Green Synthesis, Characterization, and Potential Antibacterial and Anticancer Applications of Gold Nanoparticles: Current Status and Future Prospects
Abstract
1. Introduction
2. Plant-Mediated Green Synthesis
| Plant | Used Part | Optimum Synthesis Conditions (Salt Concentration, Temperature, Incubation Time) | Size (nm) | Shape | Applications | Reference |
|---|---|---|---|---|---|---|
| Clerodendrum trichotomum | Leaf extract | 10.20 g HAuCl4 was added to 100 mL of plant extract, incubated at 65 °C, 80 min | 19.1 ± 2.2 (Average) | Spherical | Antibacterial application against Klebsiella pneumoniae and Staphylococcus aureus. Anticancer application against breast cancer cell line (MCF-7) | [2] |
| Henna | Leaf extract | 10 mM HAuCl4, room temperature, 30 min | 6 ± 2.5 (Average) | Spherical | Antibacterial activity against Staphylococcus aureus and Escherichia coli | [68] |
| Halodule uninervis | Leaf extract | HAuCl4·3H2O, 70–80 °C, 30 min | 10–50 | Spherical | Anticancer activity against human breast cancer cells MDA-MB-231 | [69] |
| Tangerine | Peel extract | 15 mM HAuCl4·3H2O, 40 °C, 60 min | 26 ± 5 (Average) | Spherical | Antibacterial activity against Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa | [70] |
| Aconitum violaceum | Plant extract | 1 mM HAuCl4, room temperature, 50 min | <100 (Average) | Spherical and triangular | Antibacterial activity against Lactobacillus acidophilus and Escherichia coli | [71] |
| Syzygium cumini | Leaf extract | HAuCl4·3H2O, ambient temperature, 24 h | 120.5 (Average) | Spherical | Antibacterial application against Aeromonas hydrophila, E. coli, Salmonella Typhimurium, P. aeruginosa, Enterococcus faecium, Pediococcus sp., and Bacillus cereus | [72] |
| Zingiber officinale, curcumin | Root extract | HAuCl4 solution (0.1 mM) and ginger extract mixture is kept boiling and stirring at 600 rpm until the solution turned purple | 20 (Average size) | Spherical and oval | Antimicrobial efficacy against, E. coli, P. aeruginosa and S. aureus | [73] |
| Abutilon indicum | Leaf extract | 1 mM HAuCl4, room temperature, 2 min | 10–20 | Spherical | Effective against human colon cancer | [12,74] |
| Artemisia vulgaris | Leaf extracts | 1 mM HAuCl4 solution, room temperature, 24 h | 50–100 | Spherical, triangular, and hexagonal | Antibacterial application against S. aureus, S. pyogenes, E. coli, P. aeruginosa, anti-fungal activity against Aspergillus niger, induced apoptosis in MCF-7 (breast cancer) | [36,40] |
| Azadirachta indica | Leaf extract | 100 ppm gold chlorate, the extract and the gold chlorate mixture are boiled till the appearance of wine-red color. | ≤121.7 | Spherical, hexagonal, and triangular | Anticancer activity on HeLa and MDCK cell | [75] |
| Areca catechu | Nut | 30 mL chloroauric acid, 10 mL aqueous nut extract, 4–5 h | 22.2 | Spherical | Anticancer activity on HeLa | [76] |
| Acacia nilotica | Bark extract | 1 mM HAuCl4, room temperature, 10 min | 10–15 | Unshaped, quasispherical | Anticancer activity on hepatic cell, antibacterial activity against B. subtilis and S. aureus | [77] |
| Acorus calamus | Rhizome extract | 2.5 mL of extract, 2.5 mL of 0.001 M chloroauric acid, stir at 240 rpm until the color turns dark brown. | 10 | Spherical | Antibacterial activity S. aureus and E. coli | [33,78] |
| Artocarpus hirsutus | Leaf extract | 1 mM HAuCl4, at 80 °C, about 12 h. | 5–40 | Spherical | Efficacy against human cancer cell lines (HeLa, RKO and A549). | [34,79] |
| Abelmoschus esculentus | Seed and pulp extract | (1 mM) HAuCl4 × H2O (95 mL) at room temperature, 1 h | 45–75 | Spherical, uneven shape | Antibacterial activity against E. coli, P. aeruginosa, B. cereus, and B. subtilis. Antifungal activity against A. niger, Puccinia graminis tritci, and C. albicans | [50,51] |
| Butea monosperma | Leaf extract | 0.01 M of HAuCl4, room temperature, 35 min | 20–80 | Mainly spherical but with a few rods; irregular and hexagonal | Anticancer activity on B16F10, MCF-7, HNGC2, A549, HUVEC and ECV-304 | [12,80] |
| Cassia auriculata | Leaf extract | 1 mM auric chloride solution, room temperature, 10 min. | 15–25 | Spherical, triangular, and hexagonal | Antibacterial efficacy against Bacillus subtilis, K. pneumonia, P. aeruginosa | [81] |
| Caesalpinia crista | Seed extract | 1 mM HAuCl4, room temperature (25 °C ± 2 °C) for 24 h | 15.13 | Spherical | Antibacterial efficacy against B. subtilis, S. aureus, E. coli, K. pneumoniae Anticancer activity against human cancer cell lines (HeLa, MCF-7), | [52] |
| Citrus (lemon, tangerine, orange) | Fruit extract | 1 mM HAuCl43H2O, room temperature | 32.3, 43.4, 56.7 | Spherical and triangular | Anticancer effect on the growth of HepG2 (liver cancer cell line) | [42,43] |
| Citrus maxima | Fruit extract | (1%, w/v) HAuCl4·4H2O, room temperature, 5 min. | 15–35 | Spherical | Antibacterial efficacy against Staphylococcus aureus | [44,45] |
| Clitoria ternatea | Leaf extract | 99 mL of 10−3 aqueous HAuCl4, room temperature for (0 min–24 h) | 100 | Rod | Antibacterial activity against E. coli, K. pneumoniae, S. aureus, and S. pyogenes | [82,83] |
| Curcuma longa | Rhizome extract | 0.01 mL HAuCl4, room temperature, overnight culture | 5–60 | Oblong and spherical | Anticancer effect on the lung cancer cells | [41] |
| Curcumae Kwangsiensis | Leaf extracts | (1 mM) HAuCl4·H2O, 25 °C, 1 h | 8–25 | Spherical | Anticancer effect on ovarian cancer cell lines i.e., PA-1, SW-626, and SK-OV-3. | [84] |
| Dendropanax morbifera | Leaf extract | 1 mM chloroauric acid, 80 °C for 10 min | 10–20 | Polygonal and hexagonal | Anticancer activity on HaCaT and A549 | [85] |
| Dracocephalum kotschyi | Leaf extract | 1 mM HAuCl4, room temperature for 10 min. | 11 | Spherical | Anticancer activity on K562 and HeLa | [86] |
| Ecklonia cava (marine brown alga) | Seaweed extract | 1 mM chloroauric acid, 80 °C, 10 min | 20–50 | Spherical and triangular | Anticancer activity on HaCaT, MCF-7 | [87,88] |
| Genipa americana | Fruit extract | 0.5 mM AuCl4 solution and kept at 22–25 °C, 15 min | 30.4 ± 14.9 | Spherical | Anticancer activity on A-549 and Hela | [46,47] |
| Guazuma ulmifolia | Bark extract | 1 mM HAuCl4·3H2O, room temperature, 1 h | 20–25 | Spherical | Antibacterial properties against Staphylococcus aureus and anticancer activity | [89,90] |
| Hibiscus sabdariffa | Leaf extract | (1 mM) HAuCl4 × H2O (100 mL), room temperature, 30 min | 10–30 | Near spherical | Antifungal potentials against C. krusei, C. guilliermondii, C. glabrata, and C. albicans, antibacterial effects against Streptococcus pneumonia, Staphylococcus aureus, Bacillus subtilis, Salmonella typhimurium, Escherichia coli, and Pseudomonas aeruginosa. Anticancer activity on U87 and HEK 293 | [91,92] |
| Justicia glauca | Leaf extract | 1 mM chloroauric acid, room temperature, 10 min | 32 | Hexagonal and spherical | Antimicrobial effects against E. coli, Streptococcus mutans, Micrococcus luteus, S. aureus, S. cerevisiae, Bacillus subtilis, L. acidophilus, P. aeruginosa, and C. albicans | [93,94] |
| Lantana camara | Fruit extract | 0.2 mM AuCl4 room temperature (22–25 °C), 72 h | 150–300 | Triangular | Antibacterial efficacy against S. aureus, E. coli, Propionibacterium acnes, and P. aeruginosa | [48,49] |
| Linum usitatissimum | Seed extract | 1 mM HAuCl4·3H2O, room temperature (30 °C), 6 h | 3.4–5.7 | Spherical and triangular | Anticancer activity on MCF-7, HepG-2, HCT-116 | [53] |
| Lonicera japonica | Flower extract | HAuCl4 concentration (0.125, 0.5, 1, 1.5, and 2 mM), reaction temperature (40, 50, 60, 70, and 80 °C), reaction time (1, 1.5, 2, 2.5, and 3 min) | 8 | Triangular and tetrahedral | Anticancer activity on HeLa cells | [95] |
| Mangifera indica | Leaf extract | (5 × 10−4 M) HAuCl4·3H2O, room temperature, 2 min | 17–20 | Spherical | Anticancer activity on HeLa, MCF-7, Normal fibroblast | [56] |
| Mangifera indica Linn (Mango) | Peel extract | HAuCl4 (1.0 mM), incubation at 100 °C, 15 min | 3.26–21.68 | Quasi-spherical | Anticancer activity on CV-1 and WI-38 | [57] |
