Mucinous Ovarian Carcinoma: Integrating Molecular Stratification into Surgical and Therapeutic Management
Abstract
1. Introduction
2. Surgical and Therapeutic Management of Mucinous Ovarian Carcinoma: A Comprehensive Review
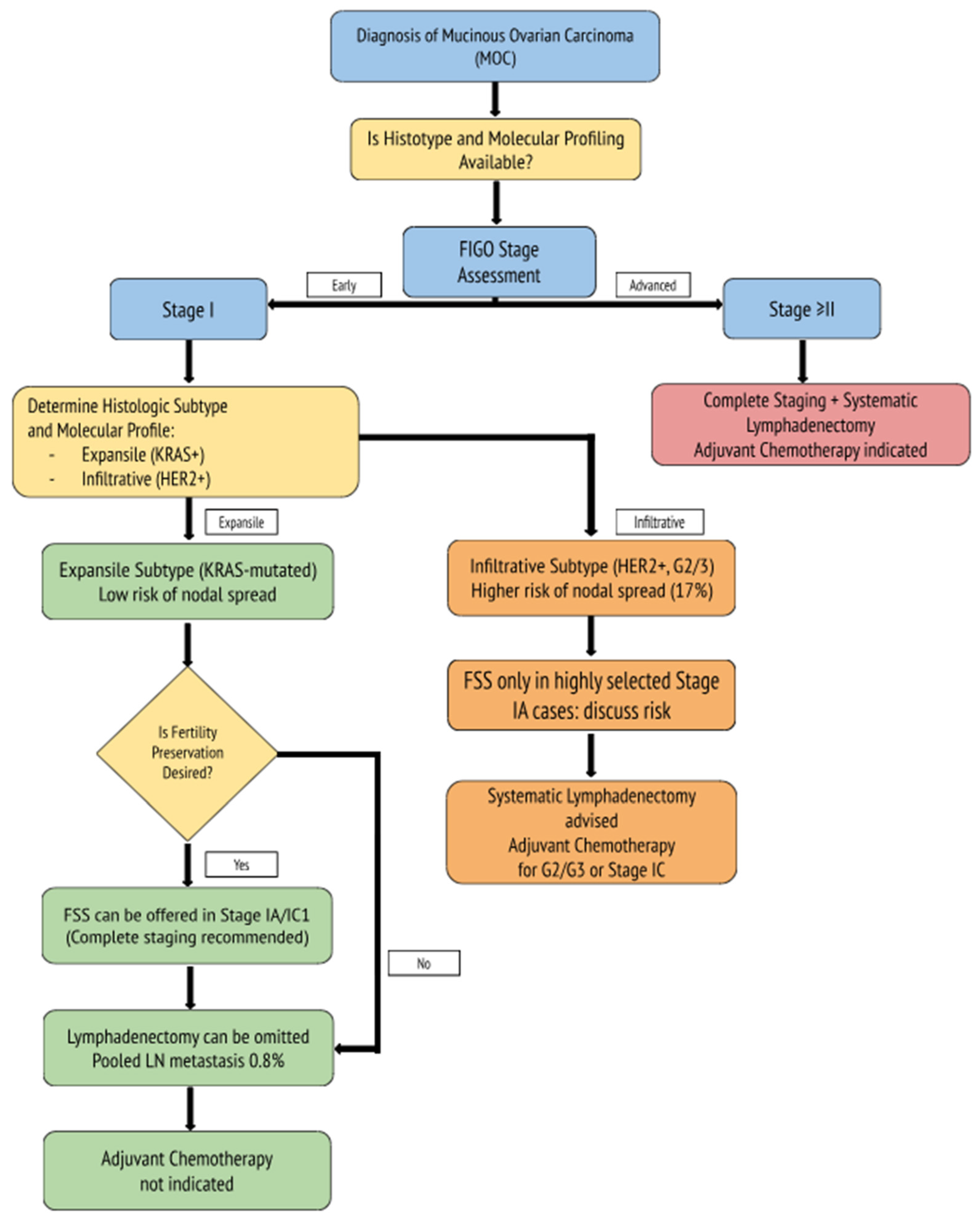
2.1. Surgical Staging and the Role of Lymphadenectomy
2.2. The Importance of Complete Surgical Staging
2.3. Fertility-Sparing Surgery in MOC
2.4. Oncologic Outcomes and Prognostic Factors
2.5. The Role of Adjuvant Therapy in Early-Stage MOC
2.6. Molecular Considerations and Emerging Targeted Therapies in Mucinous Ovarian Carcinoma
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Liu, L.; Yu, Y. Mucins and mucinous ovarian carcinoma: Development, differential diagnosis, and treatment. Heliyon 2023, 9, e19221. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Kajiyama, H.; Mizuno, M.; Shibata, K.; Kawai, M.; Nagasaka, T.; Kikkawa, F. Clinicopathologic features of epithelial ovarian carcinoma in younger vs. older patients: Analysis in Japanese women. J. Gynecol. Oncol. 2014, 25, 118–123. [Google Scholar] [CrossRef]
- Babaier, A.; Ghatage, P. Mucinous Cancer of the Ovary: Overview and Current Status. Diagnostics 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuo, K.; Machida, H.; Mandelbaum, R.S.; Grubbs, B.H.; Roman, L.D.; Sood, A.K.; Gershenson, D.M. Mucinous Borderline Ovarian Tumor Versus Invasive Well-Differentiated Mucinous Ovarian Cancer: Difference in Characteristics and Outcomes. Gynecol. Oncol. 2019, 153, 230–237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hollis, R.L. Molecular characteristics and clinical behaviour of epithelial ovarian cancers. Cancer Lett. 2023, 555, 216057. [Google Scholar] [CrossRef] [PubMed]
- Therachiyil, L.; Anand, A.; Azmi, A.; Bhat, A.; Korashy, H.M.; Uddin, S. Role of RAS signaling in ovarian cancer. F1000Res 2022, 11, 1253. [Google Scholar] [CrossRef]
- Meagher, N.S.; Köbel, M.; Karnezis, A.N.; Talhouk, A.; Anglesio, M.S.; Berchuck, A.; Gayther, S.A.; Pharoah, P.P.; Webb, P.M.; Ramus, S.J.; et al. Cellular origins of mucinous ovarian carcinoma. J. Pathol. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Cheasley, D.; Wakefield, M.J.; Ryland, G.L.; Allan, P.E.; Alsop, K.; Amarasinghe, K.C.; Gorringe, K.L. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat. Commun. 2019, 10, 3935. [Google Scholar] [CrossRef]
- Winder, T.; Lenz, H.J. Mucinous adenocarcinomas with intra-abdominal dissemination: A review of current therapy. Oncologist 2010, 15, 836–844. [Google Scholar] [CrossRef]
- Borella, F.; Mitidieri, M.; Cosma, S.; Benedetto, C.; Bertero, L.; Fucina, S.; Ray-Coquard, I.; Carapezzi, A.; Ferraioli, D. Update on Prognostic and Predictive Markers in Mucinous Ovarian Cancer. Cancers 2023, 15, 1172. [Google Scholar] [CrossRef]
