Abstract
Fibrosis is a pathological process characterized by excessive extracellular matrix (ECM) deposition, leading to tissue stiffening and organ dysfunction. It is a major contributor to chronic diseases affecting various organs, with limited therapeutic options available. Among the different forms of fibrosis, cardiac fibrosis is particularly relevant due to its impact on cardiovascular diseases (CVDs), which remain the leading cause of morbidity and mortality worldwide. This process is driven by activated cardiac fibroblasts (CFs), which promote ECM accumulation in response to chronic stressors. Epigenetic mechanisms, including DNA methylation, histone modifications, and chromatin remodeling, are key regulators of fibroblast activation and fibrotic gene expression. Enzymes such as DNA methyltransferases (DNMTs), histone methyltransferases (HMTs), histone acetyltransferases (HATs), and histone deacetylases (HDACs) have emerged as potential therapeutic targets, and epigenetic inhibitors have shown promise in modulating these enzymes to attenuate fibrosis by controlling fibroblast function and ECM deposition. These small-molecule compounds offer advantages such as reversibility and precise temporal control, making them attractive candidates for therapeutic intervention. This review aims to provide a comprehensive overview of the mechanisms by which epigenetic regulators influence cardiac fibrosis and examines the latest advances in preclinical epigenetic therapies. By integrating recent data from functional studies, single-cell profiling, and drug development, it highlights key molecular targets, emerging therapeutic strategies, and current limitations, offering a critical framework to guide future research and clinical translation.
1. Introduction
Fibrosis is a major health burden worldwide, contributing to approximately 45% of global deaths annually [1]. This high prevalence is due to the fact that fibrosis is a common consequence of numerous chronic diseases affecting different organ systems, including the heart, lungs, liver, kidneys, and skin. Among fibrotic diseases, ischemic heart disease stands out as a leading cause of mortality, responsible for nearly 9 million deaths per year [2]. Similarly, liver cirrhosis, chronic kidney disease, and interstitial lung diseases contribute significantly to global morbidity and mortality, with limited treatment options available [3]. Despite its widespread impact, fibrosis remains a poorly addressed clinical problem, with no effective therapies capable of halting or reversing its progression.
This pathological process is characterized by excessive deposition of extracellular matrix (ECM) components, primarily collagen and fibronectin, which leads to tissue stiffening and organ dysfunction [4]. Fibroblasts, the key cellular mediators of fibrosis, become activated upon injury, differentiating into myofibroblasts that produce ECM components necessary for wound healing [5]. Under physiological conditions, fibrosis is an essential repair mechanism activated in response to tissue injury. However, when the fibrotic response is exaggerated or sustained over time, ECM accumulation leads to structural damage and loss of organ function. Various molecular pathways, including transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), and reactive oxygen species (ROS), regulate fibroblast activation and ECM remodeling across different organs [6]. Although fibrosis shares common molecular mechanisms, its clinical manifestations and progression vary significantly depending on the affected organ.
Among the different forms of fibrosis, cardiac fibrosis is particularly relevant due to its impact on cardiovascular diseases (CVDs), which remain the leading cause of morbidity and mortality worldwide. Given the critical role of fibrosis in cardiac pathology, understanding its mechanisms, classifications, and implications is essential for the development of targeted therapeutic strategies. In this review, we highlight the role of epigenetic regulation in cardiac fibrosis and discuss emerging therapeutic strategies based on epigenetic modulation. A better understanding of these mechanisms may help pave the way for more effective targeted anti-fibrotic therapies.
2. Classification and Progression of Cardiac Fibrosis
Cardiac fibrosis represents a key pathological process that disrupts normal heart function. This condition results from the excessive accumulation of ECM proteins, primarily collagen, within the myocardium. Unlike other tissues, the heart has a limited regenerative capacity, making fibrosis a major contributor to cardiac dysfunction and progression to heart failure (HF) [7]. Depending on its etiology and distribution, cardiac fibrosis can be classified into different types, each with distinct pathological consequences.
Reactive fibrosis occurs in response to chronic stressors such as hypertension, obesity, diabetes, or aging [8]. Unlike replacement fibrosis, reactive fibrosis is not associated with massive cardiomyocyte death. Instead, it manifests as an excessive accumulation of ECM in the interstitial and perivascular spaces, leading to increased myocardial stiffness and impaired diastolic function. In experimental models of left ventricular pressure overload, reactive fibrosis initially serves as an adaptive response to maintain cardiac structure and function. However, if the pathological stimulus persists, this process may evolve into maladaptive remodeling, ultimately leading to cardiac dysfunction and failure [9]. Moreover, reactive fibrosis is commonly observed in remote zones following myocardial infarction (MI), where it extends beyond the initial ischemic injury, further compromising cardiac function [10].
