Evaluating the Causal Role of Genetically Inferred Immune Cells and Inflammatory Cytokines on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.2.1. Datasets for Circulating Inflammatory Cytokines and Immune Cell
2.2.2. Datasets for ME/CFS
2.3. IV Selection
2.4. MR Analysis
2.5. Mediation Analysis
3. Results
3.1. Effects of Immune Cell on ME/CFS
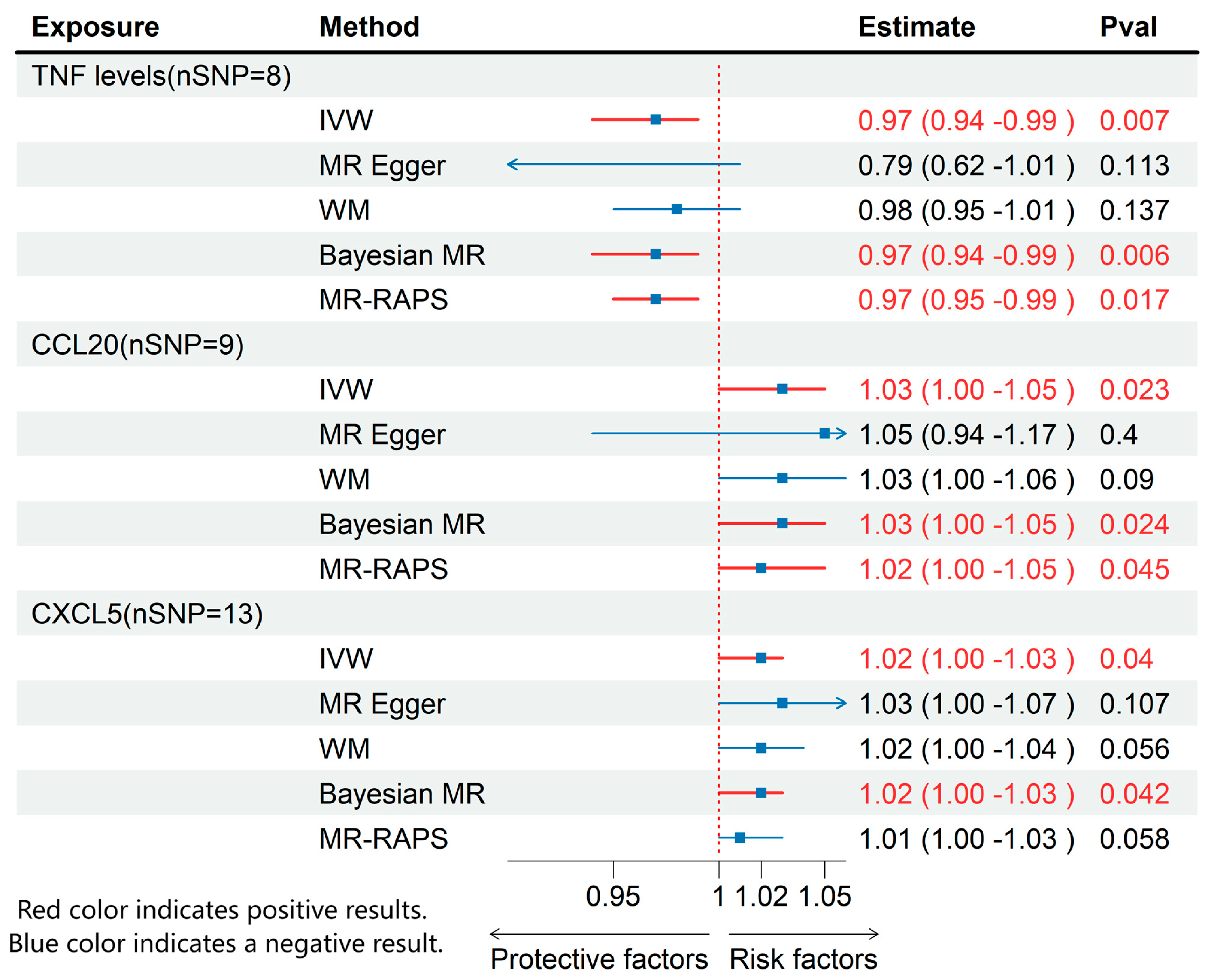
3.2. Effects of Inflammatory Cytokines on ME/CFS
3.3. MR Results of Effects of Immune Cell on Inflammatory Cytokines
3.4. Mediating Role of Circulating Inflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MR | Mendelian randomization |
| GWAS | Genome-wide association study |
| IV | Instrumental variable |
| SNP | Single nucleotide polymorphisms |
| IVW | Inverse variance weighted |
| WM | Weighted median |
| BWMR | Bayesian weighted MR |
| (ME/CFS) | Myalgic encephalomyelitis/chronic fatigue syndrome |
| OR | Odds ratios |
| CI | Confidence interval |
References
- Grach, S.L.; Seltzer, J.; Chon, T.Y.; Ganesh, R. Diagnosis and Management of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Mayo Clin. Proc. 2023, 98, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Kalka, I.N.; Klompus, S.; Leviatan, S.; Weinberger, A.; Segal, E. Systemic Antibody Responses against Human Microbiota Flagellins Are Overrepresented in Chronic Fatigue Syndrome Patients. Sci. Adv. 2022, 8, eabq2422. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.W. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An IOM Report on Redefining an Illness. JAMA 2015, 313, 1101. [Google Scholar] [CrossRef]
- Kingdon, C.C.; Bowman, E.W.; Curran, H.; Nacul, L.; Lacerda, E.M. Functional Status and Well-Being in People with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Compared with People with Multiple Sclerosis and Healthy Controls. PharmacoEcon. Open 2018, 2, 381–392. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. Mayo Clin. Proc. 2021, 96, 2861–2878. [Google Scholar] [CrossRef]
- Vu, L.T.; Ahmed, F.; Zhu, H.; Iu, D.S.H.; Fogarty, E.A.; Kwak, Y.; Chen, W.; Franconi, C.J.; Munn, P.R.; Tate, A.E.; et al. Single-Cell Transcriptomics of the Immune System in ME/CFS at Baseline and Following Symptom Provocation. Cell Rep. Med. 2024, 5, 101373. [Google Scholar] [CrossRef]
- Maya, J. Surveying the Metabolic and Dysfunctional Profiles of T Cells and NK Cells in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Int. J. Mol. Sci. 2023, 24, 11937. [Google Scholar] [CrossRef]
- Jahanbani, F.; Maynard, R.D.; Sing, J.C.; Jahanbani, S.; Perrino, J.J.; Spacek, D.V.; Davis, R.W.; Snyder, M.P. Phenotypic Characteristics of Peripheral Immune Cells of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome via Transmission Electron Microscopy: A Pilot Study. PLoS ONE 2022, 17, e0272703. [Google Scholar] [CrossRef]
