Co-Occurrence of Helicobacter pylori and Candida spp. Infections in the Pathogenesis of Gastrointestinal Diseases
Abstract
1. Introduction
2. Methodology
3. Helicobacter pylori
4. Candida albicans and Candida spp.
5. Epidemiology of H. pylori Infections
| Global Prevalence of H. pylori | |
|---|---|
| Region | Prevalence Estimates, % |
| African region | 56.5 |
| Eastern Mediterranean region | 56.5 |
| European region | 45.7 |
| Region of the Americas | 49.0 |
| Southeast Asia region | 44.2 |
| Western Pacific region | 49.4 |
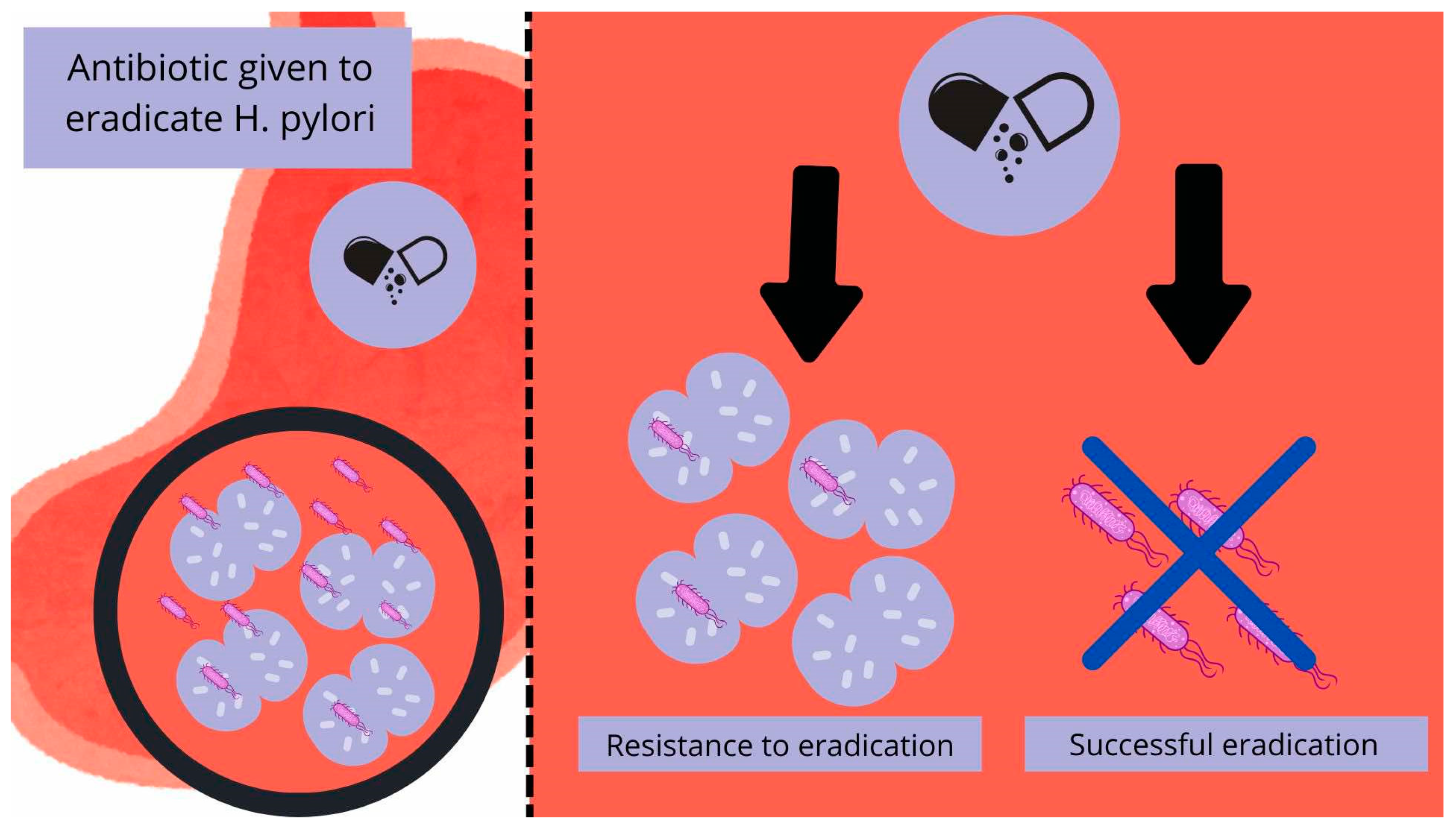
6. H. pylori Eradication and Its Resistance to Antibiotics
7. Interplay Between Candida spp. and H. pylori Infection
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siavoshi, F.; Saniee, P. Vacuoles of Candida Yeast as a Specialized Niche for Helicobacter pylori. World J. Gastroenterol. 2014, 20, 5263–5273. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.W.; Emerson, L.E.; Clapp, B.; Sheppe, A.E.; Sharma, J.; del Castillo, J.; Ou, M.; Maegawa, G.H.B.; Hoffman, C.; Larkin, J.; et al. The Cross-Kingdom Interaction between Helicobacter pylori and Candida Albicans. PLoS Pathog. 2021, 17, e1009515. [Google Scholar] [CrossRef]
- Ali, A.; AlHussaini, K.I. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms 2024, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, A.; Dighe, O.R.; Lamture, Y. Role of Helicobacter pylori in Gastric Carcinoma: A Review. Cureus 2023, 15, e37205. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori Virulence Factors—Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef]
- Azevedo, N.F.; Huntington, J.; Goodman, K.J. The Epidemiology of Helicobacter pylori and Public Health Implications. Helicobacter 2009, 14 (Suppl. 1), 1–7. [Google Scholar] [CrossRef]
- Sachs, G.; Scott, D.R. Helicobacter pylori: Eradication or Preservation. F1000 Med. Rep. 2012, 4, 7. [Google Scholar] [CrossRef]
- Salvatori, S.; Marafini, I.; Laudisi, F.; Monteleone, G.; Stolfi, C. Helicobacter pylori and Gastric Cancer: Pathogenetic Mechanisms. Int. J. Mol. Sci. 2023, 24, 2895. [Google Scholar] [CrossRef]
- Goni, E.; Franceschi, F. Helicobacter pylori and Extragastric Diseases. Helicobacter 2016, 21 (Suppl. 1), 45–48. [Google Scholar] [CrossRef]
- Maiorana, F.; Neschuk, M.; Caronia, M.V.; Elizondo, K.; Robledo, M.L.; Schneider, A.; Veron, G.; Zapata, P.D.; Barreyro, F.J. The Interplay between Helicobacter pylori Infection and Rs738409 PNPLA3 in Metabolic Dysfunction-Associated Steatotic Liver Disease. PLoS ONE 2024, 19, e0310361. [Google Scholar] [CrossRef]
- Keikha, M.; Karbalaei, M. Potential Association between Bacterial Infections and Ischemic Stroke Based on Fifty Case-Control Studies: A Systematic Review and Meta-Analysis. New Microbes New Infect. 2022, 47, 100980. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Karbalaei, M. A Comprehensive Survey of the Relationship between Helicobacter pylori Infection and Atherosclerosis in the Iranian Population: A Systematic Review and Meta-Analysis. Arch. Iran Med. 2022, 25, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Arellano, L. Helicobacter pylori and Neurological Diseases: Married by the Laws of Inflammation. World J. Gastrointest. Pathophysiol. 2014, 5, 400. [Google Scholar] [CrossRef]