Replacement fibrosis, also referred to as reparative fibrosis, is characterized by scar formation following extensive cardiomyocyte loss, as seen in MI [11]. Due to the heart’s inability to regenerate lost cardiomyocytes, fibroblasts proliferate and deposit ECM components to preserve myocardial integrity [12]. While this process is necessary to prevent ventricular rupture and maintain structural stability, it severely impairs cardiac function by increasing tissue rigidity and disrupting electrical conduction, predisposing the heart to arrhythmias.
The progression of cardiac fibrosis occurs through a series of overlapping phases, particularly well-characterized in the context of ischemic injury, such as in MI. Following MI, the fibrotic response unfolds in three main stages: the inflammatory phase, the proliferative phase, and the maturation phase [13].
The inflammatory phase is triggered by hypoxic injury and subsequent cardiomyocyte death, which leads to the release of damage-associated molecular patterns (DAMPs) [14]. These molecules activate resident immune cells, including macrophages, as well as fibroblasts, initiating an acute inflammatory response [15]. Neutrophils rapidly infiltrate the injured myocardium, releasing ROS and proteolytic enzymes that degrade damaged tissue while amplifying inflammation [16]. Macrophages play a dual role in this phase, initially adopting a pro-inflammatory phenotype (M1) to clear necrotic debris before transitioning into an anti-inflammatory and reparative phenotype (M2) that promotes fibrosis and tissue healing [17].
The proliferative phase, occurring between days 4 and 14 post-MI, is characterized by a shift from a pro-inflammatory to an anti-inflammatory environment, allowing fibroblasts to become activated and differentiate into myofibroblasts [18]. The activation of fibroblasts is largely mediated by TGF-β and other profibrotic cytokines. These myofibroblasts migrate to the infarcted region and deposit ECM proteins, particularly type III collagen, to replace the fibrin scaffold formed during the inflammatory phase [19]. At the same time, angiogenesis is initiated, with endothelial cells (ECs) responding to hypoxic signals by forming new capillaries that facilitate oxygen delivery and tissue repair [20].
The maturation phase begins around two weeks post-MI and is marked by a transition from a loose type III collagen matrix to a more rigid and cross-linked type I collagen network [21]. Lysyl oxidase (LOX), an enzyme produced by myofibroblasts, plays a critical role in stabilizing the fibrotic scar by catalyzing collagen cross-linking [22]. Additionally, myofibroblasts progressively lose their contractile properties and transition into matrifibrocytes, a specialized fibroblast subset involved in ECM maintenance [23]. While this process ensures scar stability, it also contributes to myocardial stiffening and electrical conduction abnormalities, increasing the risk of arrhythmias and HF.
In addition to ventricular remodeling, atrial fibrosis has emerged as a relevant pathological process with important clinical implications, particularly in the context of atrial fibrillation (AF). While ventricular fibrosis is typically associated with ischemic injury, atrial fibrosis often results from chronic hemodynamic or inflammatory stress and involves endomysial collagen deposition that disrupts electrical conduction. Epigenetic dysregulation has been implicated in the pathogenesis of AF through multiple mechanisms, including altered expression of specific microRNAs, dysregulated histone acetylation, and increased activity of HMTs such as EZH2, which promotes fibrotic gene expression [24]. These findings support the concept of atrial cardiomyopathy as an epigenetically driven substrate for AF and further emphasize the importance of cardiac fibroblasts (CFs) as central mediators of fibrotic remodeling.
CFs are the primary effectors of cardiac fibrosis and play a crucial role in ECM homeostasis. Under physiological conditions, CFs maintain the structural integrity of the myocardium, regulate ECM turnover, and modulate intercellular communications with cardiomyocytes and ECs [25]. However, in response to pathological stimuli, CFs become activated, transitioning into myofibroblasts that secrete excessive ECM components, perpetuating fibrosis and impairing cardiac function [26].
Despite their essential role in cardiac fibrosis, identifying CFs remains a challenge due to their plasticity and heterogeneity. Their dynamic nature has been extensively studied using single-cell RNA sequencing (scRNA-seq), which has revealed distinct fibroblast subpopulations, their transitions, and their roles in cardiac injury and repair. Murine studies were instrumental in uncovering these diverse fibroblast states. One of the first to apply scRNA-seq in the heart was Gladka et al., who identified distinct fibroblast clusters and proposed Ckap4 as a novel marker of activated fibroblasts following ischemia-reperfusion injury [27]. As fibroblast heterogeneity became increasingly evident, Ruiz-Villalba et al. took a step further by integrating scRNA-seq with single-cell Assay for Transposase-Accessible Chromatin-sequencing (ATAC-seq) to investigate not only transcriptional diversity but also the underlying regulatory mechanisms. Their study identified a reparative Cthrc1⁺ fibroblast population involved in ECM remodeling and highlighted key transcription factors (TFs) such as RUNX1 and TEAD as regulators of fibroblast activation through chromatin accessibility profiling [28]. Forte et al. further expanded this work by analyzing post-MI fibroblast dynamics over time. They showed how specific fibroblast states emerged sequentially during repair, from homeostatic fibroblasts in healthy hearts to inflammatory, myofibroblastic, and late-response fibroblasts post-injury, each contributing differently to fibrosis [29]. In a broader context, Buechler et al. compared fibroblasts across tissues and disease models, identifying clusters such as Lrrc15⁺, Cxcl5⁺, and Adamdec1⁺ associated with fibrosis, while Dpt⁺ fibroblasts were linked to early activation states [30].
