Loss of miRNA-Mediated VEGFA Regulation by SNP-Induced Impairment: A Bioinformatic Analysis in Diabetic Complications
Abstract
1. Introduction
2. Materials and Methods
2.1. miRNA VEGFA Target
2.2. miRNA Expression Profile in Diabetes-Related Tissues
2.3. miRNA SNPs Associated with VEGF Regulation Alteration
2.4. miRNA–mRNA Interaction
2.5. Evaluation of Physiological Implications
3. Results
3.1. microRNA Predicted and Validated for VEGFA Regulation
3.2. miRNA Expression Profiles in Diabetes-Related Tissues
3.3. miRNA SNPs Associated with the VEGFA Loss-of-Function Effect
3.4. miRNA–mRNA Interaction
3.5. Physiological Implications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilimova, M.; Pfeffer, S. Post-transcriptional Regulation of Polycistronic microRNAs. Wiley Interdiscip. Rev. RNA 2023, 14, e1749. [Google Scholar] [CrossRef] [PubMed]
- Titov, I.I.; Vorozheykin, P.S. Comparing MiRNA Structure of Mirtrons and Non-Mirtrons. BMC Genom. 2018, 19, 114. [Google Scholar] [CrossRef]
- Wang, Y.; Ru, J.; Meng, X.; Song, J.; Jiang, Q.; Li, S.; Jiang, J.; Li, Y. Role of SNPs in the Biogenesis of Mature MiRNAs. Biomed Res. Int. 2021, 2021, 2403418. [Google Scholar] [CrossRef] [PubMed]
- Bahreini, F.; Rayzan, E.; Rezaei, N. MicroRNA-related Single-nucleotide Polymorphisms and Breast Cancer. J. Cell. Physiol. 2021, 236, 1593–1605. [Google Scholar] [CrossRef]
- Machowska, M.; Galka-Marciniak, P.; Kozlowski, P. Consequences of Genetic Variants in MiRNA Genes. Comput. Struct. Biotechnol. J. 2022, 20, 6443–6457. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, T.; Morales-Pison, S.; Maldonado, E.; Jara, L. Association between Single-Nucleotide Polymorphisms in MiRNA and Breast Cancer Risk: An Updated Review. Biol. Res. 2021, 54, 26. [Google Scholar] [CrossRef]
- Huang, W. MicroRNAs: Biomarkers, Diagnostics, and Therapeutics. Methods Mol. Biol. 2017, 1617, 57–67. [Google Scholar]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic Vascular Diseases: Molecular Mechanisms and Therapeutic Strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Babel, R.A.; Dandekar, M.P. A Review on Cellular and Molecular Mechanisms Linked to the Development of Diabetes Complications. Curr. Diabetes Rev. 2021, 17, 457–473. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef]
