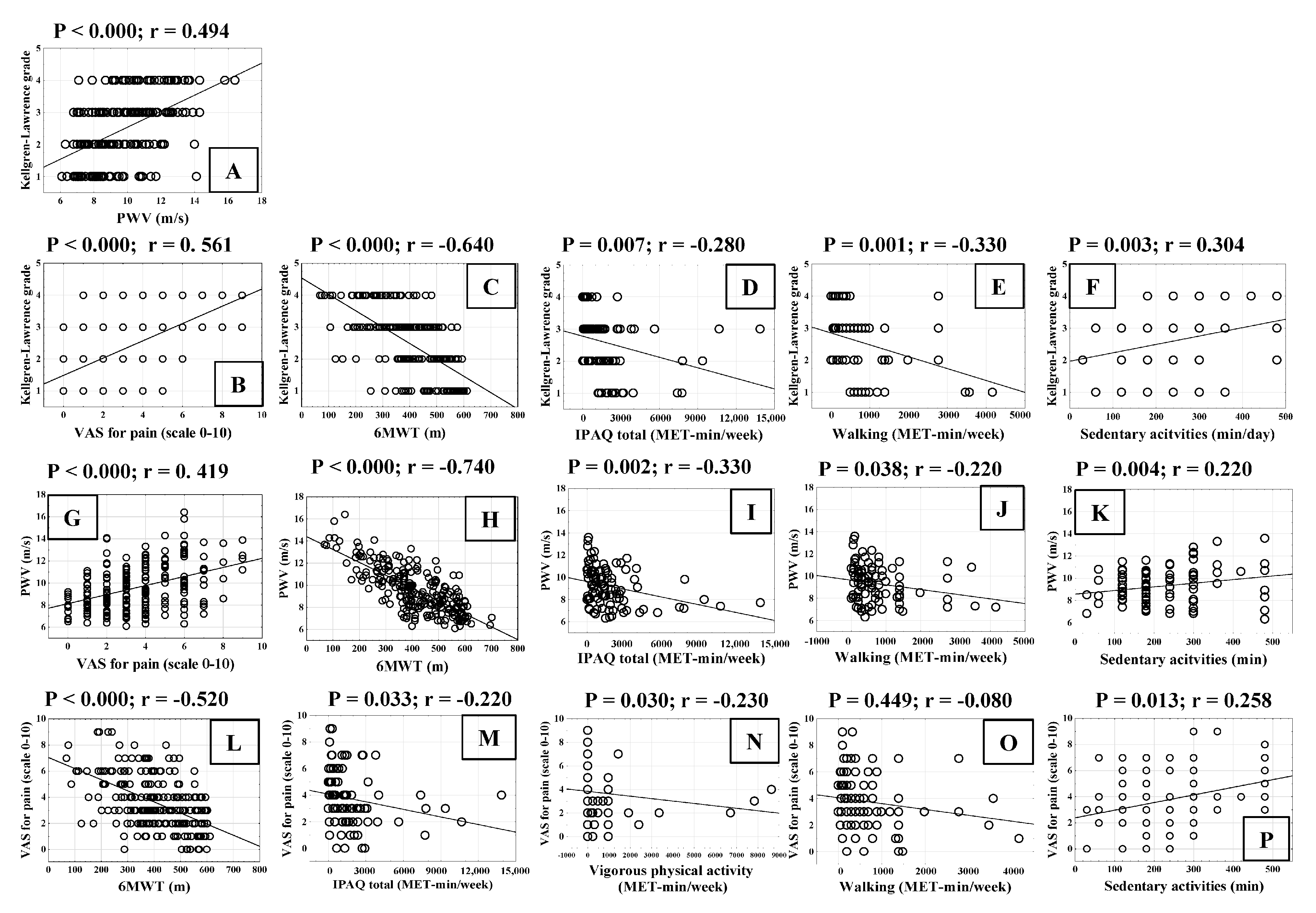
3.1.3. Correlation of Cardiovascular Risk Factors with Pulse Wave Velocity
3.1.4. Correlation of Functional Capacity of Patients with Radiological Grade of Knee Osteoarthritis and Pulse Wave Velocity
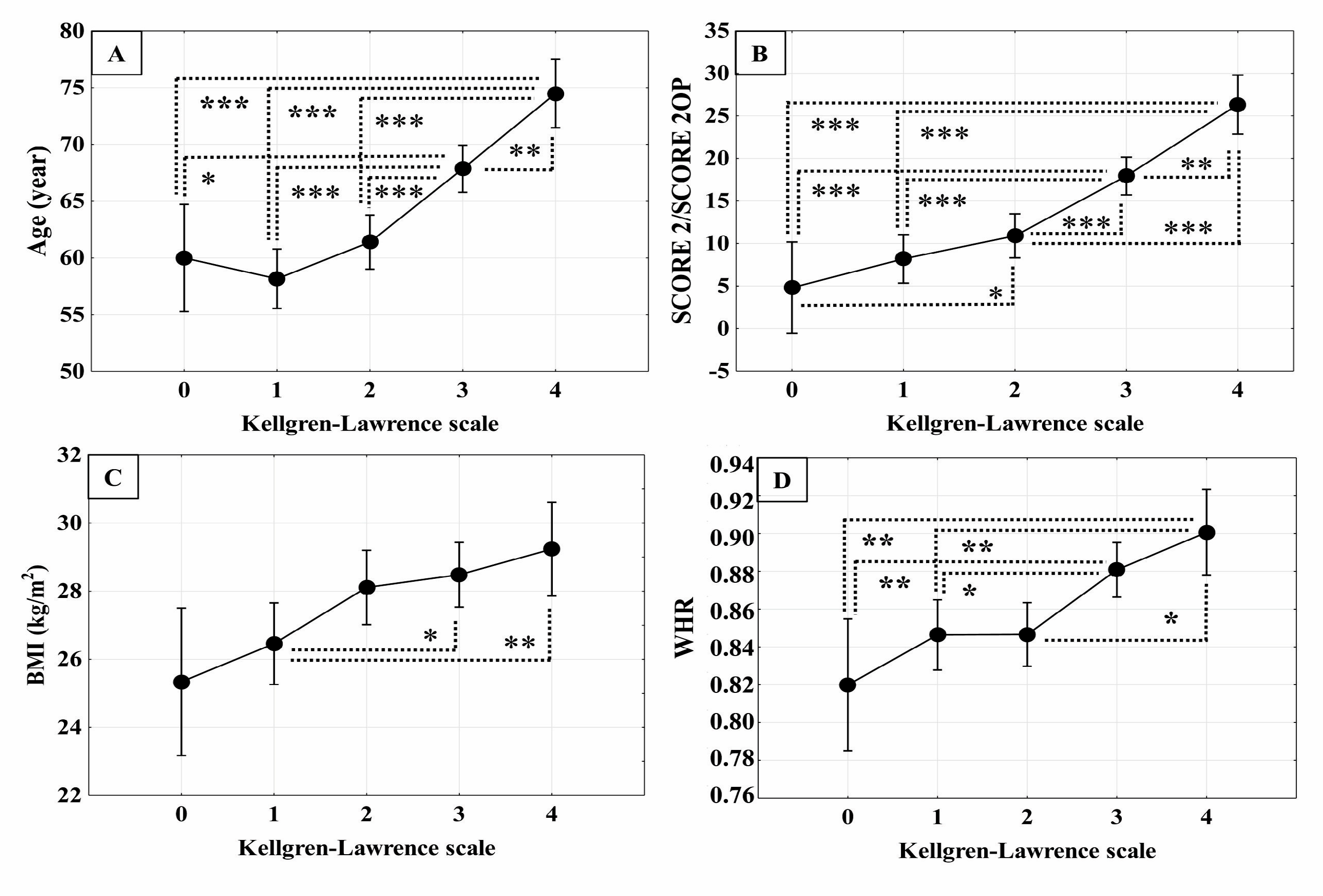
3.1.5. Comparison of Cardiovascular Risk Assessment Between Mild and Severe Knee Osteoarthritis Groups
3.1.6. Difference in Pulse Wave Velocity Between Mild and Severe Forms of Knee Osteoarthritis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferre, I.M.; Roof, M.A.; Anoushiravani, A.A.; Wasterlain, A.S.; Lajam, C.M. Understanding the Observed Sex Discrepancy in the Prevalence of Osteoarthritis. JBJS Rev. 2019, 7, e6. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Osteoarthritis. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/osteoarthritis (accessed on 21 February 2025).
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New Insights. Part 1: The Disease and Its Risk Factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef]
- Wluka, A.E.; Lombard, C.B.; Cicuttini, F.M. Tackling Obesity in Knee Osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 225–235. [Google Scholar] [CrossRef]
- Grazio, S.; Balen, D. Obesity: Risk factor and predictor of osteoarthritis. Liječnički Vjesn. 2009, 131, 22–26. [Google Scholar]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; González-Gay, M.Á.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the Interplay of Inflammation, Metabolism and Immune System Disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Kroon, F.P.B.; Veenbrink, A.I.; de Mutsert, R.; Visser, A.W.; van Dijk, K.W.; le Cessie, S.; Rosendaal, F.R.; Kloppenburg, M. The Role of Leptin and Adiponectin as Mediators in the Relationship between Adiposity and Hand and Knee Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1761–1767. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Kato, J.; Svensson, C.I. Role of extracellular damage-associated molecular pattern molecules (DAMPs) as mediators of persistent pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 251–279. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Yang, Y.X.; Li, J.; Fei, Y.; Guo, H.; Sun, Z.; Lu, J.; Xu, X.; Jiang, Q.; Ikegawa, S.; et al. Molecular Classification of Knee Osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 725568. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic Signaling Pathways and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Laskarin, G.; Persic, V.; Kukic, S.R.; Massari, D.; Legovic, A.; Boban, M.; Miskulin, R.; Rogoznica, M.; Kehler, T. Can pain intensity in osteoarthritis joint be indicator of the impairment of endothelial function? Med. Hypotheses. 2016, 94, 15–19. [Google Scholar] [CrossRef]