scRNA-seq has also been applied to human hearts, providing critical insights into disease-specific fibroblast programs. Koenig et al. profiled samples from healthy donors and patients with dilated cardiomyopathy (DCM), revealing nine fibroblast subtypes. While some were common to both conditions (Fb1, Fb2), others were enriched in DCM and linked to fibrotic remodeling (Fb5–Fb9) [31]. In a separate study, Chaffin et al. identified an activated fibroblast population expressing POSTN, THBS4, COL1A1, and FAP that was nearly absent in non-failing hearts but prevalent in both DCM and hypertrophic cardiomyopathy (HCM), underscoring disease-specific pathogenic transitions [32].
Further work by Kuppe et al. used an integrative approach combining single-nucleus RNA-seq, single-nucleus ATAC-seq, and spatial transcriptomics to define four fibroblast subtypes in post-MI human hearts. They identified a progenitor-like population (SCARA5⁺) in healthy regions and terminally differentiated myofibroblasts (POSTN⁺, COL1A1⁺) localized in fibrotic areas, driven by TFs such as TEAD and RUNX1 [33]. In the context of HF, Amrute et al. identified fibrotic subpopulations (POSTN⁺, THBS4⁺, FAP⁺) linked to inflammation and cytoskeletal remodeling. Recovery was marked by partial reversion to quiescence and downregulation of inflammatory and TGF-β pathways [34]. Fu et al. investigated arrhythmogenic right ventricular cardiomyopathy (ARVC), identifying fibroblast subtypes with distinct roles: FB0 and FB5 were enriched in fibrotic hearts and associated with pro-fibrotic activity, while FB3 and FB6 showed adipogenic signatures [35].
While previous studies provided detailed transcriptional and spatial maps of fibroblast diversity in cardiac fibrosis, none had addressed the epigenetic regulation underlying these cellular states. Recently, Aguado-Álvaro et al. published a preprint reporting the first large-scale functional interrogation of chromatin regulators during fibroblast transformation using combined bulk and single-cell CRISPR screens. A pooled screen in primary CFs under TGF-β stimulation identified pro- and anti-fibrotic chromatin factors, including Kat5, Wdr82, and components of the SRCAP and NSL complexes, based on their influence over fibroblast activation. To understand their role at the subpopulation level, the top regulators were further profiled using Perturb-seq under resting, fibrotic (TGF-β), and inflammatory (IL-1β) conditions. This approach revealed that specific chromatin regulators are key determinants of fibroblast plasticity, as they enable the expansion of pro-fibrotic myofibroblast subpopulations, while their depletion redirects cells toward alternative, less pathogenic or deactivated states, ultimately modulating the balance between reparative and fibrotic phenotypes in response to external cues [36].
Together, these studies not only reinforce the importance of fibroblast heterogeneity in cardiac disease progression but also underscore the need to understand how chromatin dynamics and epigenetic regulators shape fibroblast identity. This lays the foundation for exploring the role of chromatin factors in cardiovascular pathophysiology, which is discussed in the following section.
3. Chromatin Factors and Epigenetic Regulators in Cardiovascular Diseases
Epigenetics refers to the mechanisms that regulate gene expression without altering the DNA sequence. These mechanisms include DNA methylation, histone modifications, and chromatin remodeling, which collectively influence chromatin structure and control DNA accessibility [37]. Epigenetic modifications introduce biochemical marks that impact transcription, while chromatin remodelers reorganize nucleosomes to modulate DNA accessibility.
DNA methylation is a key epigenetic modification that involves the addition of methyl groups to cytosine residues, typically at cytosine-phosphodiester bond-guanine (CpG) dinucleotides, using DNA methyltransferases (DNMTs). This modification is generally associated with transcriptional repression, as it prevents the binding of TFs or recruits repressor complexes [38].
Histone modifications, including methylation and acetylation, regulate gene expression by altering chromatin compaction and accessibility [39]. Histone methylation is catalyzed by histone methyltransferases (HMTs), which use S-adenosylmethionine (SAM) as a methyl donor. Histone demethylation is carried out by histone demethylases (HDMs), which remove these marks and modulate gene expression [40]. Histone acetylation, catalyzed by histone acetyltransferases (HATs), relaxes chromatin and facilitates transcription, whereas histone deacetylases (HDACs) remove these marks, leading to chromatin condensation and transcriptional repression [41]. Moreover, histone acetylation on lysine residues is recognized by bromodomains (BRDs), which act as “readers” of these marks [42].