- Lungu, C.; Mehedinti, M. Molecular Motifs in Vascular Morphogenesis: Vascular Endothelial Growth Factor A (VEGFA) as the Leading Promoter of Angiogenesis. Int. J. Mol. Sci. 2023, 24, 12169. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhu, D.; Fang, Y.; Chen, Y.; Yu, G.; Pan, W.; Liu, D.; Li, F.; Zhong, T.P. Vegfa Signaling Regulates Diverse Artery/Vein Formation in Vertebrate Vasculatures. J. Genet. Genom. 2017, 44, 483–492. [Google Scholar] [CrossRef]
- Tan, S.; Zang, G.; Wang, Y.; Sun, Z.; Li, Y.; Lu, C.; Wang, Z. Differences of Angiogenesis Factors in Tumor and Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2021, 14, 3375–3388. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, J.R.S.; da Costa Aguiar Alves, B.; Encinas, J.F.A.; Siqueira, A.M.; de Gois, K.C.; Perez, M.M.; Petri, G.; dos Santos, J.F.R.; Fonseca, F.L.A.; da Veiga, G.L. Expression of TNFR1, VEGFA, CD147 and MCT1 as Early Biomarkers of Diabetes Complications and the Impact of Aging on This Profile. Sci. Rep. 2023, 13, 17927. [Google Scholar] [CrossRef]
- Yang, D.-R.; Wang, M.-Y.; Zhang, C.-L.; Wang, Y. Endothelial Dysfunction in Vascular Complications of Diabetes: A Comprehensive Review of Mechanisms and Implications. Front. Endocrinol. 2024, 15, 1359255. [Google Scholar] [CrossRef]
- Simo, R.; Carrasco, E.; Garcia-Ramirez, M.; Hernandez, C. Angiogenic and Antiangiogenic Factors in Proliferative Diabetic Retinopathy. Curr. Diabetes Rev. 2006, 2, 71–98. [Google Scholar] [CrossRef]
- Kordkheyli, V.A.; Mishan, M.A.; Tarsi, A.K.; Mahrooz, A.; Kanavi, M.R.; Hafezi-Moghadam, A.; Bagheri, A. MicroRNAs May Provide New Strategies in the Treatment and Diagnosis of Diabetic Retinopathy: Importance of VEGF. Iran J. Basic Med. Sci. 2021, 24, 267–279. [Google Scholar] [CrossRef]
- Chen, B.; Chen, J.; Du, Q.; Zhou, D.; Wang, L.; Xie, J.; Li, Y.; Zhang, D. Genetic Variants in MicroRNA Biogenesis Genes as Novel Indicators for Secondary Growth in Populus. New Phytol. 2018, 219, 1263–1282. [Google Scholar] [CrossRef]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The MultiMiR R Package and Database: Integration of MicroRNA–Target Interactions along with Their Disease and Drug Associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef]
- Rishik, S.; Hirsch, P.; Grandke, F.; Fehlmann, T.; Keller, A. MiRNATissueAtlas 2025: An Update to the Uniformly Processed and Annotated Human and Mouse Non-Coding RNA Tissue Atlas. Nucleic Acids Res. 2025, 53, D129–D137. [Google Scholar] [CrossRef]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009; ISBN 1441412697. [Google Scholar]
