Cytokines Meet Phages: A Revolutionary Pathway to Modulating Immunity and Microbial Balance
Abstract
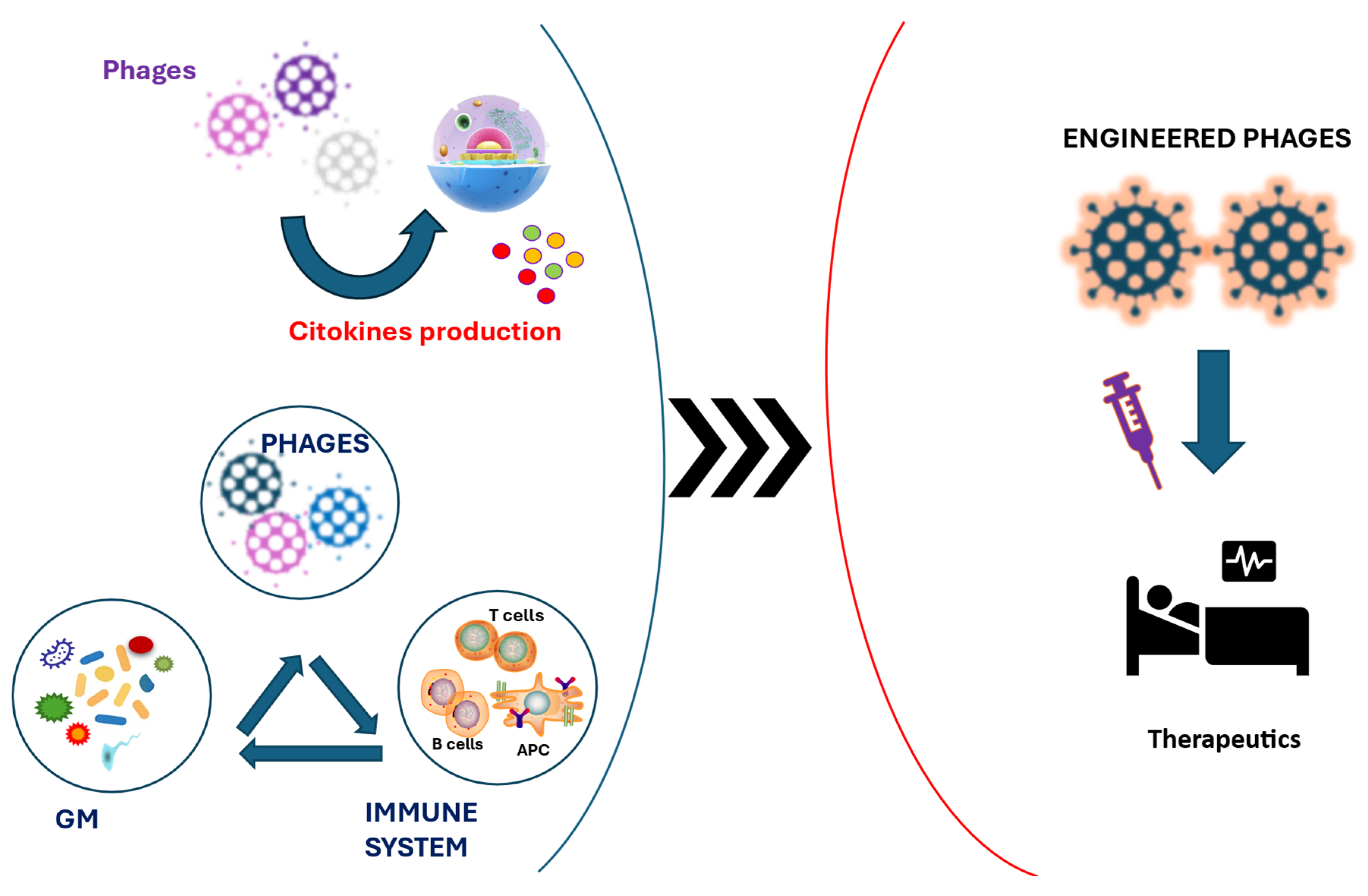
1. Introduction
2. Mechanisms of Immune Modulation
3. Direct Interactions with Human Cells
4. Drivers of Microbiota Composition
5. Phages and Cancer
6. Therapeutic Applications
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- D’Herelle, F. On an Invisible Microbe Antagonistic Toward Dysenteric Bacilli: Brief Note by Mr. F. D’Herelle, Presented by Mr. Roux. 1917. Res. Microbiol. 2007, 158, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Salmond, G.P.C.; Fineran, P.C. A Century of the Phage: Past, Present and Future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Chan, B.K.; Stanley, G.; Modak, M.; Koff, J.L.; Turner, P.E. Bacteriophage Therapy for Infections in CF. Pediatr. Pulmonol. 2021, 56 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Pope, W.H.; Bowman, C.A.; Russell, D.A.; Jacobs-Sera, D.; Asai, D.J.; Cresawn, S.G.; Jacobs, W.R.; Hendrix, R.W.; Lawrence, J.G.; Hatfull, G.F.; et al. Whole Genome Comparison of a Large Collection of Mycobacteriophages Reveals a Continuum of Phage Genetic Diversity. eLife 2015, 4, e06416. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, Z.-G.; Liu, C.; Tan, S.; Lin, S.; Zhu, J.; Dai, F.-H.; Gao, J.; She, J.-L.; Mei, Z.; et al. Gut Microbiome Alterations and Gut Barrier Dysfunction Are Associated with Host Immune Homeostasis in COVID-19 Patients. BMC Med. 2022, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Ranveer, S.A.; Dasriya, V.; Ahmad, M.F.; Dhillon, H.S.; Samtiya, M.; Shama, E.; Anand, T.; Dhewa, T.; Chaudhary, V.; Chaudhary, P.; et al. Positive and Negative Aspects of Bacteriophages and Their Immense Role in the Food Chain. NPJ Sci. Food 2024, 8, 1. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Sato, A.K. Phage Display Technology and Its Impact in the Discovery of Novel Protein-Based Drugs. Expert Opin. Drug Discov. 2024, 19, 887–915. [Google Scholar] [CrossRef]
- de Vries, C.R.; Chen, Q.; Demirdjian, S.; Kaber, G.; Khosravi, A.; Liu, D.; Van Belleghem, J.D.; Bollyky, P.L. Phages in Vaccine Design and Immunity; Mechanisms and Mysteries. Curr. Opin. Biotechnol. 2021, 68, 160–165. [Google Scholar] [CrossRef]
- Ghaznavi, G.; Vosough, P.; Ghasemian, A.; Tabar, M.M.M.; Tayebi, L.; Taghizadeh, S.; Savardashtaki, A. Engineering Bacteriophages for Targeted Superbug Eradication. Mol. Biol. Rep. 2025, 52, 221. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Łobocka, M.; Głowacka-Rutkowska, A.; Bednarek, A.; Borysowski, J.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Bagińska, N.; et al. Phage Therapy: What Have We Learned? Viruses 2018, 10, 288. [Google Scholar] [CrossRef]
- Lavilla, M.; Domingo-Calap, P.; Sevilla-Navarro, S.; Lasagabaster, A. Natural Killers: Opportunities and Challenges for the Use of Bacteriophages in Microbial Food Safety from the One Health Perspective. Foods 2023, 12, 552. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Broad, S.; Caine, J.; Clout, M.; Dicks, L.V.; Doran, H.; Entwistle, A.C.; Fleishman, E.; Gibbons, D.W.; Keim, B.; et al. A Horizon Scan of Global Conservation Issues for 2016. Trends Ecol. Evol. 2016, 31, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.d.S.; Fan, J.; Pan, F. The Power of Phages: Revolutionizing Cancer Treatment. Front. Oncol. 2023, 13, 1290296. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Park, E.-J.; Roh, S.W.; Bae, J.-W. Diversity and Abundance of Single-Stranded DNA Viruses in Human Feces. Appl. Environ. Microbiol. 2011, 77, 8062–8070. [Google Scholar] [CrossRef]