Chromatin remodeling is mediated by ATP-dependent chromatin remodelers, such as the ISWI, CHD, SWI/SNF, and INO80 complexes, which regulate nucleosome positioning, histone exchange, and chromatin accessibility [43]. These processes directly impact DNA accessibility, allowing TFs and other regulatory proteins to bind specific regions of the genome.
Beyond influencing gene expression at a cellular level, these mechanisms are increasingly recognized as key contributors to cardiovascular pathology, offering insights into their potential as therapeutic targets in cardiovascular conditions (Figure 1).
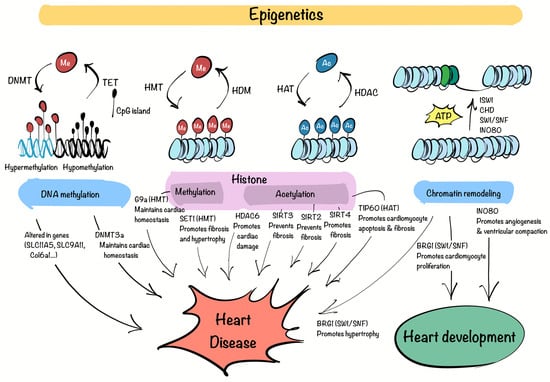
Figure 1.
Epigenetic regulation in CVD. The complex interaction between DNA methyltransferases (DNMT), tet methylcytosine dioxygenases (TET) (DNA methylation), histone methyltransferases (HMT), histone demethylases (HDM), histone deacetylases (HDAC), histone acetyltransferases (HAT), bromodomain (Histone modifications) and ISWI, CHD, SWI/SNF, INO80 complexes (Chromatin remodeling), govern methylation (Me) of DNA and histones, acetylation (Ac) of histones, and changes in nucleosomes, consequently impacting the regulation of cardiac development, homeostasis and disease. This figure was created with Procreate (version 5.3.15).
DNA methylation plays a vital role in preserving genomic integrity and regulating gene expression. Disruptions in this process can lead to gene expression abnormalities associated with disease. In coronary heart disease, genes such as SLC1A5 and SLC9A1, involved in glutamine metabolism and ionic balance, respectively, show altered methylation patterns, disrupting myocardial function [44,45]. Research using an MI mouse model revealed significant changes in methylation of genes like Ptpn6, Csf1r, Col6a1, Cyba, and Map3k14, which play key roles in disease progression. These genes may serve as biomarkers for the early detection of MI [46]. In HF, the analysis of ventricular septal tissues identified 195 regions with differential methylation, with some genes hypermethylated and others hypomethylated, impacting pathways related to cardiac remodeling, fibrosis, and mitochondrial function [47]. Additionally, a study demonstrated that DNMT3a is critical for maintaining cardiomyocyte homeostasis. Its knockout alters the expression of contractile proteins and damages mitochondrial integrity [48].
Histone modifications, such as methylation and acetylation, play a pivotal role in regulating gene expression and have been implicated in the development of CVDs. A study demonstrated that the HMT G9a is essential for maintaining cardiac function by regulating gene silencing through H3K9 dimethylation. Loss of G9a was linked to impaired cardiac contractility, increased fibrosis, and dysregulated expression of genes involved in calcium handling and hypertrophy, suggesting its potential as a therapeutic target [49]. Another example is the deletion of the H3K4 methyltransferase SET1 in a hypertensive mouse model, which reduced cardiac fibrosis and attenuated hypertrophy by modulating endothelin-1 transcription in ECs [50]. The activity of HDAC6 has been shown to worsen ischemia-reperfusion injury by increasing oxidative stress and promoting cardiomyocyte damage [51]. Conversely, SIRT3, an HDAC, protects the heart by deacetylating cyclophilin D, preventing mitochondrial dysfunction and reducing cell death [52]. In HF, a deficiency in SIRT2 has been shown to worsen myocardial fibrosis, indicating its role in counteracting fibrotic responses triggered by angiotensin II (Ang II) [53]. SIRT3, on the other hand, deacetylates GSK3β at lysine 15, enhancing its activity. This activation promotes the phosphorylation and subsequent degradation of SMAD3, thereby inhibiting fibrosis driven by TGF-β signaling [54]. In contrast, the depletion of SIRT4 has been found to reduce Ang II-induced myocardial fibrosis [55].