- Sae-Jung, T.; Leearamwat, N.; Chaiseema, N.; Sengprasert, P.; Ngarmukos, S.; Yuktananda, P.; Tanavalee, A.; Hirankarn, N.; Reantragoon, R. The infrapatellar fat pad produces interleukin-6-secreting T cells in response to a proteoglycan aggrecan peptide and provides dominant soluble mediators different from that present in synovial fluid. Int. J. Rheum. Dis. 2021, 24, 834–846. [Google Scholar] [CrossRef]
- Weinbaum, S.; Cancel, L.M.; Fu, B.M.; Tarbell, J.M. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc. Eng. Technol. 2021, 12, 37–71. [Google Scholar] [CrossRef]
- Jain, S.; Khera, R.; Corrales-Medina, V.F.; Townsend, R.R.; Chirinos, J.A. Inflammation and arterial stiffness in humans. Atherosclerosis 2014, 237, 381–390. [Google Scholar] [CrossRef]
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial Glycocalyx. Compr. Physiol. 2022, 12, 3781–3811. [Google Scholar] [CrossRef]
- Tootsi, K.; Kals, J.; Zilmer, M.; Paapstel, K.; Märtson, A. Severity of Osteoarthritis Is Associated with Increased Arterial Stiffness. Int. J. Rheumatol. 2016, 2016, 6402963. [Google Scholar] [CrossRef]
- Lanzer, P.; Hannan, F.M.; Lanzer, J.D.; Janzen, J.; Raggi, P.; Furniss, D.; Schuchardt, M.; Thakker, R.; Fok, P.W.; Saez-Rodriguez, J.; et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1145–1165. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.; Veerasingam, D.; Meyer, M.L.; Heffernan, K.S.; Higgins, S.; Maria Bruno, R.; Bueno, C.A.; Döerr, M.; Schmidt-Trucksäss, A.; Terentes-Printzios, D.; et al. Network for Research in Vascular Ageing (VascAgeNet). Reimagining the Value of Brachial-Ankle Pulse Wave Velocity as a Biomarker of Cardiovascular Disease Risk-A Call to Action on Behalf of VascAgeNet. Hypertension 2023, 80, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.; Rabkin, S.W. The relationship between arterial stiffness and heart failure with preserved ejection fraction: A systemic meta-analysis. Heart Fail. Rev. 2015, 20, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of Smooth Muscle Cells in Vascular Calcification: Implications in Atherosclerosis and Arterial Stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, X.; Luo, H.; Cheng, Y.X.; Guo, Y.; Wu, P.; Huang, Y.L.; Zhang, J.P.; Wang, Z. Assessment of aortic and peripheral arterial stiffness in patients with knee osteoarthritis by ultrasound Doppler derived pulse wave velocity. Sci. Rep. 2024, 14, 1346. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cífková, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement from the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef]
- Park, K.H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef]
- Knights, A.J.; Redding, S.J.; Maerz, T. Inflammation in osteoarthritis: The latest progress and ongoing challenges. Curr. Opin. Rheumatol. 2023, 35, 128–134. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Akkoc, N.; Brandt, J.; Chou, C.T.; Dougados, M.; Huang, F.; Gu, J.; Kirazli, Y.; et al. The Assessment of SpondyloArthritis International Society Classification Criteria for Peripheral Spondyloarthritis and for Spondyloarthritis in General. Ann. Rheum. Dis. 2011, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.; Verschueren, P.; Van Hoovels, L.; Studenic, P.; Bossuyt, X. Classification of Rheumatoid Arthritis: Is It Time to Revise the Criteria? RMD Open 2024, 10, e003851. