Immunomodulation and Immunotherapy for Patients with Prostate Cancer: An Up-to-Date Review
Abstract
1. Introduction
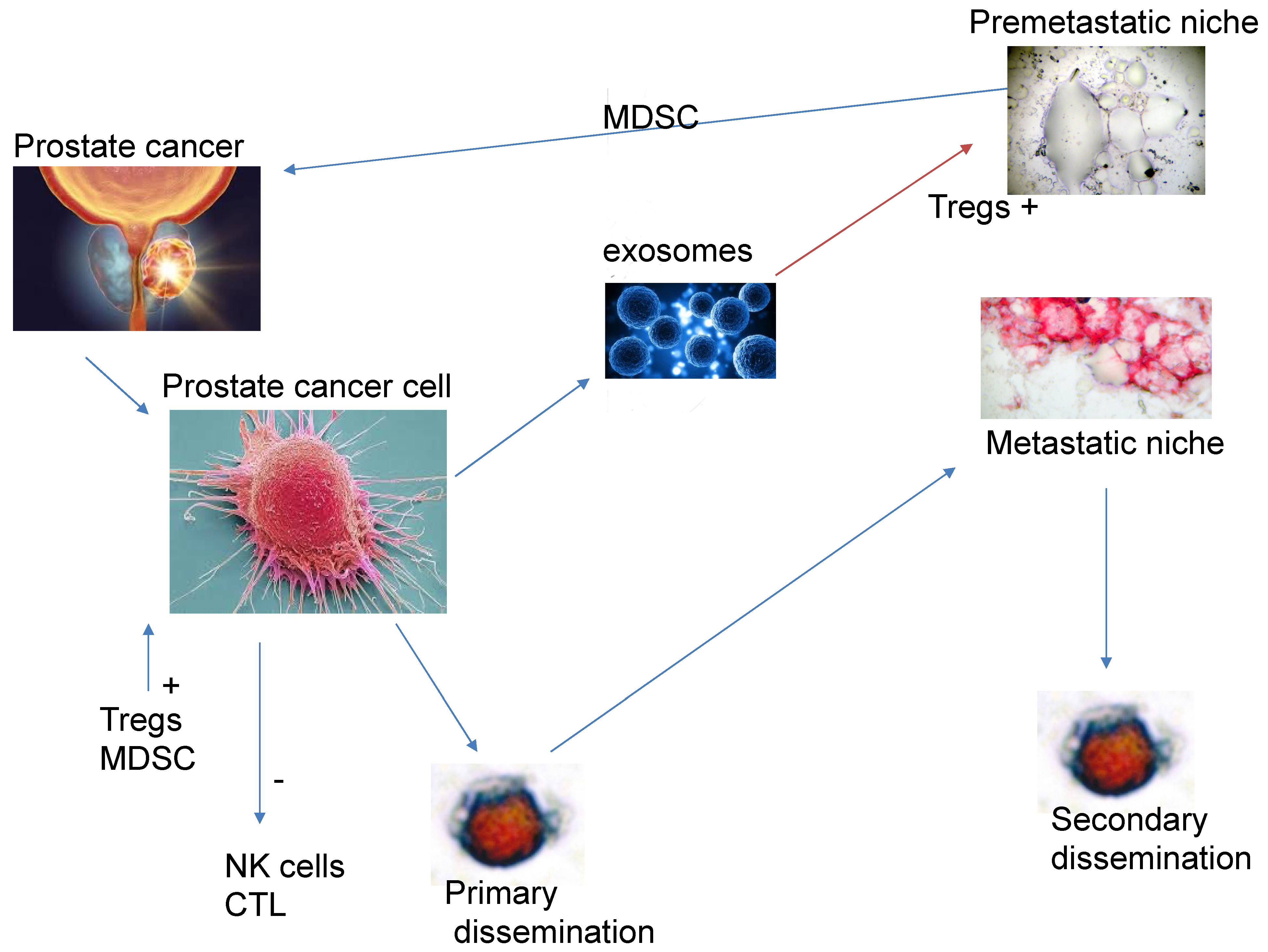
2. Modulation of the Immune System by the Primary Tumour
3. The Role of Immunotherapy and Immunomodulation
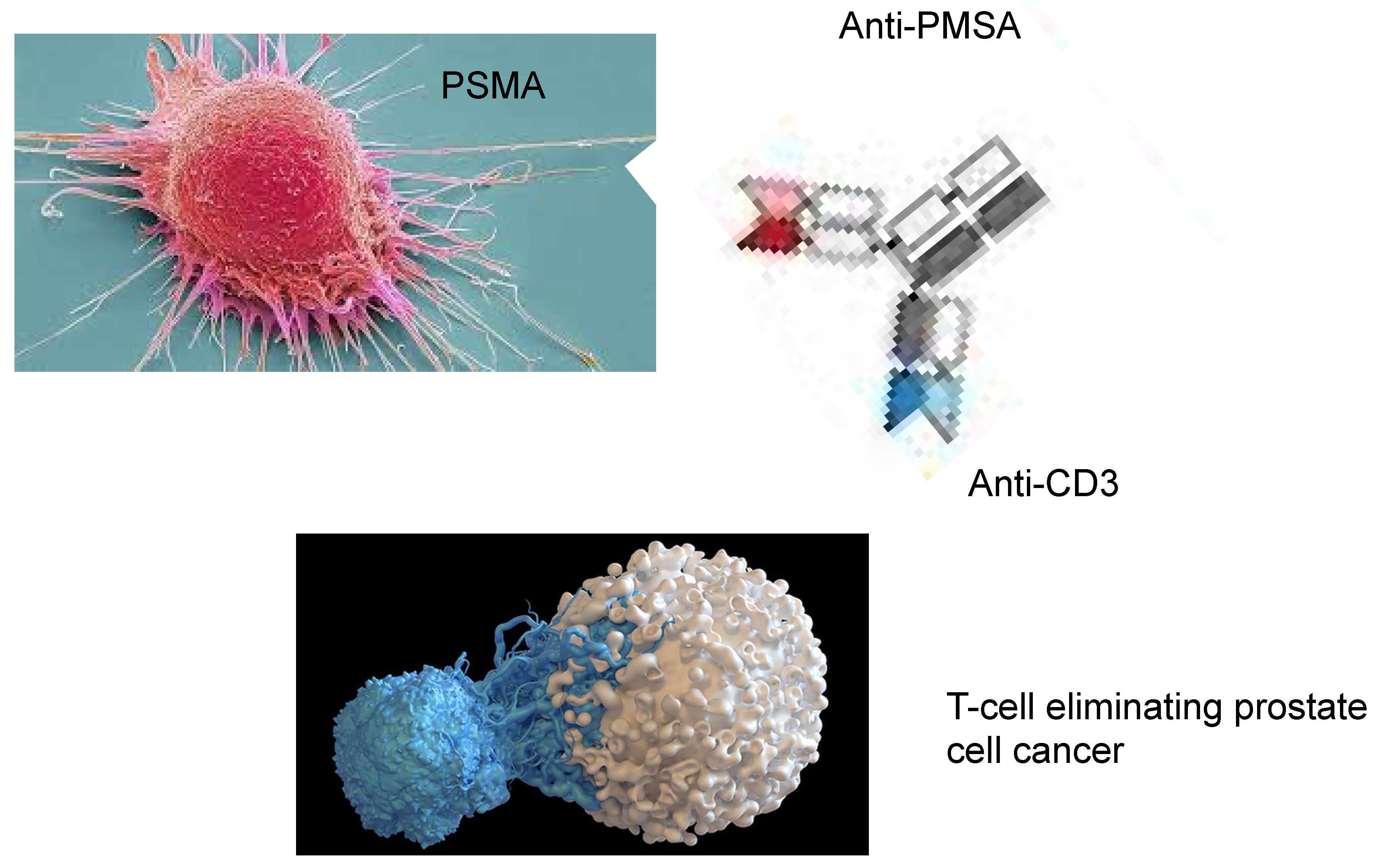
4. Bispecific Antibodies That Target Costimulatory Receptors of T-Cells
5. Chimeric Antigen Receptor (CAR) T-Cell Therapy
6. PMSA-Linked Radionuclides
7. Androgen Deprivation Therapy
8. Mechanisms of Resistance to Immunotherapy
9. Immunotherapy in the Context of Previous Knowledge
10. Should Immunotherapy Be Used as Frontline Treatment for Biochemical Relapse? Are We Not Seeing the Wood for the Trees?