- Bested, A.C.; Marshall, L.M. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Evidence-Based Approach to Diagnosis and Management by Clinicians. Rev. Environ. Health 2015, 30, 223–249. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian Randomization: Genetic Anchors for Causal Inference in Epidemiological Studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; Davey Smith, G. Mendelian Randomization: Can Genetic Epidemiology Help Redress the Failures of Observational Epidemiology? Hum. Genet. 2007, 123, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Orrù, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex Genetic Signatures in Immune Cells Underlie Autoimmunity and Inform Therapy. Nat. Genet. 2020, 52, 1036–1045. [Google Scholar] [CrossRef]
- Zhao, J.H.; Stacey, D.; Eriksson, N.; Macdonald-Dunlop, E.; Hedman, Å.K.; Kalnapenkis, A.; Enroth, S.; Cozzetto, D.; Digby-Bell, J.; Marten, J.; et al. Genetics of Circulating Inflammatory Proteins Identifies Drivers of Immune-Mediated Disease Risk and Therapeutic Targets. Nat. Immunol. 2023, 24, 1540–1551. [Google Scholar] [CrossRef]
- He, G.; Cao, Y.; Ma, H.; Guo, S.; Xu, W.; Wang, D.; Chen, Y.; Wang, H. Causal Effects between Gut Microbiome and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Two-Sample Mendelian Randomization Study. Front. Microbiol. 2023, 14, 1190894. [Google Scholar] [CrossRef]
- Song, W.; Hou, X.; Wu, M.; Zhu, L. Relationship between Major Depressive Disorder and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Two-Sample Mendelian Randomization Study Analysis. Sci. Rep. 2025, 15, 1155. [Google Scholar] [CrossRef]
- Un, H.; Wusimanjiang, W.; Zhan, W.; Zhang, X.; Li, M.; Lei, J.; Lin, R.; Zhang, Y.; Chen, J.; Wang, Z. Understanding Bladder Cancer Risk: Mendelian Randomization Analysis of Immune Cell and Inflammatory Factor Influence. Front. Immunol. 2024, 15, 1460275. [Google Scholar] [CrossRef]
- Huang, H.; Chen, B.; Feng, C.; Chen, W.; Wu, D. Exploring the Mediating Role of Immune Cells in the Pathogenesis of IgA Nephropathy through the Inflammatory Axis of Gut Microbiota from a Genomic Perspective. Mamm. Genome 2024, 36, 306–316. [Google Scholar] [CrossRef]
- Palmer, T.M.; Lawlor, D.A.; Harbord, R.M.; Sheehan, N.A.; Tobias, J.H.; Timpson, N.J.; Davey Smith, G.; Sterne, J.A.C. Using Multiple Genetic Variants as Instrumental Variables for Modifiable Risk Factors. Stat. Methods Med. Res. 2012, 21, 223–242. [Google Scholar] [CrossRef]