- Kusters, J.G.; Van Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of Helicobacter pylori Infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori Infection: An Overview of Bacterial Virulence Factors and Pathogenesis. Biomed. J. 2016, 39, 14. [Google Scholar] [CrossRef]
- Yang, T.; Li, J.; Zhang, Y.; Deng, Z.; Cui, G.; Yuan, J.; Sun, J.; Wu, X.; Hua, D.; Xiang, S.; et al. Intracellular Presence of Helicobacter pylori Antigen and Genes within Gastric and Vaginal Candida. PLoS ONE 2024, 19, e0298442. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Sun, Y.; Li, P.; Xu, M.; Fang, P.; Zhang, S.; Yuan, G.; Deng, X.; Hu, H. Antibiotics-Free Nanoparticles Eradicate Helicobacter pylori Biofilms and Intracellular Bacteria. J. Control. Release 2022, 348, 370–385. [Google Scholar] [CrossRef]
- Sánchez-Alonzo, K.; Matamala-Valdés, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. Intracellular Presence of Helicobacter pylori and Its Virulence-Associated Genotypes within the Vaginal Yeast of Term Pregnant Women. Microorganisms 2021, 9, 131. [Google Scholar] [CrossRef]
- Tang, Z.; Fu, L.; Liu, R.; Chen, Y.; Bie, M.; Wang, B. Mechanisms of intracellular Helicobacter pylori infection and clinical considerations. J. Sichuan Univ. 2023, 54, 1300. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.L.; Cheng, D.D.; Xu, W.T.; Lu, N.H. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front. Cell. Infect. Microbiol. 2016, 6, 227385. [Google Scholar] [CrossRef]
- McBain, A.J.; O’Neill, C.A.; Oates, A. Skin Microbiology. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Dowd, F.J. Candida Albicans Infections. In Reference Module in Biomedical Research; Creighton University: Omaha, NE, USA, 2014. [Google Scholar] [CrossRef]
- Wiles, C.M.; Mackenzie, D.W.R. Fungal Diseases of the Central Nervous System. Infect. Nerv. Syst. 1987, 93–117. [Google Scholar] [CrossRef]
- Hiengrach, P.; Panpetch, W.; Chindamporn, A.; Leelahavanichkul, A. Helicobacter pylori, Protected from Antibiotics and Stresses Inside Candida Albicans Vacuoles, Cause Gastritis in Mice. Int. J. Mol. Sci. 2022, 23, 8568. [Google Scholar] [CrossRef] [PubMed]
- The ALS Gene Family of Candida Albicans. Trends Microbiol. 2001, 9, 176–180. [CrossRef] [PubMed]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida Albicans Secreted Aspartyl Proteinases in Virulence and Pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Hernández-Santos, N.; Peterson, A.C. IL-17 Signaling in Host Defense Against Candida Albicans. Immunol. Res. 2011, 50, 181. [Google Scholar] [CrossRef]
- Borka Balas, R.; Meliț, L.E.; Mărginean, C.O. Worldwide Prevalence and Risk Factors of Helicobacter pylori Infection in Children. Children 2022, 9, 1359. [Google Scholar] [CrossRef]
- Tran, V.; Saad, T.; Tesfaye, M.; Walelign, S.; Wordofa, M.; Abera, D.; Desta, K.; Tsegaye, A.; Ay, A.; Taye, B. Helicobacter pylori (H. pylori) Risk Factor Analysis and Prevalence Prediction: A Machine Learning-Based Approach. BMC Infect. Dis. 2022, 22, 655. [Google Scholar] [CrossRef]