11. Conclusions: Future Developments and the Use of Immunotherapy in Patients with Prostate Cancer
Funding
Conflicts of Interest
References
- Sridaran, D.; Bradshaw, E.; DeSelm, C.; Pachynski, R.; Mahajan, K.; Mahajan, N.P. Prostate cancer immunotherapy: Improving clinical outcomes with a multi-pronged approach. Cell Rep. Rep. Med. 2023, 4, 101199. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.I.; Miller, K.D.; Jemal, A. 2018 Cancer Statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.S. Molecular states underlying androgen receptor activation: A framework for therapeutics targeting androgen signaling in prostate cancer. J. Clin. Oncol. 2023, 48, 644–6436. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.C.; Li, J.; Klink, J.C.; Kattan, M.W.; Klein, E.; Fang, D.C.; Wu, L.F.; Chen, L.X.; Tang, C.H.; Wang, Y.; et al. Optimal definition of biochemical recurrence after radical prostatectomy depends on pathological risk factors: Identifying candidates for early salvage therapy. Eur. Urol. 2013, 66, 204–210. [Google Scholar] [CrossRef]
- Roach, M.; Hanks, G.; Thames, H., Jr.; Schllhammer, P.; Shipley, W.V.; Sokel, G.H.; Sandler, H. Defining biochemical failure following radiotherapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kendal, W.S.; Mai, K.T. Histological subtypes of prostate cancer: A comparative survival study. Can. J. Oncol. 2010, 17, 5355–5539. [Google Scholar]
- Fang, D.C.; Wu, L.F.; Chen, L.X.; Tang, C.H.; Wang, Y.; Li, H.; Wang, H.; Zhang, K.; Sun, S.Q.; Gao, Q.; et al. Analysis of prostate cancer screening results and exploration of screening model for elderly males in Songjiang rural areas of Shanghai City based on PSA preliminary screening under the community linkage mode. Zhonghua Yu Fang Yi Xua Za Zhi 2025, 59, 230–234. [Google Scholar]
- Siech, C.; de Angelis, M.; Janello, L.M.I.; Di Bello, F.; Rodriguez-Peñaranda, N.; Goyal, J.A.; Tian, Z.; Saad, F.; Shariat, S.F.; Puliatti, S.; et al. Rare histological prostate cancer subtypes: Cancer specific and other cause mortality. Prostate Cancer Prostate Dis. 2024, 1–7. [Google Scholar] [CrossRef]
- Cozzi, S.; Bardoscia, L.; Najafi, M.; Igdem, S.; Triggiani, L.; Magrini, S.M.; Botti, A.; Guedea, F.; Melocchi, L.; Ciammello, P.; et al. Ductal prostate cancer: Clinical features and outcomes from a multicenter retrospective analysis and overview of the current literature. Curr. Urol. 2022, 16, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Ranasinha, N.; Omer, A.; Philippou, Y.; Harriss, E.; Davies, L.; Chow, K.; Chetta, P.M.; Erickson, A.; Rajakumar, T.; Mills, I.G.; et al. Ductal adenocarcinoma of the prostate and meta-analysis of incidence, presentation, prognosis and management. BJUI 2011, 2, 13–23. [Google Scholar] [CrossRef]
- Cui, Y.; Lin, J.; Sun, D.; Zhang, H.; Diao, T.; Fu, Q. Nonogram for predicting survival and cancer-specific survival of patients with intraductal carcinoma of the prostate. J. Cancer Res. Clin. Oncol. 2024, 150, 45. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.Y.; Sheng, Q.; Hesterberg, A.B.; Croessmann, S.; Rios, B.L.; Giri, K.; Jackson, J.; Miranda, A.X.; Watlins, E.; Schaffer, K.R.; et al. Single cell analysis of cribriform prostate cancer reveals intrinsic and tumor microenvironment pathwats of aggressive disease. Nat. Commun. 2022, 13, 6036. [Google Scholar] [CrossRef]
- Kryvenko, O.N.; Lakymenko, O.A.; De Lima Guido, L.P.; Bhattu, A.S.; Merhe, A.; Mouzannar, A.; Briski, L.M.; Oymagil, I.; Lugo, I.; Nemov, A.; et al. Prostate ductal adenocarcinoma controlled for tumor grade, stage and margin status does not independently influence the likelihood of biochemical recurrence in localized prostate cancer after radical prostatectomy. Arch. Pathol. Lab. Med. 2022, 146, 1012–1017. [Google Scholar] [CrossRef]
- Diop, M.K.; Molina, O.E.; Birlea, M.; LaRue, H.; Hovington, H.; Tetu, B.; Lacombe, L.; Bergeron, A.; Fradet, Y.; Trudel, T. Leukocytic infiltration of intraductal carcinoma of the prostate: An exploratory study. Cancers 2023, 15, 2217. [Google Scholar] [CrossRef] [PubMed]
- Coa, F.; Li, Q.; Xiong, T.; Zheng, Y.; Zhang, T.; Jin, M.; Song, L.; Xing, N.; Niu, Y. Prognostic value of intraductal carcinoma subtypes and postoperative radiotherapy for localized prostate dancer. BMC Urol. 2025, 25, 10. [Google Scholar]
- Nguyen, N.; Franz, I.I.R.D.; Mohammed, O.; Huynh, R.; Son, C.K.; Khan, R.N.; Ahmed, B. A systemic review of primary large cell neuroendocrine carcinoma of the prostate. Front. Oncol. 2024, 14, 1341794. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Alabi, B.R.; Yin, Q.; Stoyanova, T. Molecular mechanisms underlying the development of neuroendocrine prostate cancer. Semin. Cancer Biol. 2022, 86, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Ormomendia, C.; Eng, K.W.; Bareja, R.; Sigouros, M.; Molina, A.; Faltas, B.M.; Sboner, A.; Mosquer, J.M.; Elemento, O.; et al. Clinical features of neuroendocrine prostate cancer. Eur. J. Cancer Oxf. Eng. 2019, 121, 7–18. [Google Scholar] [CrossRef] [PubMed]
- de Kouchkovsky, I.; Chan, E.; Schloss, C.; Poehlein, C.; Aggarwal, R. Diagnosis and management of neuroendocrine prostate cancer. Prostate 2024, 84, 426–440. [Google Scholar] [CrossRef]
- Seguir, D.; Parent, P.; Duterque-Coquillaud, M.; Labreuche, J.; Fromont-Hankard, G.; Dariane, C.; Penel, N.; Villers, A.; Turpin, A.; Olivier, J. Emergence of neuroendocrine tumors in patients treated with androgen receptor pathway inhibitors for metastatic prostate cancer: A systemic review and meta-analysis. Eur. Urol. Oncol. 2025, 8, 581–590. [Google Scholar] [CrossRef]
- Maylin, Z.R.; Smith, C.; Classen, A.; Asim, M.; Pandha, H.; Wang, Y. Therapeutic exploitation of neuroendocrine trans-differentation drivers in prostate cancer. Cells 2024, 13, 1999. [Google Scholar] [CrossRef] [PubMed]
- Arman, T.; Nelson, P.S. Endocrine and paracrine characteristics of neuroendocrine prostate cancer. Front. Endocrinl. 2022, 13, 1012005. [Google Scholar] [CrossRef] [PubMed]
- Abufaraj, M.; Ramadan, R.; Alkhatib, A. Paraneoplastic syndromes in neuroendocrine prostate cancer: A systematic review. Curr. Oncol. 2024, 31, 1618–1632. [Google Scholar] [CrossRef]