Among the numerous HATs, Kat5/TIP60 has recently gained attention due to its versatile and critical functions in cellular processes. TIP60 is primarily recognized for acetylating histones, but it also targets non-histone proteins, positioning itself as a key epigenetic and transcriptional regulator [56]. Building on this knowledge, researchers have begun to investigate TIP60 in cardiovascular biology, particularly given its abundant expression in myocardial tissue [57]. Recent studies have revealed its significance in ischemic heart diseases. For example, conditional deletion of TIP60 in cardiomyocytes following MI has been associated with improved cardiac function, reduced fibrosis, and decreased apoptosis in the injured heart [58]. Another study demonstrated that TIP60 acetylates ataxia-telangiectasia mutated (ATM) protein at birth, triggering a DNA damage response (DDR) that induces cardiomyocyte senescence. Suppressing TIP60 reduces DNA damage markers, reactivates the cell cycle, and restores a fetal-like proliferative state in cardiomyocytes, improving cardiac function after neonatal MI [59]. Furthermore, TIP60 haploinsufficiency in adult cardiomyocytes under hypertrophic stress has been shown to enhance cell cycle activation, leading to increased proliferative activity and reduced apoptosis [60]. Building on their previous findings, the same authors investigated the long-term effects of TIP60 depletion in cardiomyocytes. While initial results showed increased cell density and reduced apoptosis, prolonged TIP60 loss led to progressive cardiac dysfunction, fibrosis, extensive cell death, and ultimately HF [61]. Moreover, TIP60 has also been recently identified as a pro-fibrotic factor in CFs through large-scale functional CRISPR screens, supporting its role beyond cardiomyocytes and reinforcing its relevance as a therapeutic target in fibrotic remodeling [36].
Chromatin remodelers are essential for regulating gene expression in the heart during both development and disease. Among the four main ATP-dependent chromatin remodeling families, the SWI/SNF complex has been extensively examined for its role in cardiac development. This complex, which includes the ATPase subunits BRG1 and BRM, reorganizes nucleosomes to control transcription [62]. BRG1 is critical for heart development, promoting cardiomyocyte proliferation and sustaining fetal gene expression programs [63]. Additionally, BRG1 becomes reactivated in adult cardiomyocytes under pathological conditions, where it drives the re-expression of fetal gene patterns associated with cardiac hypertrophy [64]. The INO80 chromatin remodeler also plays a pivotal role in heart development, particularly in coronary angiogenesis and ventricular compaction. Its deletion in endothelial cells impairs these processes, resulting in defective myocardial growth and congenital heart abnormalities, underscoring its importance in cardiovascular health [65].
In summary, epigenetic regulation is emerging as a crucial factor in the understanding of CVDs. The advances discussed highlight the significant role of epigenetic mechanisms in shaping cardiac health and pathology. These interconnected mechanisms influence fibroblast activation and fibrosis progression, making them promising targets for therapeutic intervention.
4. Epigenetic Therapies for Cardiac Fibrosis
Despite advances in understanding cardiac fibrosis, no therapy has been able to fully reverse it [66]. This challenge stems from the heart’s limited regenerative capacity, the diverse causes of fibrosis, and the heterogeneity of CFs, which complicates targeted treatments.
Current therapeutic strategies for cardiac fibrosis aim to mitigate its progression rather than completely reverse it. While these therapies do not directly target fibrosis, they play a pivotal role in reducing the factors that exacerbate its development, such as neurohormonal activation, inflammation, and hemodynamic stress. Drugs such as renin-angiotensin-aldosterone system (RAAS) inhibitors [67], including angiotensin-converting enzyme (ACE) inhibitors [68], angiotensin receptor blockers (ARBs) [69], and mineralocorticoid receptor antagonists (MRAs) [70], have shown antifibrotic effects by modulating fibroblast activation and reducing collagen deposition. Beta-blockers, commonly used in HF, also help by attenuating sympathetic overactivation, which contributes to fibrotic remodeling [71]. Statins, primarily prescribed for cholesterol control, have demonstrated pleiotropic effects in reducing oxidative stress and inflammation, indirectly influencing fibrosis progression [72].
Beyond conventional therapies, efforts to directly target fibrotic pathways have focused on key signaling mediators such as TGF-β. However, direct TGF-β inhibition has been associated with severe side effects, including increased mortality and adverse cardiac remodeling, limiting its clinical applicability [73]. Recent antifibrotic drug developments have included agents originally approved by the Food and Drug Administration (FDA) for pulmonary fibrosis, such as pirfenidone [74] and nintedanib [75]. These drugs exhibit antifibrotic properties by modulating oxidative stress, inflammation, and fibroblast activity. While promising, their efficacy in cardiac fibrosis remains under investigation, and challenges such as off-target effects and limited myocardial penetration hinder their widespread use.
Given these complexities, current therapies remain largely palliative, and in severe cases, heart transplantation is the only option, highlighting the need for novel therapeutic approaches such as epigenetic therapies [76]. Recent advancements in the understanding of epigenetic-modifying enzymes have highlighted the reversibility of many epigenetic modifications, emphasizing their role in regulating gene expression. This reversibility forms a solid foundation for developing therapeutic strategies aimed at modulating these mechanisms.