- McKinney, W. Python for Data Analysis: Data Wrangling with Pandas, NumPy, and Jupyter, 3rd ed.; O’Reilly Media: Sebastopol, CA, USA, 2022. [Google Scholar]
- Liu, C.-J.; Fu, X.; Xia, M.; Zhang, Q.; Gu, Z.; Guo, A.-Y. MiRNASNP-v3: A Comprehensive Database for SNPs and Disease-Related Variations in MiRNAs and MiRNA Targets. Nucleic Acids Res. 2021, 49, D1276–D1281. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. DbSNP: The NCBI Database of Genetic Variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y. Statistical Notes for Clinical Researchers: Chi-Squared Test and Fisher’s Exact Test. Restor. Dent. Endod. 2017, 42, 152. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Vejnar, C.E.; Zdobnov, E.M. MiRmap: Comprehensive Prediction of MicroRNA Target Repression Strength. Nucleic Acids Res. 2012, 40, 11673–11683. [Google Scholar] [CrossRef]
- Rappaport, N.; Twik, M.; Plaschkes, I.; Nudel, R.; Stein, T.I.; Levitt, J.; Gershoni, M.; Morrey, C.P.; Safran, M.; Lancet, D. MalaCards: An Amalgamated Human Disease Compendium with Diverse Clinical and Genetic Annotation and Structured Search. Nucleic Acids Res. 2017, 45, D877–D887. [Google Scholar] [CrossRef]
- Thillaiyampalam, G.; Cristino, A.S. MicroRNA Target Prediction and Validation. In MicroRNA; Elsevier: Amsterdam, The Netherlands, 2022; pp. 53–67. [Google Scholar]
- Riolo, G.; Cantara, S.; Marzocchi, C.; Ricci, C. MiRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc. 2020, 4, 1. [Google Scholar] [CrossRef]
- Kariuki, D.; Asam, K.; Aouizerat, B.E.; Lewis, K.A.; Florez, J.C.; Flowers, E. Review of Databases for Experimentally Validated Human MicroRNA-MRNA Interactions. Database 2023, 2023, baad014. [Google Scholar] [CrossRef] [PubMed]
- Quillet, A.; Anouar, Y.; Lecroq, T.; Dubessy, C. Prediction Methods for MicroRNA Targets in Bilaterian Animals: Toward a Better Understanding by Biologists. Comput. Struct. Biotechnol. J. 2021, 19, 5811–5825. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases: An Updated Overview. In Methods in Molecular Biology; Humana: New York, NY, USA, 2023; pp. 1–12. [Google Scholar]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Zlotorynski, E. Insights into the Kinetics of MicroRNA Biogenesis and Turnover. Nat. Rev. Mol. Cell Biol. 2019, 20, 511. [Google Scholar] [CrossRef]