- Brown, J.; Frumovitz, M. Mucinous tumors of the ovary: Current thoughts on diagnosis and management. Curr. Oncol. Rep. 2014, 16, 389. [Google Scholar] [CrossRef] [PubMed]
- Hada, T.; Miyamoto, M.; Ishibashi, H.; Matsuura, H.; Sakamoto, T.; Kakimoto, S.; Iwahashi, H.; Tsuda, H.; Takano, M. Survival and Biomarker Analysis for Ovarian Mucinous Carcinoma According to Invasive Patterns: Retrospective Analysis and Review Literature. J. Ovarian Res. 2021, 14, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dundr, P.; Bártů, M.; Bosse, T.; Bui, Q.H.; Cibula, D.; Drozenová, J.; Fabian, P.; Fadare, O.; Hausnerová, J.; Hojný, J.; et al. Primary Mucinous Tumors of the Ovary: An Interobserver Reproducibility and Detailed Molecular Study Reveals Significant Overlap Between Diagnostic Categories. Mod Pathol. 2023, 36, 100040. [Google Scholar] [CrossRef] [PubMed]
- Gorringe, K.L.; Cheasley, D.; Wakefield, M.J.; Ryland, G.L.; Allan, P.E.; Alsop, K.; Scott, C.L. Therapeutic options for mucinous ovarian carcinoma. Gynecol. Oncol. 2020, 156, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, W.J. Surgical staging and cytoreductive surgery of epithelial ovarian cancer. Cancer 1993, 71, 1534–1540. [Google Scholar] [CrossRef]
- van Baal, J.; Van de Vijver, K.K.; Coffelt, S.B.; Van der Noort, V.; Van Driel, W.J.; Kenter, G.G.; Lok, C.A.R. Incidence of lymph node metastases in clinical early-stage mucinous and seromucinous ovarian carcinoma: A retrospective cohort study. BJOG 2017, 124, 486–494. [Google Scholar] [CrossRef]
- Gouy, S.; Saidani, M.; Maulard, A.; Faron, M.; Bach-Hamba, S.; Bentivegna, E.; Morice, P. Staging surgery in early-stage ovarian mucinous tumors according to expansile and infiltrative types. Gynecol. Oncol. Rep. 2017, 22, 21–25. [Google Scholar] [CrossRef]
- Chen, M.; Han, L.; Wang, Y.; Qiu, Q.; Chen, Y.; Zheng, A. The Prognostic Value of Growth Pattern-Based Grading for Mucinous Ovarian Carcinoma (MOC): A Systematic Review and Meta-Analysis. Front. Oncol. 2025, 15, 1541572. [Google Scholar] [CrossRef]
- Canlorbe, G.; Chabbert-Buffet, N.; Uzan, C. Fertility-Sparing Surgery for Ovarian Cancer. J. Clin. Med. 2021, 10, 4235. [Google Scholar] [CrossRef]
- Craig, O.; Salazar, C.; Gorringe, K.L. Options for the Treatment of Mucinous Ovarian Carcinoma. Curr. Treat. Options Oncol. 2021, 22, 114. [Google Scholar] [CrossRef]
- Ben Ltaief, S.; Ghalleb, M.; Chabchoub, A.; Slimen, M.; Hadj Kacem, L.; Zenzeri, Y.; Chargui, R.; Dhieb, T. Fertility-Sparing Surgery in Infiltrative Mucinous Carcinoma of the Ovary. Case Rep. Oncol. 2023, 16, 1494–1499. [Google Scholar] [CrossRef]