| Mimosa pudica | Leaf extract | 1 mM HAuCl4. 3H2O, 55 °C, 30 min. | 12 | Spherical | Anticancer activity on MDA-MB-231, MCF-7 and HMEC | [96] |
| Musa paradisiaca | Peel extract | 1 mM HAuCl4, 20 min | 50 | Spherical | Anticancer activity on human lung cancer cells (A549) | [97] |
| Murraya koenigii | Seed extract | 1 mM HAuCl4, 50 °C, room temperature, 10 min | 20–40 | Spherical | Antibacterial efficacy against S. aureus. P. aeruginosa and Enterococci | [22,54] |
| Nerium oleander | Stem/bark extract | 1 mM HAuCl4 room temperature (25 °C ± 2 °C), 24 h | 20–40 | Spherical, hexagonal, triangular, and rod shaped | Anticancer activity on MCF-7 cell lines | [58] |
| Padina gymnospora (marine Macroalgae) | Leaf extract | 1 mM HAuCl4, 30 °C, 45 °C, 55 °C, 65 °C, 75 °C, 85 °C and 95 °C, few minutes to hours | 14.10 ± 1.5 | Spherical | Anticancer activity on HepG2, A549, and 3T3 cell line | [98,99] |
| Platycodon grandiflorum | Leaf extract | HAuCl4·3H2O (1 mM), (20, 37, and 50 °C), 10 min | 15 | Spherical | Antibacterial application against E. coli, B. subtilis | [100] |
| Phragmites australis | Leaf extract | 1 mM HAuCl4, 85 °C for 1 h | 18 | Spherical | Anticancer activity on A549 cell line | [101] |
| Ricinus ommunis | Leaf extract | HAuCl4 (0.5 mM), 60 °C, 5 min | 40–80 | Spherical | Antibacterial activity against S. aureus, E. coli, P. mirabilis, S. flexneri, C. albicans. Anticancer activity on HT29 and SW480 Cancer Cell | [102] |
| Pistacia integerrima | Gall extract | 1 mM HAuCl4·3H2O, 37 °C, 24–72 h | 20–200 | Grain-like | Antibacterial activity against K. pneumonia | [103] |
| Sargassum swartzii | Seaweed | Chloroauric acid (1 mM HAuCl4), 60 °C, 5 min | 20–60 | Spherical and few hexagonal | Anticancer activity on HeLa | [104] |
| Terminalia arjuna | Peel extract | 1 mM HAuCl4, 80 °C, 15 min | 60 | Triangular, hexagonal, and pentagonal | Antibacterial activity against S. aureus, P. aeruginosa, S. typhimurium | [105] |
| Theobromo cacao | Seed extract | 1 mM HAuCl4, (30, 40, 50, 60, and 70 °C), 15 min | 150–200 | Spherical | Antibacterial activity against A431 cell line | [55,106] |
| Zataria multiflora | Leaf extract | 1 mM chloroauric acid (HAuCl4), room temperature, few minutes | 10–50 | Different shapes | Anticancer activity on HeLa and BMSCs cell line | [107] |
3. Microbe-Mediated Green Synthesis
| Microbes Used for Synthesis | Synthesis Method | Optimum Synthesis Conditions (Salt Concentration, Temperature, Incubation Time) | Size (nm) | Shape | Applications | Reference |
|---|---|---|---|---|---|---|
| Streptomyces sp. ASM19 | Extracellular | 1 mM HAuCl4, 37 °C for 24 h | 6.28 ± 0.78 to 100.2 ± 0.25 | Sphere-like form | Antimicrobial activity against Staphylococcus aureus and Escherichia coli, anticancer potency against liver, colon, breast, and oral carcinoma | [121] |
| Streptomyces monashensis MSK03 | Extracellular | 1 mM HAuCl4, 37 °C for 72 h | 7.1–40.0 | Spherical | Antibacterial activity against Pseudomonas aeruginosa and Acinetobacter baumannii | [122] |
| Alternaria alternate | Extracellular | 1 mM HAuCl4, room temperature for 24 h | 12–29 | Spherical, triangular, and hexagonal | Antibacterial application against E. coli and S. aureus | [123] |
| Aspergillus flavus | Extracellular | 10 mM HAuCl4, 30 °C for 2 min | 12 | Spherical | Anticancer agent against HepG2 and A549 cell lines | [124] |
| Aspergillus clavatus | Extracellular | 1 mM HAuCl4, room temperature for 48–72 h | 24.4 ± 11 | Triangular, spherical, and hexagonal | Antibacterial application against E. coli and S. aureus | [125] |
| Aspergillus foetidus | Extracellular | 1 mM HAuCl4, 75 ± 2 °C for 4 h | 30–50 | Spherical | Anticancer effect on A549 | [126,127] |
| Aspergillus niger | Extracellular | 1 mM HAuCl4, 25 °C for 72 h | 5.6 ± 12.8 | Spherical | Antibacterial application against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Anti larval application against mosquito larvae | [119,120] |
| Aspergillus sydowii | Extracellular | 3 mM HAuCl4, 27 °C for 72 h | 8.7–15.6 | Spherical | Antibacterial application against Staphylococcus aureus, Staphylococcus epidermidis | [128] |
| Bacillus flexus | Extracellular | 1 mM aqueous HAuCl4, room temperature for 2 h | 20 | Different shapes (irregular, spherical, and triangular) | Anticancer effect on MCF-7 | [129] |
| Bacillus megatherium | Extracellular | 10 mg/mL HAuCl4, 9 h for 26 °C | 1.9 ± 0.8 | Spherical | Antibacterial application against Staphylococcus aureus and Bacillus subtilis | [115] |
| Brevibacillus formosus | Extracellular | 1 mM HAuCl4, 37 °C for 24 h | 5–12 | Spherical | Antibacterial application against Escherichia coli, Staphylococcus aureus | [130] |
| Cladosporium sp. | Extracellular | 1 mM Chloroauric acid (HAuCl4), 12 h for 37 °C | 5–10 | Spherical | Anticancer application against MCF-7 | [131] |
| Caldicellulosiruptor changbaiensis | Extracellular | 500 μM HAuCl4·3H2O, 12 h for 75 °C | 20–60 | Spherical | Antibacterial efficacy against S. aureus and E. coli | [132,133] |
| Enterococcus sp. | Extracellular | 1 mM gold chloride, room temperature for 24–48 h | 6–13 | Spherical | Anticancer application against HepG2 and A549 cell | [134] |
| Fusarium solani | Extracellular | 1 mM HAuCl4, 28 °C, for 48 h | 15–35 | Spherical | Anticancer application against HEp2 and Vero cells | [135] |
| Fusarium oxysporum | Extracellular | 0.5 mM HAuCl4, 30 °C for 24 to 96 h | 10–40 | Spherical | Anticancer application against ZR-75-1, Daudi and PBMC | [136,137] |
| Humicola spp. | Extracellular | 1 mM HAuCl4, 50 °C for 96 h | 18–24 | Spherical | Anticancer application against NIH3T3 and MDA-MB-231 | [138,139] |
| Micrococcus yunnanensis | Extracellular | 1 mM HAuCl4, 30 °C for 24 h | 15–55 | Spherical | Anticancer application against U87, HT1080, PC12, Caco-2, MCF7, A549. Antibacterial application against B. subtilis, S. typhi, Micrococcus luteus, E. coli, K. pneumoniae, | [113] |
| Pseudomonas aeruginosa | Extracellular | 1 mM HAuCl4, 37 °C for 24 h | 40 ± 10 | Spherical | Antibacterial application against Enterococcus faecalis, S. aureus, and E. coli | [116] |
| Penicillium brevicompactum | Extracellular | 1 mM HAuCl4, 30 °C for 12–72 h | 10–120 | Spherical, Triangular, and hexagonal | Anticancer application against C2C12 | [140,141] |
| Pleurotus ostreatus | Extracellular | 2.5 mM HAuCl4, 37 °C for 24 h | 10–30 | Spherical and prism-shaped | Anticancer and synergistic antimicrobial activity against C. albicans, P. aeruginosa and S. aureus | [142,143] |
| Paracoccus haeundaensis | Extracellular | 2 mM HAuCl4·3H2O, 25 °C for 48 h | ~20 | Spherical | Anticancer application against HaCaT A549 | [144] |
| Rhodopseudomonas capsulata | Extracellular | 1 mM aqueous HAuCl4, room temperature for 48 h | 10–20 | Spherical | Antibacterial application against E. coli and S. aureus | [141,145] |
| Streptomyces sp. | Extracellular | 1 mM aqueous HAuCl4, 80 °C for 30 min | 10–50 | Spherical and triangular | Anticancer application against HeLa cell | [146] |
| Streptomyces viridogens | Intracellular | 1 mM HAuCl4, 28 °C for 120 h | 18–20 | Spherical | Antibacterial application against Escherichia coli, and S. aureus | [1,117] |
| Streptomyces hygroscopicus | Extracellular | 1 mM HAuCl4, 30 °C for 48 h | 10–20 | Antibacterial application against E. coli, S. typhimurium and S. aureus. | [147] | |
| Shewanella oneidensis | Extra cellular | 1 mM HAuCl4, 30 °C for 48 h | 12 ± 5 | Spherical | Antibacterial application against E. coli and S. aureus | [108,148] |
| Vibrio alginolyticus | Intracellular | 1 mM aqueous chloroauric acid (HAuCl4), 40 °C for 24 h | 50–100 | Irregular | Anticancer application against HCA-7 | [118] |
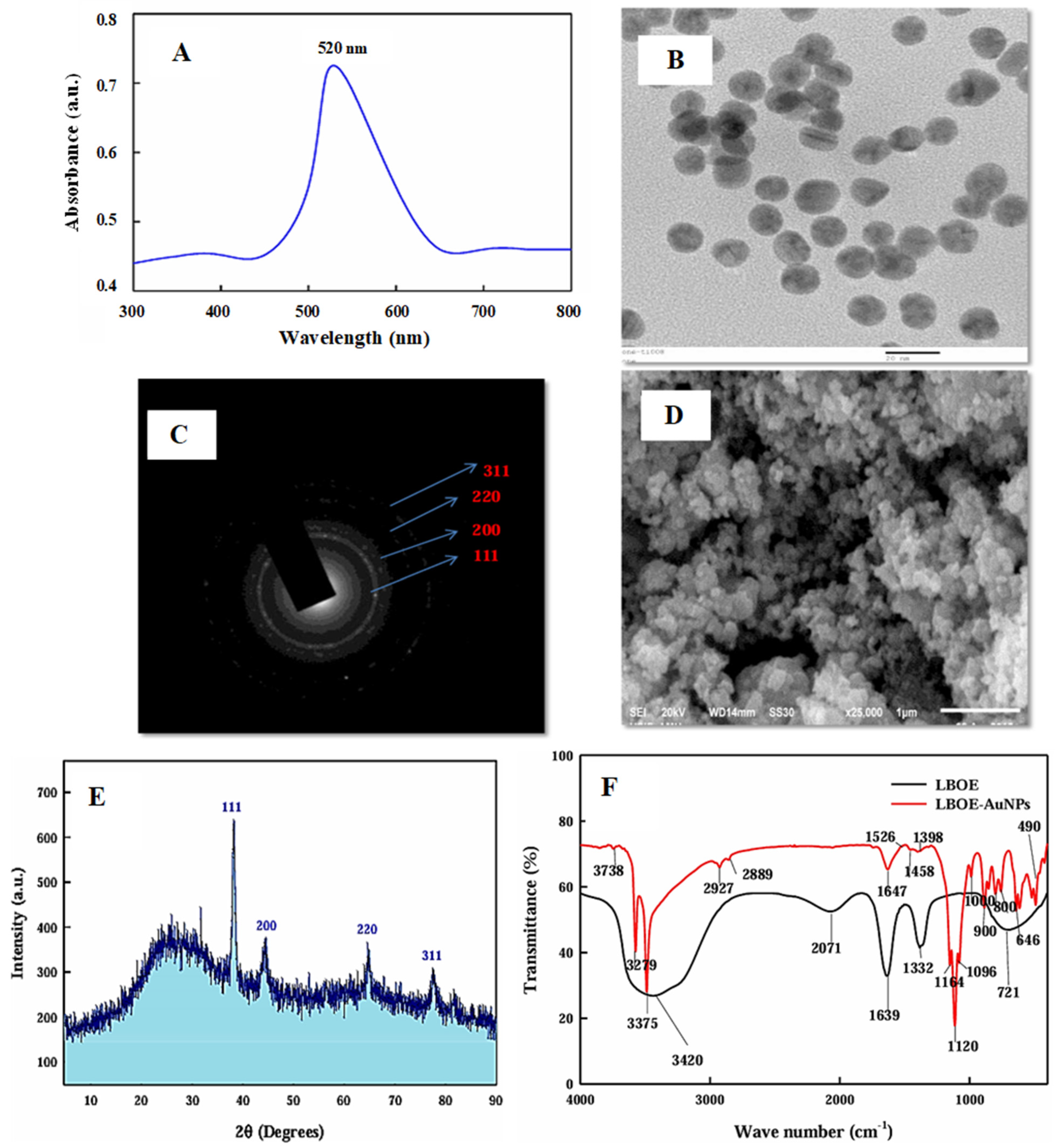
4. Characterization of Green Synthesized AuNPs
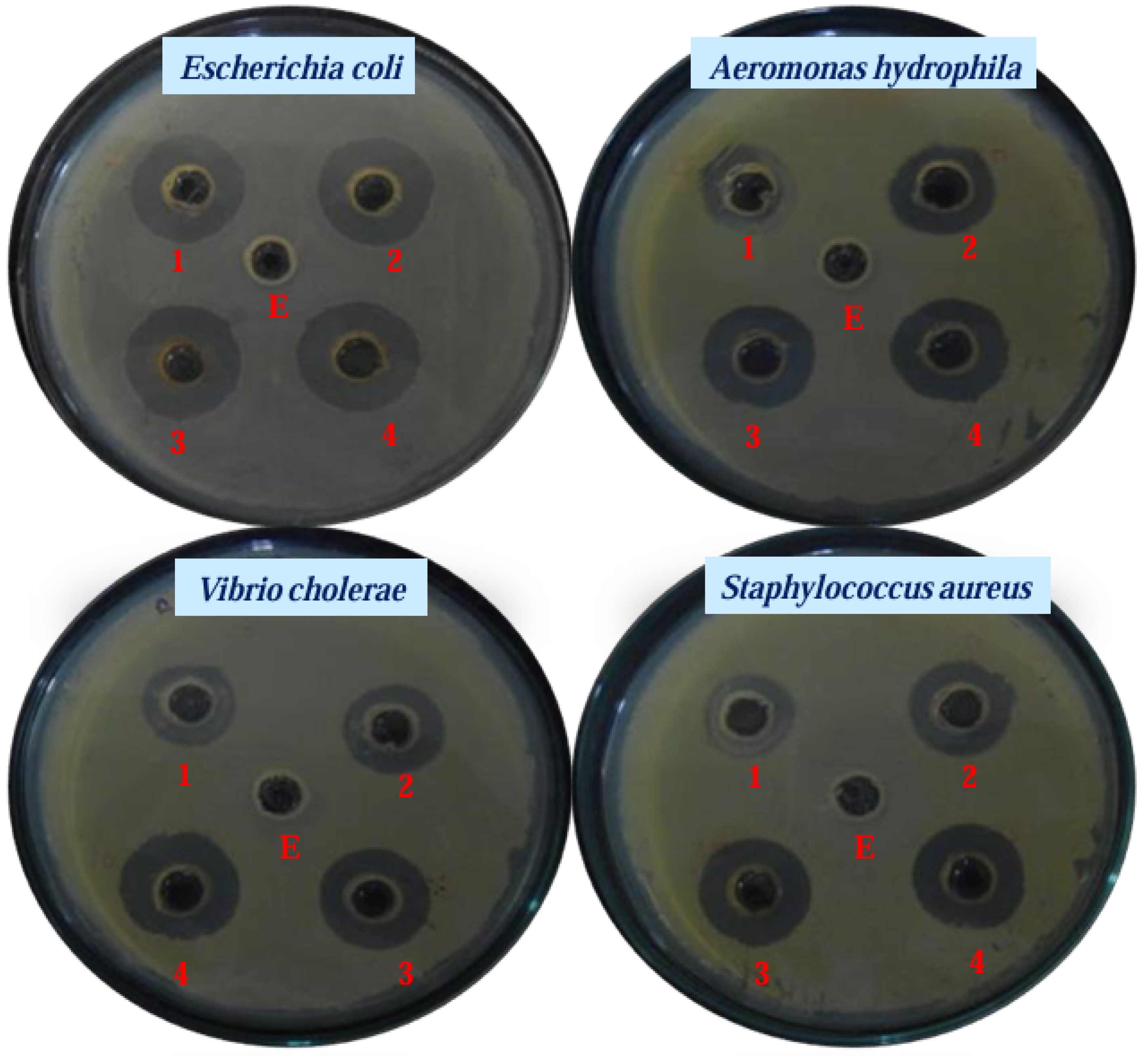
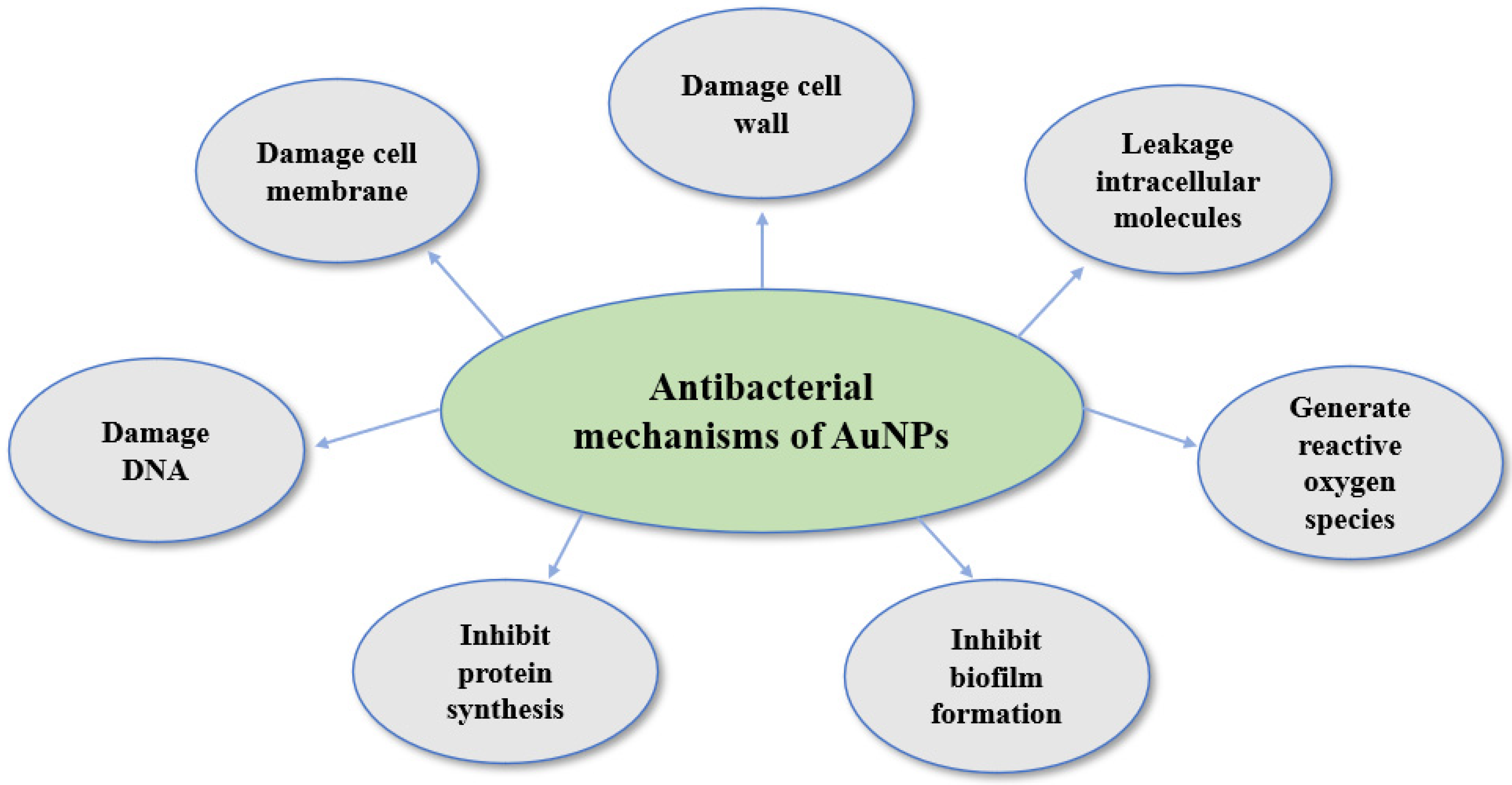
5. Antibacterial Applications and Mechanisms of Green-Synthesized AuNPs
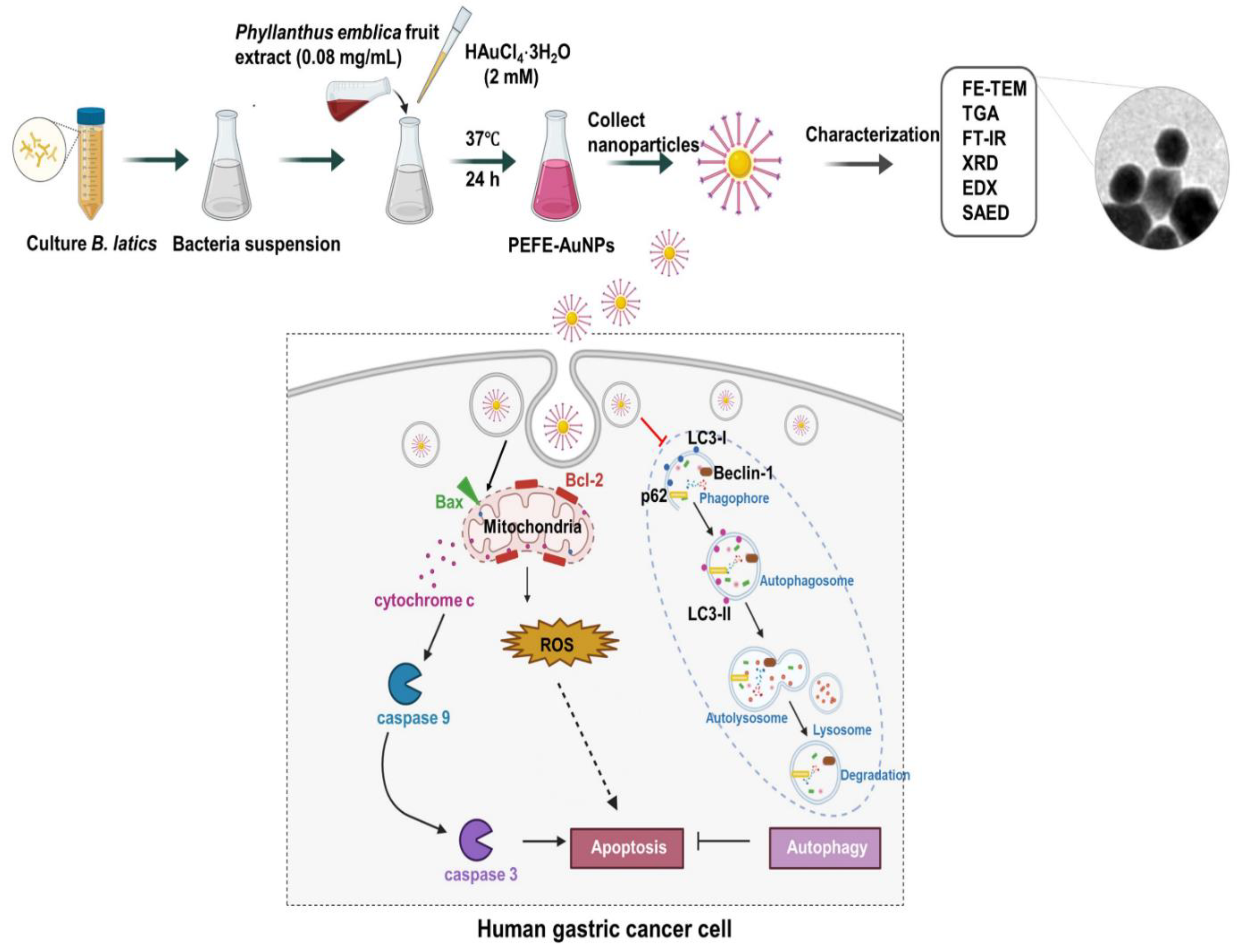
6. Anticancer Applications and Mechanisms of Green Synthesized AuNPs
7. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Abirami, B.; Akshata, V.; Radhakrishnan, M.; Namitha, R.; Govindaraju, K.; Gopikrishnan, V.; Manigundan, K. Characterization of biosynthesized gold nanoparticles from Streptomyces misionensis PYA9 with biomedical and environmental applications. Int. J. Agric. Technol. 2023, 19, 323–338. [Google Scholar]
- Shakoor, A.; Ferdous, U.T.; Khan, S.A.; Gulzar, M.M. Green Synthesis of Gold Nanoparticles Using Clerodendrum trichotomum Thunberg for Antibacterial and Anticancer Applications. Int. J. Nanomed. 2025, 20, 2645–2658. [Google Scholar] [CrossRef] [PubMed]
- Varshan, G.A.; Namasivayam, S.K.R. A Critical Review on Sustainable Formulation of Anti-quorum Sensing Compounds Using Nanotechnology Principles Against Candida albicans. BioNanoScience 2025, 15, 161. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: A review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Cherian, T.; Maity, D.; Rajendra Kumar, R.T.; Balasubramani, G.; Ragavendran, C.; Yalla, S.; Mohanraju, R.; Peijnenburg, W.J. Green chemistry based gold nanoparticles synthesis using the marine bacterium Lysinibacillus odysseyi PBCW2 and their multitudinous activities. Nanomaterials 2022, 12, 2940. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Wang, R.; Xu, X.; Puja, A.M.; Perumalsamy, H.; Balusamy, S.R.; Kim, H.; Kim, Y.-J. Gold nanoparticles prepared with Phyllanthus emblica fruit extract and Bifidobacterium animalis subsp. lactis can induce apoptosis via mitochondrial impairment with inhibition of autophagy in the human gastric carcinoma cell line AGS. Nanomaterials 2021, 11, 1260. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Huq, M.A.; Apu, M.A.I.; Ashrafudoulla, M.; Rahman, M.M.; Parvez, M.A.K.; Balusamy, S.R.; Akter, S.; Rahman, M.S. Bioactive ZnO nanoparticles: Biosynthesis, characterization and potential antimicrobial applications. Pharmaceutics 2023, 15, 2634. [Google Scholar] [CrossRef]
- Deepak, P.; Amutha, V.; Kamaraj, C.; Balasubramani, G.; Aiswarya, D.; Perumal, P. Chemical and green synthesis of nanoparticles and their efficacy on cancer cells. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–387. [Google Scholar]
- Huang, X.; Devi, S.; Bordiga, M.; Brennan, C.S.; Xu, B. Phenolic compounds mediated biosynthesis of gold nanoparticles and evaluation of their bioactivities: A review. Int. J. Food Sci. Technol. 2023, 58, 1673–1694. [Google Scholar] [CrossRef]
- Taha, R.H. Green synthesis of silver and gold nanoparticles and their potential applications as therapeutics in cancer therapy; a review. Inorg. Chem. Commun. 2022, 143, 109610. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A.; Akter, S. Biosynthesis, characterization and antibacterial application of novel silver nanoparticles against drug resistant pathogenic Klebsiella pneumoniae and Salmonella enteritidis. Molecules 2021, 26, 5996. [Google Scholar] [CrossRef]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef]
- Akter, S.; Lee, S.-Y.; Siddiqi, M.Z.; Balusamy, S.R.; Ashrafudoulla, M.; Rupa, E.J.; Huq, M.A. Eco-friendly synthesis of silver nanoparticles by Terrabacter humi sp. nov. and their antibacterial application against antibiotic-resistant pathogens. Int. J. Mol. Sci. 2020, 21, 9746. [Google Scholar] [CrossRef]
- Wang, X.; Lee, S.-Y.; Akter, S.; Huq, M.A. Probiotic-mediated biosynthesis of silver nanoparticles and their antibacterial applications against pathogenic strains of Escherichia coli O157:H7. Polymers 2022, 14, 1834. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Huq, M.A.; Akter, S. Characterization and genome analysis of Arthrobacter bangladeshi sp. nov., applied for the green synthesis of silver nanoparticles and their antibacterial efficacy against drug-resistant human pathogens. Pharmaceutics 2021, 13, 1691. [Google Scholar] [CrossRef]
- Pal, G.; Rai, P.; Pandey, A. Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Bhagat, D.S.; Gurnule, W.B.; Bumbrah, G.S.; Koinkar, P.; Chawla, P.A. Recent advances in biomedical applications of biogenic nanomaterials. Curr. Pharm. Biotechnol. 2023, 24, 86–100. [Google Scholar]
- Khatami, M.; Sharifi, I.; Nobre, M.A.; Zafarnia, N.; Aflatoonian, M.R. Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green. Chem. Lett. Rev. 2018, 11, 125–134. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Huq, M.A. Biologically rapid synthesis of silver nanoparticles by Sphingobium sp. MAH-11T and their antibacterial activity and mechanisms investigation against drug-resistant pathogenic microbes. Artif. Cells Nanomed. Biotechnol. 2020, 48, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Mayegowda, S.B.; Sarma, G.; Gadilingappa, M.N.; Alghamdi, S.; Aslam, A.; Refaat, B.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A. Green-synthesized nanoparticles and their therapeutic applications: A review. Green. Process. Synth. 2023, 12, 20230001. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Negi, A.; Bhattacharya, B.; Dey, T.; Mitra, P.; Preetam, S.; Kumar, L.; Kar, S.; Das, S.S.; Iqbal, D. Nanotheranostics to target antibiotic-resistant bacteria: Strategies and applications. OpenNano 2023, 11, 100138. [Google Scholar] [CrossRef]
- Huq, M.A. Biogenic silver nanoparticles synthesized by Lysinibacillus xylanilyticus MAHUQ-40 to control antibiotic-resistant human pathogens Vibrio parahaemolyticus and Salmonella Typhimurium. Front. Bioeng. Biotechnol. 2020, 8, 597502. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green synthesized silver nanoparticles: Antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Elgamouz, A.; Idriss, H.; Nassab, C.; Bihi, A.; Bajou, K.; Hasan, K.; Abu Haija, M.; Patole, S.P. Green synthesis, characterization, antimicrobial, anti-cancer, and optimization of colorimetric sensing of hydrogen peroxide of algae extract capped silver nanoparticles. Nanomaterials 2020, 10, 1861. [Google Scholar] [CrossRef]
- Mohamad Sukri, S.N.A.; Shameli, K.; Teow, S.-Y.; Chew, J.; Ooi, L.-T.; Lee-Kiun Soon, M.; Ismail, N.A.; Moeini, H. Enhanced antibacterial and anticancer activities of plant extract mediated green synthesized zinc oxide-silver nanoparticles. Front. Microbiol. 2023, 14, 1194292. [Google Scholar] [CrossRef]
- Qamar, M.; Ahmad, N.; Ismail, T.; Esatbeyoglu, T.; Akhtar, S.; Mubarak, M.S. Medicinal Uses, Phytochemistry, and Pharmacological Properties of Acorus calamus. In Aquatic Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2023; pp. 89–106. [Google Scholar]
- Muddapur, U.M.; Alshehri, S.; Ghoneim, M.M.; Mahnashi, M.H.; Alshahrani, M.A.; Khan, A.A.; Iqubal, S.S.; Bahafi, A.; More, S.S.; Shaikh, I.A. Plant-based synthesis of gold nanoparticles and theranostic applications: A review. Molecules 2022, 27, 1391. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A.; Ashrafudoulla, M.; Parvez, M.A.K.; Balusamy, S.R.; Rahman, M.M.; Kim, J.H.; Akter, S. Chitosan-coated polymeric silver and gold nanoparticles: Biosynthesis, characterization and potential antibacterial applications: A review. Polymers 2022, 14, 5302. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Tabassum, A.; Aftab, A.; Zahid, N.; Jamal, A.; Sajini, A.A.; Gul, A.; Ahmad, B. Artemisia vulgaris reduced and stabilized titanium oxide nanoparticles for anti-microbial, anti-fungal and anti-cancer activity. Appl. Nanosci. 2023, 13, 6165–6175. [Google Scholar] [CrossRef]
- Rani, N.; Singh, P.; Kumar, S.; Kumar, P.; Bhankar, V.; Kumar, K. Plant-mediated synthesis of nanoparticles and their applications: A review. Mater. Res. Bull. 2023, 163, 112233. [Google Scholar] [CrossRef]
- Dhaka, A.; Mali, S.C.; Sharma, S.; Trivedi, R. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Kumar, N.; Devra, V. Plant extract mediated synthesis of transition metal nanoparticles: A review. Int. J. Res. Appl. Sci. Eng. Technol. 2021, 9, 1988–1994. [Google Scholar] [CrossRef]