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, J.; Magnussen, C.G.; Würtz, P.; Soininen, P.; Kangas, A.J.; Viikari, J.S.; Kähönen, M.; Loo, B.M.; Jula, A.; Ahotupa, M.; et al. Apolipoprotein B, oxidized low-density lipoprotein, and LDL particle size in predicting the incidence of metabolic syndrome: The Cardiovascular Risk in Young Finns study. Eur. J. Prev. Cardiol. 2012, 19, 1296–1303. [Google Scholar] [CrossRef]
- SCORE2 Working Group and ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic Index of Plasma (AIP): A Marker of Cardiovascular Disease. Med. J. Islam. Repub. Iran 2015, 29, 240. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Pereira, T.; Paulino, E.; Maximiano, S.; Rosa, M.; Pinto, A.L.; Mendes, M.J.; Brito, J.; Soares, P.; Risse, J.; Gose, S. Measurement of arterial stiffness and vascular aging in community pharmacies-the ASINPHAR@2action project. J. Clin. Hypertens. 2019, 21, 813–821. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef] [PubMed]
- Wluka, A.E.; Cicuttini, F.M.; Spector, T.D. Menopause, oestrogens and arthritis. Maturitas 2000, 35, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Franceković, P.; Gliemann, L. Endothelial Glycocalyx Preservation—Impact of Nutrition and Lifestyle. Nutrients 2023, 15, 2573. [Google Scholar] [CrossRef] [PubMed]
- Karaali, E.; Çiloğlu, O.; Yücel, C.; Ekiz, T. The Relationship Between Primary Knee Osteoarthritis and Aortic Stiffness, Distensibility, and Valve Calcifications: A Case-Control Study. J. Clin. Rheumatol. 2022, 28, e9–e12. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef]
- Groh, L.; Keating, S.T.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P. Monocyte and macrophage immunometabolism in atherosclerosis. Semin. Immunopathol. 2018, 40, 203–214. [Google Scholar] [CrossRef]
- Villalvilla, A.; Larrañaga-Vera, A.; Lamuedra, A.; Pérez-Baos, S.; López-Reyes, A.G.; Herrero-Beaumont, G.; Largo, R. Modulation of the Inflammatory Process by Hypercholesterolemia in Osteoarthritis. Front. Med. 2020, 7, 566250. [Google Scholar] [CrossRef]
- Niki, E. Biomarkers of lipid peroxidation in clinical material. Biochim. Biophys. Acta 2014, 1840, 809–817. [Google Scholar] [CrossRef]
- Hashimoto, K.; Akagi, M. The role of oxidation of low-density lipids in pathogenesis of osteoarthritis: A narrative review. J. Int. Med. Res. 2020, 48, 300060520931609. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, C.; Xu, P.; Zhang, S.; Song, J.H.; Wang, D.; Yuan, S.; Lee, H.J.; Zhang, M.; Wang, Z.; et al. Osteoarthritis and cardiovascular disease: A Mendelian randomization study. Front. Cardiovasc. Med. 2022, 9, 1025063. [Google Scholar] [CrossRef]