- Lin, W.; Cao, D.; Shi, X.; You, Y.; Yang, J.; Shen, K. Oncological and Reproductive Outcomes After Fertility-Sparing Surgery for Stage I Mucinous Ovarian Carcinoma. Front. Oncol. 2022, 12, 856818. [Google Scholar] [CrossRef] [PubMed]
- Hoogendam, J.P.; Vlek, C.A.; Witteveen, P.O.; Verheijen, R.; Zweemer, R.P. Surgical lymph node assessment in mucinous ovarian carcinoma staging: A systematic review and meta-analysis. BJOG 2017, 124, 370–378. [Google Scholar] [CrossRef]
- Huin, M.; Lorenzini, J.; Arbion, F.; Carcopino, X.; Touboul, C.; Dabi, Y.; Ouldamer, L. Presentation and Prognosis of Primary Expansile and Infiltrative Mucinous Carcinomas of the Ovary. J. Clin. Med. 2022, 11, 6120. [Google Scholar] [CrossRef] [PubMed]
- Schmeler, K.M.; Tao, X.; Frumovitz, M.; Deavers, M.T.; Sun, C.C.; Sood, A.K.; Brown, J.; Gershenson, D.M.; Ramirez, P.T. Prevalence of lymph node metastasis in primary mucinous carcinoma of the ovary. Obstet. Gynecol. 2010, 116, 269–273. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; Fagotti, A. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Y.; Cao, D.; Shen, K. Surgical staging of apparent early-stage ovarian mucinous carcinoma. World J. Surg. Oncol. 2022, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Madariaga, A.; Hogen, L.; Vicus, D.; Covens, A.; Parra-Herran, C.; Gien, L.T. Impact of lymphadenectomy and intra-operative tumor rupture on survival in early-stage mucinous ovarian cancers. Int. J. Gynecol. Cancer 2023, 33, 755–760. [Google Scholar] [CrossRef]
- Matsuo, K.; Machida, H.; Yamagami, W.; Ebina, Y.; Kobayashi, Y.; Tabata, T.; Mikami, M. Intraoperative Capsule Rupture, Postoperative Chemotherapy, and Survival of Women With Stage I Epithelial Ovarian Cancer. Obstet. Gynecol. 2019, 134, 1017–1026. [Google Scholar] [CrossRef]
- Kim, S.R.; Madariaga, A.; Hogen, L.; Vicus, D.; Covens, A.; Parra-Herran, C.; Gien, L.T. Safety of fertility sparing management in invasive mucinous ovarian carcinoma. Gynecol. Oncol. 2023, 174, 129–132. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Frumovitz, M. Primary mucinous ovarian cancer: Options for surgery and chemotherapy. Int. J. Gynecol. Cancer 2022, 32, 1455–1462. [Google Scholar] [CrossRef]
- Richardson, M.T.; Mysona, D.P.; Klein, D.A.; Mann, A.; Liao, C.I.; Diver, E.J.; Chan, J.K. Long term survival outcomes of stage I mucinous ovarian cancer—A clinical calculator predictive of chemotherapy benefit. Gynecol. Oncol. 2020, 159, 118–128. [Google Scholar] [CrossRef]