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and Their Potential to Modulate the Microbiome and Immunity. Cell. Mol. Immunol. 2021, 18, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.; Brown, N.; Redgwell, T.; Rihtman, B.; Barnes, M.; Clokie, M.; Stekel, D.J.; Hobman, J.; Jones, M.A.; Millard, A. INfrastructure for a PHAge REference Database: Identification of Large-Scale Biases in the Current Collection of Cultured Phage Genomes. Phage 2021, 2, 214–223. [Google Scholar] [CrossRef]
- Chiang, M.-C.; Chen, C.-L.; Feng, Y.; Chen, C.-C.; Lien, R.; Chiu, C.-H. Lactobacillus Rhamnosus Sepsis Associated with Probiotic Therapy in an Extremely Preterm Infant: Pathogenesis and a Review for Clinicians. J. Microbiol. Immunol. Infect. 2021, 54, 575–580. [Google Scholar] [CrossRef]
- Zuo, T. Gut Bacteriophages Ignite Mammalian Immunity. Nat. Rev. Microbiol. 2023, 21, 634. [Google Scholar] [CrossRef]
- Adiliaghdam, F.; Amatullah, H.; Digumarthi, S.; Saunders, T.L.; Rahman, R.-U.; Wong, L.P.; Sadreyev, R.; Droit, L.; Paquette, J.; Goyette, P.; et al. Human Enteric Viruses Autonomously Shape Inflammatory Bowel Disease Phenotype Through Divergent Innate Immunomodulation. Sci. Immunol. 2022, 7, eabn6660. [Google Scholar] [CrossRef]
- Domenjo-Vila, E.; Casella, V.; Iwabuchi, R.; Fossum, E.; Pedragosa, M.; Castellví, Q.; Rica, P.C.; Kaisho, T.; Terahara, K.; Bocharov, G.; et al. XCR1+ DCs Are Critical for T Cell-Mediated Immunotherapy of Chronic Viral Infections. Cell Rep. 2023, 42, 112123. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Jończyk-Matysiak, E.; Kniotek, M.; Letkiewicz, S. Therapeutic Phages as Modulators of the Immune Response: Practical Implications. Clin. Infect. Dis. 2023, 77, S433–S439. [Google Scholar] [CrossRef]
- Souza, E.B.d.; Pinto, A.R.; Fongaro, G. Bacteriophages as Potential Clinical Immune Modulators. Microorganisms 2023, 11, 2222. [Google Scholar] [CrossRef]
- Schauvliege, R.; Janssens, S.; Beyaert, R. Pellino Proteins Are More than Scaffold Proteins in TLR/IL-1R Signalling: A Role as Novel RING E3–Ubiquitin-Ligases. FEBS Lett. 2006, 580, 4697–4702. [Google Scholar] [CrossRef]
- Novoa, I.; Zeng, H.; Harding, H.P.; Ron, D. Feedback Inhibition of the Unfolded Protein Response by GADD34-Mediated Dephosphorylation of eIF2α. J. Cell Biol. 2001, 153, 1011–1022. [Google Scholar] [CrossRef]
- Henry, T.; Monack, D.M. Activation of the Inflammasome upon Francisella Tularensis Infection: Interplay of Innate Immune Pathways and Virulence Factors. Cell. Microbiol. 2007, 9, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.; Taniguchi, T. Cytosolic DNA Recognition for Triggering Innate Immune Responses. Adv. Drug Deliv. Rev. 2008, 60, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Animal and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Mesev, E.V.; LeDesma, R.A.; Ploss, A. Decoding Type I and III Interferon Signalling During Viral Infection. Nat. Microbiol. 2019, 4, 914–924. [Google Scholar] [CrossRef]
- Mori, K.; Kubo, T.; Kibayashi, Y.; Ohkuma, T.; Kaji, A. Anti-Vaccinia Virus Effect of M13 Bacteriophage DNA. Antivir. Res. 1996, 31, 79–86. [Google Scholar] [CrossRef]
- Sartorius, R.; D’Apice, L.; Trovato, M.; Cuccaro, F.; Costa, V.; De Leo, M.G.; Marzullo, V.M.; Biondo, C.; D’Auria, S.; De Matteis, M.A.; et al. Antigen Delivery by Filamentous Bacteriophage Fd Displaying an Anti-DEC-205 Single-Chain Variable Fragment Confers Adjuvanticity by Triggering a TLR9-Mediated Immune Response. EMBO Mol. Med. 2015, 7, 973–988. [Google Scholar] [CrossRef]
- Krieg, A.M. The Role of CpG Motifs in Innate Immunity. Curr. Opin. Immunol. 2000, 12, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef]