- Xiang, Z.; Lin, T.; Ling, J.; Xu, Z.; Huang, R.; Hu, H. MiRNA Expression Profiling and Clinical Implications in Prostate Cancer across Various Stages. Sci. Rep. 2025, 15, 7771. [Google Scholar] [CrossRef] [PubMed]
- Napoletano, S.; Dannhauser, D.; Netti, P.A.; Causa, F. Integrative Analysis of MiRNA Expression Data Reveals a Minimal Signature for Tumour Cells Classification. Comput. Struct. Biotechnol. J. 2025, 27, 233–242. [Google Scholar] [CrossRef]
- Fedorova, L.; Khrunin, A.; Khvorykh, G.; Lim, J.; Thornton, N.; Mulyar, O.A.; Limborska, S.; Fedorov, A. Analysis of Common SNPs across Continents Reveals Major Genomic Differences between Human Populations. Genes 2022, 13, 1472. [Google Scholar] [CrossRef]
- Sirugo, G.; Williams, S.M.; Tishkoff, S.A. The Missing Diversity in Human Genetic Studies. Cell 2019, 177, 26–31. [Google Scholar] [CrossRef]
- Smetana, J.; Brož, P. National Genome Initiatives in Europe and the United Kingdom in the Era of Whole-Genome Sequencing: A Comprehensive Review. Genes 2022, 13, 556. [Google Scholar] [CrossRef]
- Cao, Y.; Li, L.; Xu, M.; Feng, Z.; Sun, X.; Lu, J.; Xu, Y.; Du, P.; Wang, T.; Hu, R.; et al. The ChinaMAP Analytics of Deep Whole Genome Sequences in 10,588 Individuals. Cell Res. 2020, 30, 717–731. [Google Scholar] [CrossRef]
- Zhang, P.; Luo, H.; Li, Y.; Wang, Y.; Wang, J.; Zheng, Y.; Niu, Y.; Shi, Y.; Zhou, H.; Song, T.; et al. NyuWa Genome Resource: A Deep Whole-Genome Sequencing-Based Variation Profile and Reference Panel for the Chinese Population. Cell Rep. 2021, 37, 110017. [Google Scholar] [CrossRef] [PubMed]
- Fatumo, S.; Chikowore, T.; Choudhury, A.; Ayub, M.; Martin, A.R.; Kuchenbaecker, K. A Roadmap to Increase Diversity in Genomic Studies. Nat. Med. 2022, 28, 243–250. [Google Scholar] [CrossRef]
- Oleksyk, T.K.; Wolfsberger, W.W.; Schubelka, K.; Mangul, S.; O’Brien, S.J. The Pioneer Advantage: Filling the Blank Spots on the Map of Genome Diversity in Europe. Gigascience 2022, 11, giac081. [Google Scholar] [CrossRef] [PubMed]
- Santiago, E.; Novo, I.; Pardiñas, A.F.; Saura, M.; Wang, J.; Caballero, A. Recent Demographic History Inferred by High-Resolution Analysis of Linkage Disequilibrium. Mol. Biol. Evol. 2020, 37, 3642–3653. [Google Scholar] [CrossRef] [PubMed]
- Fournier, T.; Saada, O.A.; Hou, J.; Peter, J.; Caudal, E.; Schacherer, J. Extensive Impact of Low-Frequency Variants on the Phenotypic Landscape at Population-Scale. eLife 2019, 8, e49258. [Google Scholar] [CrossRef]