- Burgess, S.; Scott, R.A.; Timpson, N.J.; Davey Smith, G.; Thompson, S.G.; EPIC-InterAct Consortium. Using Published Data in Mendelian Randomization: A Blueprint for Efficient Identification of Causal Risk Factors. Eur. J. Epidemiol. 2015, 30, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian Randomization with Invalid Instruments: Effect Estimation and Bias Detection through Egger Regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred from Mendelian Randomization between Complex Traits and Diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Hemani, G.; Bowden, J.; Small, D.S. Statistical Inference in Two-Sample Summary-Data Mendelian Randomization Using Robust Adjusted Profile Score. Ann. Stat. 2020, 48, 1742–1769. [Google Scholar] [CrossRef]
- Zhao, J.; Ming, J.; Hu, X.; Chen, G.; Liu, J.; Yang, C. Bayesian Weighted Mendelian Randomization for Causal Inference Based on Summary Statistics. Bioinformatics 2020, 36, 1501–1508. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the Causal Relationship between Imprecisely Measured Traits Using GWAS Summary Data. PLoS Genet. 2017, 13, e1007081. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Galecki, P.; Maes, M. The Emerging Role of Autoimmunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/Cfs). Mol. Neurobiol. 2013, 49, 741–756. [Google Scholar] [CrossRef]
- Brenu, E.W.; Huth, T.K.; Hardcastle, S.L.; Fuller, K.; Kaur, M.; Johnston, S.; Ramos, S.B.; Staines, D.R.; Marshall-Gradisnik, S.M. Role of Adaptive and Innate Immune Cells in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. Int. Immunol. 2013, 26, 233–242. [Google Scholar] [CrossRef]
- Brenu, E.W.; van Driel, M.L.; Staines, D.R.; Ashton, K.J.; Ramos, S.B.; Keane, J.; Klimas, N.G.; Marshall-Gradisnik, S.M. Immunological Abnormalities as Potential Biomarkers in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. J. Transl. Med. 2011, 9, 81. [Google Scholar] [CrossRef]
- Proal, A.; Marshall, T. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in the Era of the Human Microbiome: Persistent Pathogens Drive Chronic Symptoms by Interfering With Host Metabolism, Gene Expression, and Immunity. Front. Pediatr. 2018, 6, 373. [Google Scholar] [CrossRef]
- Tan, L.; Fichtner, A.S.; Bruni, E.; Odak, I.; Sandrock, I.; Bubke, A.; Borchers, A.; Schultze-Florey, C.; Koenecke, C.; Förster, R.; et al. A Fetal Wave of Human Type 3 Effector Γδ Cells with Restricted TCR Diversity Persists into Adulthood. Sci. Immunol. 2021, 6, eabf0125. [Google Scholar] [CrossRef]
- Herrmann, T.; Karunakaran, M.M. Butyrophilins: Γδ T Cell Receptor Ligands, Immunomodulators and More. Front. Immunol. 2022, 13, 876493. [Google Scholar] [CrossRef]
- Xiang, Z.; Liu, Y.; Zheng, J.; Liu, M.; Lv, A.; Gao, Y.; Hu, H.; Lam, K.T.; Chan, G.C.; Yang, Y.; et al. Targeted Activation of Human Vγ9Vδ2-T Cells Controls Epstein-Barr Virus-Induced B Cell Lymphoproliferative Disease. Cancer Cell 2014, 26, 565–576. [Google Scholar] [CrossRef]