- Nasioudis, D.; Haggerty, A.F.; Giuntoli, R.L., 2nd; Burger, R.A.; Morgan, M.A.; Ko, E.M.; Latif, N.A. Adjuvant chemotherapy is not associated with a survival benefit for patients with early stage mucinous ovarian carcinoma. Gynecol. Oncol. 2019, 154, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Nugawela, D.; Gorringe, K.L. Targeted therapy for mucinous ovarian carcinoma: Evidence from clinical trials. Int. J. Gynecol. Cancer 2023, 33, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Affatato, R.; Carrassa, L.; Damia, G. Recent Insights into Mucinous Ovarian Carcinoma. Int. J. Mol. Sci. 2018, 19, 1569. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.L.; Lee, M.Y.; Chao, W.R.; Han, C.P. The Status of Her2 Amplification and Kras Mutations in Mucinous Ovarian Carcinoma. Hum. Genom. 2016, 10, 40. [Google Scholar] [CrossRef]
- Mackenzie, R.; Kommoss, S.; Winterhoff, B.J.; Kipp, B.R.; Garcia, J.J.; Voss, J.; Anglesio, M.S. Targeted Deep Sequencing of Mucinous Ovarian Tumors Reveals Multiple Overlapping Ras-Pathway Activating Mutations in Borderline and Cancerous Neoplasms. BMC Cancer 2015, 15, 415. [Google Scholar] [CrossRef]
- Tafe, L.J.; Muller, K.E.; Ananda, G.; Mitchell, T.; Spotlow, V.; Patterson, S.E.; Mockus, S.M. Molecular Genetic Analysis of Ovarian Brenner Tumors and Associated Mucinous Epithelial Neoplasms: High Variant Concordance and Identification of Mutually Exclusive RAS Driver Mutations and MYC Amplification. Am. J. Pathol. 2016, 186, 671–677. [Google Scholar] [CrossRef]
- Hunter, S.M.; Gorringe, K.L.; Christie, M.; Rowley, S.M.; Bowtell, D.D.; Australian Ovarian Cancer Study Group; Campbell, I.G. Pre-Invasive Ovarian Mucinous Tumors Are Characterized by CDKN2A and RAS Pathway Aberrations. Clin. Cancer Res. 2012, 18, 5267–5277. [Google Scholar] [CrossRef]
- Frumovitz, M.; Schmeler, K.M.; Malpica, A.; Sood, A.K.; Gershenson, D.M. Unmasking the Complexities of Mucinous Ovarian Carcinoma. Gynecol. Oncol. 2010, 117, 491–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drosten, M.; Barbacid, M. Targeting the MAPK Pathway in KRAS-Driven Tumors. Cancer Cell 2020, 37, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Oda, K.; Aoki, K.; Sone, K.; Ikeda, Y.; Miyasaka, A.; Kashiyama, T.; Fukuda, T.; Makii, C.; Arimoto, T.; et al. Synergistic antitumor effects of combination PI3K/mTOR and MEK inhibition (SAR245409 and pimasertib) in mucinous ovarian carcinoma cells by fluorescence resonance energy transfer imaging. Oncotarget 2016, 7, 29577–29591. Available online: https://www.oncotarget.com/article/8807/text/ (accessed on 25 March 2025). [CrossRef] [PubMed]
- Gershenson, D.M.; Miller, A.; Brady, W.E.; Paul, J.; Carty, K.; Rodgers, W.; Millan, D.; Coleman, R.L.; Moore, K.N.; Banerjee, S.; et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): An international, randomised, open-label, multicentre, phase 2/3 trial. Lancet 2022, 399, 541–553. [Google Scholar] [CrossRef]