- Smith, S.; Jolaiya, T.; Fowora, M.; Palamides, P.; Ngoka, F.; Bamidele, M.; Lesi, O.; Onyekwere, C.; Ugiagbe, R.; Agbo, I.; et al. Clinical and Socio- Demographic Risk Factors for Acquisition of Helicobacter pylori Infection in Nigeria. Asian Pac. J. Cancer Prev. 2018, 19, 1851–1857. [Google Scholar] [CrossRef]
- Chen, Y.C.; Malfertheiner, P.; Yu, H.T.; Kuo, C.L.; Chang, Y.Y.; Meng, F.T.; Wu, Y.X.; Hsiao, J.L.; Chen, M.J.; Lin, K.P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Ernst, P.B.; Gold, B.D. The Disease Spectrum of Helicobacter pylori: The Immunopathogenesis of Gastroduodenal Ulcer and Gastric Cancer. Annu. Rev. Microbiol. 2000, 54, 615–640. [Google Scholar] [CrossRef]
- McConaghy, J.R.; Decker, A.; Nair, S. Peptic Ulcer Disease and H. pylori Infection: Common Questions and Answers. Am. Fam. Physician 2023, 107, 165–172. [Google Scholar] [PubMed]
- Chen, T.H.; Cheng, H.T.; Yeh, C.T. Epidemiology Changes in Peptic Ulcer Diseases 18 Years Apart Explored from the Genetic Aspects of Helicobacter pylori. Transl. Res. 2021, 232, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zheng, M.; Jiang, J.; Cao, D.; Wu, Y.; Zhang, Y.; Fu, Y.; Cao, X. Positive H. pylori Status Predicts Better Prognosis of Non-Cardiac Gastric Cancer Patients: Results from Cohort Study and Meta-Analysis. BMC Cancer 2022, 22, 155. [Google Scholar] [CrossRef]
- He, J.J.; Hu, W.C.; Ouyang, Q.; Zhang, S.W.; He, L.J.; Chen, W.Y.; Li, X.Z.; Hu, C.J. Helicobacter pylori Infection Induces Stem Cell-like Properties in Correa Cascade of Gastric Cancer. Cancer Lett. 2022, 542, 215764. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xu, Y.; Wang, P.; Zhao, Y.; Wan, C. Research Progress on Molecular Mechanism of Pyroptosis Caused by Helicobacter pylori in Gastric Cancer. Ann. Med. Surg. 2024, 86, 2016–2022. [Google Scholar] [CrossRef]
- Kumar, S.; Metz, D.C.; Ellenberg, S.; Kaplan, D.E.; Goldberg, D.S. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020, 158, 527–536.e7. [Google Scholar] [CrossRef]
- Watari, J.; Das, K.K.; Amenta, P.S.; Tanabe, H.; Tanaka, A.; Geng, X.; Lin, J.J.C.; Kohgo, Y.; Das, K.M. Effect of Eradication of Helicobacter pylori on the Histology and Cellular Phenotype of Gastric Intestinal Metaplasia. Clin. Gastroenterol. Hepatol. 2008, 6, 409–417. [Google Scholar] [CrossRef]
- Pimanov, S.I.; Makarenko, E.V.; Voropaeva, A.V.; Matveenko, M.E.; Voropaev, E.V. Helicobacter pylori Eradication Improves Gastric Histology and Decreases Serum Gastrin, Pepsinogen I and Pepsinogen II Levels in Patients with Duodenal Ulcer. J. Gastroenterol. Hepatol. 2008, 23, 1666–1671. [Google Scholar] [CrossRef]
- Rokkas, T.; Pistiolas, D.; Sechopoulos, P.; Robotis, I.; Margantinis, G. The Long-Term Impact of Helicobacter pylori Eradication on Gastric Histology: A Systematic Review and Meta-Analysis. Helicobacter 2007, 12 (Suppl. 2), 32–38. [Google Scholar] [CrossRef]
- Li, W.Q.; Ma, J.L.; Zhang, L.; Brown, L.M.; Li, J.Y.; Shen, L.; Pan, K.F.; Liu, W.D.; Hu, Y.; Han, Z.X.; et al. Effects of Helicobacter pylori Treatment on Gastric Cancer Incidence and Mortality in Subgroups. J. Natl. Cancer Inst. 2014, 106, dju116. [Google Scholar] [CrossRef]