- Loebel, M.; Strohschein, K.; Giannini, C.; Koelsch, U.; Bauer, S.; Doebis, C.; Thomas, S.; Unterwalder, N.; von Baehr, V.; Reinke, P.; et al. Deficient EBV-Specific B- and T-Cell Response in Patients with Chronic Fatigue Syndrome. PLoS ONE 2014, 9, e85387. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Lee, J.-S.; Oh, H.-M.; Lee, E.-J.; Lim, E.-J.; Son, C.-G. Evaluation of Viral Infection as an Etiology of ME/CFS: A Systematic Review and Meta-Analysis. J. Transl. Med. 2023, 21, 763. [Google Scholar] [CrossRef]
- Lidbury, B.A. Ross River Virus Immune Evasion Strategies and the Relevance to Post-Viral Fatigue, and Myalgic Encephalomyelitis Onset. Front. Med. 2021, 8, 662513. [Google Scholar] [CrossRef]
- He, J.; Yu, Q.; Wu, C.; Sun, Z.; Wu, X.; Liu, R.; Zhang, H. Acupuncture of the Beishu Acupoint Participates in Regulatory Effects of Ginsenoside Rg1 on T Cell Subsets of Rats with Chronic Fatigue Syndrome. Ann. Palliat. Med. 2020, 9, 3436–3446. [Google Scholar] [CrossRef]
- Porter, N.; Lerch, A.; Jason, L.A.; Sorenson, M.; Fletcher, M.A.; Herrington, J. A Comparison of Immune Functionality in Viral versus Non-Viral CFS Subtypes. J. Behav. Neurosci. Res. 2010, 8, 1–8. [Google Scholar]
- Corthay, A. How Do Regulatory T Cells Work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef]
- Serra, P.; Amrani, A.; Yamanouchi, J.; Han, B.; Thiessen, S.; Utsugi, T.; Verdaguer, J.; Santamaria, P. CD40 Ligation Releases Immature Dendritic Cells from the Control of Regulatory CD4+CD25+ T Cells. Immunity 2003, 19, 877–889. [Google Scholar] [CrossRef]
- Sato, W.; Ono, H.; Matsutani, T.; Nakamura, M.; Shin, I.; Amano, K.; Suzuki, R.; Yamamura, T. Skewing of the B Cell Receptor Repertoire in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Brain Behav. Immun. 2021, 95, 245–255. [Google Scholar] [CrossRef]
- Mihaylova, I.; DeRuyter, M.; Rummens, J.L.; Bosmans, E.; Maes, M. Decreased Expression of CD69 in Chronic Fatigue Syndrome in Relation to Inflammatory Markers: Evidence for a Severe Disorder in the Early Activation of T Lymphocytes and Natural Killer Cells. Neuroendocrinol. Lett. 2007, 28, 477–483. [Google Scholar]
- Huth, T.K.; Brenu, E.W.; Ramos, S.; Nguyen, T.; Broadley, S.; Staines, D.; Marshall-Gradisnik, S. Pilot Study of Natural Killer Cells in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis and Multiple Sclerosis. Scand. J. Immunol. 2015, 83, 44–51. [Google Scholar] [CrossRef]
- Rasa-Dzelzkaleja, S.; Krumina, A.; Capenko, S.; Nora-Krukle, Z.; Gravelsina, S.; Vilmane, A.; Ievina, L.; Shoenfeld, Y.; Murovska, M.; VirA Project. The Persistent Viral Infections in the Development Severity of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Transl. Med. 2023, 21, 33. [Google Scholar] [CrossRef]