- Kufe, D. Dependence on MUC1-C in progression of neuroendocrine prostate cancer. J. Mol. Sci. 2023, 24, 3719. [Google Scholar] [CrossRef] [PubMed]
- Dalal, A.; Clark-Garvey, S.; Gdowski, A.; Zhnag, S.; Wobker, S.E.; Rowe, S.P.; Altun, E.; Beltran, H.; Milowsky, M.I. A case of rapidly progressive de novo metastatic small cell neuroendocrine prostate cancer. Case Rep. Oncol Med 2024, 7998149. [Google Scholar] [CrossRef]
- Cao, B.; Kim, M.; Reizine, N.M.; Moreira, D.M. Adverse events and androgen signaling inhibitors in the treatment of prostate cancer: A systemic review and meta-analysis and multivariate network meta-analysis. Eur. Urol. Oncol. 2022, 6, 237–250. [Google Scholar] [CrossRef]
- Ding, T.; He, L.; Lin, G.; Xu, L.; Zhu, Y.; Wang, X.; Liu, X.; Guo, J.; Lei, F.; Zuo, Z.; et al. Integrated analysis of single cell and bulk transcriptomes uncovers clinically relevant molecular subtypes in human prostate cancer. Chin. J. Cancer Res. 2023, 37, 90–114. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kosaka, T.; Nakamura, K.; Shojo, K.; Hongo, H.; Mikami, S.; Nishihara, H.; Oya, M. A first case of ductal adenocarcinoma of the prostate having characteristics of neuroendocrine phenotype with PTEN, RB1 and TP53 alterations. BMC Med. Genom. 2021, 14, 245. [Google Scholar] [CrossRef]
- Lindh, C.; Samaratunga, H.; Delahunt, B.; Bergstrom, R.; Chellappa, V.; Yaxley, J.; Lindberg, J.; Egevad, L. Ductal and acinar components of mixed prostatic adenocarcinoma frequency have a common clonal origin. Prostate 2022, 82, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.G.; Croce, C.M.; Fischer, R.; Monne, M.; Vihko, P.; Mulholland, S.G.; Gomella, L.G. Detection of hematogenous micrometastasis in patients with prostate cancer. Cancer 1992, 52, 6110–6112. [Google Scholar]
- Murray, N.P.; Reyes, E.; Orellana, N.; Fuentealba, C.; Jacob, O. Head-to-head of the Chun Nomogram, percentage free PSA and primary circulating postate cells to predict thew presence of prostate cancer at repeat biopsy. Asian Pac. J. Cancer Prev. 2016, 17, 2559–2563. [Google Scholar]
- Chang, Y.S.; di Tomaso, E.; McDonald, D.M.; Jone, R.; Jasin, R.K.; Munn, L.L. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc. Natl. Acad. Sci. USA 2000, 97, 14608–14613. [Google Scholar] [CrossRef]
- Fidler, I.J. Metastasis: Quantitative analysis of distribution and fate of tumor micro-emboli labelled with 125-I-5-iodo 2´ deoxyuridine. J. Natl. Cancer Inst. 1970, 45, 773–782. [Google Scholar] [PubMed]
- Song, H.; Weinstein, H.N.W.; Allegakoen, P.; Wadsworth, I.I.M.H.; Xie, J.; Yang, H.; Castro, E.A.; Lu, L.; Stohr, B.A.; Feny, F.Y.; et al. Single-cell analysis of human. Primary prostate cancer reveals the heterogeneity of tumor-associated epithelial cell states. Nat. Commun. 2022, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Fildler, I.J.; Poste, G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008, 9, 8. [Google Scholar]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as a multidimension spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. The theory of tumour ecosystem. Cancer Commun. 2022, 42, 587–608. [Google Scholar] [CrossRef]
- Adorno Febles, V.R.; Hao, Y.; Ahsan, A.; Wu, J.; Qian, Y.; Zhong, H.; Loeb, S.; Makova, A.V.; Lepor, A.; Wysock, J.; et al. Single-cell analysis of localized prostate cancer patients links high Gleason score with an immunosuppressive profile. Prostate 2023, 83, 840–849. [Google Scholar] [CrossRef]
- Vidotto, T.; Saggioro, F.P.; Jamasphishvili, T.; Chesca, D.L.; Picanco de Aluquerque, C.G.; Reis, R.B.; Graham, C.H.; Berman, D.M.; Semens, A.R.; Squire, J.A.; et al. PTEN-deficient prostate cancer is associated with an immunosuppressive tumor microenvironment mediated by increased expression of IDO1 and infiltrating FoxP3 T regulatory cells. Prostate 2019, 79, 969–979. [Google Scholar] [CrossRef]
- Jenzer, M.; Keβ, P.; Nientiedt, C.; Endris, V.; Kippenberger, M.; Leichsenring, J.; Stogbauer, F.; Haimes, J.; Miskin, S.; Kudlow, B.; et al. The BRCA2 mutation status shapes the immune phenotype of prostate cancer. Cancer Immunol. Immunother. 2019, 68, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Kaur, P.; Sheehan, C.E.; Fisher, H.A.; Kaufman, R.A., Jr.; Kallakury, B.V. Prognostic significance of metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 expression in prostate cancer. Mod. Pathol. 2003, 16, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F.; Tetu, B. Significance of MMP-2 expression in prostate cancer: An immunohistochemical study. Cancer Res. 2003, 63, 8511–8515. [Google Scholar]
- Nissinen, L.; Kahari, V.M. MMPs in inflammation. Biochem. Biophys. Acta 2014, 1840, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Kim, M.J.; Jang, H.S.; Lee, H.R.; Ahn, K.M.; Lee, J.H.; Choung, P.H.; Kim, M.J. High concentrations of MMP-2 and MMP-9 reduce NK mediated cytotoxicity against oral squamous cell carcinoma line. In Vivo 2008, 22, 593–598. [Google Scholar]
- Kahlert C, Kalluri R Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 2013, 91, 431–437. [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatalo, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.P.; Goh, B.C. Exosome-mediated metastasis: From epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef]
- Xiang, X.; Poliakov, A.; Liu, C.; Liu, Y.; Deng, Z.B.; Wang, J.; Cheng, Z.; Shah, S.V.; Wang, G.J.; Zhang, L.; et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer 2009, 124, 2621–2633. [Google Scholar] [CrossRef]
- Morrisey, S.M.; Zhang, F.; Ding, C.; Montoya-Durango, D.E.; Hu, X.; Yang, C.; Wang, Z.; Yuan, F.; Fox, M.; Zhang, H.G.; et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021, 33, 2020–2058. [Google Scholar] [CrossRef]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J.; Whiteside, T.L. Tumor-derived micro-vesicles promote regulatory T-cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef]
- Figueiro, F.; Muller, L.; Funk, S.; Jackson, E.K.; Battastini, A.M.; Whiteside, T.L. Phenotypic and functional characteristics of CD39 high human regulatory B-cells (Breg). Oncoimmunology 2016, 5, e1082703. [Google Scholar] [CrossRef]