- Han, R.; Madariaga, A.; Gonzalez-Ochoa, E.; Smith, A.C.; Wang, L.; Lheureux, S.; Rouzbahman, M. HER2-low and Overexpression in Mucinous Ovarian Cancer: Analysis of ASCO/CAP and ToGA Immunohistochemical Scoring. Int. J. Gynecol. Pathol. 2024, 43, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, N.H. HER2-positive mucinous adenocarcinomas of the ovary have an expansile invasive pattern associated with a favorable prognosis. Int. J. Clin. Exp. Pathol. 2014, 7, 4222–4230. [Google Scholar]
- Stratmann, J.A.; Althoff, F.C.; Doebel, P.; Rauh, J.; Trummer, A.; Hünerlitürkoglu, A.N.; Reinmuth, N. Sotorasib in KRAS G12C-mutated non-small cell lung cancer: A multicenter real-world experience from the compassionate use program in Germany. Eur. J. Cancer 2024, 201, 113911. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar]
- Ohnishi, K.; Nakayama, K.; Ishikawa, M.; Ishibashi, T.; Yamashita, H.; Nakamura, K.; Minamoto, T.; Iida, K.; Razia, S.; Ishikawa, N.; et al. Mucinous borderline ovarian tumors with BRAFV600E mutation may have low risk for progression to invasive carcinomas. Arch. Gynecol. Obstet. 2020, 302, 487–495. [Google Scholar] [CrossRef]
| Feature | Expansile MOC | Infiltrative MOC |
|---|---|---|
| Frequency | 43% | 57% |
| Lymph Node Metastasis Rate | 0–1% | ~17% |
| Lymphadenectomy | Often omitted | Recommended |
| Prognosis | Generally favorable | Less favorable |
| FSS Suitability | High (IA) | Controversial/Selected IA |
| Chemotherapy Sensitivity | Low | Low |
| KRAS Mutation | ~70–85% | ~40–60% |
| HER2 Amplification | ~20–30% | >30% |
| TP53 Mutation | Rare | May occur |
| MUC1/MUC16 Overexpression | Common | Common |
| Study (Year) | Sample Size | Outcome(s) and Molecular Insights | Key Results |
|---|---|---|---|
| Chen et al. (2025) [18] | 1185 | Prognostic value of growth pattern-based grading in MOC. | Expansile MOC: Death rate 10.5%, Recurrence rate 6.9%, and FIGO I rate 89.8%. Infiltrative MOC: Death rate 31.1%, Recurrence rate 24.5%, and FIGO I rate 56.2%. Infiltrative pattern linked to poorer prognosis. Complete surgical staging recommended for infiltrative MOC. |
| Richardson et al. (2020) [32] | 2041 patients | Impact of adjuvant chemotherapy on survival in stage I MOC. | 10-year OS rate: 79% (no CHT) vs. 81% (CHT). CHT improved survival only in high-risk patients (HR = 1.58, p = 0.03). |
| Gouy et al. (2017) [17] | 68 patients (29 expansile and 39 infiltrative) | Lymphadenectomy necessity, peritoneal spread, and upstaging rates in early-stage MOC. | Lymphadenectomy in 31 patients (8 expansile, 23 infiltrative). Nodal metastases in four infiltrative cases (17%). Microscopic peritoneal spread in two cases. One patient upstaged from IA to IC3 due to positive cytology. |