- Arroll, M.A. Allostatic Overload in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Med. Hypotheses 2013, 81, 506–508. [Google Scholar] [CrossRef]
- Clark, L.V.; Buckland, M.; Murphy, G.; Taylor, N.; Vleck, V.; Mein, C.; Wozniak, E.; Smuk, M.; White, P.D. Cytokine Responses to Exercise and Activity in Patients with Chronic Fatigue Syndrome: Case-Control Study. Clin. Exp. Immunol. 2017, 190, 360–371. [Google Scholar] [CrossRef]
- Montoya, J.G.; Holmes, T.H.; Anderson, J.N.; Maecker, H.T.; Rosenberg-Hasson, Y.; Valencia, I.J.; Chu, L.; Younger, J.W.; Tato, C.M.; Davis, M.M. Cytokine Signature Associated with Disease Severity in Chronic Fatigue Syndrome Patients. Proc. Natl. Acad. Sci. USA 2017, 114, E7150–E7158. [Google Scholar] [CrossRef]
- Strawbridge, R.; Sartor, M.L.; Scott, F.; Cleare, A.J. Inflammatory Proteins Are Altered in Chronic Fatigue Syndrome-A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2019, 107, 69–83. [Google Scholar] [CrossRef]
- Jonsjö, M.A.; Olsson, G.L.; Wicksell, R.K.; Alving, K.; Holmström, L.; Andreasson, A. The Role of Low-Grade Inflammation in ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome)—Associations with Symptoms. Psychoneuroendocrinology 2020, 113, 104578. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Increased Nuclear Factor-κB and Loss of P53 Are Key Mechanisms in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Med. Hypotheses 2012, 79, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Maes, M. Mitochondrial Dysfunctions in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Explained by Activated Immuno-Inflammatory, Oxidative and Nitrosative Stress Pathways. Metab. Brain Dis. 2013, 29, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.Y.; Li, M.; Breunis, H.; Timilshina, N.; Minden, M.D.; Alibhai, S.M.H. Correlation between Cytokine Levels and Changes in Fatigue and Quality of Life in Patients with Acute Myeloid Leukemia. Leuk. Res. 2013, 37, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Pedraz-Petrozzi, B.; Neumann, E.; Sammer, G. Pro-Inflammatory Markers and Fatigue in Patients with Depression: A Case-Control Study. Sci. Rep. 2020, 10, 9494. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Du, K.; Chen, J.; Ding, H.; Petersen, F.; Ye, S.; Lin, Z.; Yu, X. Serum Levels of CXCL5 Are Decreased and Correlate with Circulating Platelet Counts in Systemic Lupus Erythematosus. Int. J. Rheum. Dis. 2024, 27, e15089. [Google Scholar] [CrossRef]
- Du, L.Y.; Zhang, M.J.; Liu, P.F.; Xiao, G.; Lyu, X.M. Correlation between serum CCL20 level and disease severity in patients with rheumatoid arthritis. Zhonghua Yu Fang Yi Xue Za Zhi 2021, 55, 226–232. [Google Scholar]