- Shiley, J.R.; Comfort, K.K.; Robinson, J.B. Immunogenicity and Antimicrobial Effectiveness of Pseudomonas Aeruginosa Specific Bacteriophage in a Human Lung in Vitro Model. Appl. Microbiol. Biotechnol. 2017, 101, 7977–7985. [Google Scholar] [CrossRef]
- Huang, H.-H.; Furuta, M.; Nasu, T.; Hirono, M.; Pruet, J.; Duc, H.M.; Zhang, Y.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Inhibition of Phage-Resistant Bacterial Pathogen Re-Growth with the Combined Use of Bacteriophages and EDTA. Food Microbiol. 2021, 100, 103853. [Google Scholar] [CrossRef] [PubMed]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe 2019, 25, 285–299.e8. [Google Scholar] [CrossRef]
- Miernikiewicz, P.; Dąbrowska, K.; Piotrowicz, A.; Owczarek, B.; Wojas-Turek, J.; Kicielińska, J.; Rossowska, J.; Pajtasz-Piasecka, E.; Hodyra, K.; Macegoniuk, K.; et al. T4 Phage and Its Head Surface Proteins Do Not Stimulate Inflammatory Mediator Production. PLoS ONE 2013, 8, e71036. [Google Scholar] [CrossRef] [PubMed]
- Chechushkov, A.; Kozlova, Y.; Baykov, I.; Morozova, V.; Kravchuk, B.; Ushakova, T.; Bardasheva, A.; Zelentsova, E.; Allaf, L.A.; Tikunov, A.; et al. Influence of Caudovirales Phages on Humoral Immunity in Mice. Viruses 2021, 13, 1241. [Google Scholar] [CrossRef]
- Żaczek, M.; Łusiak-Szelachowska, M.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Owczarek, B.; Kopciuch, A.; Fortuna, W.; Rogóż, P.; Górski, A. Antibody Production in Response to Staphylococcal MS-1 Phage Cocktail in Patients Undergoing Phage Therapy. Front. Microbiol. 2016, 7, 1681. [Google Scholar] [CrossRef]
- Krut, O.; Bekeredjian-Ding, I. Contribution of the Immune Response to Phage Therapy. J. Immunol. 2018, 200, 3037–3044. [Google Scholar] [CrossRef]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Champagne-Jorgensen, K.; Luong, T.; Darby, T.; Roach, D.R. Immunogenicity of Bacteriophages. Trends Microbiol. 2023, 31, 1058–1071. [Google Scholar] [CrossRef]
- Barr, J.J. A Bacteriophages Journey Through the Human Body. Immunol. Rev. 2017, 279, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the Human Gut: The “Known Unknown” of the Microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Stockdale, S.R.; Lavelle, A.; Kondova, I.; Heuston, C.; Upadrasta, A.; Khokhlova, E.V.; van der Kamp, I.; Ouwerling, B.; Draper, L.A.; et al. Viral Biogeography of the Mammalian Gut and Parenchymal Organs. Nat. Microbiol. 2022, 7, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.; Wong, S.; St Jean, J.; Slavcev, R. Bacteriophage Interactions with Mammalian Tissue: Therapeutic Applications. Adv. Drug Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Bichet, M.C.; Chin, W.H.; Richards, W.; Lin, Y.-W.; Avellaneda-Franco, L.; Hernandez, C.A.; Oddo, A.; Chernyavskiy, O.; Hilsenstein, V.; Neild, A.; et al. Bacteriophage Uptake by Mammalian Cell Layers Represents a Potential Sink That May Impact Phage Therapy. iScience 2021, 24, 102287. [Google Scholar] [CrossRef]
- Sweere, J.M.; Van Belleghem, J.D.; Ishak, H.; Bach, M.S.; Popescu, M.; Sunkari, V.; Kaber, G.; Manasherob, R.; Suh, G.A.; Cao, X.; et al. Bacteriophage Trigger Antiviral Immunity and Prevent Clearance of Bacterial Infection. Science 2019, 363, eaat9691. [Google Scholar] [CrossRef]
- Yang, J.H.; Bhargava, P.; McCloskey, D.; Mao, N.; Palsson, B.O.; Collins, J.J. Antibiotic-Induced Changes to the Host Metabolic Environment Inhibit Drug Efficacy and Alter Immune Function. Cell Host Microbe 2017, 22, 757–765.e3. [Google Scholar] [CrossRef]