| Huin et al. (2022) [24] | 94 patients (35 expansile and 59 infiltrative) | Clinical presentation and prognosis by histologic subtype. | Lymph node metastases: 21% in infiltrative vs. 0% in expansile. 5-year recurrence-free survival (RFS): 90% (expansile) vs. 60% (infiltrative). Adjuvant chemotherapy used in 46% of infiltrative vs. 20% of expansile cases. |
| Schmeler et al. (2010) [25] | 107 patients with primary MOC (51 with lymphadenectomy) | Prevalence of lymph node metastases and staging outcomes in early-stage MOC. | 51 patients with tumors confined to the ovary: 0% nodal metastases. No significant difference in 5-year OS (83% vs. 69%) or PFS (80% vs. 63%) between patients with and without lymphadenectomy. Routine lymphadenectomy may be omitted in clinically early-stage MOC. |
| Yuan et al. (2022) [27] | 163 patients | Upstaging rates after complete surgical staging. | 9.2% upstaged to FIGO stage II-IVB; risk factors: bilateral ovarian involvement (OR = 9.739, p = 0.005) and history of MOC (OR = 4.745, p = 0.033). |
| Lin et al. (2022) [22] | 159 patients | Oncologic and reproductive outcomes after FSS. | 5-year DFS rate: 82.5% (FSS) vs. 94.5% (RS) (p = 0.207). Pregnancy success rate 91.3%. Live birth rate 88.9%. |
| Frumovitz et al. (2010) [40] | Multiple cohorts | Prognosis and treatment response KRAS ~50%, TP53 ~16%, and BRCA <2%. | Poor CHT response; OS in advanced MOC: ~12–15 months vs. ~36–45 in serous carcinoma. |
| Cheasley et al. (2019) [8] | 255 MOC cases (134 sequenced) | KRAS/TP53 64%, HER2 amp 26%, and CDKN2A loss 76%. | Copy number burden linked to high-grade progression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiorano, M.F.P.; Maiorano, B.A.; Cormio, G.; Loizzi, V. Mucinous Ovarian Carcinoma: Integrating Molecular Stratification into Surgical and Therapeutic Management. Biomedicines 2025, 13, 1198. https://doi.org/10.3390/biomedicines13051198
Maiorano MFP, Maiorano BA, Cormio G, Loizzi V. Mucinous Ovarian Carcinoma: Integrating Molecular Stratification into Surgical and Therapeutic Management. Biomedicines. 2025; 13(5):1198. https://doi.org/10.3390/biomedicines13051198
Chicago/Turabian StyleMaiorano, Mauro Francesco Pio, Brigida Anna Maiorano, Gennaro Cormio, and Vera Loizzi. 2025. "Mucinous Ovarian Carcinoma: Integrating Molecular Stratification into Surgical and Therapeutic Management" Biomedicines 13, no. 5: 1198. https://doi.org/10.3390/biomedicines13051198
APA StyleMaiorano, M. F. P., Maiorano, B. A., Cormio, G., & Loizzi, V. (2025). Mucinous Ovarian Carcinoma: Integrating Molecular Stratification into Surgical and Therapeutic Management. Biomedicines, 13(5), 1198. https://doi.org/10.3390/biomedicines13051198