- Shuler, P.J.; Saze, Z.; Hong, C.S.; Muller, L.; Gillespie, D.G.; Cheng, D.; Harasymczuk, A.; Mandapathi, M.; Jackson, E.K.; Lang, S.; et al. Human CD4+ CD39+ regulatory T cells producer adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin. Exp. Immunol. 2014, 177, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Bakouny, Z.; Hirsch, L.; Flippot, R.; van Allen, E.M.; Wu, C.J.; Choueiri, T.K. Beyond conventional immune checkpoint inhibition-novel immunotherapies for renal cell carcinoma. Nat. Rev. Clin. Oncol. 2021, 18, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Rhea, L.P.; Mendez-Marti, S.; Kim, D.; Aragon-Ching, J.B. Role of immunotherapy in bladder cancer. Cancer Treat. Res. Commun. 2021, 26, 100296. [Google Scholar] [CrossRef]
- Bansal, D.; Reimers, M.A.; Knoche, E.M.; Pachynski, R.K. Immunotherapy and immunotherapy combinations in metastatic castration resistant prostate cancer. Cancers 2021, 13, 334. [Google Scholar] [CrossRef]
- Movassaghi, M.; Chung, R.; Anderson, C.B.; Stein, M.; Saenger, Y.; Faiena, I. Overcoming immune resistance in prostate cancer: Challenges and advances. Cancers 2021, 13, 4757. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Jiao, S.; Subudhi, S.H.; Aparicio, A.; Ge, Z.; Guan, B.; Miura, Y.; Sharma, P. Differences in tumour microenvironment dictate T helper lineage polarization and response to immune checkpoint Therapy. Cell 2019, 179, 1177–1190. [Google Scholar] [CrossRef]
- Lopez-Campos, F.; Gajate, P.; Romero-Laorden, N.; Zafra-Martin, J.; Juan, M.; Hernando-Polo, S.; Conde Moreno, A.; Couñago, F. Immunotherapy in advance prostate cancer: Current knowledge and future directions. Biomedicines 2022, 10, 537. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.P.; et al. Sipuleucel-T immunotherapy for castration resistant prostate cancer. N. Eng. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Prostate Cancer Version 1. Available online: http://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 24 November 2024).
- McNeel, D.G.; Bander, N.H.; Beer, T.M.; Drake, C.G.; Fang, L.; Harrelson, S.; Kanttoff, P.V.; Mdan, R.A.; Oh, W.K.; Peace, D.J.; et al. The Society for Immunotherapy for the treatment of prostate carcinoma. J. Immunother. Cancer 2016, 4, 92. [Google Scholar] [CrossRef]
- Cookson, M.S.; Roth, B.J.; Dahm, P.; Engstrom, C.; Freedland, S.J.; Hussain, M.; Lin, D.W.; Lawrance, W.T.; Murad, M.H.; Oh, W.K.; et al. Castration-Resistant Prostate Cancer: AUA Guidelines. J. Urol. 2013, 190, 429–438. [Google Scholar] [CrossRef]
- Basch, E.; Loblaw, D.A.; Oliver, T.K.; Carducci, M.; Chen, R.C.; Frame, J.N.; Gorrels, K.; Hotte, S.; Kattan, M.W.; Raghavan, D.; et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guidelines. J. Clin. Oncol. 2014, 32, 3436–3448. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Gillessen, S.; Heidenreich, A.; Horwich, A.; ESMO Guidelines Committee. Cancer of the prostate: ESMO guidelines clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v69–v77. [Google Scholar] [CrossRef]
- Sims, R.B. Development of sipuleucel-T: Autologous cellular immunotherapy for the treatment of metastatic castrate resistant prostate cancer. Vaccine 2012, 30, 4394–4397. [Google Scholar] [CrossRef]
- Stewart, F.P.; Dela Rosa, C.; Sheikh, N.A.; McNeel, D.G.; Frohlich, M.W.; Urdal, D.L.; Provost, N.M. Correlation between product parameters and overall survival in three trials of sipuleucel-T, an autologous active cancer chemotherapy for the treatment of prostate cancer. J. Clin. Oncol. 2010, 28, 4552. [Google Scholar] [CrossRef]
- Fong, L.; Carroll, P.; Weinberg, V.; Chan, S.; Lewis, J.; Corman, J.; Amling, C.L.; Stephenson, R.A.; Simko, J.; Sheikh, N.A.; et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative Sipuleucel-T for localized prostate cancer. J. Natl. Cancer Inst. 2014, 106, dju268. [Google Scholar] [CrossRef]
- Marshall, C.H.; Fu, W.; Wang, H.; Park, J.C.; DeWeeese, T.L.; Tran, P.T.; Song, D.Y.; King, S.; Afful, M.; Hurrelbrink, J.; et al. Randomized phase II trial of sipuleucel T with or without radium-223 in men with bone metastatic castration resistant Prostate cancer. Clin. Cancer Res. 2021, 27, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Rosser, C.J.; Hirasawa, Y.; Acaba, J.D.; Tamura, D.J.; Pal, S.K.; Huang, J.; Scholz, M.C.; Dorffet, T.B. Phase 1b study assessing different sequencing regimens of atezolizumab (anti-PD-L1) and spipuleucin T (Sip-T) in patients who have asymptomatic or minimally symptomatic metastatic castrate. resistant prostate cancer. J. Clin. Oncol. 2020, 38, e17564. [Google Scholar] [CrossRef]
- Warren, T.L.; Weiner, G.J. Uses of granulocyte-macrophage colony stimulating factor in vaccine development. Curr. Opin. Hematol. 2000, 7, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Joniau, S.; Abrahamsson, P.A.; Bellmunt, J.; Figdor, C.; Hamdy, F.; Verhagen, P.; Vogelzang, N.J.; Wirth, M.; Van Poppel, H.; Osanto, S. Current vaccination strategies for prostate cancer. Eur. Urol. 2012, 61, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Hans, B.; Goel, S.; Bashorun, H.O.; Dovey, Z.; Tewari, A. Immunotherapy in prostate cancer: Current state and future perspectives. Ther. Adv. Urol. 2020, 12, 1756287220951404. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Borre, M.; Vogelzang, N.J.; Siobhan, N.; Agarwal, N.; Parker, C.C.; Pook, D.W.; Rathenborg, P.; Flaig, T.W.; Carles, J.; et al. Phase III trial of PROSTVAC in. asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef]
- Arlen, P.M.; Culley, J.L.; Parker, C.; Skarupa, L.; Pazdur, M.; Panicali, D.; Beetham, P.; Tsang, K.Y.; Grosenbach, D.W.; Feldman, J.; et al. A randomized phase II study of concurrent docetaxol plus vaccine versus vaccine alone in metastatic androgen independente prostate cancer. Clin. Cancer Res. 2006, 12, 1260–126958. [Google Scholar] [CrossRef] [PubMed]
- Granier, C.; Badoual, C.; De Guillebon, E.; Blanc, C.; Roussel, H.S.; Colin, E.; Saldmann, A.; Gey, A.; Oudard, S.; Tartour, E. Mechanisms of action and use of checkpoint inhibitors in cancer. ESMO Open 2017, 2, e000213. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Cong, Z.; Amoozgar, Z.; Kiner, E.; Xing, D.; Orsulic, S.; Matulonis, U.; Goldberg, M.S. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in BRCA-1 −/− murine model of ovarian cancer. Biochem. Biophys. Res. Commun. 2015, 463, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Farhangnia, P.; Ghomi, S.M.; Akbarpour, M.; Delbandi, A.A. Bispecific antibodies targeting CTLA-4, Game changer troopers in cancer immunotherapy. Font. Immunol. 2023, 14, 1155778. [Google Scholar] [CrossRef] [PubMed]