- Bichet, M.C.; Adderley, J.; Avellaneda-Franco, L.; Magnin-Bougma, I.; Torriero-Smith, N.; Gearing, L.J.; Deffrasnes, C.; David, C.; Pepin, G.; Gantier, M.P.; et al. Mammalian Cells Internalize Bacteriophages and Use Them as a Resource to Enhance Cellular Growth and Survival. PLoS Biol. 2023, 21, e3002341. [Google Scholar] [CrossRef]
- Harhala, M.; Gembara, K.; Miernikiewicz, P.; Owczarek, B.; Kaźmierczak, Z.; Majewska, J.; Nelson, D.C.; Dąbrowska, K. DNA Dye Sytox Green in Detection of Bacteriolytic Activity: High Speed, Precision and Sensitivity Demonstrated with Endolysins. Front. Microbiol. 2021, 12, 752282. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage Transcytosis Provides a Mechanism To Cross Epithelial Cell Layers. mBio 2017, 8, e01874-17. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Clement, F.; Merabishvili, M.; Lavigne, R.; Vaneechoutte, M. Pro- and Anti-Inflammatory Responses of Peripheral Blood Mononuclear Cells Induced by Staphylococcus Aureus and Pseudomonas Aeruginosa Phages. Sci. Rep. 2017, 7, 8004. [Google Scholar] [CrossRef]
- Lehti, T.A.; Pajunen, M.I.; Skog, M.S.; Finne, J. Internalization of a Polysialic Acid-Binding Escherichia Coli Bacteriophage into Eukaryotic Neuroblastoma Cells. Nat. Commun. 2017, 8, 1915. [Google Scholar] [CrossRef]
- Costantini, T.W.; Putnam, J.G.; Sawada, R.; Baird, A.; Loomis, W.H.; Eliceiri, B.P.; Bansal, V.; Coimbra, R. Targeting the Gut Barrier: Identification of a Homing Peptide Sequence for Delivery into the Injured Intestinal Epithelial Cell. Surgery 2009, 146, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ivanenkov, V.; Felici, F.; Menon, A.G. Uptake and Intracellular Fate of Phage Display Vectors in Mammalian Cells. Biochim. Biophys. Acta 1999, 1448, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Zamora, P.F.; Reidy, T.G.; Armbruster, C.R.; Sun, M.; Van Tyne, D.; Turner, P.E.; Koff, J.L.; Bomberger, J.M. Lytic Bacteriophages Induce the Secretion of Antiviral and Proinflammatory Cytokines from Human Respiratory Epithelial Cells. PLoS Biol. 2024, 22, e3002566. [Google Scholar] [CrossRef]
- Wiest, R.; Garcia-Tsao, G. Bacterial Translocation (BT) in Cirrhosis. Hepatology 2005, 41, 422–433. [Google Scholar] [CrossRef]
- Jaiswal, A.; Koley, H.; Mitra, S.; Saha, D.R.; Sarkar, B. Comparative Analysis of Different Oral Approaches to Treat Vibrio Cholerae Infection in Adult Mice. Int. J. Med. Microbiol. 2014, 304, 422–430. [Google Scholar] [CrossRef]
- Jun, J.W.; Shin, T.H.; Kim, J.H.; Shin, S.P.; Han, J.E.; Heo, G.J.; De Zoysa, M.; Shin, G.W.; Chai, J.Y.; Park, S.C. Bacteriophage Therapy of a Vibrio Parahaemolyticus Infection Caused by a Multiple-Antibiotic-Resistant O3:K6 Pandemic Clinical Strain. J. Infect. Dis. 2014, 210, 72–78. [Google Scholar] [CrossRef]
- Letarova, M.; Strelkova, D.; Nevolina, S.; Letarov, A. A Test for the “Physiological Phagemia” Hypothesis-Natural Intestinal Coliphages Do Not Penetrate to the Blood in Horses. Folia Microbiol. 2012, 57, 81–83. [Google Scholar] [CrossRef] [PubMed]
- McCallin, S.; Alam Sarker, S.; Barretto, C.; Sultana, S.; Berger, B.; Huq, S.; Krause, L.; Bibiloni, R.; Schmitt, B.; Reuteler, G.; et al. Safety Analysis of a Russian Phage Cocktail: From Metagenomic Analysis to Oral Application in Healthy Human Subjects. Virology 2013, 443, 187–196. [Google Scholar] [CrossRef]
- Majewska, J.; Beta, W.; Lecion, D.; Hodyra-Stefaniak, K.; Kłopot, A.; Kaźmierczak, Z.; Miernikiewicz, P.; Piotrowicz, A.; Ciekot, J.; Owczarek, B.; et al. Oral Application of T4 Phage Induces Weak Antibody Production in the Gut and in the Blood. Viruses 2015, 7, 4783–4799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, L.; Wei, R.; Gao, Q.; He, T.; Xu, C.; Liu, X.; Wang, R. Intracellular Staphylococcus Aureus Control by Virulent Bacteriophages Within MAC-T Bovine Mammary Epithelial Cells. Antimicrob. Agents Chemother. 2017, 61, e01990-16. [Google Scholar] [CrossRef]