- Lo, K.; Au, M.; Ni, J.; Wen, C. Association between hypertension and osteoarthritis: A systematic review and meta-analysis of observational studies. J. Orthop. Transl. 2021, 32, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wu, Z.; Zheng, J.; Chu, J.; Zhu, J.; Qi, Y.; Zhang, Z.; Wang, Z.; Zhang, M.; Mo, Z.; et al. Arterial Stiffness and Obesity as Predictors of Diabetes: Longitudinal Cohort Study. JMIR Public Health Surveill. 2024, 10, e46088. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, H.; Luo, L.; Fan, S.; Zhou, L.; Liu, Z.; Yao, S.; Zhang, X.; Zhong, K.; Zhao, H.; et al. Toll-like receptor 4 promotes high glucose-induced catabolic and inflammatory responses in chondrocytes in an NF-κB-dependent manner. Life Sci. 2019, 228, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.C.; Yan, C.H.; Chiu, K.Y.; Wei, Q.; Zhao, J.; Guo, X.E.; Leung, F.; Lu, W.W. Abnormal subchondral bone remodeling and its association with articular cartilage degradation in knees of type 2 diabetes patients. Bone Res. 2017, 5, 17034. [Google Scholar] [CrossRef]
- Savvoulidis, P.; Kalogeropoulos, A.P.; Raptis, V.; Rafailidis, V.; Georgianos, P.I.; Balaskas, E.V.; Kouskouras, K.; Karvounis, H.; Hadjimiltiades, S. Calcification of coronary arteries and aortic valve and circulating a-klotho levels in patients with chronic kidney disease. J. Thorac. Dis. 2020, 12, 431–437. [Google Scholar] [CrossRef]
- Kimura, Y.; Tsukui, D.; Kono, H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 12394. [Google Scholar] [CrossRef]
- Neogi, T.; Krasnokutsky, S.; Pillinger, M.H. Urate and osteoarthritis: Evidence for a reciprocal relationship. Jt. Bone Spine 2019, 86, 576–582. [Google Scholar] [CrossRef]
- Chen, D.; Xu, H.; Sun, L.; Li, Y.; Wang, T.; Li, Y. Assessing causality between osteoarthritis with urate levels and gout: A bidirectional Mendelian randomization study. Osteoarthr. Cartil. 2022, 30, 551–558. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, R. Mechanistic insights into CKD-MBD-related vascular calcification and its clinical implications. Life Sci. 2022, 311, 121148. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef]
- Lu, X.; Hu, M.C. Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease. Kidney Dis. 2017, 3, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.C.; Lu, L.; Cao, Y.; Sun, R.R.; Chen, S.; Zhang, P.Y. Cardiovascular disease and its relationship with chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2918–2926. [Google Scholar] [PubMed]
- Eitner, A.; Hofmann, G.O.; Schaible, H.G. Mechanisms of Osteoarthritic Pain. Studies in Humans and Experimental Models. Front. Mol. Neurosci. 2017, 10, 349. [Google Scholar] [CrossRef]
- Joseph, K.L.; Hagen, K.B.; Tveter, A.T.; Magnusson, K.; Provan, S.A.; Dagfinrud, H. Osteoarthritis-Related Walking Disability and Arterial Stiffness: Results from a Cross-Sectional Study. Arthritis Care Res. 2019, 71, 252–258. [Google Scholar] [CrossRef] [PubMed]


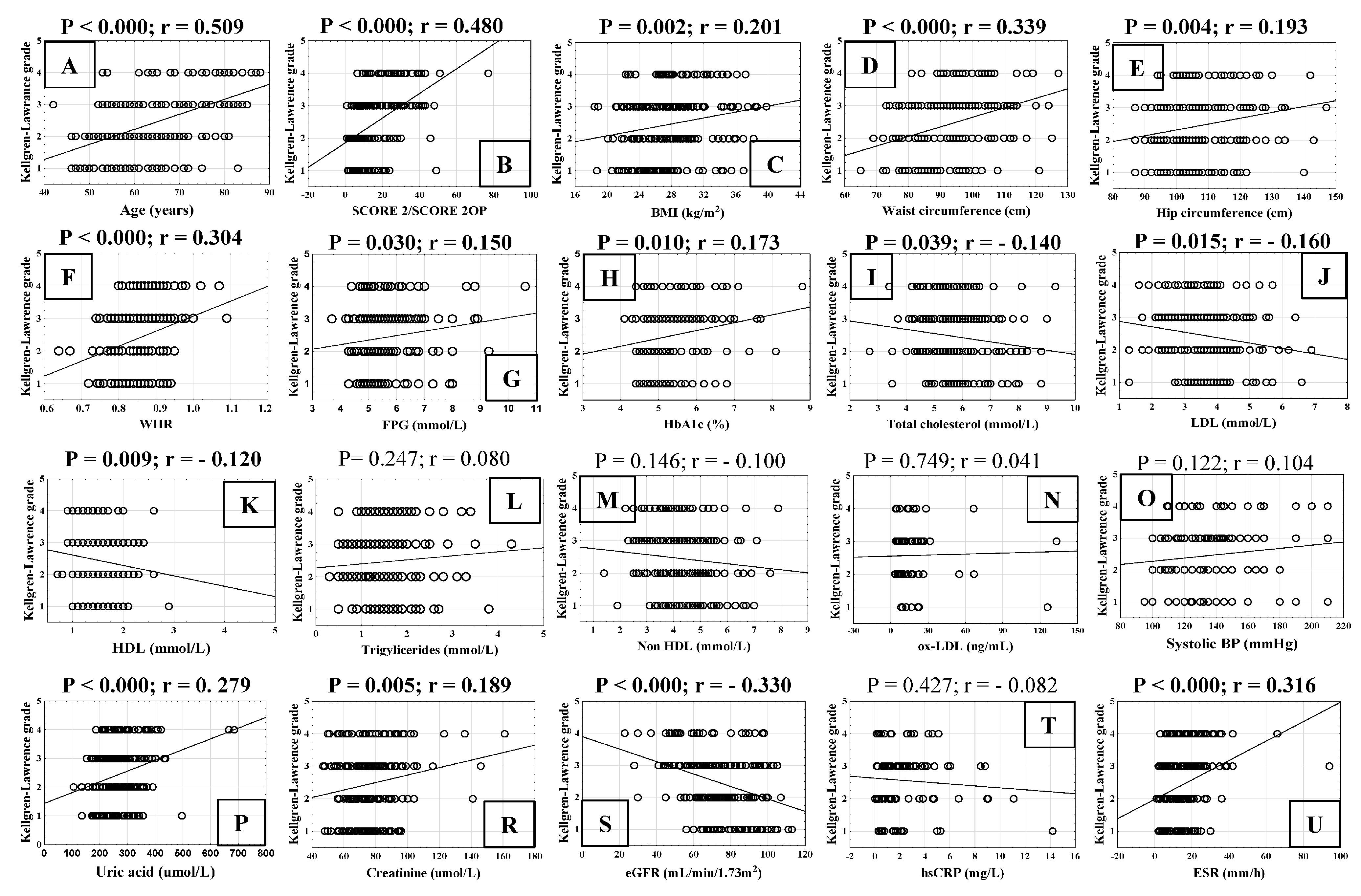