- Sundararajan, B.; Kumari, B.R. Novel synthesis of gold nanoparticles using Artemisia vulgaris L. leaf extract and their efficacy of larvicidal activity against dengue fever vector Aedes aegypti L. J. Trace Elem. Med. Biol. 2017, 43, 187–196. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Iyanna, N.; Lalley, J.; Han, C.; Dionysiou, D.D.; Varma, R.S. Synthesis of silver and gold nanoparticles using antioxidants from blackberry, blueberry, pomegranate, and turmeric extracts. ACS Sustain. Chem. Eng. 2014, 2, 1717–1723. [Google Scholar] [CrossRef]
- Majumdar, M.; Khan, S.A.; Biswas, S.C.; Roy, D.N.; Panja, A.S.; Misra, T.K. In vitro and in silico investigation of anti-biofilm activity of Citrus macroptera fruit extract mediated silver nanoparticles. J. Mol. Liq. 2020, 302, 112586. [Google Scholar] [CrossRef]
- Sujitha, M.V.; Kannan, S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 15–23. [Google Scholar] [CrossRef]
- Sengupta, S.; Saha, M.; Ghosh, N.R.; Bhattacharya, M.; Chatterjee, S.; Ghosh, R. Green synthesis of gold nano-conjugates using commonly used citrus species and evaluation of its In-vitro antibacterial efficacy against Staphylococcus aureus: A comparative study. Int. J. Herb. Med. 2023, 11, 38–43. [Google Scholar] [CrossRef]
- Yu, J.; Xu, D.; Guan, H.N.; Wang, C.; Huang, L.K.; Chi, D.F. Facile one-step green synthesis of gold nanoparticles using Citrus maxima aqueous extracts and its catalytic activity. Mater. Lett. 2016, 166, 110–112. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Camacho, J.; Hernández-Gallegos, E.; de Guadalupe Chávez-López, M.; Grijalva, M.; Andrade, K. One pot phytosynthesis of gold nanoparticles using Genipa americana fruit extract and its biological applications. Mater. Sci. Eng. C 2016, 62, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Dipankar, C.; Murugan, S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef]
- Hidayat, H.; Purwiandono, G.; Tohari, T.; Nugroho, B.H.; Jauhari, M.H.; Widyaputra, S.B.; Fatimah, I. Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract. Green. Process. Synth. 2022, 11, 1072–1082. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Extracellular biofabrication of gold nanoparticles by using Lantana camara berry extract. Inorg. Nano-Met. Chem. 2017, 47, 138–142. [Google Scholar] [CrossRef]
- Rahaman Mollick, M.M.; Bhowmick, B.; Mondal, D.; Maity, D.; Rana, D.; Dash, S.K.; Chattopadhyay, S.; Roy, S.; Sarkar, J.; Acharya, K.; et al. Anticancer (in vitro) and antimicrobial effect of gold nanoparticles synthesized using Abelmoschus esculentus (L.) pulp extract via a green route. RSC Adv. 2014, 4, 37838–37848. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Ramkumar, R.; Rahuman, A.A.; Perumal, P. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind. Crops Prod. 2013, 45, 423–429. [Google Scholar] [CrossRef]
- Donga, S.; Bhadu, G.R.; Chanda, S. Facile, Low Cost and Eco-Friendly Synthesis of Gold Nanoparticles Using Caesalpinia Crista Seed Extract and Evaluation of their Antimicrobial, Antioxidant and Anticancer Efficacies. In Applications of Gold Nanoparticles; Morrow, G.L., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2022; pp. 73–100. [Google Scholar]
- Al-Radadi, N.S. Green biosynthesis of flaxseed gold nanoparticles (Au-NPs) as potent anti-cancer agent against breast cancer cells. J. Saudi Chem. Soc. 2021, 25, 101243. [Google Scholar] [CrossRef]
- Ananth, S.; Induja, M.; Thangamathi, P.; Prabha, D.; Vinotha, K. In vitro antibacterial activity of biogenic gold nanoparticles from Murraya koenigii seed extract against pathogens associated with traumatic wound infections. Int. J. Fauna Biol. Stud. 2018, 5, 137–144. [Google Scholar]
- Dwivedi, K.; Mandal, A.K.; Afzal, O.; Altamimi, A.S.A.; Sahoo, A.; Alossaimi, M.A.; Almalki, W.H.; Alzahrani, A.; Barkat, M.A.; Almeleebia, T.M. Emergence of nano-based formulations for effective delivery of flavonoids against topical infectious disorders. Gels 2023, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; WeiHong, L.; Hao, L. Biosynthesis of Au nanoparticles using agricultural waste mango peel extract and its in vitro cytotoxic effect on two normal cells. Mater. Lett. 2014, 134, 67–70. [Google Scholar] [CrossRef]
- Barai, A.C.; Paul, K.; Dey, A.; Manna, S.; Roy, S.; Bag, B.G.; Mukhopadhyay, C. Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Converg. 2018, 5, 10. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Timoszyk, A.; Grochowalska, R. Mechanism and antibacterial activity of gold nanoparticles (AuNPs) functionalized with natural compounds from plants. Pharmaceutics 2022, 14, 2599. [Google Scholar] [CrossRef]
- Ahmad Kuthi, N.; Chandren, S.; Basar, N.; Jamil, M.S.S. Biosynthesis of gold nanoisotrops using Carallia brachiata leaf extract and their catalytic application in the reduction of 4-nitrophenol. Front. Chem. 2022, 9, 800145. [Google Scholar] [CrossRef]
- Mariychuk, R.; Grulova, D.; Grishchenko, L.M.; Linnik, R.P.; Lisnyak, V.V. Green synthesis of non-spherical gold nanoparticles using Solidago canadensis L. extract. Appl. Nanosci. 2020, 10, 4817–4826. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef]
- Princy, K.; Gopinath, A. Optimization of physicochemical parameters in the biofabrication of gold nanoparticles using marine macroalgae Padina tetrastromatica and its catalytic efficacy in the degradation of organic dyes. J. Nanostructure Chem. 2018, 8, 333–342. [Google Scholar] [CrossRef]
- Diksha, D.; Gupta, S.K.; Gupta, P.; Banerjee, U.C.; Kalita, D.; Gupta, S. Antibacterial potential of gold nanoparticles synthesized from leaf extract of Syzygium cumini against multidrug-resistant urinary tract pathogens. Cureus 2023, 15, e34830. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, F.; Momeni, M.; Barghbani, M.; Far, B.F.; Hosseinian, S.; Hosseini, S.M. Extract-mediated biosynthesis and characterization of gold nanoparticles: Exploring their protective effect against cyclophosphamide-induced oxidative stress in rat testis. J. Drug Deliv. Sci. Technol. 2022, 71, 103306. [Google Scholar] [CrossRef]
- Can, M. Green gold nanoparticles from plant-derived materials: An overview of the reaction synthesis types, conditions, and applications. Rev. Chem. Eng. 2020, 36, 859–877. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Labbaf, S.; Kermanpur, A. Eco-friendly synthesis of gold nanoparticles using henna extract: Toward medical applications. Mater. Lett. 2025, 388, 138310. [Google Scholar] [CrossRef]
- Wehbe, N.; Mesmar, J.E.; El Kurdi, R.; Al-Sawalmih, A.; Badran, A.; Patra, D.; Baydoun, E. Halodule uninervis extract facilitates the green synthesis of gold nanoparticles with anticancer activity. Sci. Rep. 2025, 15, 4286. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Mortazavi-Derazkola, S. Eco-friendly synthesis of gold nanoparticles via tangerine peel extract: Unveiling their multifaceted biological and catalytic potentials. Heliyon 2025, 11, e40104. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Xu, Q.; Khan, I.; Cao, X.; Yang, R.; Yan, H. Green synthesis of gold and silver nanoparticles using crude extract of Aconitum violaceum and evaluation of their antibacterial, antioxidant and photocatalytic activities. Front. Bioeng. Biotechnol. 2024, 11, 1320739. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.-S.; Lim, K.-J.; Patra, J.K. Bio-inspired synthesis of gold nanoparticles using leaf extract of Jamun and research on its biomedical potential. Int. J. Nanomed. 2024, 19, 12257–12286. [Google Scholar] [CrossRef]
- Kalantari, H.; Turner, R.J. Structural and antimicrobial properties of synthesized gold nanoparticles using biological and chemical approaches. Front. Chem. 2024, 12, 1482102. [Google Scholar] [CrossRef]
- Mata, R.; Nakkala, J.R.; Sadras, S.R. Biogenic silver nanoparticles from Abutilon indicum: Their antioxidant, antibacterial and cytotoxic effects in vitro. Colloids Surf. B Biointerfaces 2015, 128, 276–286. [Google Scholar] [CrossRef]
- Dharmatti, R.; Phadke, C.; Mewada, A.; Thakur, M.; Pandey, S.; Sharon, M. Biogenic gold nano-triangles: Cargos for anticancer drug delivery. Mater. Sci. Eng. C 2014, 44, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Vilas, V.; Philip, D. Studies on catalytic, antioxidant, antibacterial and anticancer activities of biogenic gold nanoparticles. J. Mol. Liq. 2015, 212, 331–339. [Google Scholar] [CrossRef]