- Isaacsson, V.; Antonarkis, E.S. PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin. Pharmacol. 2018, 11, 475–486. [Google Scholar] [CrossRef]
- Xu, Y.; Song, G.; Xie, S.; Jiang, W.; Chen, X.; Chu, M.; Hu, X.; Wang, Z.W. The roles of PD-1/PD-L1 in the prognosis and immunotherapy of prostate cancer. Mol. Ther. 2021, 29, 1958–1969. [Google Scholar] [CrossRef]
- Leng, C.; Li, Y.; Qin, J.; Ma, J.; Liu, X.; Cui, Y.; Sun, H.; Wang, Z.; Hua, X.; Yu, Y. Relationship between expression of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8+ T-cells. Oncol. Rep. 2016, 35, 699–708. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimandani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell Phsiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef] [PubMed]
- Lotfinejad, P.; Kazemi, T.; Mokhtarzadeh, A.; Shanebandi, D.; Niaragh, F.J.; Safaei, S.; Asadi, M.; Baradaran, B. PD-1/PD-L1 axis importance and tumor microenvironment immune cells. Life Sci. 2020, 259, 118297.87. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.J.; Liu, Z.; Cheng, Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; Van der Eeertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H. Ipilimumab versus placebo after radiotherapy in patients with metastastic castration-resistant prostate cancer that had progressed after docetaxol chemotherapy (CA184-03): A multi-centre randomized double blind phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, L.K.; Drake, C.G.; Beer, T.M.; Kwon, E.D.; Scher, H.I.; Gerritsen, W.R.; Bossi, A.; van der Eertwegh, A.J.M.; Krainer, M.; Houede, N.; et al. Final analysis of the Ipilimumab versus placebo following radiotherapy Phase III trial in post docetaxol metastatic castration resistant prostate cancer identifies an excess of long-term survivors. Eur. Urol. 2020, 78, 822–830.90. [Google Scholar] [CrossRef]
- Beer, T.M.; Logothetis, C.; Sharma, A.; McHenry, B.; Fairchild, J.P.; Gagnier, P.; Chin, K.M.; Cuillerot, J.M.; Fizazi, K. CA184-095 A randomized double blind phase III trial to compare the efficacy of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy naïve castration resistant prostate cancer. J. Clin. Oncol. 2012, 30, TPS4691. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, I.Y.; Su, R.; Shen, K.; Pan, J.; Wang, Q.; Xue, W. Docetaxel remodels prostate cancer immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. Theranostics 2012, 12, 4965–4979. [Google Scholar] [CrossRef]
- Topalin, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, S.A.; Atkins, M.B.; et al. Safety, activity and immune correlates of Anti-PD-1 antibody in cancer. N. Eng. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Fakhrejehani, F.; Madan, R.A.; Dahut, W.L.; Karzai, F.; Cordes, L.M.; Schlom, J.; Liow, E.; Bennett, C.; Zheng, T.; Yu, J.; et al. Avelumab in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2017, 35, 159. [Google Scholar] [CrossRef]
- Holmes, C.J.; Graff, J.N.; Tagawa, S.T.; Hwang, C.; Kilari, D.; Ten Tije, A.J.; Omlin, A.U.; McDermott, R.S.; Vaishampayan, U.N.; Elliot, T.; et al. KEYNOTE-199 cohort (C) Phase II study of pembrolizumam (pembro) plus enzalutamide (enzo) for enzo resistant metastatic castration resistant prostate cancer. J. Cin. Oncol. 2020, 38, 5543. [Google Scholar]
- Antonarakis, E.; Piulats, J.; Gross-Goupil, M.; Goh, J.; Vaishampayan, U.; De Wit, R.; Alanko, T.; Fukasawan, S.; Tabata, T.; Feyerabend, S. Pembrolizumab monotherapy for treatment-refractory for docetaxol-pretreated metastatic castration resistant prostate cancer. Updated analyses with 4 years of follow-up from cohorts 1-3 of the KEYNOTE-199 study. Ann. Oncol. 2021, 32, S651–S652. [Google Scholar] [CrossRef]
- Graff, J.N.; Liang, L.W.; Kim, J.; Stenzel, A. Keynote-641, A phase 3 study of pembrolizumumab plus enzalutamide for metastatic castration resistant prostate cancer. Future Oncol. 2021, 17, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Yuen, K.C.; Gillessen, S.; Kadel, E.E., 3rd; Rathkopf, D.; Matsubara, N.; Drake, C.G.; Fizazi, K.; Piulats, J.M.; Wysocki, P.J.; et al. Atezolizumab with enzautamide versus enzautamide alone in metastatic castration resistant prostate cancer. A randomized phase 3 trial. Nat. Med. 2022, 28, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Cicala, C.M.; Musacchio, L.; Scambia, G.; Lorusso, D. Dostarlimab: From preclinical investigation to drug approval and future directions. Hum. Vaccin Immunother. 2023, 19, 2178220. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhnag, J.; Li, L.; Wqng, Z.; Yang, C. Biomarkers in metastatic castration resistant prostate cancer for efficiency of immune checkpoint inhibitors. Ann. Med. 2025, 57, 2426755. [Google Scholar] [CrossRef] [PubMed]
- Farzeen, Z.; Khan, R.R.M.; Chaudry, A.R.; Pervaiz, M.; Saeed, Z.; Rsheed, S.; Shehzad, B.; Kantoff, P.W.; Adan, A.; Summer, M. Dostarliab: A promising new PD-1 inhibitor for cancer immunotherapy. J. Oncol. Pharm. Pract. 2024, 30, 1411–1431. [Google Scholar] [CrossRef]
- Yadav, R.; Mathur, I.; Haokip, H.R.; Pandey, A.K.; Kumar, V.; Jain, N. Dostarlimab: Review on success story and clinical trials. Crit. Rev. Oncol. Hematol. 2024, 198, 104374. [Google Scholar] [CrossRef]
- Blanco, B.; Dominguez-Alonso, C.; Alvarez -Vallina, L. Bispecific immunomodulatory antibodies for cancer immunotherapy. Clin. Cancer Res. 2021, 27, 5457–5464. [Google Scholar] [CrossRef]
- Heuls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef]
- Zhou, S.J.; Wei, J.; Su, S.; Chen, E.J.; Qui, Y.D.; Liu, B.R. Strategies for bispecific single chain antibody in cancer immunotherapy. J. Cancer 2017, 8, 3689. [Google Scholar] [CrossRef][Green Version]