| Control (N 15) | Patients with KOA (N 223) | Man-Whitney U Test p Value | Student’s t Test p Value | |||
|---|---|---|---|---|---|---|
| Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | |||
| Kellgren-Lawrence grade | - | - | 3 (1–4) | |||
| Age (years) | 61 (50–72) | 60 ± 7 | 65 (42–96) | 65 ± 11 | 0.073 | 0.070 |
| BMI (kg/m2) | 25 (20–32) | 25.60 ± 3.10 | 28 (18–45) | 28 ± 4.40 | 0.012 | 0.010 |
| VAS for pain (scale 0–10) | 1 (0–5) | 1.60 ± 2 | 3 (0–9) | 3.5 ± 2 | 0.010 | <0.001 |
| 6MWT (m) | 588 (356–702) | 575 ± 94 | 401 (68–612) | 406 ± 126 | <0.001 | <0.001 |
| PWV (m/s) | 7.30 (6.40–10.90) | 7.70 ± 1.30 | 9.40 (6.10–16.40) | 9.70 ± 2 | <0.001 | <0.001 |
| SCORE 2/SCORE 2OP | 4 (1–14) | 4.80 ± 3.80 | 11 (1–77) | 15.70 ± 12.70 | <0.001 | 0.002 |
| Cigarette smoking (years) | 0 (0–45) | 4.10 ± 12 | 0 (0–46.5) | 6 ± 11 | 0.638 | - |
| Systolic BP (mmHg) | 120 (103–160) | 127 ± 20 | 138 (95–210) | 138 ± 21 | 0.069 | 0.063 |
| Diastolic BP (mmHg) | 80 (64–90) | 79 ± 9.50 | 80 (55–110) | 80 ± 11.40 | 0.810 | 0.864 |
| FPG (mmol/L) | 5.50 (4.50–6.50) | 5.40 ± 0.62 | 5.40 (3.70–12) | 5.70 ± 1 | 0.300 | 0.180 |
| HbA1c (%) | 4.95 (4.40–5.40) | 4.93 ± 0.29 | 5 (4.10–8.80) | 5.23 ± 0.72 | 0.104 | 0.062 |
| Total cholesterol (mmol/L) | 5 (4.60–8.30) | 6 ± 1.20 | 5.70 (2.70–9.30) | 5.80 ± 1.10 | 0.422 | 0.295 |
| LDL (mmol/L) | 3.70 (2.60–6.50) | 3.90 ± 1.10 | 3.50 (1.30–6.90) | 3.60 ± 0.99 | 0.264 | 0.174 |
| Triglycerides (mmol/L) | 1 (0.60–1.90) | 1.10 ± 0.40 | 1.30 (0.30–4.30) | 1.45 ± 0.65 | 0.060 | 0.069 |
| HDL (mmol/L) | 1.70 (7–2) | 1.60 ± 0.30 | 1.40 (0.70–2.90) | 1.5 ± 0.36 | 0.160 | 0.325 |
| Non-HDL (mmol/L) | 4.30 (2.90–2.70) | 4.50 ± 1.30 | 4.20 (1.40–7.90) | 4.30 ± 1 | 0.640 | 0.467 |
| Atherosclerosis index | 2.06 (1.40–5) | 2.43 ± 0.99 | 2.40 (0.90–5.75) | 2.50 ± 0.82 | 0.991 | 0.748 |
| oxLDL (pg/mL) | 12.80 (7.20–162.50) | 48.80 ± 75.90 | 13 (3.40–202.80) | 24.20 ± 36.30 | 0.800 | 0.223 |
| Uric acid (μmol/L) | 225 (178–401) | 240 ± 58 | 261 (105–685) | 271 ± 77 | 0.031 | 0.091 |
| Creatinine (μmol/L) | 77.50 (61–88) | 76 ± 7.20 | 75 (47–161) | 76.70 ± 16.80 | 0.090 | 0.781 |
| eGFR (mL/min/1.73 m2) | 79 (63–101) | 80.60 ± 11.70 | 73 (23–113) | 73.80 ± 17.50 | 0.070 | 0.008 |
| ESR (mm/h) | 12 (2–37) | 12.60 ± 9.50 | 14 (1–94) | 16 ± 11 | 0.080 | 0.160 |
| hsCRP (mg/L) | 1.30 (0.60–2.60) | 1.34 ± 0.80 | 1.45 (0–14.20) | 2.37 ± 2.50 | 0.460 | 0.326 |
| Comorbidities and Medication | Control (N 15) N (%) | KOA (N 223) N (%) | Chi-Squere Test | Yates’s Correction | Fisher’s Exact Test |
|---|---|---|---|---|---|
| Cardiovascular diseases | 5 (33.33) | 112 (50.22) | 0.205 | 0.206 | 0.158 |
| Diabetes mellitus | 3 (20.00) | 79 (35.42) | 0.223 | 0.349 | 0.175 |
| Chronic respiratory diseases | 4 (26.66) | 53 (23.76) | 0.548 | 0.787 | 0.373 |
| Gastrointestinal disorders | 6 (40.00) | 126 (56.50) | 0.213 | 0.328 | 0.164 |
| Neurological diseases | 2 (13.33) | 43 (19.28) | 0.569 | 0.818 | 0.434 |