- Dogara, A.M.; Hama, H.A.; Ozdemir, M. Biological evaluation of Acacia nilotica (L.) Willd. ex Delile: A systematic review. Adv. Tradit. Med. 2024, 24, 1–39. [Google Scholar] [CrossRef]
- Ganesan, R.; Prabu, H.G. Synthesis of gold nanoparticles using herbal Acorus calamus rhizome extract and coating on cotton fabric for antibacterial and UV blocking applications. Arab. J. Chem. 2019, 12, 2166–2174. [Google Scholar] [CrossRef]
- Vijayashree, I.; Niranjana, P.; Prabhu, G.; Sureshbabu, V.; Manjanna, J. Conjugation of Au nanoparticles with chlorambucil for improved anticancer activity. J. Clust. Sci. 2017, 28, 133–148. [Google Scholar] [CrossRef]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef]
- Kumar, V.G.; Gokavarapu, S.D.; Rajeswari, A.; Dhas, T.S.; Karthick, V.; Kapadia, Z.; Shrestha, T.; Barathy, I.; Roy, A.; Sinha, S. Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata. Colloids Surf. B Biointerfaces 2011, 87, 159–163. [Google Scholar] [CrossRef]
- Fatimah, I.; Citradewi, P.W.; Yahya, A.; Nugroho, B.H.; Hidayat, H.; Purwiandono, G.; Sagadevan, S.; Ghazali, S.A.I.S.M.; Ibrahim, S. Biosynthesized gold nanoparticles-doped hydroxyapatite as antibacterial and antioxidant nanocomposite. Mater. Res. Express 2021, 8, 115003. [Google Scholar] [CrossRef]
- Vanaraj, S.; Jabastin, J.; Sathiskumar, S.; Preethi, K. Production and characterization of bio-AuNPs to induce synergistic effect against multidrug resistant bacterial biofilm. J. Clust. Sci. 2017, 28, 227–244. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Fang, G.; Cao, Z.; Shang, Y.; Alfarraj, S.; Alharbi, S.A.; Li, J.; Yang, S.; Duan, X. Green synthesis, characterization, cytotoxicity, antioxidant, and anti-human ovarian cancer activities of Curcumae kwangsiensis leaf aqueous extract green-synthesized gold nanoparticles. Arab. J. Chem. 2021, 14, 103000. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of gold and silver nanoparticles using phytochemical compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, N.; Jamshidi, F. Plant-mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: Synthesis, characterization, and evaluation of anticancer and antibacterial activity. J. Appl. Biomed. 2016, 14, 235–245. [Google Scholar] [CrossRef]
- Yang, S.; Li, D.; Liu, W.; Chen, X. Polysaccharides from marine biological resources and their anticancer activity on breast cancer. RSC Med. Chem. 2023, 14, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Manivasagan, P.; Kim, S.-K.; Kirthi, A.V.; Marimuthu, S.; Rahuman, A.A. Marine algae-mediated synthesis of gold nanoparticles using a novel Ecklonia cava. Bioprocess. Biosyst. Eng. 2014, 37, 1591–1597. [Google Scholar] [CrossRef]
- Patel, A. Metal nanoparticles produced by plants with antibacterial properties against Staphylococcus aureus. Braz. J. Biol. 2023, 82, e268052. [Google Scholar] [CrossRef]
- Karthika, V.; Arumugam, A.; Gopinath, K.; Kaleeswarran, P.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Guazuma ulmifolia bark-synthesized Ag, Au and Ag/Au alloy nanoparticles: Photocatalytic potential, DNA/protein interactions, anticancer activity and toxicity against 14 species of microbial pathogens. J. Photochem. Photobiol. B Biol. 2017, 167, 189–199. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Zangeneh, A. Novel green synthesis of Hibiscus sabdariffa flower extract conjugated gold nanoparticles with excellent anti-acute myeloid leukemia effect in comparison to daunorubicin in a leukemic rodent model. Appl. Organomet. Chem. 2020, 34, e5271. [Google Scholar] [CrossRef]
- Mishra, P.; Ray, S.; Sinha, S.; Das, B.; Khan, M.I.; Behera, S.K.; Yun, S.-I.; Tripathy, S.K.; Mishra, A. Facile bio-synthesis of gold nanoparticles by using extract of Hibiscus sabdariffa and evaluation of its cytotoxicity against U87 glioblastoma cells under hyperglycemic condition. Biochem. Eng. J. 2016, 105, 264–272. [Google Scholar] [CrossRef]
- Balkrishna, A.; Rohela, A.; Kumar, A.; Mishra, S.; Arya, V.; Kala, V.; Thakur, N.; Thakur, N.; Kumari, A.; Khan, N. Elucidating the Role of Plant Extracts Mediated Gold Nanoparticles as Smart Antimicrobials: Two-Way Attack. J. Nanomater. 2023, 2023, 4085090. [Google Scholar] [CrossRef]
- Karuppiah, C.; Palanisamy, S.; Chen, S.-M.; Emmanuel, R.; Muthupandi, K.; Prakash, P. Green synthesis of gold nanoparticles and its application for the trace level determination of painter’s colic. RSC Adv. 2015, 5, 16284–16291. [Google Scholar] [CrossRef]
- Patil, M.P.; Bayaraa, E.; Subedi, P.; Piad, L.L.A.; Tarte, N.H.; Kim, G.-D. Biogenic synthesis, characterization of gold nanoparticles using Lonicera japonica and their anticancer activity on HeLa cells. J. Drug Deliv. Sci. Technol. 2019, 51, 83–90. [Google Scholar] [CrossRef]
- KS, U.S.; Govindaraju, K.; Prabhu, D.; Arulvasu, C.; Karthick, V.; Changmai, N. Anti-proliferative effect of biogenic gold nanoparticles against breast cancer cell lines (MDA-MB-231 & MCF-7). Appl. Surf. Sci. 2016, 371, 415–424. [Google Scholar]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Gopi, N.; Ekambaram, P.; Pachaiappan, R.; Velusamy, P.; Murugan, K.; Benelli, G.; Kumar, R.S. Therapeutic effects of gold nanoparticles synthesized using Musa paradisiaca peel extract against multiple antibiotic resistant Enterococcus faecalis biofilms and human lung cancer cells (A549). Microb. Pathog. 2017, 102, 173–183. [Google Scholar] [CrossRef]
- Chaudhary, V.; Chowdhury, R.; Thukral, P.; Pathania, D.; Saklani, S.; Rustagi, S.; Gautam, A.; Mishra, Y.K.; Singh, P.; Kaushik, A. Biogenic green metal nano systems as efficient anti-cancer agents. Environ. Res. 2023, 229, 115933. [Google Scholar] [CrossRef]
- Singh, M.; Saurav, K.; Majouga, A.; Kumari, M.; Kumar, M.; Manikandan, S.; Kumaraguru, A. The cytotoxicity and cellular stress by temperature-fabricated polyshaped gold nanoparticles using marine macroalgae, Padina gymnospora. Biotechnol. Appl. Biochem. 2015, 62, 424–432. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.; Jayanthi, S. Synthesis of gold nanoparticles using Platycodon grandiflorum extract and its antipathogenic activity under optimal conditions. Nanomater. Nanotechnol. 2020, 10, 1847980420961697. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Iku, S.I.; Ntwasa, M.; Nkambule, T.T.; Mamba, B.B.; Msagati, T.A. Doxorubicin conjugated hydrophilic AuPt bimetallic nanoparticles fabricated from Phragmites australis: Characterization and cytotoxic activity against human cancer cells. J. Drug Deliv. Sci. Technol. 2020, 57, 101749. [Google Scholar] [CrossRef]
- Soto, K.M.; Luzardo-Ocampo, I.; López-Romero, J.M.; Mendoza, S.; Loarca-Piña, G.; Rivera-Muñoz, E.M.; Manzano-Ramírez, A. Gold nanoparticles synthesized with common mullein (Verbascum thapsus) and castor bean (Ricinus communis) ethanolic extracts displayed antiproliferative effects and induced caspase 3 activity in human HT29 and SW480 cancer cells. Pharmaceutics 2022, 14, 2069. [Google Scholar] [CrossRef]
- Islam, N.U.; Jalil, K.; Shahid, M.; Muhammad, N.; Rauf, A. Pistacia integerrima gall extract mediated green synthesis of gold nanoparticles and their biological activities. Arab. J. Chem. 2019, 12, 2310–2319. [Google Scholar] [CrossRef]
- Dhas, T.S.; Kumar, V.G.; Karthick, V.; Govindaraju, K.; Narayana, T.S. Biosynthesis of gold nanoparticles using Sargassum swartzii and its cytotoxicity effect on HeLa cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 102–106. [Google Scholar] [CrossRef]
- Suganthy, N.; Sri Ramkumar, V.; Pugazhendhi, A.; Benelli, G.; Archunan, G. Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: Assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ. Sci. Pollut. Res. 2018, 25, 10418–10433. [Google Scholar] [CrossRef] [PubMed]
- Fazal, S.; Jayasree, A.; Sasidharan, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Green synthesis of anisotropic gold nanoparticles for photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2014, 6, 8080–8089. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Ramezani, T.; Divsalar, A.; Mousavi, M.; Seyedarabi, A. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J. Med. Biotechnol. 2016, 8, 75. [Google Scholar]