Notably, FDA-approved epigenetic drugs, such as DNMT inhibitors (azacitidine and decitabine) and HDAC inhibitors (vorinostat, romidepsin, and belinostat), have proven effective in treating cancers like myelodysplastic syndrome, leukemia, and T-cell lymphomas [77]. While epigenetic therapies have primarily been explored in oncology, their application in CVDs, including cardiac fibrosis, is an area of growing interest. Although none have gained FDA approval yet for cardiovascular conditions, epigenetic inhibitors represent a promising avenue for modulating fibrosis. The potential of these drugs is illustrated in Figure 2 by their diverse targets and mechanisms, which offer innovative approaches for the treatment of cardiac fibrosis.
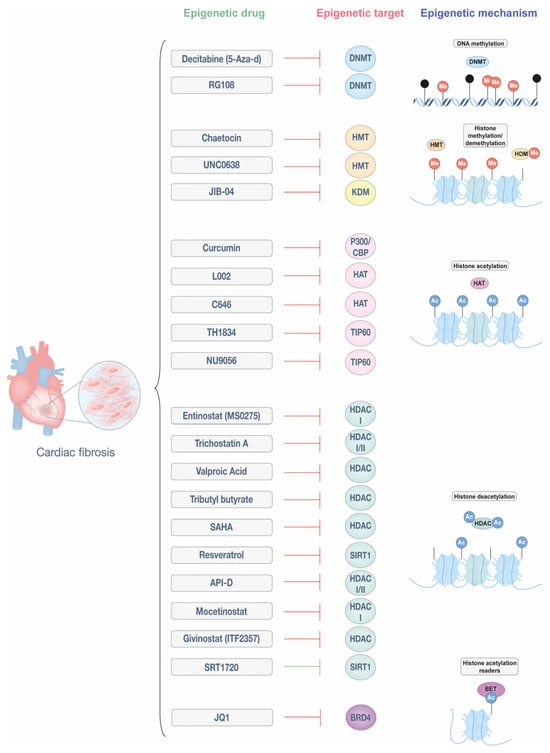
Figure 2.
Epigenetic drugs targeting cardiac fibrosis. Epigenetic modifications regulate fibroblast activation and ECM deposition, playing a key role in the progression of cardiac fibrosis. This figure illustrates different classes of epigenetic drugs and their molecular targets, including DNA methyltransferases (DNMTs), histone methyltransferases (HMTs), histone/lysine demethylases (HDMs/KDMs), histone acetyltransferases (HATs), histone deacetylases (HDACs), bromodomains (BRD), and bromodomain and extra-terminal motif (BET) proteins. By modulating these mechanisms, epigenetic therapies hold potential for reversing or mitigating cardiac fibrosis. This figure was created with Adobe Illustrator (version 29.5).
DNMT inhibitors, such as 5-azacytidine (also known as decitabine) and RG108, have shown potential in reducing cardiac fibrosis. For example, 5-aza-d improves cardiac remodeling and reduces fibrosis in hypertensive rats by blocking the expression of HCM genes [78,79]. Similarly, RG108 effectively prevented fibrosis and hypertrophy in mouse models of pressure overload [80,81]. While 5-aza-d integrates into DNA, potentially limiting its clinical use, small molecules like RG108 offer a more flexible approach, leveraging the reversible nature of DNA methylation to alter gene expression and promote antifibrotic effects.
Inhibitors of histone-modifying enzymes have demonstrated potential in addressing cardiac fibrosis. Chaetocin, an HMT inhibitor targeting H3K9 methyltransferase SUV39H, reduced cardiac fibrosis in a mouse model of MI [82]. UNC0638, an HMT inhibitor, reduced infarct size in an acute MI model by suppressing fibrosis, inflammation, and apoptosis through the downregulation of SMAD3 and TGF-β signaling [83]. Additionally, the demethylation of the histone mark H3K9me2 by KDM3a has been implicated in fibrosis progression, a process counteracted by the pan-KDM inhibitor JIB-04, which mitigated fibrosis in a mouse model of pressure overload [84].
Histone acetylation regulators, particularly HDAC inhibitors, are well-studied epigenetic drugs, while HAT inhibitors are emerging as promising treatments for cardiac fibrosis. Among HAT inhibitors, curcumin, a natural polyphenol with specificity for p300/CBP, reduces infarct size and cardiac fibrosis in MI models by inhibiting macrophage-fibroblast crosstalk and restraining IL18-TGFβ1-SMAD2/3 signaling. It also suppresses collagen deposition, reduces TGF-β1 production, and enhances SMAD7 expression, contributing to its antifibrotic effects [85,86,87]. Small molecule inhibitors C646 and L002 also target p300 and have been effective in attenuating hypertrophy and fibrosis in hypertension-induced HF models [88,89]. Beyond p300, the Kat5/TIP60 family has gained attention for its roles in cancer and potential in cardiac fibrosis. The experimental Kat5/TIP60 inhibitor TH1834 reduced apoptosis and fibrosis while improving systolic function in a post-MI model [90]. Similarly, another Kat5/TIP60 inhibitor, NU9056, has recently been shown to reduce fibrotic gene expression, α-SMA levels, and collagen deposition in both murine and human CFs, further supporting the therapeutic relevance of targeting Kat5 in cardiac fibrosis [36].