| ACE inhibitors | 3 (20.00) | 74 (33.18) | 0.290 | 0.440 | 0.224 |
| ARB | 1 (6.66) | 15 (6.72) | 0.992 | 0.600 | 0.733 |
| Beta-blockers | 4 (26.66) | 82 (36.77) | 0.430 | 0.609 | 0.311 |
| Calcium channel blocker | 1 (3.66) | 17 (7.62) | 0.892 | 0.712 | 0.684 |
| Diuretics | 1 (3.66) | 11 (4.93) | 0.766 | 0.754 | 0.551 |
| Statins | 3 (20.00) | 76 (34.08) | 0.262 | 0.402 | 0.204 |
| Metformin | 3 (20.00) | 77 (34.52) | 0.235 | 0.365 | 0.184 |
| Dipeptidyl Peptidase IV | 1 (6.66) | 46 (20.62) | 0.188 | 0.327 | 0.164 |
| Allopurinol | 2 (13.33) | 28 (12.55) | 0.930 | 0.753 | 0.588 |
| Proton pump inhibitors | 6 (40.00) | 104 (46.63) | 0.617 | 0.816 | 0.411 |
| Paracetamol | 5 (33.33) | 120 (53.81) | 0.545 | 0.739 | 0.375 |
| Tramadol | 2 (13.33) | 49 (21.97) | 0.429 | 0.642 | 0.338 |
| KOA 1–2 (N 108) | KOA 3–4 (N 115) | p Value | ||||
|---|---|---|---|---|---|---|
| Median (Range) | Mean SD | Median (Range) | Mean SD | Mann-Whitney U Test | Student’s t Test | |
| PWV (m/s) | 8.50 (6.10–14.10) | 8.40 ± 1.63 | 10.40 (6.80–16.40) | 10.50 ± 1.96 | <0.001 | <0.001 |
| Age (years) | 58 (46–83) | 59.96 ± 8.76 | 71 (42–88) | 69.99 ± 10.50 | <0.001 | <0.001 |
| SCORE 2/SCORE 2OP | 7.25 (1–49) | 7.00 ± 6.65 | 19 (1–77) | 20.92 ± 13.78 | <0.001 | <0.001 |
| VAS for pain (scale 0–10) | 2 (0–6) | 2 ± 1.44 | 5 (0–9) | 4.70 ± 2.03 | <0.001 | <0.001 |
| WHR | 0.86 (0.64–0.95) | 0.84 ± 0.06 | 0.89 (0.74–1.09) | 0.89 ± 0.06 | <0.001 | <0.001 |
| Waist circumference (cm) | 89 (65–125) | 90.25 ± 11.28 | 97 (73–127) | 97.40 ± 11.01 | <0.001 | <0.001 |
| Hip circumference (cm) | 104 (87–143) | 105.70 ± 11 | 108 (87–147) | 110.30 ± 10.61 | 0.005 | 0.002 |
| BMI (kg/m2) | 27.10 (18.70–38.20) | 27.36 ± 4.14 | 28.20 (18.40–44.80) | 28.73 ± 4.52 | 0.013 | 0.002 |
| Cigarette smoking (years) | 0 (0–46.50) | 0.72 ± 13.31 | 0 (0–40) | 2.29 ± 7.10 | 0.002 | <0.001 |
| Diastolic BP (mmHg) | 80 (60–110) | 88.80 ± 11.60 | 80 (55–105) | 77.77 ± 10.61 | 0.039 | 0.021 |
| Systolic BP (mmHg) | 130 (95–210) | 136 ± 20.50 | 140 (100–210) | 139 ± 21.17 | 0.360 | 0.388 |
| FPG (mmol/L) | 5.40 (4.30–9.30) | 5.53 ± 0.86 | 5.60 (3.70–12.10) | 5.87 ± 1.22 | 0.019 | 0.021 |
| HbA1c (%) | 4.90 (4.40–8.10) | 5.13 ± 0.64 | 5.20 (4.10–8.80) | 5.37 ± 0.77 | 0.009 | 0.014 |
| Uric acid (µmol/L) | 254 (105–496) | 257.30 ± 63 | 275 (152–685) | 290.30 ± 82.52 | 0.003 | 0.001 |
| Creatinine (µmol/L) | 73 (48–141) | 74.68 ± 13.55 | 78 (47–161) | 79.17 ± 18.75 | 0.046 | 0.043 |
| eGFR (mL/min/1.73 m2) | 79 (30–113) | 78.85 ± 14.85 | 67 (23–105) | 69 ± 18.26 | <0.001 | <0.001 |
| ESR (mm/h) | 12 (1–36) | 12.68 ± 7.23 | 18 (2–94) | 19.35 ± 12.32 | <0.001 | <0.001 |
| hsCRP (mg/L) | 1.25 (0–24.10) | 2.45 ± 3.41 | 2.30 (0.10–6.40) | 4 ± 7.23 | 0.001 | 0.035 |
| Total cholesterol (mmol/L) | 5.80 (2.70–8.80) | 5.90 ± 1.13 | 5.60 (3.40–9.30) | 5.65 ± 1.07 | 0.075 | 0.972 |
| LDL (mmol/L) | 3.60 (1.30–6.90) | 3.71 ± 1.01 | 3.40 (1.60–6.40) | 3.42 ± 0.95 | 0.022 | 0.028 |