- Nadeem, M.; Pervez, L.; Khan, A.M.; Burton, R.A.; Ullah, S.; Nadhman, A.; Celli, J. Microbial-mediated synthesis of gold nanoparticles—Current insights and future vistas. Gold. Bull. 2023, 56, 69–81. [Google Scholar] [CrossRef]
- Singh, N.A.; Narang, J.; Garg, D.; Jain, V.; Payasi, D.; Suleman, S.; Swami, R.K. Nanoparticles synthesis via microorganisms and their prospective applications in agriculture. Plant Nano Biol. 2023, 5, 100047. [Google Scholar] [CrossRef]
- Varimadugu, A.; CVS, A.; Kansoth, A.N.; Mokkapati, V.; Koodalingam, D.; Salla, S. Microbial synthesis of gold nanoparticles. In Microbial Processes for Synthesizing Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 29–59. [Google Scholar]
- Menon, K.G.; Reddy, K.V.; Ranjit, P.; Sree, N.R.S. Microbial enzymes in nanoparticle synthesis. In Microbial Processes for Synthesizing Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 153–175. [Google Scholar]
- Patil, T.; Gambhir, R.; Vibhute, A.; Tiwari, A.P. Gold nanoparticles: Synthesis methods, functionalization and biological applications. J. Clust. Sci. 2023, 34, 705–725. [Google Scholar] [CrossRef]
- Jafari, M.; Rokhbakhsh-Zamin, F.; Shakibaie, M.; Moshafi, M.H.; Ameri, A.; Rahimi, H.R.; Forootanfar, H. Cytotoxic and antibacterial activities of biologically synthesized gold nanoparticles assisted by Micrococcus yunnanensis strain J2. Biocatal. Agric. Biotechnol. 2018, 15, 245–253. [Google Scholar] [CrossRef]
- Malarkodi, C.; Rajeshkumar, S.; Vanaja, M.; Paulkumar, K.; Gnanajobitha, G.; Annadurai, G. Eco-friendly synthesis and characterization of gold nanoparticles using Klebsiella pneumoniae. J. Nanostructure Chem. 2013, 3, 30. [Google Scholar] [CrossRef]
- Wen, L.; Lin, Z.; Gu, P.; Zhou, J.; Yao, B.; Chen, G.; Fu, J. Extracellular biosynthesis of monodispersed gold nanoparticles by a SAM capping route. J. Nanoparticle Res. 2009, 11, 279–288. [Google Scholar] [CrossRef]
- Husseiny, M.; Abd El-Aziz, M.; Badr, Y.; Mahmoud, M. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef]
- Balagurunathan, R.; Radhakrishnan, M.; Rajendran, R.B.; Velmurugan, D. Biosynthesis of gold nanoparticles by actinomycete Streptomyces viridogens strain HM10. Indian J. Biochem. Biophys. 2011, 48, 331–335. [Google Scholar] [PubMed]
- Shunmugam, R.; Balusamy, S.R.; Kumar, V.; Menon, S.; Lakshmi, T.; Perumalsamy, H. Biosynthesis of gold nanoparticles using marine microbe (Vibrio alginolyticus) and its anticancer and antioxidant analysis. J. King Saud. Univ. Sci. 2021, 33, 101260. [Google Scholar] [CrossRef]
- Alzamily, I.A.; Al-Shibly, M.K.; Ali, Z.A.H. Biosynthesis of Gold Nanoparticles by Aspergillus Niger And Their Role Against Pathogenic Bacteria. Clin. Med. Health Res. J. 2023, 3, 513–518. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Synthesis of gold nanoparticles by the fungus Aspergillus niger and its efficacy against mosquito larvae. Rep. Parasitol. 2012, 2, 1–7. [Google Scholar]
- Aati, S.; Aati, H.Y.; Hamed, A.A.; El-Shamy, S.; Aati, S.H.; Abdelmohsen, U.R.; Bringmann, G.; Taha, M.N.; Hassan, H.M.; Bahr, H.S. Gold nanoparticles synthesized from soil-derived Streptomyces sp. ASM19: Characterization, antimicrobial, anticancer potency targeted G2/M phase cell-cycle arrest, and in silico studies. RSC Adv. 2025, 15, 3954–3968. [Google Scholar] [CrossRef]
- Kerdtoob, S.; Chanthasena, P.; Rosyidah, A.l.; Limphirat, W.; Penkhrue, W.; Ganta, P.; Srisakvarangkool, W.; Yasawong, M.; Nantapong, N. Streptomyces monashensis MSK03-mediated synthesis of gold nanoparticles: Characterization and antibacterial activity. RSC Adv. 2024, 14, 4778–4787. [Google Scholar] [CrossRef]
- Kamal, M.; Abdel-Raouf, N.; Sonbol, H.; Abdel-Tawab, H.; Abdelhameed, M.S.; Hammouda, O.; Elsayed, K.N. In vitro assessment of antimicrobial, anti-inflammatory, and schistolarvicidal activity of macroalgae-based gold nanoparticles. Front. Mar. Sci. 2022, 9, 1075832. [Google Scholar] [CrossRef]
- Abu-Tahon, M.A.; Ghareib, M.; Abdallah, W.E. Environmentally benign rapid biosynthesis of extracellular gold nanoparticles using Aspergillus flavus and their cytotoxic and catalytic activities. Process Biochem. 2020, 95, 1–11. [Google Scholar] [CrossRef]
- Verma, V.C.; Singh, S.K.; Solanki, R.; Prakash, S. Biofabrication of anisotropic gold nanotriangles using extract of endophytic Aspergillus clavatus as a dual functional reductant and stabilizer. Nanoscale Res. Lett. 2011, 6, 1–7. [Google Scholar] [CrossRef]
- Brandelli, A.; Veras, F.F. Biosynthesis of Gold Nanoparticles by Fungi. In Mycosynthesis of Nanomaterials: Perspectives and Challenges; Rai, M., Golinska, P., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 143–169. [Google Scholar]
- Roy, S.; Das, T.K.; Maiti, G.P.; Basu, U. Microbial biosynthesis of nontoxic gold nanoparticles. Mater. Sci. Eng. B 2016, 203, 41–51. [Google Scholar] [CrossRef]
- Vala, A.K. Exploration on green synthesis of gold nanoparticles by a marine-derived fungus Aspergillus sydowii. Environ. Prog. Sustain. Energy 2015, 34, 194–197. [Google Scholar] [CrossRef]
- Murugan, M.; Anthony, K.J.P.; Jeyaraj, M.; Rathinam, N.K.; Gurunathan, S. Biofabrication of gold nanoparticles and its biocompatibility in human breast adenocarcinoma cells (MCF-7). J. Ind. Eng. Chem. 2014, 20, 1713–1719. [Google Scholar] [CrossRef]
- Srinath, B.; Namratha, K.; Byrappa, K. Eco-friendly synthesis of gold nanoparticles by gold mine bacteria Brevibacillus formosus and their antibacterial and biocompatible studies. IOSR J. Pharm. 2017, 7, 53–60. [Google Scholar]
- Munawer, U.; Raghavendra, V.B.; Ningaraju, S.; Krishna, K.L.; Ghosh, A.R.; Melappa, G.; Pugazhendhi, A. Biofabrication of gold nanoparticles mediated by the endophytic Cladosporium species: Photodegradation, in vitro anticancer activity and in vivo antitumor studies. Int. J. Pharm. 2020, 588, 119729. [Google Scholar] [CrossRef]
- Bing, W.; Sun, H.; Wang, F.; Song, Y.; Ren, J. Hydrogen-producing hyperthermophilic bacteria synthesized size-controllable fine gold nanoparticles with excellence for eradicating biofilm and antibacterial applications. J. Mater. Chem. B 2018, 6, 4602–4609. [Google Scholar] [CrossRef]
- Facal Marina, P.; Kaul, L.; Mischer, N.; Richter, K. Metal-Based Nanoparticles for Biofilm Treatment and Infection Control: From Basic Research to Clinical Translation. Antibiofilm Strateg. Curr. Future Appl. Prev. Control Eradicate Biofilms 2022, 11, 467–500. [Google Scholar]
- Rajeshkumar, S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genet. Eng. Biotechnol. 2016, 14, 195–202. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.-C.; Al Khulaifi, M.M.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K. Mycosynthesis of metal-based nanoparticles and their perspectives in agri-food and veterinary/medical applications. In Fungal Cell Factories for Sustainable Nanomaterials Productions and Agricultural Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 423–482. [Google Scholar]
- Naimi-Shamel, N.; Pourali, P.; Dolatabadi, S. Green synthesis of gold nanoparticles using Fusarium oxysporum and antibacterial activity of its tetracycline conjugant. J. Mycol. Medicale 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Syed, A.; Al Saedi, M.H.; Bahkali, A.H.; Elgorgan, A.M.; Kharat, M.; Pai, K.; Pichtel, J.; Ahmad, A. αAu2S nanoparticles: Fungal-mediated synthesis, structural characterization and bioassay. Green. Chem. Lett. Rev. 2022, 15, 61–70. [Google Scholar] [CrossRef]
- Santra, T.S.; Tseng, F.-G.; Barik, T.K. Green biosynthesis of gold nanoparticles and biomedical applications. Am. J. Nano Res. Appl. 2014, 2, 5–12. [Google Scholar]
- Mishra, A.; Tripathy, S.K.; Wahab, R.; Jeong, S.-H.; Hwang, I.; Yang, Y.-B.; Kim, Y.-S.; Shin, H.-S.; Yun, S.-I. Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C2C12 cells. Appl. Microbiol. Biotechnol. 2011, 92, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, N.; Lakshmi, L.V.; Shankar, A.S.; Basha, P.O. Biosynthesis, Characterization and Applications of Gold Nanoparticles. In Microbial Processes for Synthesizing Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 61–79. [Google Scholar]