- Gisbert, J.P.; McNicholl, A.G. Optimization Strategies Aimed to Increase the Efficacy of H. pylori Eradication Therapies. Helicobacter 2017, 22, e12392. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H. Management of Helicobacter pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.O.; Cardenas, V.M.; Reddy, R.K.; Dominguez, D.C.; Snyder, L.K.; Graham, D.Y. Greater than 95% Success with 14-Day Bismuth Quadruple Anti- Helicobacter pylori Therapy: A Pilot Study in US Hispanics. Helicobacter 2012, 17, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ford, A.C.; Khan, K.J.; Gisbert, J.P.; Forman, D.; Leontiadis, G.I.; Tse, F.; Calvet, X.; Fallone, C.; Fischbach, L.; et al. Optimum Duration of Regimens for Helicobacter pylori Eradication. Cochrane Database Syst. Rev. 2013, 2013, 1–205. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review Article: The Global Emergence of Helicobacter pylori Antibiotic Resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef]
- ACG. American College of Gastroenterology, Guideline on Treatment of Helicobacter pylori: New Recommendations… Will Practice Change? Available online: https://gi.org/journals-publications/ebgi/schoenfeld_sep2024/ (accessed on 22 April 2025).
- McNicholl, A.G.; Linares, P.M.; Nyssen, O.P.; Calvet, X.; Gisbert, J.P. Meta-Analysis: Esomeprazole or Rabeprazole vs. First-Generation Pump Inhibitors in the Treatment of Helicobacter pylori Infection. Aliment. Pharmacol. Ther. 2012, 36, 414–425. [Google Scholar] [CrossRef]
- Gong, Y.; Yuan, Y. Resistance Mechanisms of Helicobacter pylori and Its Dual Target Precise Therapy. Crit. Rev. Microbiol. 2018, 44, 371–392. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori Infection and Antibiotic Resistance—from Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-Analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Salahi-Niri, A.; Nabavi-Rad, A.; Monaghan, T.M.; Rokkas, T.; Doulberis, M.; Sadeghi, A.; Zali, M.R.; Yamaoka, Y.; Tacconelli, E.; Yadegar, A. Global Prevalence of Helicobacter pylori Antibiotic Resistance among Children in the World Health Organization Regions between 2000 and 2023: A Systematic Review and Meta-Analysis. BMC Med. 2024, 22, 1–17. [Google Scholar] [CrossRef]
- Katzenstein, A.L.A.; Maksem, J. Candidal Infection of Gastric Ulcers. Histology, Incidence, and Clinical Significance. Am. J. Clin. Pathol. 1979, 71, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.B.; Jenkins, D. Gastro-Oesophageal Candidiasis. Gut 1982, 23, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Robin Warren, J.; Marshall, B. Unidentified Curved Bacilli On Gastric Epithelium In Active Chronic Gastritis. Lancet 1983, 321, 1273–1275. [Google Scholar] [CrossRef]
- Zwolińska-Wcisło, M.; Budak, A.; Trojanowska, D.; Bogdał, J.; Stachura, J. Fungal Colonization of the Stomach and Its Clinical Relevance. Mycoses 1998, 41, 327–334. [Google Scholar] [CrossRef]
- Medical Science Monitor, Effect of Fungal Colonization of Gastric Mucosa on the Course of Gastric Ulcers Healing. Article Abstract #421150. Available online: https://medscimonit.com/abstract/index/idArt/421150 (accessed on 23 March 2025).