- Wang, L.; Hong, X.; Du, H. Association Between Serum Chemokine Ligand 20 Levels and Disease Activity and Th1/Th2/Th17-Related Cytokine Levels in Rheumatoid Arthritis. J. Interferon Cytokine Res. 2023, 43, 512–517. [Google Scholar] [CrossRef]
- Marino, Y.; Arangia, A.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Cupi, R.; Peritore, A.F.; Gugliandolo, E.; Fusco, R.; et al. Analysis of the Influence of IL-6 and the Activation of the Jak/Stat3 Pathway in Fibromyalgia. Biomedicines 2023, 11, 792. [Google Scholar] [CrossRef]
- Yang, T.; Yang, Y.; Wang, D.; Li, C.; Qu, Y.; Guo, J.; Shi, T.; Bo, W.; Sun, Z.; Asakawa, T. The Clinical Value of Cytokines in Chronic Fatigue Syndrome. J. Transl. Med. 2019, 17, 213. [Google Scholar] [CrossRef]



| Phenotype | Data Source | Sample Size | PubMed ID | GWAS ID | Ancestry |
|---|---|---|---|---|---|
| Immune cell | Orrù V et al. [14] | 3757 | 32929287 | GCST90001391-GCST90002121 | European |
| Inflammatory cytokines | Zhao JH et al. [15] | 14,824 | 37563310 | GCST90274758-GCST90274848 | European |
| ME/CFS | UKB | 462,933 | NA | ukb-b-8961 | European |
| Exposure | Mediation | Outcome | Mediated Effect | Mediated Proportion | p |
|---|---|---|---|---|---|
| CD3 on naive CD8+ T cell | CCL20 | ME/CFS | −0.000659 (−0.00152, 0.000197) | 10.2% (23.3%, −3.04%) | 0.13 |
| CD3 on CD28+ CD45RA- CD8+ T cell | CCL20 | ME/CFS | −0.00111 (−0.00275, 0.00053) | 12.3% (30.6%, −5.88%) | 0.18 |
| CD80 on CD62L+ myeloid Dendritic Cell | CXCL5 | ME/CFS | −0.000442 (−0.00118, 3 × 10−4) | −3.48% (−9.31%, 2.35%) | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, L.; Yang, J.; Zhao, J.; Chen, Z.; Yang, H.; Cai, D. Evaluating the Causal Role of Genetically Inferred Immune Cells and Inflammatory Cytokines on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines 2025, 13, 1200. https://doi.org/10.3390/biomedicines13051200
Duan L, Yang J, Zhao J, Chen Z, Yang H, Cai D. Evaluating the Causal Role of Genetically Inferred Immune Cells and Inflammatory Cytokines on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines. 2025; 13(5):1200. https://doi.org/10.3390/biomedicines13051200
Chicago/Turabian StyleDuan, Lincheng, Jingyi Yang, Junxin Zhao, Zhuoyang Chen, Hong Yang, and Dingjun Cai. 2025. "Evaluating the Causal Role of Genetically Inferred Immune Cells and Inflammatory Cytokines on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome" Biomedicines 13, no. 5: 1200. https://doi.org/10.3390/biomedicines13051200
APA StyleDuan, L., Yang, J., Zhao, J., Chen, Z., Yang, H., & Cai, D. (2025). Evaluating the Causal Role of Genetically Inferred Immune Cells and Inflammatory Cytokines on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines, 13(5), 1200. https://doi.org/10.3390/biomedicines13051200