- Gaspar, M.; Pravin, J.; Rodriques, L.; Uhlenbroich, S.; Everett, K.L.; Wollerton, F.; Morrow, M.; Tuna, M.; Brevis, N. CD137/OX40 bi-specific antibody induces potent antitumor activity that is dependent on target co-engagement. Cancer Immunol. Res. 2020, 8, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Ma, J.S.; Kim, S.C.; Laborda, E.; Choi, S.H.; Hampton, E.N.; Yun, H.; Nunez, V.; Muldong, M.T.; Wu, C.N. A PMSA targeted bispecific antibody for prostate cancer driven by a small-moecular targeting ligand. Sci. Adv. 2021, 7, eab8193. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.; Tavara, R.; Haber, L.; Aina, O.H.; Vazzana, K.; Ram, P.; Danton, M.; Finney, J.; Jalal, S.; Krueger, P. A PMSA targeting CD3 bispecific antibody induces antitumor responses that are enhanced by 4-1BB co-stimulation. Cancer Immunol. Res. 2020, 8, 595–608. [Google Scholar] [CrossRef]
- Deegan, P.; Thomas, O.; Noal-Stevaux, O.; Li, S.; Wahl, J.; Bogner, P.; Aeffner, F.; Friedrich, M.; Liao, M.Z.; Mattes, K. The PSMA targeting half-life extended BiTE therapy AMG 160 has potent antitumoral activity in pre-clinical models of metastatic castration resistant prostate cancer. Clin. Cancer Res. 2021, 27, 2928–2937. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, A.K.; Soule, H.R. The 27th Annual Prostate Cancer Foundation Scientific Retreat Report. Prostate 2021, 81, 1107–1124. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Soper, B.; Shopland, L. Cytokine release syndrome and cancer immunotherapies-historical challenges and promising futures. Front. Oncol. 2023, 14, 1190379. [Google Scholar] [CrossRef]
- Dhillon, S. Tartalamab: First approval. Drugs 2024, 84, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Piazza, L.; Carollo, A.; Di Martino, E.; Novara, M.E.; Cutaia, S.; Provenzani, A.; Rizzo, S. Cardiotoxicity associated with immune checkpoint inhibitors: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2025, 206, 104587. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhu, L.; Chen, J. Current advances and challenges in CAR-T therapy for solid tumors. Tumor associated antigens and the tumor microenvironment. Exp. Hematol. Oncol. 2023, 12, 14. [Google Scholar] [CrossRef]
- George, P.; Dasyam, N.; Giunti, G.; Mester, B.; Bauer, E.; Andrews, B.; Perera, T.; Ostapowicz, T.; Frampton, C.; Li, P. Third generation anti CD19 chimeric antigen receptor T-cells incorporating a TRL2 domain for relapsed or refractory B-cell lymphoma: A phase I clinical trial protocol (ENABLE). BMJ Open 2020, 10, e034629. [Google Scholar] [CrossRef]
- Roselli, E.; Boucher, J.C.; Li, G.; Kotani, H.; Spitler, K.; Reid, K.; Cervantes, E.V.; Bulliard, Y.; Tu, N.; Lee, S.B. 4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells. J. Immunother. Cancer 2021, 9, e003354. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Rouce, R.; Robertson, C.S.; Reyna, A.; Narala, N.; Vys, G.; Mehta, B.; Zhang, H.; Dakhova, O.; Carrum, G. In vivo fate and activity of second versus third generation CD19 specific CAR-T cells in B cell non-Hodgkins lymphomas. Mol. Ther. 2018, 26, 2727–2737. [Google Scholar] [CrossRef] [PubMed]
- Kloss, C.C.; Lee, J.; Zhang, A.; Chen, F.; Melenhorst, J.J.; Lacey, S.F.; Maus, M.V.; Fraietta, J.A.; Zhao, Y.; June, C.H. Dominant- negative TGF-β receptor enhances PSMA targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 2018, 26, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Frieling, J.S.; Tordesilla, L.; Bustos, X.E.; Ramello, M.C.; Bishop, R.T.; Cianne, J.E.; Snedal, S.A.; Li, T.; Lo, C.H.; de la Iglesia, J. gamma-delta enriched CAR-T therapy for bone metastastic castrate resistant prostate cancer. Sci. Adv. 2023, 9, eadf0108. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Q.; Wang, F.; Zhang, C.; Xu, C.; Gu, A.; Zhong, W.H.; Wu, Y.; Zhong, X. Co-expression IL-15 receptor alpha with IL-15 reduces toxicity via limiting IL-15 systemic exposure during CAR-T immunotherapy. J. Transl. Med. 2022, 20, 432. [Google Scholar] [CrossRef]
- Dorff, T.B.; Blanchard, S.; Martirosyan, H.; Adkins, L.; Dhapola, G.; Moriarty, A.; Wagner, J.R.; Chaudhry, A.D.; Apuzzo, M.; Kuhn, P. Phase 1 study of PSCA-targeted CAR T cell therapy for metastatic Castration resistant prostate cancer (mCRPC). J. Clin. Oncol. 2022, 40, 91. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Vallabhajosula, S.; Goldsmith, S.J.; Bander, N.H. Phase I trial of yttrium-90-labelled anti prostate specific membrane antigen monoclonal antibody J591 for androgen independent prostate cancer. J. Clin. Oncol. 2004, 22, 2522–2531. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhust, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patient’s metastatic resistant prostate cancer (luPSMA trial); a single center, single arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Niaz, M.J.; Batra, J.S.; Walsh, R.D.; Ramirez-Font, M.K.; Vallabhaajosulo, S.; Jhanwar, Y.S.; Molina, A.M.; Nanus, D.M.; Osborne, J.R.; Bando, N.H.; et al. Pilot study of hyper-fractionated dosing of lutetium-177-labelled anti-prostate specific membrane antigen monoclonal antibody J591 1777Lu-J5911 for metastatic castration resistant prostate cancer. Oncologist 2020, 25, 477-e895. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Niaz, M.J.; Skafida, M.; Mossallaie, S.A.; Thomas, C.; Christos, P.J.; Osborne, J.R.; Molina, A.M.; Nanus, D.M.; Bander, N.H.; et al. Imaging expression of prostate specific membrane antigen and response to PMSA targeted beta-emitting radionuclide therapies in metastatic castration resistant prostate cancer. Prostate 2021, 81, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Vilet, J.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. TheraP: A randomized phase 2 trial of 177 Lu-PMSA-617 theranostics vs cabazitaxel in progressive castration resistant prostate cancer, (Clinical trial protocol ANZUP 1603). BJU Int. 2019, 124 (Suppl. S1), 5–13. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA). FDA Approves Pluvicto for Metastatic Castratin Resistant Prostate Cancer. News Release 23 March 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer (accessed on 15 December 2024).
- European Medicines Agency (EMA). Pluvicto: EPAR-Medicine Overview. European Public Assessment Report. Available online: https://www.ema.europa.eu/en/documents/product-information/pluvicto-epar-product-information_en.pdf (accessed on 21 December 2024).