- Mirzaei, M.K.; Maurice, C.F. Ménage à Trois in the Human Gut: Interactions Between Host, Bacteria and Phages. Nat. Rev. Microbiol. 2017, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Cornuault, J.K.; Petit, M.-A.; Mariadassou, M.; Benevides, L.; Moncaut, E.; Langella, P.; Sokol, H.; De Paepe, M. Phages Infecting Faecalibacterium Prausnitzii Belong to Novel Viral Genera That Help to Decipher Intestinal Viromes. Microbiome 2018, 6, 65. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, H.; Si, Y.; Gan, R.; Yu, L.; Chen, C.; Ren, C.; Wu, J.; Zhang, F. PhageTailFinder: A Tool for Phage Tail Module Detection and Annotation. Front. Genet. 2023, 14, 947466. [Google Scholar] [CrossRef]
- Zhang, R.; Mirdita, M.; Levy Karin, E.; Norroy, C.; Galiez, C.; Söding, J. SpacePHARER: Sensitive Identification of Phages from CRISPR Spacers in Prokaryotic Hosts. Bioinformatics 2021, 37, 3364–3366. [Google Scholar] [CrossRef]
- Scanlan, P.D. Bacteria-Bacteriophage Coevolution in the Human Gut: Implications for Microbial Diversity and Functionality. Trends Microbiol. 2017, 25, 614–623. [Google Scholar] [CrossRef]
- Zhu, A.; Sunagawa, S.; Mende, D.R.; Bork, P. Inter-Individual Differences in the Gene Content of Human Gut Bacterial Species. Genome Biol. 2015, 16, 82. [Google Scholar] [CrossRef]
- Stern, A.; Mick, E.; Tirosh, I.; Sagy, O.; Sorek, R. CRISPR Targeting Reveals a Reservoir of Common Phages Associated with the Human Gut Microbiome. Genome Res. 2012, 22, 1985–1994. [Google Scholar] [CrossRef]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting Mechanisms of Tailed Bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef]
- Sausset, R.; Petit, M.A.; Gaboriau-Routhiau, V.; De Paepe, M. New Insights into Intestinal Phages. Mucosal Immunol. 2020, 13, 205–215. [Google Scholar] [CrossRef]
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage Adhering to Mucus Provide a Non-Host-Derived Immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776. [Google Scholar] [CrossRef]
- De Sordi, L.; Lourenço, M.; Debarbieux, L. The Battle Within: Interactions of Bacteriophages and Bacteria in the Gastrointestinal Tract. Cell Host Microbe 2019, 25, 210–218. [Google Scholar] [CrossRef]
- Howard-Varona, C.; Hargreaves, K.R.; Abedon, S.T.; Sullivan, M.B. Lysogeny in Nature: Mechanisms, Impact and Ecology of Temperate Phages. ISME J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef]
- Lourenço, M.; Chaffringeon, L.; Lamy-Besnier, Q.; Pédron, T.; Campagne, P.; Eberl, C.; Bérard, M.; Stecher, B.; Debarbieux, L.; De Sordi, L. The Spatial Heterogeneity of the Gut Limits Predation and Fosters Coexistence of Bacteria and Bacteriophages. Cell Host Microbe 2020, 28, 390–401.e5. [Google Scholar] [CrossRef]
- Galtier, M.; De Sordi, L.; Sivignon, A.; de Vallée, A.; Maura, D.; Neut, C.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages Targeting Adherent Invasive Escherichia Coli Strains as a Promising New Treatment for Crohn’s Disease. J. Crohns Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef]
- Frazão, N.; Sousa, A.; Lässig, M.; Gordo, I. Horizontal Gene Transfer Overrides Mutation in Escherichia Coli Colonizing the Mammalian Gut. Proc. Natl. Acad. Sci. USA 2019, 116, 17906–17915. [Google Scholar] [CrossRef]
- Acheson, D.W.; Reidl, J.; Zhang, X.; Keusch, G.T.; Mekalanos, J.J.; Waldor, M.K. In Vivo Transduction with Shiga Toxin 1-Encoding Phage. Infect. Immun. 1998, 66, 4496–4498. [Google Scholar] [CrossRef]