- Rai, S.N.; Mishra, D.; Singh, P.; Singh, M.P.; Vamanu, E.; Petre, A. Biosynthesis and bioapplications of nanomaterials from mushroom products. Curr. Pharm. Des. 2023, 29, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- El Domany, E.B.; Essam, T.M.; Ahmed, A.E.; Farghali, A.A. Biosynthesis physico-chemical optimization of gold nanoparticles as anti-cancer and synergetic antimicrobial activity using Pleurotus ostreatus fungus. J. Appl. Pharm. Sci. 2018, 8, 119–128. [Google Scholar]
- Patil, M.P.; Kang, M.-j.; Niyonizigiye, I.; Singh, A.; Kim, J.-O.; Seo, Y.B.; Kim, G.-D. Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf. B Biointerfaces 2019, 183, 110455. [Google Scholar] [CrossRef]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Manivasagan, P.; Oh, J. Production of a novel fucoidanase for the green synthesis of gold nanoparticles by Streptomyces sp. and its cytotoxic effect on HeLa cells. Mar. Drugs 2015, 13, 6818–6837. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Shanmugam, P.; Veerapandian, M.; Subbiah, R.; Yun, K. Biogenic synthesis of multidimensional gold nanoparticles assisted by Streptomyces hygroscopicus and its electrochemical and antibacterial properties. Biometals 2012, 25, 351–360. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Broich, M.L.; Moon, J.-W.; Gu, B.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater. 2011, 7, 2148–2152. [Google Scholar] [CrossRef]
- Huq, M.A.; Khan, A.A.; Alshehri, J.M.; Rahman, M.S.; Balusamy, S.R.; Akter, S. Bacterial mediated green synthesis of silver nanoparticles and their antibacterial and antifungal activities against drug-resistant pathogens. R. Soc. Open Sci. 2023, 10, 230796. [Google Scholar] [CrossRef]
- Abdulwahed, S.H.; Alias, M.; MohammedHasan, Z. Green Synthesis and Characterization of Gold Nanoparticles from Malus viridisand Capsicum annuum as AnticancerAgent. J. Phys. Conf. Ser. 2021, 2114, 012088. [Google Scholar] [CrossRef]
- Kureshi, A.A.; Vaghela, H.M.; Kumar, S.; Singh, R.; Kumari, P. Green synthesis of gold nanoparticles mediated by Garcinia fruits and their biological applications. Pharm. Sci. 2020, 27, 238–250. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Lokina, S.; Suresh, R.; Giribabu, K.; Stephen, A.; Sundaram, R.L.; Narayanan, V. Spectroscopic investigations, antimicrobial, and cytotoxic activity of green synthesized gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 484–490. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Lee, B.; Lee, D.G. Synergistic antibacterial activity of gold nanoparticles caused by apoptosis-like death. J. Appl. Microbiol. 2019, 127, 701–712. [Google Scholar] [CrossRef]
- Mahdi, H.S.; Parveen, A. Biosynthesis, Characterization and antibacterial activity of gold nanoparticles (Au-NPs) using black lemon extract. Mater. Today Proc. 2019, 18, 5164–5169. [Google Scholar] [CrossRef]
- Dasari, T.S.; Zhang, Y.; Yu, H. Antibacterial activity and cytotoxicity of gold (I) and (III) ions and gold nanoparticles. Biochem. Pharmacol. Open Access 2015, 4, 199. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Q.; Wang, X.; Li, L. Preparation of hybrid gold/polymer nanocomposites and their application in a controlled antibacterial assay. ACS Appl. Mater. Interfaces 2016, 8, 29101–29109. [Google Scholar] [CrossRef]
- Khan, a.S.; Bakht, J.; Syed, F. Green synthesis of gold nanoparticles using Acer pentapomicum leaves extract its characterization, antibacterial, antifungal and antioxidant bioassay. Dig. J. Nanomater. Biostruct 2018, 13, 579–589. [Google Scholar]
- Mahmoud, N.N.; Alhusban, A.A.; Ali, J.I.; Al-Bakri, A.G.; Hamed, R.; Khalil, E.A. Preferential accumulation of phospholipid-PEG and cholesterol-PEG decorated gold nanorods into human skin layers and their photothermal-based antibacterial activity. Sci. Rep. 2019, 9, 5796. [Google Scholar] [CrossRef] [PubMed]
- Khedr, W.E.; Shaheen, M.N.; Elmahdy, E.M.; El-Bendary, M.A.; Hamed, A.A.; Mohamedin, A.H. Silver and gold nanoparticles: Eco-friendly synthesis, antibiofilm, antiviral, and anticancer bioactivities. Prep. Biochem. Biotechnol. 2024, 54, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Aljarba, N.H.; Imtiaz, S.; Anwar, N.; Alanazi, I.S.; Alkahtani, S. Anticancer and microbial activities of gold nanoparticles: A mechanistic review. J. King Saud. Univ.-Sci. 2022, 34, 101907. [Google Scholar] [CrossRef]
- Mi, X.-j.; Park, H.-R.; Dhandapani, S.; Lee, S.; Kim, Y.-J. Biologically synthesis of gold nanoparticles using Cirsium japonicum var. maackii extract and the study of anti-cancer properties on AGS gastric cancer cells. Int. J. Biol. Sci. 2022, 18, 5809. [Google Scholar] [CrossRef]
- Muthukumar, T.; Sambandam, B.; Aravinthan, A.; Sastry, T.P.; Kim, J.-H. Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem. 2016, 51, 384–391. [Google Scholar] [CrossRef]
- Majumdar, M.; Biswas, S.C.; Choudhury, R.; Upadhyay, P.; Adhikary, A.; Roy, D.N.; Misra, T.K. Synthesis of gold nanoparticles using citrus macroptera fruit extract: Anti-biofilm and anticancer activity. ChemistrySelect 2019, 4, 5714–5723. [Google Scholar] [CrossRef]
- Arunachalam, K.D.; Arun, L.B.; Annamalai, S.K.; Arunachalam, A.M. Biofunctionalized gold nanoparticles synthesis from Gymnema sylvestre and its preliminary anticancer activity. Int. J. Pharm. Pharm. Sci. 2014, 6, 423–430. [Google Scholar]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Li, F. Synthesis of gold nanoparticles from leaf Panax notoginseng and its anticancer activity in pancreatic cancer PANC-1 cell lines. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1216–1223. [Google Scholar] [CrossRef]
- Patil, M.P.; Jin, X.; Simeon, N.C.; Palma, J.; Kim, D.; Ngabire, D.; Kim, N.-H.; Tarte, N.H.; Kim, G.-D. Anticancer activity of Sasa borealis leaf extract-mediated gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 82–88. [Google Scholar] [CrossRef]
- Sun, B.; Hu, N.; Han, L.; Pi, Y.; Gao, Y.; Chen, K. Anticancer activity of green synthesised gold nanoparticles from Marsdenia tenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4012–4019. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Duan, X.; Hu, C.; Wu, C.; Chen, X.; Huang, J.; Liu, J.; Cui, S. Synthesis and characterization of gold nanoparticles from Abies spectabilis extract and its anticancer activity on bladder cancer T24 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Su, W.; Wang, Y.; Dang, M.; Zhang, W.; Wang, C. Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, K.; Vasantharaja, R.; Suganya, K.U.; Anbarasu, S.; Revathy, K.; Pugazhendhi, A.; Karthickeyan, D.; Singaravelu, G. Unveiling the anticancer and antimycobacterial potentials of bioengineered gold nanoparticles. Process Biochem. 2020, 96, 213–219. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Razmjou, A. Gold nanoparticles as potent anticancer agent: Green synthesis, characterization, and in vitro study. RSC Adv. 2016, 6, 63973–63983. [Google Scholar] [CrossRef]
- Tan, G.; Tevlek, A.; Aydin, H.M. Comparison of garlic and onion extract-derived gold nanoparticles: Characterization and anticancer activity. J. Drug Deliv. Sci. Technol. 2023, 84, 104542. [Google Scholar] [CrossRef]
- Palaniyandi, T.; Viswanathan, S.; Prabhakaran, P.; Baskar, G.; Wahab, M.R.A.; Sivaji, A.; Ravi, M.; Rajendran, B.K.; Moovendhan, M.; Surendran, H. Green synthesis of gold nanoparticles using Halymenia pseudofloresii extracts and their antioxidant, antimicrobial, and anti-cancer activities. Biomass Convers. Biorefinery 2023, 1–12. [Google Scholar] [CrossRef]
- Abdulateef, S.; Raypah, M.E.; Omar, A.; Jafri, M.M.; Ahmed, N.M.; Kaus, N.H.M.; Seeni, A.; Mail, M.H.; Tabana, Y.; Ahmed, M. Rapid synthesis of bovine serum albumin-conjugated gold nanoparticles using pulsed laser ablation and their anticancer activity on hela cells. Arab. J. Chem. 2023, 16, 104395. [Google Scholar] [CrossRef]