- Bomba, L.; Walter, K.; Soranzo, N. The Impact of Rare and Low-Frequency Genetic Variants in Common Disease. Genome Biol. 2017, 18, 77. [Google Scholar] [CrossRef]
- Hajibabaie, F.; Abedpoor, N.; Assareh, N.; Tabatabaiefar, M.A.; Shariati, L.; Zarrabi, A. The Importance of SNPs at MiRNA Binding Sites as Biomarkers of Gastric and Colorectal Cancers: A Systematic Review. J. Pers. Med. 2022, 12, 456. [Google Scholar] [CrossRef]
- Gottmann, P.; Ouni, M.; Zellner, L.; Jähnert, M.; Rittig, K.; Walther, D.; Schürmann, A. Polymorphisms in MiRNA Binding Sites Involved in Metabolic Diseases in Mice and Humans. Sci. Rep. 2020, 10, 7202. [Google Scholar] [CrossRef]
- Kilikevicius, A.; Meister, G.; Corey, D.R. Reexamining Assumptions about MiRNA-Guided Gene Silencing. Nucleic Acids Res. 2022, 50, 617–634. [Google Scholar] [CrossRef]
- Das, R.P.; Konkimalla, V.B.; Rath, S.N.; Hansa, J.; Jagdeb, M. Elucidation of the Molecular Interaction between MiRNAs and the HOXA9 Gene, Involved in Acute Myeloid Leukemia, by the Assistance of Argonaute Protein through a Computational Approach. Genom. Inform. 2015, 13, 45. [Google Scholar] [CrossRef]
- Hassan, M.; Iqbal, M.S.; Naqvi, S.; Alashwal, H.; Moustafa, A.A.; Kloczkowski, A. Prediction of Site Directed MiRNAs as Key Players of Transcriptional Regulators Against Influenza C Virus Infection Through Computational Approaches. Front. Mol. Biosci. 2022, 9, 866072. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.N.; Das, D.; Konkimalla, V.B.; Pradhan, S.K. In Silico Study of MiRNA Based Gene Regulation, Involved in Solid Cancer, by the Assistance of Argonaute Protein. Genom. Inform. 2016, 14, 112–124. [Google Scholar] [CrossRef]
- Mendonca, A.; Thandapani, P.; Swaminathan, P.; Sundaresan, S. In Silico Screening and Analysis of Candidate MicroRNA-Target Interaction Involved in Progression of Prediabetes to Type II Diabetes Mellitus. J. Appl. Biol. Biotechnol. 2023, 12, 77–85. [Google Scholar] [CrossRef]
- Li, Y.E.; Xiao, M.; Shi, B.; Yang, Y.-C.T.; Wang, D.; Wang, F.; Marcia, M.; Lu, Z.J. Identification of High-Confidence RNA Regulatory Elements by Combinatorial Classification of RNA–Protein Binding Sites. Genome Biol. 2017, 18, 169. [Google Scholar] [CrossRef]