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S.; et al. Communication Between Viruses Guides Lysis-Lysogeny Decisions. Nature 2017, 541, 488–493. [Google Scholar] [CrossRef]
- Davidson, A.R. Virology: Phages Make a Group Decision. Nature 2017, 541, 466–467. [Google Scholar] [CrossRef]
- Harms, A.; Diard, M. Crowd Controlled-Host Quorum Sensing Drives Phage Decision. Cell Host Microbe 2019, 25, 179–181. [Google Scholar] [CrossRef]
- Hynes, A.P.; Moineau, S. Phagebook: The Social Network. Mol. Cell 2017, 65, 963–964. [Google Scholar] [CrossRef][Green Version]
- Bellas, C.M.; Anesio, A.M.; Barker, G. Analysis of Virus Genomes from Glacial Environments Reveals Novel Virus Groups with Unusual Host Interactions. Front. Microbiol. 2015, 6, 656. [Google Scholar] [CrossRef]
- Silpe, J.E.; Bassler, B.L. A Host-Produced Quorum-Sensing Autoinducer Controls a Phage Lysis-Lysogeny Decision. Cell 2019, 176, 268–280.e13. [Google Scholar] [CrossRef]
- Oh, J.-H.; Alexander, L.M.; Pan, M.; Schueler, K.L.; Keller, M.P.; Attie, A.D.; Walter, J.; van Pijkeren, J.-P. Dietary Fructose and Microbiota-Derived Short-Chain Fatty Acids Promote Bacteriophage Production in the Gut Symbiont Lactobacillus Reuteri. Cell Host Microbe 2019, 25, 273–284.e6. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 2019, 25, 803–814.e5. [Google Scholar] [CrossRef]
- Diard, M.; Bakkeren, E.; Cornuault, J.K.; Moor, K.; Hausmann, A.; Sellin, M.E.; Loverdo, C.; Aertsen, A.; Ackermann, M.; De Paepe, M.; et al. Inflammation Boosts Bacteriophage Transfer Between Salmonella spp. Science 2017, 355, 1211–1215. [Google Scholar] [CrossRef]
- Gheorghe, C.E.; Ritz, N.L.; Martin, J.A.; Wardill, H.R.; Cryan, J.F.; Clarke, G. Investigating Causality with Fecal Microbiota Transplantation in Rodents: Applications, Recommendations and Pitfalls. Gut Microbes 2021, 13, 1941711. [Google Scholar] [CrossRef]
- Draper, L.A.; Ryan, F.J.; Smith, M.K.; Jalanka, J.; Mattila, E.; Arkkila, P.A.; Ross, R.P.; Satokari, R.; Hill, C. Long-Term Colonisation with Donor Bacteriophages Following Successful Faecal Microbial Transplantation. Microbiome 2018, 6, 220. [Google Scholar] [CrossRef]
- Rasmussen, T.S.; Mentzel, C.M.J.; Kot, W.; Castro-Mejía, J.L.; Zuffa, S.; Swann, J.R.; Hansen, L.H.; Vogensen, F.K.; Hansen, A.K.; Nielsen, D.S. Faecal Virome Transplantation Decreases Symptoms of Type 2 Diabetes and Obesity in a Murine Model. Gut 2020, 69, 2122–2130. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, J.; Li, Y.; Yang, C.-T.; Zhou, X. Modified Bacteriophage for Tumor Detection and Targeted Therapy. Nanomaterials 2023, 13, 665. [Google Scholar] [CrossRef]
- Bortot, B.; Apollonio, M.; Baj, G.; Andolfi, L.; Zupin, L.; Crovella, S.; di Giosia, M.; Cantelli, A.; Saporetti, R.; Ulfo, L.; et al. Advanced Photodynamic Therapy with an Engineered M13 Phage Targeting EGFR: Mitochondrial Localization and Autophagy Induction in Ovarian Cancer Cell Lines. Free Radic. Biol. Med. 2022, 179, 242–251. [Google Scholar] [CrossRef]
- Jakobsen, C.G.; Rasmussen, N.; Laenkholm, A.-V.; Ditzel, H.J. Phage Display–Derived Human Monoclonal Antibodies Isolated by Binding to the Surface of Live Primary Breast Cancer Cells Recognize GRP78. Cancer Res. 2007, 67, 9507–9517. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and Cons of Phage Therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- ALTUN, İ.; SONKAYA, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran. J. Public Health 2018, 47, 1218–1219. [Google Scholar]
- Ragothaman, M.; Yoo, S.Y. Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions. Vaccines 2023, 11, 919. [Google Scholar] [CrossRef]