Despite the promising effects of curcumin and other HAT inhibitors on cardiac fibrosis, their pharmacological application remains limited by poor bioavailability, rapid metabolism, and low systemic concentrations. Curcumin, in particular, displays limited aqueous solubility and is rapidly metabolized into less active conjugates, which restricts its therapeutic efficacy after oral administration [91]. Further studies are required to enhance its bioavailability and avoid unwanted effects on other tissues or cardiac cell types. In this regard, targeted delivery systems such as polymeric nanoparticles or lipid-based carriers have emerged as optimal strategies. These nanocarriers can improve solubility, protect curcumin from premature degradation, and enable controlled release while facilitating tissue-specific accumulation, especially in fibrotic cardiac regions. Moreover, engineered nanoparticles can be functionalized to minimize systemic exposure and maximize antifibrotic activity in the heart, potentially overcoming the limitations associated with conventional administration routes [92].
HDAC inhibitors are among the most extensively studied epigenetic therapies for cardiac fibrosis, targeting different HDAC classes. Entinostat (MS-0275), a class I-specific HDAC inhibitor, reduces infarct size and improves left ventricular function after ischemia-reperfusion by decreasing oxidative damage and preserving myocardial viability. It also prevents cardiac fibrosis and mitigates electrical remodeling caused by rapid ventricular stimulation [93,94]. Similarly, Trichostatin A, an inhibitor of class I and II HDACs, prevents cardiomyocyte hypertrophy, reduces MI size, and attenuates Ang II-induced fibroblast activity by regulating the expression of matrix metalloproteinase (MMP) 9 and IL-18, enhancing its protective effects in MI [95,96,97]. Valproic acid, tributyl butyrate, and vorinostat (SAHA), all HDAC inhibitors, collectively mitigate ventricular remodeling and reduce infarct size, contributing to improved cardiac outcomes [98,99,100]. Vorinostat, in particular, has demonstrated anti-fibrotic effects across various conditions, including cardiac fibrosis. A preclinical study in an MI-induced cardiac fibrosis model showed that SAHA treatment improved cardiac function by suppressing fibrosis and reversing the activation of the TGFβ1/P38 pathway [101]. Resveratrol, a modulator of SIRT1/SMAD3 deacetylation, reduced myocardial fibrosis in a DCM model. Additionally, in the HF model, it has been shown to decrease MMP-2 activity, contributing to enhanced cardiac function and fibrosis [102,103]. API-D, a dual class I and II HDAC inhibitor, and Mocetinostat, a class I-specific inhibitor, both effectively reverse myocardial fibrosis [104,105], with Mocetinostat inducing fibroblast apoptosis and cell cycle arrest [106]. Givinostat (ITF2357) mitigates endothelial-to-mesenchymal transition and inflammation, improving heart performance and reducing fibrosis in MI models [107,108,109]. Additionally, SRT1720, a SIRT1 activator, has shown efficacy in alleviating both cardiac hypertrophy and fibrosis in a model of pressure overload. Another study demonstrated its ability to reduce fibrosis by modulating SMAD2/3 signaling [108,109].
Finally, the BRD4 inhibitor JQ1 has shown potential in reducing cardiac fibrosis by suppressing key fibrotic markers and fibroblast contractility [110,111]. A recent single-cell epigenomic study revealed that JQ1 inhibitors regulate fibroblast activation by shifting them from an activated state to a quiescent state, correlating with improved cardiac function. This effect involves the regulation of MEOX1, a TF driving fibrosis, further emphasizing JQ1’s therapeutic promise in HF and MI [111].
Overall, the exploration of epigenetic therapies has demonstrated promising results in preclinical studies, highlighting their potential to modulate key mechanisms involved in cardiac fibrosis. These findings underscore the need for further research to refine these approaches and assess their translational potential for clinical application.
5. Conclusions and Future Directions
In this review, we have summarized the emerging role of epigenetic therapies in cardiac fibrosis and their potential for clinical application.
Epigenetic inhibitors offer several advantages as therapeutic agents, including their reversibility, precise temporal control, and potential for clinical translation. Unlike genetic modifications, which are often permanent and associated with ethical concerns, small-molecule inhibitors allow the transient and controlled modulation of epigenetic targets. These characteristics make them particularly attractive for therapeutic applications. In cancer, epigenetic drugs have already reached clinical trials and, in some cases, received FDA approval [77]. However, the application of epigenetic therapies in CVDs is still in its early stages, and no epigenetic drug has been specifically tested in clinical trials for cardiac fibrosis.
This lack of clinical translation may be attributed to key limitations that must be addressed before these therapies can become viable treatment options. One of the major challenges in translating epigenetic therapies into clinical practice is ensuring precise delivery to the affected tissue and specific targeting of the relevant cell populations. Systemic administration of epigenetic drugs risks affecting multiple organs and cell populations, leading to off-target effects and undesirable consequences. Moreover, ensuring sufficient drug bioavailability within fibrotic myocardial tissue remains a significant hurdle.