- PubMed, Assessment of Co-Existence of Helicobacter pylori and Candida Fungi in Diseases of the Upper Gastrointestinal Tract. Available online: https://pubmed.ncbi.nlm.nih.gov/20224149/ (accessed on 9 March 2025).
- Wang, Y.H.; Lv, Z.F.; Zhong, Y.; Liu, D.S.; Chen, S.P.; Xie, Y. The Internalization of Helicobacter pylori Plays a Role in the Failure of H. pylori Eradication. Helicobacter 2017, 22, e12324. [Google Scholar] [CrossRef]
- D’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The Impact of the Fungus-Host-Microbiota Interplay upon Candida Albicans Infections: Current Knowledge and New Perspectives. FEMS Microbiol. Rev. 2021, 45, 14. [Google Scholar] [CrossRef]
- Heydari, S.; Siavoshi, F.; Jazayeri, M.H.; Sarrafnejad, A.; Saniee, P. Helicobacter pylori Release from Yeast as a Vesicle-Encased or Free Bacterium. Helicobacter 2020, 25, e12725. [Google Scholar] [CrossRef]
- Plant cell biology, Volume 1, PWN. Available online: https://ksiegarnia.pwn.pl/Biologia-komorki-roslinnej-Tom-1-Struktura,68710268,p.html?srsltid=AfmBOopJzuyr-7vIiLAziGblmX-O8gomgJuV5XVfUzCd4WosnmEx6E7J (accessed on 9 March 2025).
- Siavoshi, F.; Heydari, S.; Shafiee, M.; Ahmadi, S.; Saniee, P.; Sarrafnejad, A.; Kolahdoozan, S. Sequestration inside the Yeast Vacuole May Enhance Helicobacter pylori Survival against Stressful Condition. Infect. Genet. Evol. 2019, 69, 127–133. [Google Scholar] [CrossRef]
- Scott, D.R.; Sachs, G.; Marcus, E.A. The Role of Acid Inhibition in Helicobacter pylori Eradication. F1000Research 2016, 5, 1747. [Google Scholar] [CrossRef]
- Yang, J.C.; Lu, C.W.; Lin, C.J. Treatment of Helicobacter pylori Infection: Current Status and Future Concepts. World J. Gastroenterol. 2014, 20, 5283–5293. [Google Scholar] [CrossRef]
- Siavoshi, F.; Sahraee, M.; Ebrahimi, H.; Sarrafnejad, A.; Saniee, P. Natural Fruits, Flowers, Honey, and Honeybees Harbor Helicobacter pylori-Positive Yeasts. Helicobacter 2018, 23, e12471. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonzo, K.; Silva-Mieres, F.; Arellano-Arriagada, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Smith, C.T.; Campos, V.L.; García-Cancino, A. Nutrient Deficiency Promotes the Entry of Helicobacter pylori Cells into Candida Yeast Cells. Biology 2021, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vega, S.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. In Vitro Incorporation of Helicobacter pylori into Candida Albicans Caused by Acidic PH Stress. Pathogens 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonzo, K.; Belmar, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. Antibiotics as a Stressing Factor Triggering the Harboring of Helicobacter pylori J99 within Candida Albicans ATCC10231. Pathogens 2021, 10, 382. [Google Scholar] [CrossRef]
- Hildebrandt, E.; McGee, D.J. Helicobacter pylori Lipopolysaccharide Modification, Lewis Antigen Expression, and Gastric Colonization Are Cholesterol-Dependent. BMC Microbiol. 2009, 9, 258. [Google Scholar] [CrossRef]