- Chi, S.W.; Zang, J.B.; Mele, A.; Darnell, R.B. Argonaute HITS-CLIP Decodes MicroRNA–MRNA Interaction Maps. Nature 2009, 460, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Chen, S.; Dou, W.; Xie, R.; Gao, J. MiR-654-3p Predicts the Prognosis of Hepatocellular Carcinoma and Inhibits the Proliferation, Migration, and Invasion of Cancer Cells. Cancer Biomark. 2020, 28, 73–79. [Google Scholar] [CrossRef]
- Deng, G.; Mou, T.; He, J.; Chen, D.; Lv, D.; Liu, H.; Yu, J.; Wang, S.; Li, G. Circular RNA CircRHOBTB3 Acts as a Sponge for MiR-654-3p Inhibiting Gastric Cancer Growth. J. Exp. Clin. Cancer Res. 2020, 39, 1. [Google Scholar] [CrossRef] [PubMed]
- Formosa, A.; Markert, E.K.; Lena, A.M.; Italiano, D.; Finazzi-Agro’, E.; Levine, A.J.; Bernardini, S.; Garabadgiu, A.V.; Melino, G.; Candi, E. MicroRNAs, MiR-154, MiR-299-5p, MiR-376a, MiR-376c, MiR-377, MiR-381, MiR-487b, MiR-485-3p, MiR-495 and MiR-654-3p, Mapped to the 14q32.31 Locus, Regulate Proliferation, Apoptosis, Migration and Invasion in Metastatic Prostate Cancer Cells. Oncogene 2014, 33, 5173–5182. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.-C.; Chen, L.-R.; Huang, H.-Z.; Wu, W.-Y.; Wang, Y.; Li, G. Pyroptosis and Mitochondrial Function Participated in MiR-654-3p-Protected against Myocardial Infarction. Cell Death Dis. 2024, 15, 393. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, H.; Chen, J.; He, L.; Chen, Y. Role of VEGF-A and LRG1 in Abnormal Angiogenesis Associated With Diabetic Nephropathy. Front. Physiol. 2020, 11, 1064. [Google Scholar] [CrossRef]
- Biselli-Chicote, P.M.; Biselli, J.M.; Cunha, B.R.; Castro, R.; Maniglia, J.V.; de Santi Neto, D.; Tajara, E.H.; de Góis Filho, J.F.; Fukuyama, E.E.; Pavarino, É.C.; et al. Overexpression of Antiangiogenic Vascular Endothelial Growth Factor Isoform and Splicing Regulatory Factors in Oral, Laryngeal and Pharyngeal Squamous Cell Carcinomas. Asian Pac. J. Cancer Prev. 2017, 18, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Rehn, M.; Olsson, A.; Reckzeh, K.; Diffner, E.; Carmeliet, P.; Landberg, G.; Cammenga, J. Hypoxic Induction of Vascular Endothelial Growth Factor Regulates Murine Hematopoietic Stem Cell Function in the Low-Oxygenic Niche. Blood 2011, 118, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Abou Khouzam, R.; Brodaczewska, K.; Filipiak, A.; Zeinelabdin, N.A.; Buart, S.; Szczylik, C.; Kieda, C.; Chouaib, S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front. Immunol. 2021, 11, 613114. [Google Scholar] [CrossRef]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia Regulates Vascular Endothelial Growth Factor Gene Expression in Endothelial Cells. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid. Med. Cell Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef]