- Viswanathan, S.; Palaniyandi, T.; Chellam, D.C.; Ahmed, M.F.; Shoban, N.; Pushpakumar, M.; Wahab, M.R.A.; Baskar, G.; Ravi, M.; Sivaji, A. Anti-cancer activity of Hypnea valentiae seaweed loaded gold nanoparticles through EMT signaling pathway in A549 cells. Biochem. Syst. Ecol. 2023, 107, 104606. [Google Scholar] [CrossRef]
- Gawali, P.; Ramteke, L.; Jadhav, B.; Khade, B.S. Trypsin conjugated Au nanoparticles using Sonneratia alba fruits: Interaction and binding studies with antioxidant, anti-inflammatory, and anticancer activities. J. Clust. Sci. 2023, 34, 2759–2779. [Google Scholar] [CrossRef]
- Nisa, S.A.; Govindaraju, K.; Vasantharaja, R.; Kannan, M.; Karthickeyan, D. Biosynthesis of gold nanoparticles using jellyfish nematocyst venom protein and evaluation of their anticancer activity against breast cancer cells: In-vitro study. Chem. Phys. Lett. 2023, 823, 140523. [Google Scholar] [CrossRef]
- Pomal, N.C.; Bhatt, K.D.; Kundariya, D.S.; Desai, R.A.; Bhatt, V.; Kongor, A. Calix [4] pyrrole-Grafted Gold Nanoparticles as a Turn-On Fluorescence Sensor for Noxious Fungicide Dimoxystrobin and Their Anti-Cancer Activity against the KB-3-1 Cell Line. ChemistrySelect 2023, 8, e202204252. [Google Scholar] [CrossRef]
- Susanna, D.; Balakrishnan, R.M.; Ettiyappan, J.P. Ultrasonication-assisted green synthesis and characterization of gold nanoparticles from Nothapodytes foetida: An assessment of their antioxidant, antibacterial, anticancer and wound healing potential. J. Drug Deliv. Sci. Technol. 2023, 87, 104740. [Google Scholar] [CrossRef]
- Hammad, S.E.; El-Rouby, M.N.; Abdel-Aziz, M.M.; El-Sayyad, G.S.; Elshikh, H.H. Endophytic fungi–assisted biomass synthesis of gold, and zinc oxide nanoparticles for increasing antibacterial, and anticancer activities. Biomass Convers. Biorefinery 2025, 15, 2285–2302. [Google Scholar] [CrossRef]
- Das, C.A.; Kumar, V.G.; Dharani, G.; Dhas, T.S.; Karthick, V.; Kumar, C.V.; Embrandiri, A. Macroalgae-associated halotolerant marine bacteria Exiguobacterium aestuarii ADCG SIST3 synthesized gold nanoparticles and its anticancer activity in breast cancer cell line (MCF-7). J. Mol. Liq. 2023, 383, 122061. [Google Scholar]
- Singh, A.K.; Tiwari, R.; Singh, V.K.; Singh, P.; Khadim, S.R.; Singh, U.; Srivastava, V.; Hasan, S.; Asthana, R. Green synthesis of gold nanoparticles from Dunaliella salina, its characterization and in vitro anticancer activity on breast cancer cell line. J. Drug Deliv. Sci. Technol. 2019, 51, 164–176. [Google Scholar] [CrossRef]
- Ajdari, Z.; Rahman, H.; Shameli, K.; Abdullah, R.; Abd Ghani, M.; Yeap, S.; Abbasiliasi, S.; Ajdari, D.; Ariff, A. Novel gold nanoparticles reduced by Sargassum glaucescens: Preparation, characterization and anticancer activity. Molecules 2016, 21, 123. [Google Scholar] [CrossRef]
- Mmola, M.; Roes-Hill, M.L.; Durrell, K.; Bolton, J.J.; Sibuyi, N.; Meyer, M.E.; Beukes, D.R.; Antunes, E. Enhanced antimicrobial and anticancer activity of silver and gold nanoparticles synthesised using Sargassum incisifolium aqueous extracts. Molecules 2016, 21, 1633. [Google Scholar] [CrossRef]
- Ye, H.; Shen, Z.; Yu, L.; Wei, M.; Li, Y. Manipulating nanoparticle transport within blood flow through external forces: An exemplar of mechanics in nanomedicine. Proc. R. Soc. A Math. Phys. Eng. Sci. 2018, 474, 20170845. [Google Scholar] [CrossRef]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.-J.; Menichetti, S.; Rotello, V.M. Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [Google Scholar] [CrossRef]
- Puja, A.M.; Xu, X.; Wang, R.; Kim, H.; Kim, Y.-J. Ginsenoside compound K-loaded gold nanoparticles synthesized from Curtobacterium proimmune K3 exerts anti-gastric cancer effect via promoting PI3K/Akt-mediated apoptosis. Cancer Nanotechnol. 2022, 13, 27. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, G.-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, R.; Song, J.G.; Patil, B.R.; Lee, S.H.; Noh, H.-M.; Kim, D.-H.; Kim, G.-L.; Seo, S.-H.; Park, J.-W.-W.; Jeong, S.H. Functional ligands for improving anticancer drug therapy: Current status and applications to drug delivery systems. Drug Deliv. 2022, 29, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.F.; In, L.L.A.; Vijayaraj Kumar, P. Surface functionalization of gold nanoparticles for targeting the tumor microenvironment to improve antitumor efficiency. ACS Appl. Bio Mater. 2023, 6, 2944–2981. [Google Scholar] [CrossRef]
- Nisha; Sachan, R.S.K.; Singh, A.; Karnwal, A.; Shidiki, A.; Kumar, G. Plant-mediated gold nanoparticles in cancer therapy: Exploring anti-cancer mechanisms, drug delivery applications, and future prospects. Front. Nanotechnol. 2024, 6, 1490980. [Google Scholar] [CrossRef]
- Magogotya, M.; Vetten, M.; Roux-van der Merwe, M.; Badenhorst, J.; Gulumian, M. In vitro toxicity and internalization of gold nanoparticles (AuNPs) in human epithelial colorectal adenocarcinoma (Caco-2) cells and the human skin keratinocyte (HaCaT) cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2022, 883, 503556. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Gong, N.; Chen, S.; Jin, S.; Zhang, J.; Wang, P.C.; Liang, X.-J. Effects of the physicochemical properties of gold nanostructures on cellular internalization. Regen. Biomater. 2015, 2, 273–280. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Zhang, Y.; Rong, H.; Lu, W.; Jiang, L. Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 46–53. [Google Scholar] [CrossRef]
- Dhandapani, S.; Xu, X.; Wang, R.; Puja, A.M.; Kim, H.; Perumalsamy, H.; Balusamy, S.R.; Kim, Y.-J. Biosynthesis of gold nanoparticles using Nigella sativa and Curtobacterium proimmune K3 and evaluation of their anticancer activity. Mater. Sci. Eng. C 2021, 127, 112214. [Google Scholar] [CrossRef]
- Patil, M.P.; Ngabire, D.; Thi, H.H.P.; Kim, M.-D.; Kim, G.-D. Eco-friendly synthesis of gold nanoparticles and evaluation of their cytotoxic activity on cancer cells. J. Clust. Sci. 2017, 28, 119–132. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Arun, R.; Sathishkumar, G.; MubarakAli, D.; Rajesh, M.; Sivanandhan, G.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Ganapathi, A. An evidence on G2/M arrest, DNA damage and caspase mediated apoptotic effect of biosynthesized gold nanoparticles on human cervical carcinoma cells (HeLa). Mater. Res. Bull. 2014, 52, 15–24. [Google Scholar] [CrossRef]
- Tiloke, C.; Phulukdaree, A.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera gold nanoparticles modulate oncogenes, tumor suppressor genes, and caspase-9 splice variants in a549 cells. J. Cell. Biochem. 2016, 117, 2302–2314. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huq, M.A.; Rana, M.R.; Samad, A.; Rahman, M.S.; Rahman, M.M.; Ashrafudoulla, M.; Akter, S.; Park, J.-W. Green Synthesis, Characterization, and Potential Antibacterial and Anticancer Applications of Gold Nanoparticles: Current Status and Future Prospects. Biomedicines 2025, 13, 1184. https://doi.org/10.3390/biomedicines13051184
Huq MA, Rana MR, Samad A, Rahman MS, Rahman MM, Ashrafudoulla M, Akter S, Park J-W. Green Synthesis, Characterization, and Potential Antibacterial and Anticancer Applications of Gold Nanoparticles: Current Status and Future Prospects. Biomedicines. 2025; 13(5):1184. https://doi.org/10.3390/biomedicines13051184
Chicago/Turabian StyleHuq, Md. Amdadul, Md. Rasel Rana, Abdus Samad, Md. Shahedur Rahman, M. Mizanur Rahman, Md Ashrafudoulla, Shahina Akter, and Jong-Whi Park. 2025. "Green Synthesis, Characterization, and Potential Antibacterial and Anticancer Applications of Gold Nanoparticles: Current Status and Future Prospects" Biomedicines 13, no. 5: 1184. https://doi.org/10.3390/biomedicines13051184
APA StyleHuq, M. A., Rana, M. R., Samad, A., Rahman, M. S., Rahman, M. M., Ashrafudoulla, M., Akter, S., & Park, J.-W. (2025). Green Synthesis, Characterization, and Potential Antibacterial and Anticancer Applications of Gold Nanoparticles: Current Status and Future Prospects. Biomedicines, 13(5), 1184. https://doi.org/10.3390/biomedicines13051184