| Triglycerides (mmol/L) | 1.30 (0.30–3.80) | 1.40 ± 0.65 | 1.30 (0.50–4.30) | 1.49 ± 0.65 | 0.195 | 0.333 |
| HDL (mmol/L) | 1.50 (0.70–2.90) | 1.52 ± 0.38 | 1.40 (0.80–2.60) | 1.45 ± 0.33 | 0.098 | 0.143 |
| Non-HDL (mmol/L) | 4.25 (1.40–7.60) | 4.38 ± 1.08 | 4.1 (2.20–7.90) | 4.56 ± 3.77 | 0.242 | 0.642 |
| Atherosclerosis index | 2.45 (1–5.75) | 2.54 ± 0.82 | 2.40 (0.90–4.90) | 2.47 ± 0.83 | 0.529 | 0.505 |
| oxLDL (pg/mL) | 13.80 (3.35–154.55) | 25.63 ± 34.94 | 11.60 (3.90–202.80) | 23.06 ± 37.78 | 0.605 | 0.779 |
| 6MWT (m) | 501 (125–612) | 477 ± 102 | 351 (68–576) | 337 ± 110 | <0.001 | <0.001 |
| IPAQ total (MET-min/week) | 1890 (0–9373) | 2366 ± 2212 | 643.50 (0–13878) | 1430 ± 2450 | <0.001 | 0.063 |
| Vigorous physical activity (MET-min/week) | 80 (0–7840) | 564 ± 1334 | 0 (0–8640) | 422 ± 1540 | 0.009 | 0.646 |
| Moderate physical activity (MET-min/week) | 720 (0–5040) | 829 ± 909 | 120 (0–5040) | 460 ± 958 | <0.001 | 0.065 |
| Walking (MET-min/week) | 594 (0–4158) | 973 ± 1007 | 313.50 (0–2722) | 578 ± 666 | 0.014 | 0.026 |
| Sedentary activity (min/day) | 180 (30–480) | 204 ± 101 | 240 (60–480) | 251 ± 112 | 0.036 | 0.039 |
| PWV ≤ 8.4 m/s (N 72) | PWV > 8.4 m/s (N 151) | p Value | ||||
|---|---|---|---|---|---|---|
| Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Mann- Whitney U Test | Student’s t Test | |
| PWV (m/s) | 7.70 (6.10–8.40) | 7.57 ± 0.60 | 10.50 (8.50 -14.30) | 10.69 ± 1.61 | <0.001 | <0.001 |
| Kellgren-Lawrence grade | 2 (1–4) | 1.86 ± 0.87 | 3 (1–4) | 2.74 ± 0.94 | <0.001 | <0.001 |
| Age (years) | 54 (42–64) | 54.09 ± 5.02 | 70 (50–88) | 70.37 ± 8.84 | <0.001 | <0.001 |
| SCORE 2/SCORE 2OP | 5.50 (1–14) | 6.13 ± 3.32 | 17 (2.50–51) | 20.28 ± 13 | <0.001 | <0.001 |
| VAS for pain (scale 0-10) | 2 (0–7) | 2.90 ± 1.87 | 4 (0–9) | 3.93 ± 2.12 | <0.001 | <0.001 |
| WHR | 0.83 (0.73–0.98) | 0.84 ± 0.06 | 0.89 (0.64–1.09) | 0.88 ± 0.06 | <0.001 | <0.001 |
| Waist circumference (cm) | 87 (69–127) | 91.22 ± 13.19 | 95 (65–125) | 95.23 ± 10.69 | 0.002 | 0.016 |
| Hip circumference (cm) | 106 (90–140) | 108.05 ± 11.79 | 107 (87–147) | 108.28 ± 10.43 | 0.319 | 0.883 |
| BMI (kg/m2) | 27.10 (20.10–39.80) | 28 ± 4.83 | 28.20 (18.40–44.8) | 28.09 ± 4.16 | 0.299 | 0.884 |
| Cigarette smoking (years) | 2.50 (0–36) | 7.75 ± 9.80 | 0 (0–47) | 4.63 ± 10.87 | 0.002 | 0.140 |
| Diastolic BP (mmHg) | 80 (60–110) | 79.59 ± 11.36 | 80 (55–110) | 79.36 ± 11.14 | 0.818 | 0.153 |
| Systolic BP (mmHg) | 130 (95–170) | 128.38 ± 17.06 | 140 (100–210) | 141.60 ± 21.16 | <0.001 | <0.001 |
| FPG (mmol/L) | 5.20 (4.30–8) | 5.32 ± 0.63 | 5.60 (3.70–12.10) | 5.88 ± 1.19 | <0.001 | <0.001 |
| HbA1c (%) | 4.90 (4.40–6.80) | 5 ± 0.50 | 5.10 (4.10–8.80) | 5.38 ± 0.78 | <0.001 | <0.001 |
| Uric acid (μmol/L) | 249 (105–432) | 256.68 ± 67.31 | 267 (136–685) | 282.72 ± 77.79 | 0.023 | 0.016 |
| Creatinine (μmL/L) | 73 (47–96) | 72.32 ± 11.58 | 76 (48–146) | 79.22 ± 18.08 | 0.027 | 0.003 |