- Mittal, M.; Kumari, A.; Paul, B.; Varshney, A.; Bhavya; Saini, A.; Verma, C.; Mani, I. Challenges and Opportunities of Gene Therapy in Cancer. OBM Genet. 2024, 8, 1–501. [Google Scholar] [CrossRef]
- Jia, L.-T.; Chen, S.-Y.; Yang, A.-G. Cancer Gene Therapy Targeting Cellular Apoptosis Machinery. Cancer Treat. Rev. 2012, 38, 868–876. [Google Scholar] [CrossRef]
- Veeranarayanan, S.; Azam, A.H.; Kiga, K.; Watanabe, S.; Cui, L. Bacteriophages as Solid Tumor Theragnostic Agents. Int. J. Mol. Sci. 2021, 23, 402. [Google Scholar] [CrossRef]
- Lim, C.C.; Choong, Y.S.; Lim, T.S. Cognizance of Molecular Methods for the Generation of Mutagenic Phage Display Antibody Libraries for Affinity Maturation. Int. J. Mol. Sci. 2019, 20, 1861. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Mirshekari, H.; Moosavi Basri, S.M.; Bahrami, S.; Moghoofei, M.; Hamblin, M.R. Bacteriophages and Phage-Inspired Nanocarriers for Targeted Delivery of Therapeutic Cargos. Adv. Drug Deliv. Rev. 2016, 106, 45–62. [Google Scholar] [CrossRef]
- Gierlicka, I.; Rattan, S.I.S.; Wnuk, M. Perspectives on Using Bacteriophages in Biogerontology Research and Interventions. Chem. Biol. Interact. 2022, 366, 110098. [Google Scholar] [CrossRef] [PubMed]
- Foglizzo, V.; Marchiò, S. Bacteriophages as Therapeutic and Diagnostic Vehicles in Cancer. Pharmaceuticals 2021, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.T. Phage Therapy Pharmacology Phage Cocktails. Adv. Appl. Microbiol. 2012, 78, 1–23. [Google Scholar] [CrossRef]
- Tanaka, H.Y.; Nakazawa, T.; Enomoto, A.; Masamune, A.; Kano, M.R. Therapeutic Strategies to Overcome Fibrotic Barriers to Nanomedicine in the Pancreatic Tumor Microenvironment. Cancers 2023, 15, 724. [Google Scholar] [CrossRef]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.; Chaud, M.V.; Vila, M.M.D.C.; Teixeira, J.A.; Balcão, V.M. Alternatives to Overcoming Bacterial Resistances: State-of-the-Art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef]
- Durr, H.A.; Leipzig, N.D. Advancements in Bacteriophage Therapies and Delivery for Bacterial Infection. Mater. Adv. 2023, 4, 1249–1257. [Google Scholar] [CrossRef]
- Chongchai, A.; Bentayebi, K.; Chu, G.; Yan, W.; Waramit, S.; Phitak, T.; Kongtawelert, P.; Pothacharoen, P.; Suwan, K.; Hajitou, A. Targeted Treatment of Chondrosarcoma with a Bacteriophage-Based Particle Delivering a Secreted Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand. Mol. Ther. Oncol. 2024, 32, 200805. [Google Scholar] [CrossRef]
- Adhya, S.; Merril, C.R.; Biswas, B. Therapeutic and Prophylactic Applications of Bacteriophage Components in Modern Medicine. Cold Spring Harb. Perspect. Med. 2014, 4, a012518. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.; Całkosiński, I.; Gamian, A. Phage Display—A Powerful Technique for Immunotherapy: 1. Introduction and Potential of Therapeutic Applications. Hum. Vaccines Immunother. 2012, 8, 1817–1828. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, X.; Kaczanowska, K.; Sungsuwan, S.; Aragones, M.C.; Pett, C.; Yu, J.; Baniel, C.; Westerlind, U.; Finn, M.G.; et al. Antitumor Humoral and T Cell Responses by Mucin-1 Conjugates of Bacteriophage Qβ in Wild-Type Mice. ACS Chem. Biol. 2018, 13, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Peng, X.; Leal, J.; Mohanty, R. Peptides as Drug Delivery Vehicles across Biological Barriers. J. Pharm. Investig. 2018, 48, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Shadidi, M.; Sioud, M. Identification of Novel Carrier Peptides for the Specific Delivery of Therapeutics into Cancer Cells. FASEB J. 2003, 17, 256–258. [Google Scholar] [CrossRef]