- Arbuznikova, D.; Eder, M.; Grosu, A.L.; Meyer, P.T.; Gratzke, C.; Zamboglu, C.; Eder, A.C. Towards improving the efficacy of PSMA targeting radionuclide therapy for late-stage prostate cancer-combination strategies. Curr. Cancer Rep. 2023, 25, 1363–1374. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J. Urol. 2002, 168, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal therapy for prostate cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Evans, S.T.; Jani, Y.; Jansen, C.S.; Yildirim, A.; Kalemoglu, E.; Bilen, M.A. Understanding and overcoming resistance to immunotherapy in genitourinary cancer. Cancer Biol. Ther. 2024, 25, 23459924. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.M. Cancer resistance to immunotherapy: Comprehensive insights with future perspectives. Pharmaceutics 2023, 15, 1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Peng, W.; Xu, C.; Zhang, M.; Wargo, J.A.; Chen, J.Q.; Li, H.S.; Watowich, S.S.; Yang, Y.; Hwu, P. BRAF inhibition increases tumor infiltration by T-cells and enhances the antitumor activity of adaptive immumotherapy in mice. Clin. Cancer Res. 2013, 19, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi Pourmand, G.; Mehrsai, A.; Pakdel, S.; Dialameh, H.; Ahmadi, A.; Salem, S.; Salimi, E.; Mahboubi, R. Effect of PTEN gene expressions and environment risk factors on the progression and prognosis of ladder cancer. Iran J. Public Health 2014, 43, 56–61. [Google Scholar]
- Jamaspishvili, T.; Berman, D.M.; FRoss, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Koushyar, S.; Meniel, V.S.; Phesse, T.J.; Pearson, H.B. Exploring the WNT pathway as a therapeutic target for prostate cancer. Biomolecules 2022, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Spanger, S.; Bao, R.; Gajewski, T.F. Melanoma intrinsic ß-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistence to immune checkpoint inhibitors in lung cancer. Cancer Dis. 2017, 7, 1420–1435. [Google Scholar] [CrossRef]
- Taylor, B.C.; Balko, J.M. Mechanisms of MHC-I downregulation and role in immunotherapy response. Front. Oncol. 2022, 13, 844866. [Google Scholar] [CrossRef]
- Kim, J.H. Interleukin 8 in the tumor immune niche: Lessons from comparative oncology. Adv. Exp. Med. Biol. 2020, 1240, 25–33. [Google Scholar]
- Rizo, M.; Varnier, L.; Pezzicoli, G.; Pirovano, M.; Cosmai, L.; Porta, C. IL-8 and its role as a potential biomarker of resistance to anti-angiogenic agents and immune check point inhibitors in metastatic renal cell carcinoma. Front. Oncol. 2022, 12, 990568. [Google Scholar]
- Takeda, K.; Nakayama, M.; Hayakawa, Y.; Kojima, Y.; Ikeda, H.; Imai, N.; Ogasawara, K.; Okumura, K.; Thomas, D.M.; Smyth, M.J.; et al. INF-gamma is required for cytotoxic T-cell dependent cancer genome immunoediting. Nat. Commun. 2017, 8, 14607. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN gamma in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Cerezo-Wallis, D.; Contreras_Alcade, M.; Troule, K.; Catena, X.; Mucientes, C.; Calvo, T.G.; Canon, E.; Tejedo, C.; Pennacchi, P.C.; Hogan, S.; et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat. Med. 2020, 26, 1865–1877. [Google Scholar] [CrossRef]
- Marwitz, S.; Scheufele, S.; Perner, S.; Resck, M.; Ammerpohl, O.; Goldman, T. Epigenetic modifications of the immune checkpoint genes CTLA4 and PDCDI-1 in non-small cell lung cancer results in increased expression. Clin. Epignetics 2017, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Snahnicanova, Z.; Kasubova, L.; Kalma, M.; Grendar, M.; Mikolajcik, P.; Gabonova, E.; Laca, L.; Caprnda, M.; Rodrigo, L.; Ciccocioppo, R.; et al. Genetic and epigenetic analysis of the beta-2-microglobin gene in microsatellite instable colorectal cancer. Clin. Exp. Med. 2020, 20, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lyu, A.; Fan, Z.; Clark, M.; Lea, A.; Luong, D.; Setayesh, A.; Starzinski, A.; Wolters, R.; Arias-Badia, M.; Allaire, K.; et al. Evolution of myeloid mediated immunotherapy resistance in prostate cancer. Nature 2025, 637, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sfakianos, J.P.; Beaumont, K.G.; Akturk, G.; Horowitz, A.; Sebra, R.P.; Farkas, A.M.; Gnjatic, S.; Hake, A.; Izadmehr, S.; et al. Myeloid associated resistance to PD-1/PD-L1 blockade in urothelial cancer revealed through bulk and single-cell RNA sequencing. Clin. Cancer Res. 2012, 27, 4287–4300. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Yang, S.; Xia, Y.; Meng, Q.; Sun, B.; Liu, Y.; Zhou, B.; Jin, J.; Xu, H.; et al. PD-L1 positive platelets mediate resistance to immune checkpoint inhibitors with colorectal cancer. Cell Commun. Signal 2025, 23, 29. [Google Scholar] [CrossRef]
- Lockwood, G.; Taylor, M.P.H.; Canfield, S.E.; Du, X.L. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer 2009, 115, 2388–2398. [Google Scholar]
- Dallos, M.; Obradovic, A.Z.; McCann, P.; Chowdhury, N.; Pratapa, A.; Aggen, D.H.; Gaffney, C.; Autio, K.A.; Virk, R.K.; De Marzo, A.M.; et al. Androgen deprivation therapy drives a distinct immune phenotype in localized prostate cancer. Clin. Cancer Res. 2024, 30, 5218–5230. [Google Scholar] [CrossRef]
- Obradovic, A.Z.; Dallos, M.C.; Zahurak, M.L.; Partin, A.W.; Schaeffer, E.M.; Ross, A.E.; Allaf, M.E.; Nirschl, T.R.; Liu, D.; Chapman, C.G.; et al. T-cell infiltration and adaptive Treg resistance in response to androgen deprivation with or without vaccination in localized prostate cancer. Clin. Cancer Res. 2020, 26, 3182–3192. [Google Scholar] [CrossRef]
- Pu, Y.; Xu, M.; Liang, Y.; Yang, K.; Guo, Y.; Yang, X.; Fu, Y.X. Androgen receptor antagonists compromise T cell response against prostate cancer leading to early relapse. Sci. Transl. Med. 2016, 8, 333ra47. [Google Scholar] [CrossRef]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Carus, O.B.; Thompson, R.F.; Wood, M.A.; et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Alsamraae, M.; Costanzo-Garvey, D.; Teply, B.A.; Boyle, S.; Sommerville, G.; Herbert, Z.T.; Morrissey, C.; Dafferner, A.; Abdalla, M.Y.; Fallet, R.W.; et al. Androgen receptor inhibition suppresses anti-tumor neutrophil response against bone metastatic prostate cancer via regulation of tßRI expression. Cancer Lett. 2023, 579, 216468. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Selli, C.; Zhou, H.L.; Cao, J.; Wu, S.; Ma, R.Y.; Lu, Y.; Zhang, C.B.; Xun, B.; Lam, A.D.; et al. Macrophages promote anti- androgen resistance in prostate cancer bone disease. J. Exp. Med. 2023, 220, e20221007. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.I.; Chao, T.C.; Liu, C.Y.; Huang, C.C.; Tseng, L.M. A systemic review of taxanes and their side effects in metastatic breast cancer. Front. Oncol. 2022, 12, 940239. [Google Scholar] [CrossRef]
- Kulasegaran, T.; Oliveira, N. Metastatic castration resistant prostate cancer: Advances in treatment and symptom Management. Curr. Treat. Options Oncol. 2024, 25, 914–931. [Google Scholar] [CrossRef]