- An, Y.; Xu, B.; Wan, S.; Ma, X.; Long, Y.; Xu, Y.; Jiang, Z. The Role of Oxidative Stress in Diabetes Mellitus-Induced Vascular Endothelial Dysfunction. Cardiovasc. Diabetol. 2023, 22, 237. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Farrah, T.E.; Dhillon, B.; Keane, P.A.; Webb, D.J.; Dhaun, N. The Eye, the Kidney, and Cardiovascular Disease: Old Concepts, Better Tools, and New Horizons. Kidney Int. 2020, 98, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, W.; Liu, J.; Xie, M.; Liu, Q.; Li, S. Vascular Complications of Diabetes: A Narrative Review. Medicine 2023, 102, e35285. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Q.; Zhou, J. Mechanisms of Abnormal Lipid Metabolism in the Pathogenesis of Disease. Int. J. Mol. Sci. 2024, 25, 8465. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, A.R.; Toscano, A.; Pereira, P.; Girão, H.; Gonçalves, L.; Marques, C. Hyperglycemia-Induced Degradation of HIF-1α Contributes to Impaired Response of Cardiomyocytes to Hypoxia. Rev. Port. Cardiol. 2017, 36, 367–373. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Novev, J.K.; Ahnert, S.E. Changes in MiRNA Secondary Structure Can Predict Mutations Associated with Cancer and Other Diseases. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wen, S.; Liu, Y.; Yang, G.; Chen, W.; Wu, H.; Zhu, X.; Wang, Y. Author Correction: A Method for MiRNA-Disease Association Prediction Using Machine Learning Decoding of Multi-Layer Heterogeneous Graph Transformer Encoded Representations. Sci. Rep. 2024, 14, 24181. [Google Scholar] [CrossRef]
- Javanmardifard, Z.; Rahmani, S.; Bayat, H.; Mirtavoos-Mahyari, H.; Ghanei, M.; Mowla, S.J. A Comprehensive in Silico Analysis and Experimental Validation of MiRNAs Capable of Discriminating between Lung Adenocarcinoma and Squamous Cell Carcinoma. Front. Genet. 2024, 15, 1419099. [Google Scholar] [CrossRef]
- Li, J.; Wu, C. Deep Learning and Text Mining: Classifying and Extracting Key Information from Construction Accident Narratives. Appl. Sci. 2023, 13, 10599. [Google Scholar] [CrossRef]