- Ribeiro, F.C.; Rossoni, R.D.; de Barros, P.P.; Santos, J.D.; Fugisaki, L.R.O.; Leão, M.P.V.; Junqueira, J.C. Action Mechanisms of Probiotics on Candida Spp. and Candidiasis Prevention: An Update. J. Appl. Microbiol. 2020, 129, 175–185. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, X.; Shan, T.; Wang, N.; Han, Q.; Ren, B.; Cheng, L. Polymicrobial Interactions of Helicobacter pylori and Its Role in the Process of Oral Diseases. J. Oral Microbiol. 2025, 17, 2469896. [Google Scholar] [CrossRef]
- Gareayaghi, N.; Akkus, S.; Saribas, S.; Demiryas, S.; Ozbey, D.; Kepil, N.; Demirci, M.; Dinc, H.O.; Akcin, R.; Uysal, O.; et al. Epstein-Barr Virus and Helicobacter pylori Co-Infection in Patients with Gastric Cancer and Duodenale Ulcer. New Microbiol. 2021, 44, 217–226. [Google Scholar]
- Shukla, S.K.; Prasad, K.N.; Tripathi, A.; Singh, A.; Saxena, A.; Chand Ghoshal, U.; Krishnani, N.; Husain, N.; Prasad, K.N. Epstein-Barr Virus DNA Load and Its Association with Helicobacter pylori Infection in Gastroduodenal Diseases. Braz. J. Infect. Dis. 2011, 15, 583–590. [Google Scholar] [CrossRef]
- Akkus, S.; Gareayaghi, N.; Saribas, S.; Demiryas, S.; Ozbey, D.; Kepil, N.; Demirci, M.; Ziver Sarp, T.; Oyku Dinc, H.; Akcin, R.; et al. Co-Infection Relationship with Epstein-Barr Virus in Gastroduodenal Diseases with Helicobacter pylori. Quantitative PCR and EBNA-1 Gene-Based Approach. Acta Gastroenterol. Belg. 2022, 85, 301–308. [Google Scholar] [CrossRef]
| Region | Worldwide Prevalence of H. pylori Antibiotic Resistance (%) | ||||
|---|---|---|---|---|---|
| Clarithromycin | Metronidazole | Levofloxacin | Amoxicillin | Tetracycline | |
| Africa | 15 | 91 | 14 | 38 | 13 |
| Americas | 14 | 27 | 14 | 8 | 4 |
| Eastern Mediterranean region | 29 | 61 | 23 | 14 | 10 |
| European region | 32 | 38 | 14 | 0 | 0 |
| Southeast Asia | 17 | 59 | 25 | 12 | 0 |
| Western Pacific region | 34 | 55 | 24 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braksator, J.; Kofla-Dłubacz, A.; Antosz-Popiołek, K.; Szyller, H.; Koga-Batko, J.; Wrześniewska, M.; Dyda, M.; Pytrus, T. Co-Occurrence of Helicobacter pylori and Candida spp. Infections in the Pathogenesis of Gastrointestinal Diseases. Biomedicines 2025, 13, 1172. https://doi.org/10.3390/biomedicines13051172
Braksator J, Kofla-Dłubacz A, Antosz-Popiołek K, Szyller H, Koga-Batko J, Wrześniewska M, Dyda M, Pytrus T. Co-Occurrence of Helicobacter pylori and Candida spp. Infections in the Pathogenesis of Gastrointestinal Diseases. Biomedicines. 2025; 13(5):1172. https://doi.org/10.3390/biomedicines13051172
Chicago/Turabian StyleBraksator, Joanna, Anna Kofla-Dłubacz, Katarzyna Antosz-Popiołek, Hubert Szyller, Joanna Koga-Batko, Martyna Wrześniewska, Maciej Dyda, and Tomasz Pytrus. 2025. "Co-Occurrence of Helicobacter pylori and Candida spp. Infections in the Pathogenesis of Gastrointestinal Diseases" Biomedicines 13, no. 5: 1172. https://doi.org/10.3390/biomedicines13051172
APA StyleBraksator, J., Kofla-Dłubacz, A., Antosz-Popiołek, K., Szyller, H., Koga-Batko, J., Wrześniewska, M., Dyda, M., & Pytrus, T. (2025). Co-Occurrence of Helicobacter pylori and Candida spp. Infections in the Pathogenesis of Gastrointestinal Diseases. Biomedicines, 13(5), 1172. https://doi.org/10.3390/biomedicines13051172