- Yi, H.; Ghosh, D.; Ham, M.-H.; Qi, J.; Barone, P.W.; Strano, M.S.; Belcher, A.M. M13 Phage-Functionalized Single-Walled Carbon Nanotubes as Nanoprobes for Second near-Infrared Window Fluorescence Imaging of Targeted Tumors. Nano Lett. 2012, 12, 1176–1183. [Google Scholar] [CrossRef]
- Cai, X.-M.; Xie, H.-L.; Liu, M.-Z.; Zha, X.-L. Inhibition of Cell Growth and Invasion by Epidermal Growth Factor-Targeted Phagemid Particles Carrying siRNA Against Focal Adhesion Kinase in the Presence of Hydroxycamptothecin. BMC Biotechnol. 2008, 8, 74. [Google Scholar] [CrossRef]
- Cohen, B.A.; Bergkvist, M. Targeted in Vitro Photodynamic Therapy via Aptamer-Labeled, Porphyrin-Loaded Virus Capsids. J. Photochem. Photobiol. B 2013, 121, 67–74. [Google Scholar] [CrossRef]
- Gandra, N.; Abbineni, G.; Qu, X.; Huai, Y.; Wang, L.; Mao, C. Bacteriophage Bionanowire as a Carrier for Both Cancer-Targeting Peptides and Photosensitizers and Its Use in Selective Cancer Cell Killing by Photodynamic Therapy. Small 2013, 9, 215–221. [Google Scholar] [CrossRef]
- Dong, X.; Pan, P.; Zheng, D.-W.; Bao, P.; Zeng, X.; Zhang, X.-Z. Bioinorganic Hybrid Bacteriophage for Modulation of Intestinal Microbiota to Remodel Tumor-Immune Microenvironment Against Colorectal Cancer. Sci. Adv. 2020, 6, eaba1590. [Google Scholar] [CrossRef]
- Sun, Q.; Shen, L.; Zhang, B.-L.; Yu, J.; Wei, F.; Sun, Y.; Chen, W.; Wang, S. Advance on Engineering of Bacteriophages by Synthetic Biology. Infect. Drug Resist. 2023, 16, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sunderland, K.; Mao, C. Virus-Derived Peptides for Clinical Applications. Chem. Rev. 2017, 117, 10377–10402. [Google Scholar] [CrossRef] [PubMed]
- Paule, A.; Frezza, D.; Edeas, M. Microbiota and Phage Therapy: Future Challenges in Medicine. Med. Sci. 2018, 6, 86. [Google Scholar] [CrossRef]
- Lam, H.Y.P.; Lai, M.-J.; Wang, P.-C.; Wu, W.-J.; Chen, L.-K.; Fan, H.-W.; Tseng, C.-C.; Peng, S.-Y.; Chang, K.-C. A Novel Bacteriophage with the Potential to Inhibit Fusobacterium Nucleatum-Induced Proliferation of Colorectal Cancer Cells. Antibiotics 2025, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Hodyra-Stefaniak, K.; Miernikiewicz, P.; Drapała, J.; Drab, M.; Jończyk-Matysiak, E.; Lecion, D.; Kaźmierczak, Z.; Beta, W.; Majewska, J.; Harhala, M.; et al. Mammalian Host-Versus-Phage Immune Response Determines Phage Fate in Vivo. Sci. Rep. 2015, 5, 14802. [Google Scholar] [CrossRef]
- Łobocka, M.; Dąbrowska, K.; Górski, A. Engineered Bacteriophage Therapeutics: Rationale, Challenges and Future. BioDrugs 2021, 35, 255–280. [Google Scholar] [CrossRef]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianci, R.; Caldarelli, M.; Brani, P.; Bosi, A.; Ponti, A.; Giaroni, C.; Baj, A. Cytokines Meet Phages: A Revolutionary Pathway to Modulating Immunity and Microbial Balance. Biomedicines 2025, 13, 1202. https://doi.org/10.3390/biomedicines13051202
Cianci R, Caldarelli M, Brani P, Bosi A, Ponti A, Giaroni C, Baj A. Cytokines Meet Phages: A Revolutionary Pathway to Modulating Immunity and Microbial Balance. Biomedicines. 2025; 13(5):1202. https://doi.org/10.3390/biomedicines13051202
Chicago/Turabian StyleCianci, Rossella, Mario Caldarelli, Paola Brani, Annalisa Bosi, Alessandra Ponti, Cristina Giaroni, and Andreina Baj. 2025. "Cytokines Meet Phages: A Revolutionary Pathway to Modulating Immunity and Microbial Balance" Biomedicines 13, no. 5: 1202. https://doi.org/10.3390/biomedicines13051202
APA StyleCianci, R., Caldarelli, M., Brani, P., Bosi, A., Ponti, A., Giaroni, C., & Baj, A. (2025). Cytokines Meet Phages: A Revolutionary Pathway to Modulating Immunity and Microbial Balance. Biomedicines, 13(5), 1202. https://doi.org/10.3390/biomedicines13051202