| eGFR (mL/min/1.73 m2) | 81 (56–113) | 83.25 ± 14.20 | 68 (28–102) | 69.23 ± 16.97 | <0.001 | < 0.001 |
| ESR (mm/h) | 12 (1–94) | 11.75 ± 11.46 | 18 (2–66) | 18.21 ± 9.60 | <0.001 | < 0.001 |
| hsCRP (mg/L) | 1.50 (0.10–94) | 2.17 ± 2.56 | 1.60 (0–64) | 2.49 ± 2.56 | 0.282 | 0.554 |
| Total cholesterol (mmol/L) | 5.90 (4.30–9.30) | 6.08 ± 1.07 | 5.60 (2.70–8.80) | 5.62 ± 1.09 | 0.036 | 0.328 |
| LDL (mmol/L) | 3.60 (2.10–6.90) | 3.82 ± 0.96 | 3.40 (1.30–6.60) | 3.44 ± 0.98 | 0.051 | 0.491 |
| Triglycerides (mmol/L) | 1.20 (0.50–3.50) | 1.38 ± 0.61 | 1.50 (0.30–3.80) | 1.46 ± 0.62 | 0.238 | 0.490 |
| HDL (mmol/L) | 1.50 (0.90–2.90) | 1.51 ± 0.36 | 1.40 (0.70–2.60) | 1.47 ± 0.35 | 0.363 | 0.477 |
| Non-HDL (mmol/L) | 4.30 (2.60–7.90) | 4.58 ± 1.09 | 4.10 (1.40–7) | 4.42 ± 3.32 | 0.059 | 0.697 |
| Atherosclerosis index | 2.56 (1.10–5.75) | 2.66 ± 0.91 | 2.30 (1–4.90) | 2.43 ± 0.77 | 0.283 | 0.052 |
| oxLDL (pg/mL) | 16.40 (3.80–154.60) | 30.69 ± 41.42 | 11.32 (3.40–202.8) | 20.33 ± 32.85 | 0.414 | 0.269 |
| 6MWT (m) | 508 (315–612) | 499 ± 79.13 | 371 (68–600) | 360 ± 121 | < 0.001 | < 0.001 |
| IPAQ total (MET/min/week) | 1506 (0–13878) | 2762.15 ± 3374 | 735 (0–7812) | 1288.40 ± 1365 | 0.058 | 0.004 |
| Vigorous physical activity (MET/min/week) | 0 (0–8640) | 1076.36 ± 2293.04 | 0 (0–960) | 147.79 ± 307 | 0.211 | 0.003 |
| Moderate physical activity (MET/min/week) | 360 (0–5040) | 771.51 ± 1102.89 | 240 (0–5040) | 523 ± 851 | 0.299 | 0.232 |
| Walking (MET/min/week) | 594 (0–4158) | 961.54 ± 1026.01 | 396 (0–3465) | 618 ± 698 | 0.167 | 0.060 |
| Sedentary activity (min/day) | 180 (30–480) | 217 ± 110 | 240 (30–480) | 239 ± 108 | 0.417 | 0.353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavidić, T.; Babarović, E.; Drvar, V.; Ćurko-Cofek, B.; Laškarin, G. Patients with Higher Pulse Wave Velocity Are More Likely to Develop a More Severe Form of Knee Osteoarthritis: Implications for Cardiovascular Risk. Biomedicines 2025, 13, 1208. https://doi.org/10.3390/biomedicines13051208
Zavidić T, Babarović E, Drvar V, Ćurko-Cofek B, Laškarin G. Patients with Higher Pulse Wave Velocity Are More Likely to Develop a More Severe Form of Knee Osteoarthritis: Implications for Cardiovascular Risk. Biomedicines. 2025; 13(5):1208. https://doi.org/10.3390/biomedicines13051208
Chicago/Turabian StyleZavidić, Tina, Emina Babarović, Vedrana Drvar, Božena Ćurko-Cofek, and Gordana Laškarin. 2025. "Patients with Higher Pulse Wave Velocity Are More Likely to Develop a More Severe Form of Knee Osteoarthritis: Implications for Cardiovascular Risk" Biomedicines 13, no. 5: 1208. https://doi.org/10.3390/biomedicines13051208
APA StyleZavidić, T., Babarović, E., Drvar, V., Ćurko-Cofek, B., & Laškarin, G. (2025). Patients with Higher Pulse Wave Velocity Are More Likely to Develop a More Severe Form of Knee Osteoarthritis: Implications for Cardiovascular Risk. Biomedicines, 13(5), 1208. https://doi.org/10.3390/biomedicines13051208