- Schuster, J.; Superdock, M.; Agudelo, A.; Stey, P.; Padbury, J.; Sarkar, I.N.; Uzun, A. Machine Learning Approach to Literature Mining for the Genetics of Complex Diseases. Database 2019, 2019, baz124. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Ren, M.; Gao, D. A New Multifractal-Based Deep Learning Model for Text Mining. Inf. Process. Manag. 2024, 61, 103561. [Google Scholar] [CrossRef]
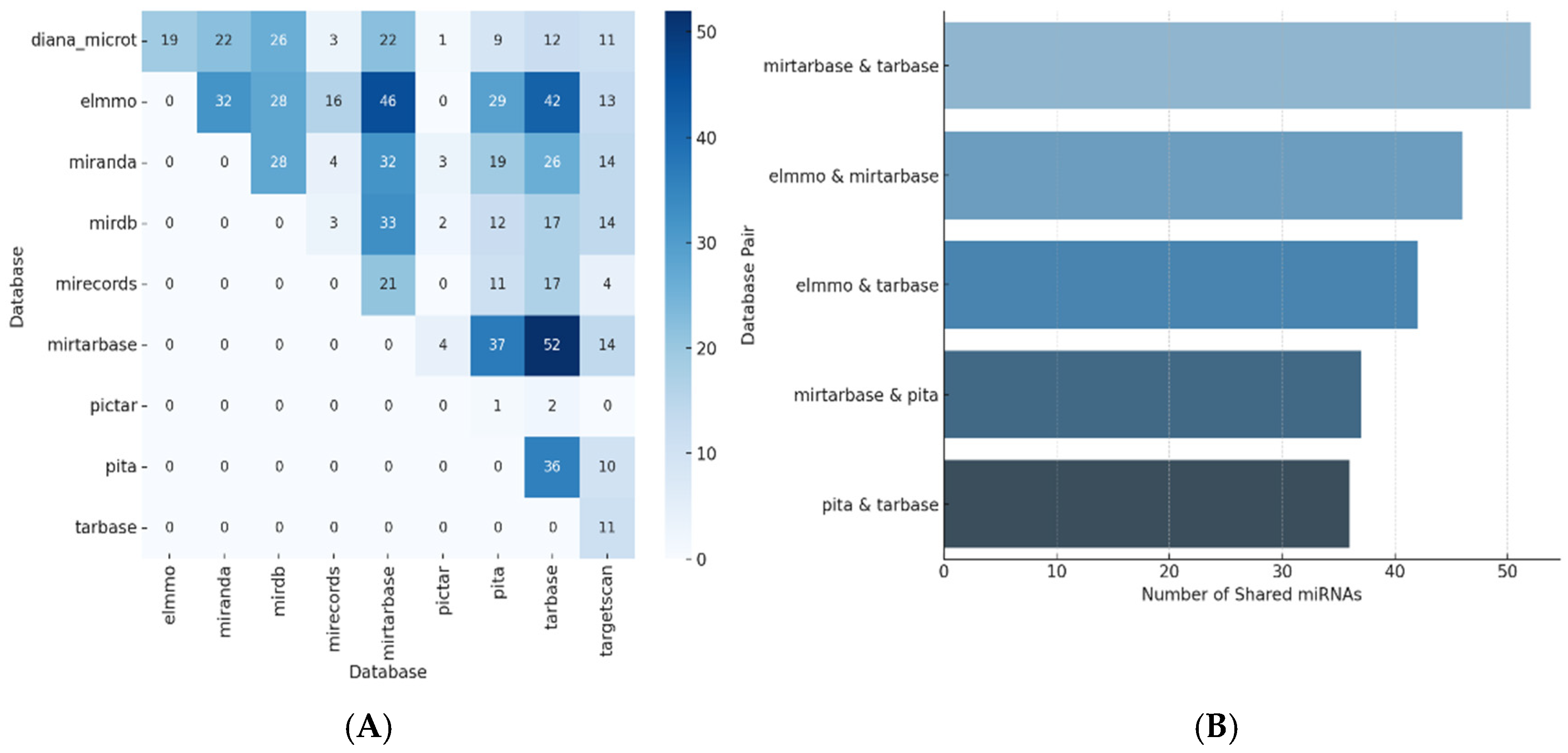
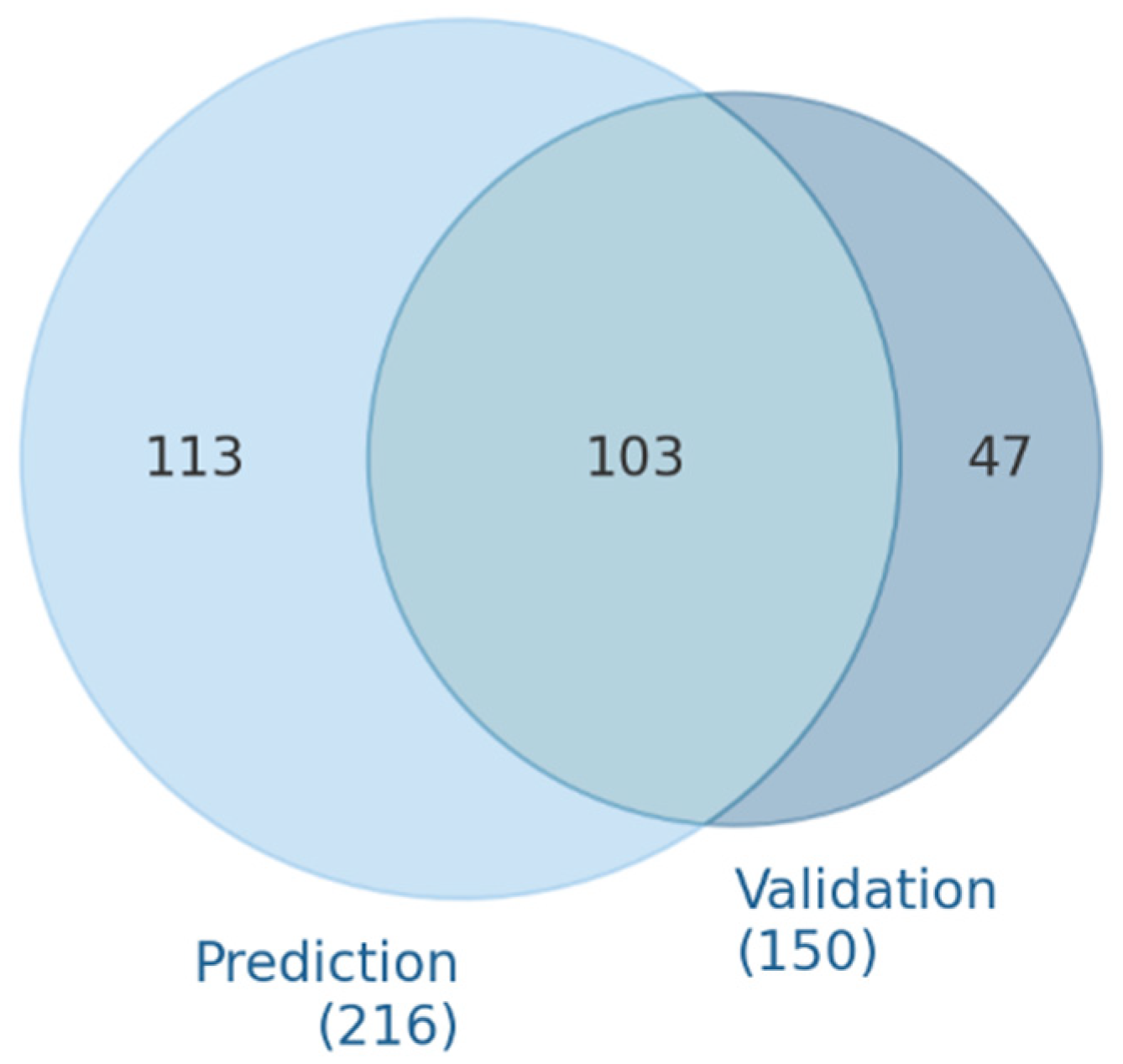
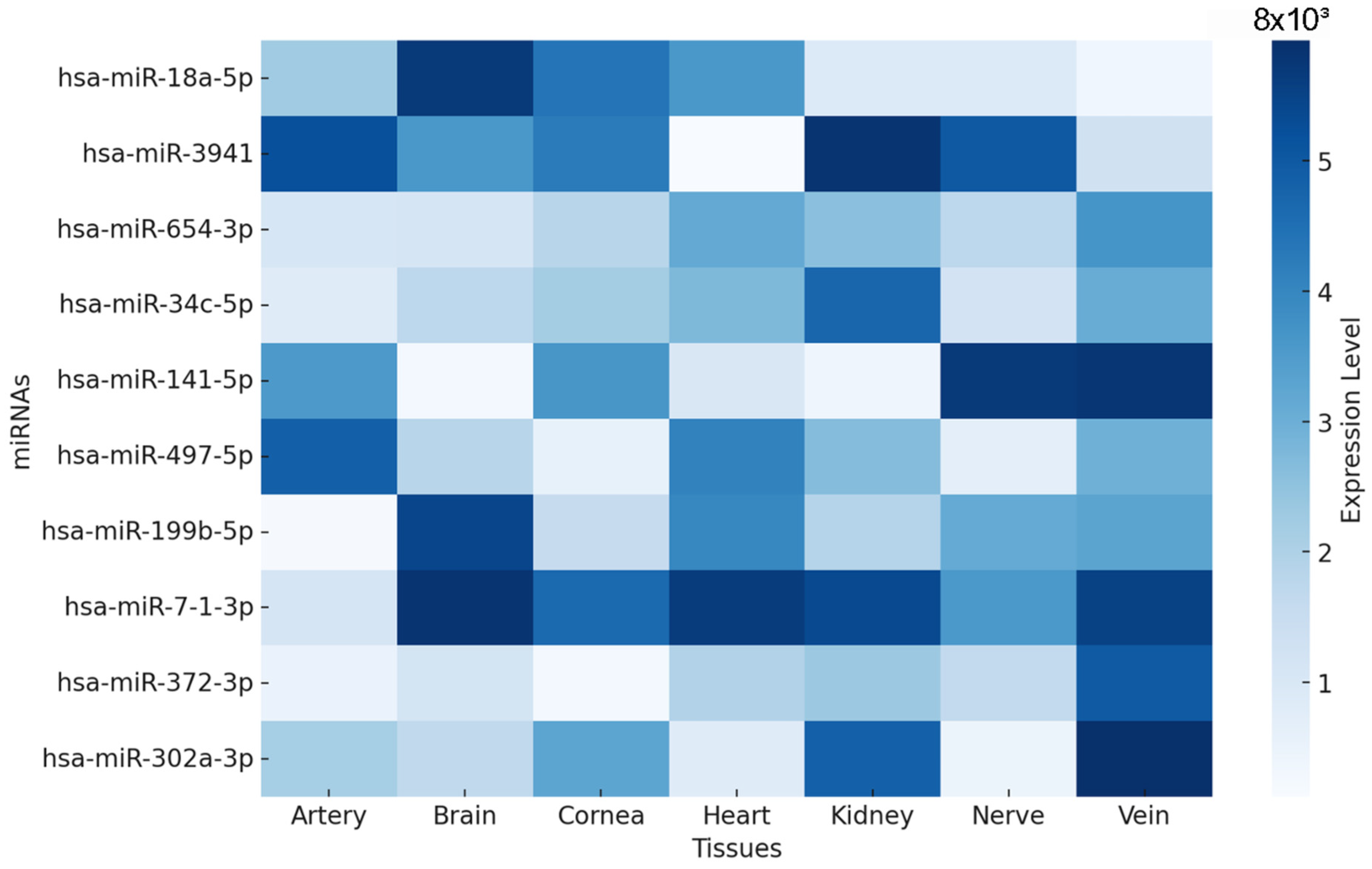
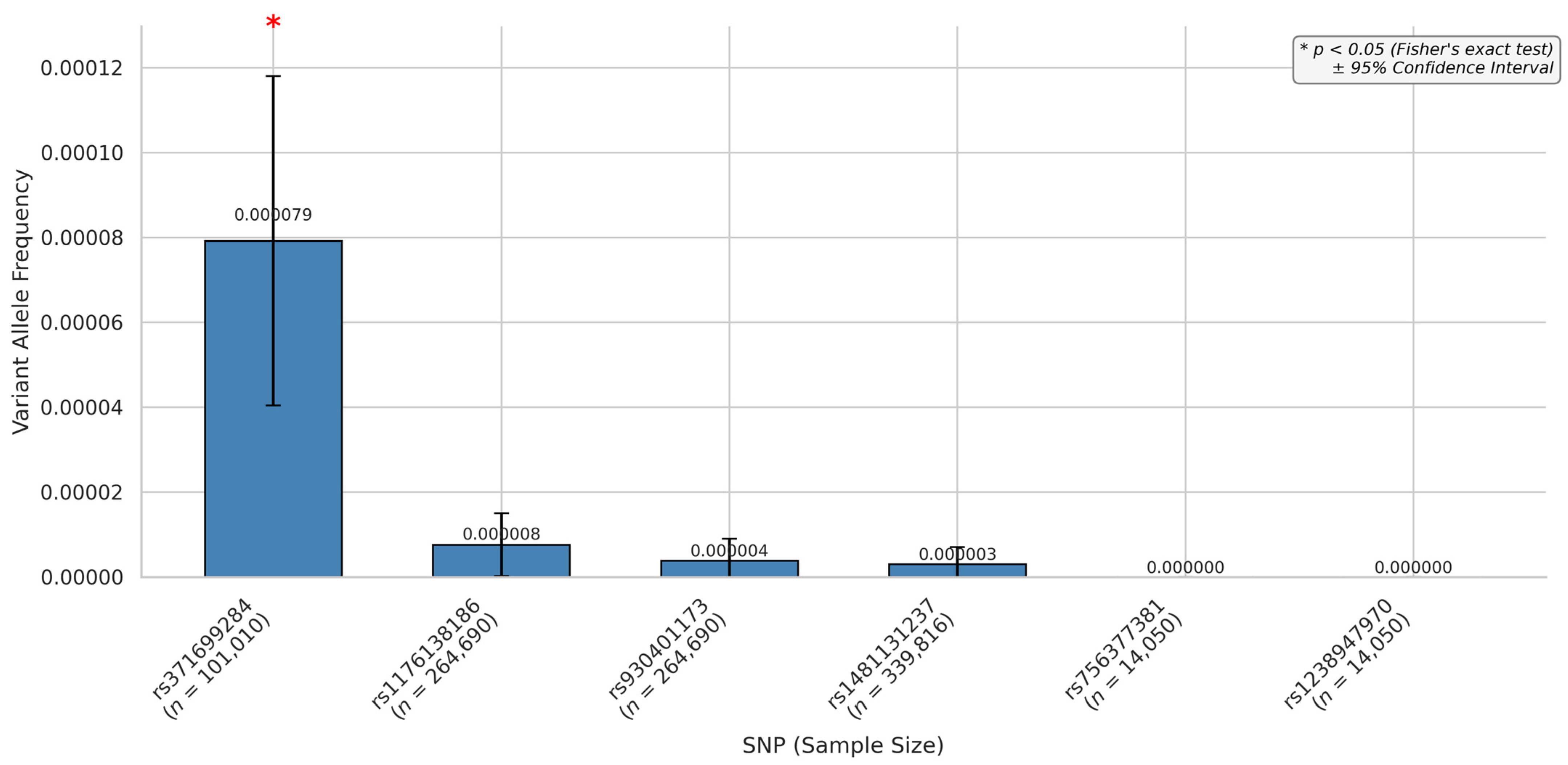
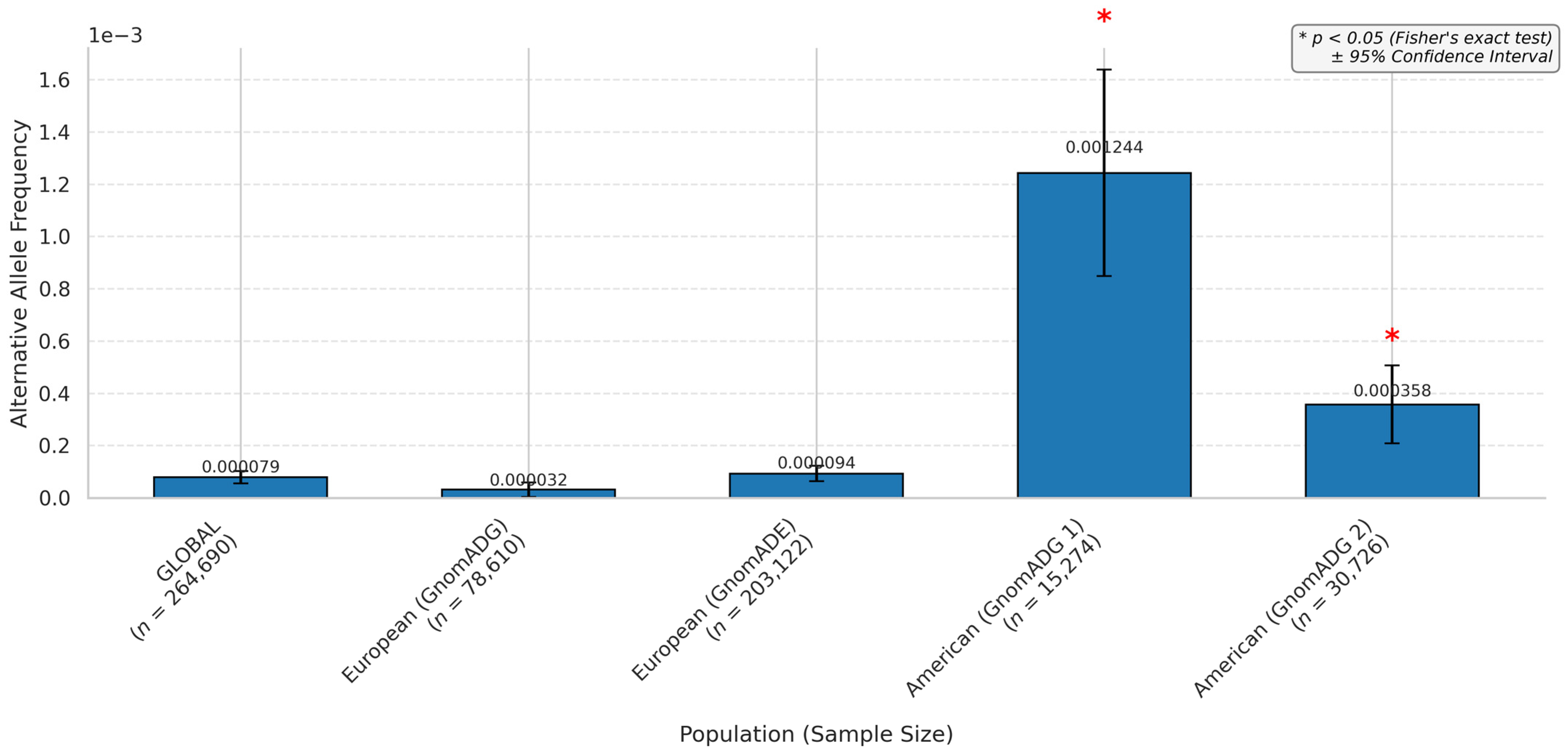
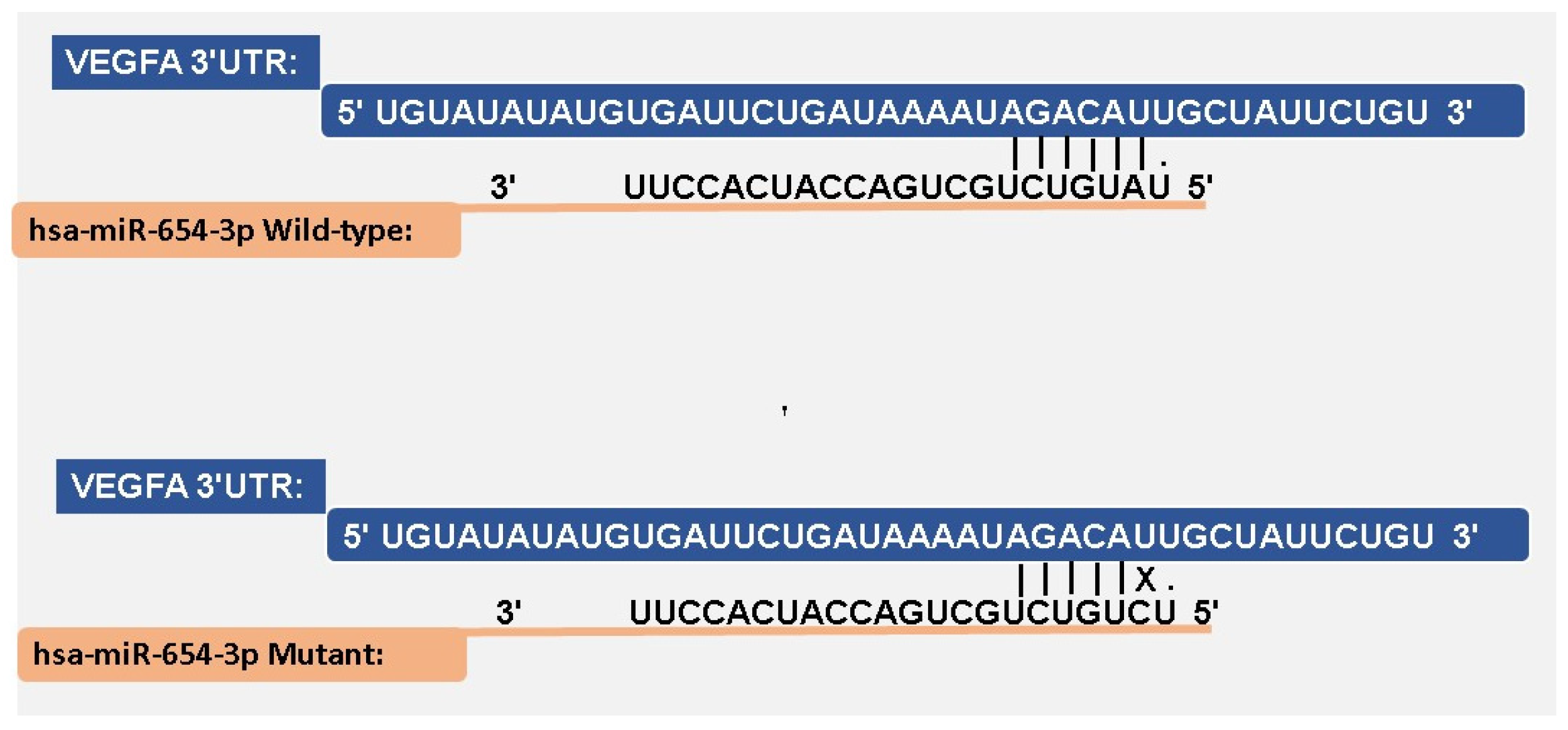
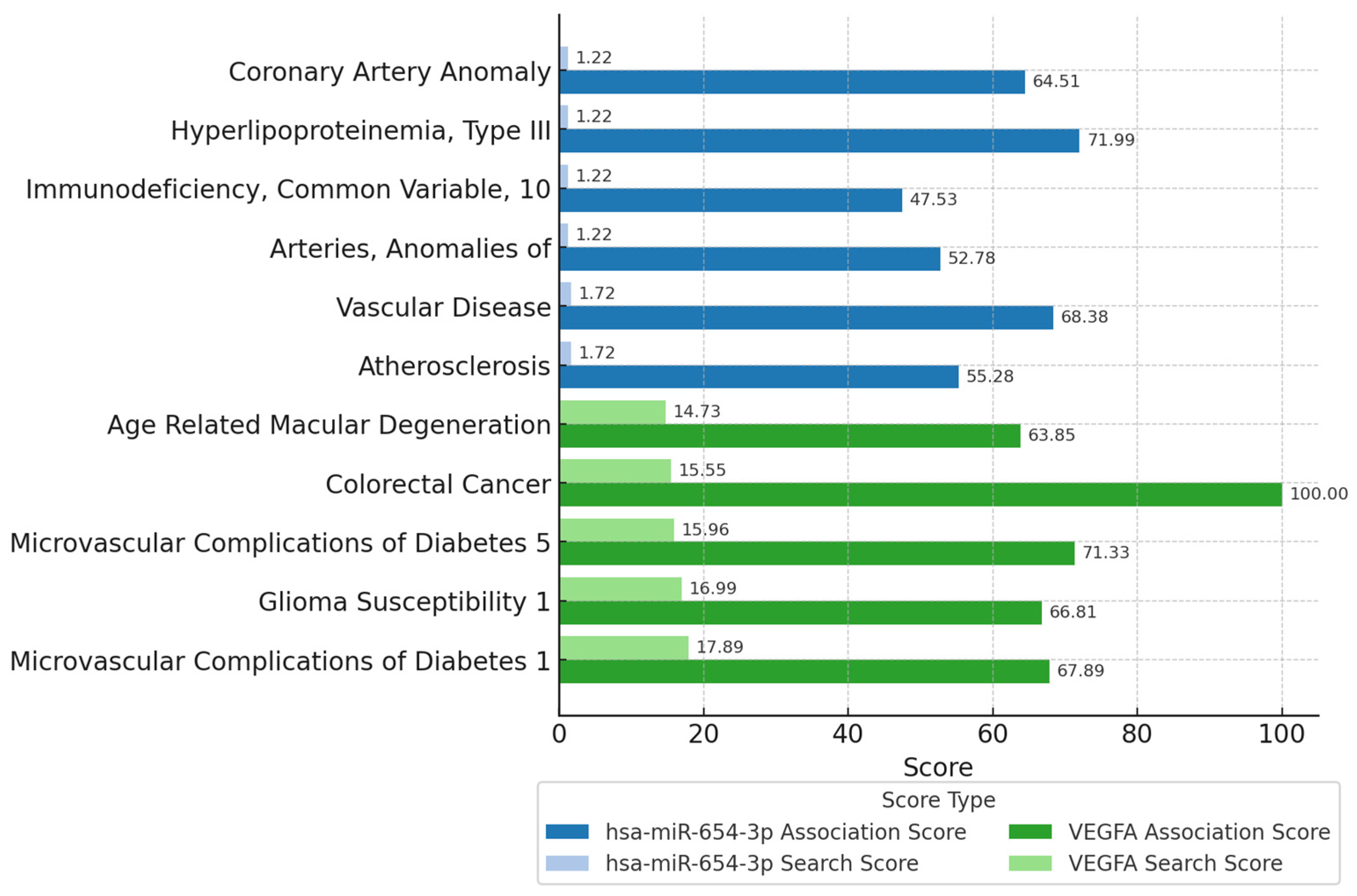
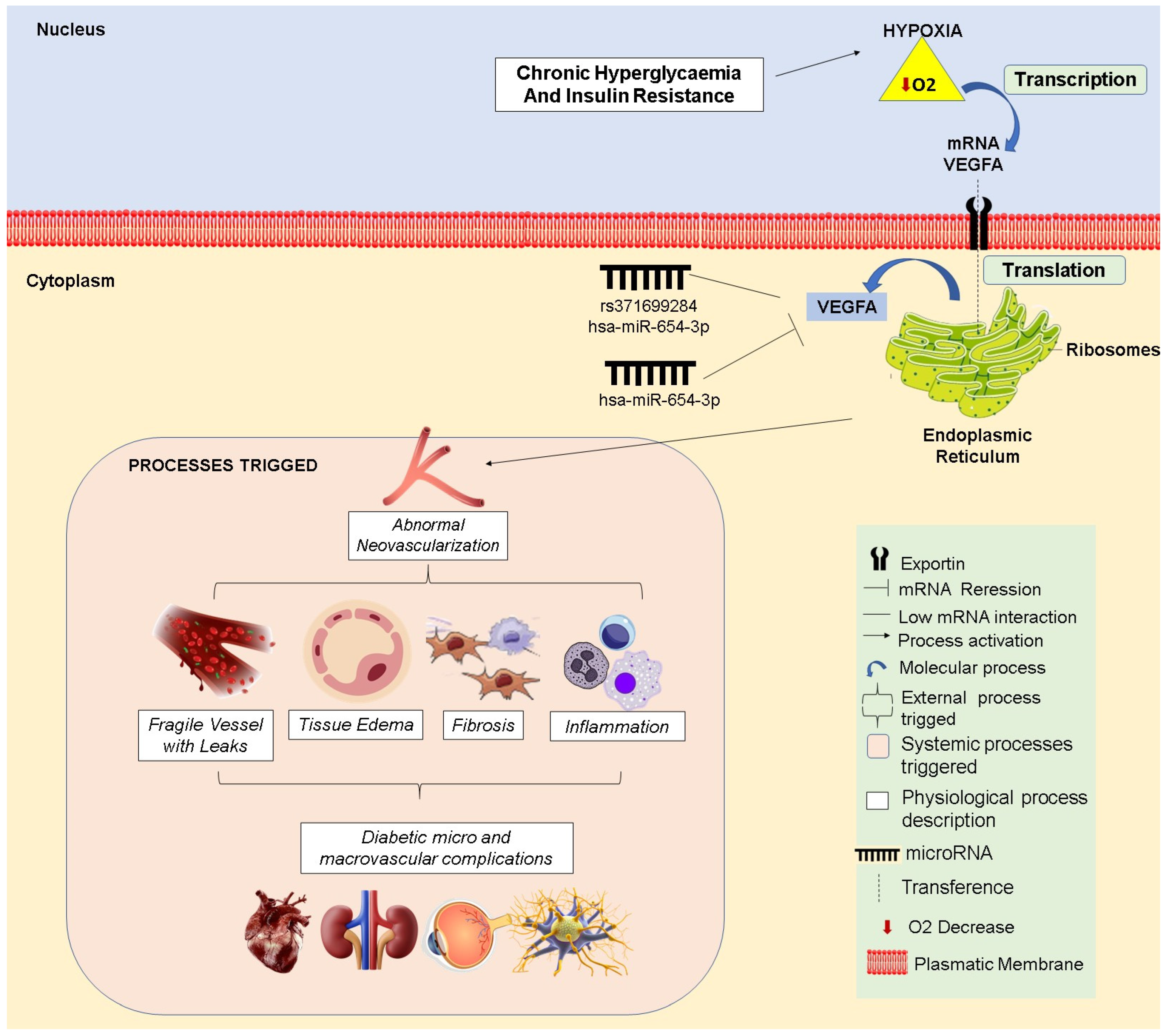
| miRNA | SNP |
|---|---|
| hsa-miR-654-3p | rs371699284 |
| hsa-miR-34c-5p | rs930401173 |
| hsa-miR-34c-5p | rs756377381 |
| hsa-miR-199b-5p | rs1176138186 |
| hsa-miR-372-3p | rs1481131237 |
| hsa-miR-302a-3p | rs1238947970 |
| miRNA Sequence | miRmap Score | ΔG Binding (kcal/mol) | Conserved Site | Seed Match |
|---|---|---|---|---|
| Wild Type | −0.412 | −23.6 | True | 8mer |
| Mutant | −0.295 | −19.8 | False | 7mer-A1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, R.; Felipe, S.; Pacheco, C.; Faria, E.; Martins, J.; Fortes, J.; Silva, D.; Oliveira, P.; Ceccatto, V. Loss of miRNA-Mediated VEGFA Regulation by SNP-Induced Impairment: A Bioinformatic Analysis in Diabetic Complications. Biomedicines 2025, 13, 1192. https://doi.org/10.3390/biomedicines13051192
Freitas R, Felipe S, Pacheco C, Faria E, Martins J, Fortes J, Silva D, Oliveira P, Ceccatto V. Loss of miRNA-Mediated VEGFA Regulation by SNP-Induced Impairment: A Bioinformatic Analysis in Diabetic Complications. Biomedicines. 2025; 13(5):1192. https://doi.org/10.3390/biomedicines13051192
Chicago/Turabian StyleFreitas, Raquel, Stela Felipe, Christina Pacheco, Emmanuelle Faria, Jonathan Martins, Jefferson Fortes, Denner Silva, Paulo Oliveira, and Vania Ceccatto. 2025. "Loss of miRNA-Mediated VEGFA Regulation by SNP-Induced Impairment: A Bioinformatic Analysis in Diabetic Complications" Biomedicines 13, no. 5: 1192. https://doi.org/10.3390/biomedicines13051192
APA StyleFreitas, R., Felipe, S., Pacheco, C., Faria, E., Martins, J., Fortes, J., Silva, D., Oliveira, P., & Ceccatto, V. (2025). Loss of miRNA-Mediated VEGFA Regulation by SNP-Induced Impairment: A Bioinformatic Analysis in Diabetic Complications. Biomedicines, 13(5), 1192. https://doi.org/10.3390/biomedicines13051192