- Maden, R.A.; Karzai, F.; Donahue, R.M.; Al-Harthy, M.; Bilusic, M.; Rosner, I.I.; Singh, H.; Arlen, P.M.; Theoret, M.R.; Marte, J.L.; et al. Clinical and immunological impact of short-course enzalutamide alone and with immunotherapy in non-metastatic Castration sensitive prostate cancer. J. Immunother. Cancer 2021, 9, e0011556. [Google Scholar]
- Schellhammer, P.F.; Chodak, G.; Whitmore, J.B.; Sims, R.; Frohlich, M.W.; Kantoff, P.W. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from Sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 2013, 81, 1297–1302. [Google Scholar] [CrossRef]
- Mateo, J.; de Bono, J.S.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Agarwal, N.; Olmos, D.; Thiery-Vuillemin, A.; et al. Olaparib for the treatment of patients with metastatic castration-resistant prostate and alterations in the BRCA1 and/or BRCA2 in the PROfound trial. J. Clin. Oncol. 2024, 42, 571–583. [Google Scholar] [CrossRef]
- Fizazi, K.; Herrmann, K.; Krause, B.J.; Rahbar, K.; Chi, K.M.; Morris, M.J.; Sartor, O.; Tagawa, S.T.; Kendi, A.T.; Vogelzang, N.; et al. Health- related quality of life and pain outcomes with [177Lu] Lu-PMSA -617 plus standard of care versus standard of care in patients with metastatic castration resistant prostate cancer (VISION): A multicentre, open label, randomized, phase 3 trial. Lancet Oncol. 2023, 24, 597–610. [Google Scholar] [CrossRef]
- Saad, F.; Clarke, N.W.; Oya, M.; Shore, N.; Procopio, G.; Guedes, J.D.; Arslan, C.; Mehra, N.; Parnis, F.; Brown, E.; et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration resistant prostate cancer (PROpel): Final prespecified overall survival results of a randomized, double blind phase 3 trial. Lancet Oncol. 2023, 24, 1094–1108. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Park, S.H.; Goh, J.C.; Shin, S.J.; Lee, J.L.; Mehra, N.; McDermott, R.; Sala-Gonzalez, N.; Fong, P.C.; Greil, R.; et al. Pembrolizumab plus Olaparib for patients with previously treated and biomarker-unselected metastatic castration resistant prostate cancer: The randomized, open label, Phase III KEYLINK-010 Trial. J. Clin. Oncol. 2023, 41, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Kockerginsky, M.; Agarwal, N.; Adra, N.; Zhang, J.; Paller, C.J.; Picus, J.; Reichert, Z.R.; Szmulewitz, R.Z.; Tagawa, S.T.; et al. Abiratrone, olaparib or abiterone + olaparib in first line metastatic castration resistant prostate cancer with DNA repair defects (BRCAAway). Clin. Cancer Res. 2024, 30, 4318–4328. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.A.; Bilusic, M.; Stein, M.N.; Donahue, R.N.; Arlen, P.M.; Karzai, F.; Plimack, E.; Wong, Y.N.; Geynisman, D.M.; Zibelman, M.; et al. Flutamide with or without PROSTVAC in non-metastatic castration resistant (M0) prostate cancer. Oncologist 2023, 28, 642-e561. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Podrazil, M.; Jarolim, L.; Bilkova, P.; Hensler, M.; Becht, E.; Gasova, Z.; Klouckova, J.; Kayserova, J.; Horvath, R.; et al. Phase I/II trial of dendritic cell-based active cellular immunotherapy with DCVAC/PCa in patients with rising PSA after primary prostatectomy or salvage radiotherapy for the treatment of prostate cancer. Cancer Immunol. Immunother. 2018, 67, 89–100. [Google Scholar] [CrossRef]
- Tryggestad, A.M.A.; Axcrona, K.; Axcona, U.; Bigalke, I.; Brennhovd, B.; Inderberg, E.M.; Honnashagen, T.K.; Skoge, L.J.; Solum, G.; Saeboe-Larssen, S.; et al. Long term first in man Phase I/II study of an adjuvant dendritic cell vaccine in patients with high risk prostate cancer after radical prostatectomy. Prostate 2022, 82, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Autio, K.A.; Higano, C.S.; Nordquist, L.; Appleman, L.J.; Zhang, T.; Zhu, X.H.; Babiker, H.; Vogelzang, N.J.; Prasad, S.M.; Schweizer, M.T.; et al. First in human phase I study of PF-06753512, a vaccine-based immunotherapy regimen (VBIR), in patients with non-metastatic hormone sensitive biochemical recurrence and metastatic castration resistant prostate cancer. J. Immunother. Cancer 2023, 11, e005702. [Google Scholar] [CrossRef]
- Yin, C.; Wang, Y.; Ji, J.; Cai, B.; Chen, H.; Wang, Z.; Wang, K.; Luo, C.; Zhang, W.; Yuan, C.; et al. Molecular profiling of pooled circulating tumor cells from prostate cancer patients using a dual-antibody-functional microfluidic device. Anal. Chem. 2018, 90, 3744–3751. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres Zhang, J. Bone marrow as a reservoir for dissemination tumor cells: A special source for liquid biopsy in cancer patients. Bonekey Rep. 2014, 3, 584. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.T.; Zhang, J.; Burner, D.N.; Liss, M.; Pittman, E.; Muldong, M.; Shabaik, A.; Woo, J.; Basler, N.; Cunha, J.; et al. Neoadjuvant rituximab modulates the tumour immune environment in patients with high-risk prostate cancer. J. Trans. Med. 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Flammiger, A.; Bayer, F.; Cirugeda-Kuhneert, A.; Huland, H.; Tennstedt, P.; Simon, R.; Trepel, M. Intratumoral T but not B lymphocytes are related to clinical outcome in prostate cancer. APMIS 2012, 120, 901–908. [Google Scholar] [CrossRef]
- Derlin, T.; Riethdorf, S.; Schumacher, U.; Lfaos, M.; Peine, S.; Coith, C.; Ross, T.L.; Pantel, K.; Bengel, F.M. PMSA heterogeneity in metastatic castraction resistant prostate cancer: Circulating tumour cells, metastatic tumor burden, and response to targeted radioligand therapy. Prostate 2023, 83, 1076–1088. [Google Scholar] [CrossRef]
- Wong, C.H.M.; Ko, I.C.H.; Ng, C.F. Liquid biomarkers in prostate cancer: Recent advancements and future directions. Curr.Opin. Urol. 2025, 35, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Agarwal, A.; Almquist, R.G.; Runyambo, D.; Park, S.; Bronson, E.; Boominathan, R.; Roa, C.; Anand, M.; Oyekunle, T.; et al. Expression of immune checkpoints on circulating tumor cells in men with metastatic prostate cancer. Bioma 2012, 9, 14rker. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray, N.P. Immunomodulation and Immunotherapy for Patients with Prostate Cancer: An Up-to-Date Review. Biomedicines 2025, 13, 1179. https://doi.org/10.3390/biomedicines13051179
Murray NP. Immunomodulation and Immunotherapy for Patients with Prostate Cancer: An Up-to-Date Review. Biomedicines. 2025; 13(5):1179. https://doi.org/10.3390/biomedicines13051179
Chicago/Turabian StyleMurray, Nigel P. 2025. "Immunomodulation and Immunotherapy for Patients with Prostate Cancer: An Up-to-Date Review" Biomedicines 13, no. 5: 1179. https://doi.org/10.3390/biomedicines13051179
APA StyleMurray, N. P. (2025). Immunomodulation and Immunotherapy for Patients with Prostate Cancer: An Up-to-Date Review. Biomedicines, 13(5), 1179. https://doi.org/10.3390/biomedicines13051179