To address these limitations, novel delivery strategies are being explored. Functionalized nanoparticles and biomaterial-based carriers have shown potential for enhancing drug accumulation in fibrotic cardiac tissue while minimizing systemic exposure. Recent studies have demonstrated that ECM-binding nanoparticles and fibroblast-specific delivery systems improve drug retention at the site of fibrosis, increasing therapeutic efficacy. For instance, MnO2-based nanozymes functionalized with tannic acid, which enhanced their affinity for ECM, have been shown to selectively accumulate in infarcted myocardium, enhancing antifibrotic effects [112]. Similarly, calcium phosphate nanoparticles modified with a cardiac-targeting peptide have enabled more efficient drug delivery via inhalation, improving ventricular remodeling and reducing fibrosis in HF models [113]. These approaches highlight the potential of targeted nanoparticle therapies to optimize epigenetic drug delivery and improve clinical outcomes.
Another promising strategy is the use of single-cell transcriptomics and epigenomics to refine the specificity of epigenetic interventions. Recent advances in single-cell technologies have revealed significant heterogeneity among CF populations, with distinct subtypes playing differential roles in fibrosis progression. Identifying and characterizing these subpopulations will allow for the development of therapies that selectively target the most pathogenic fibroblast subsets while sparing others that may contribute to tissue homeostasis.
Beyond delivery and specificity challenges, regulatory and safety considerations also limit the clinical implementation of epigenetic therapies in CVDs. One major concern is the potential risk of oncogenic activation due to global chromatin remodeling [114]. Epigenetic modifications, such as histone acetylation or methylation, can alter gene expression patterns in ways that may unintentionally activate oncogenes or suppress tumor suppressor genes. For instance, histone acetylation generally promotes gene transcription by loosening chromatin structure, which could unintentionally activate genes associated with oncogenesis. Conversely, histone deacetylation can lead to gene silencing, potentially suppressing tumor suppressor genes [115]. These off-target effects raise significant safety concerns, especially in the context of chronic treatment. Moreover, many current epigenetic drugs lack sufficient selectivity, affecting multiple pathways beyond their intended targets. This broad activity may compromise both efficacy and safety. Regulatory approval for epigenetic drugs in CVDs requires extensive preclinical and clinical evaluation and is further hindered by the absence of standardized methods to assess epigenetic changes [111]. Addressing these issues through improved drug design, delivery technologies, and biomarker-guided strategies will be essential to safely translate epigenetic therapies into the cardiovascular field.
In conclusion, the combination of emerging delivery technologies with a deeper understanding of fibroblast heterogeneity and epigenetic regulation holds promise for developing more precise and effective antifibrotic therapies. These advancements will be essential for translating epigenetic therapies into viable treatments for cardiac fibrosis, ultimately improving patient outcomes. Importantly, this review provides a comprehensive and integrative perspective that connects fibroblast heterogeneity, epigenetic regulation, and therapeutic innovation in cardiac fibrosis. By combining insights from transcriptomic and functional studies with advances in targeted epigenetic therapies, it offers a novel framework to understand disease mechanisms and guide translational research in the field.
Author Contributions
Writing—original draft preparation, N.G. and L.P.A.-A.; review and editing, N.G., L.P.A.-A. and B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto Salud Carlos III (ISCIII) and co-funded by the European Regional Development Fund-FEDER (PI22/00029) and Gobierno de Navarra-Salud (20-2022) and Gobierno de Navarra-Universidad, Innovación y Transformación Digital (PC24-FIBROS-X-007-015-003) to B.P., and Gobierno de Navarra Doctorados Industriales 0011-1408-2021-000013 to N.G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We deeply acknowledge Iñigo Izal of the University of Navarra for his artistic contribution to the cartoon in Figure 1.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE | Angiotensin-converting enzyme |
| AF | Atrial fibrillation |
| Ang II | Angiotensin II |
| ARB | Angiotensin receptor blocker |
| ARVC | Arrhythmogenic right ventricular cardiomyopathy |
| ATAC-seq | Assay for Transposase-Accessible Chromatin-sequencing |
| ATM | Ataxia telangiectasia mutated |
| BET | Bromodomain and extra-terminal motif |
| BRD | Bromodomain |
| CF | Cardiac fibroblast |
| CpG | Cytosine-phosphodiester bond-guanine |
| CVD | Cardiovascular disease |
| DAMPS | Damage-associated molecular patterns |
| DCM | Dilated cardiomyopathy |
| DDR | DNA damage response |
| DNMT | DNA methyltransferase |
| ECM | Extracellular matrix |
| FDA | Food and Drug Administration |
| HAT | Histone acetyltransferase |
| HCM | Hypertrophic cardiomyopathy |
| HDAC | Histone deacetylase |
| HDM | Histone demethylase |
| HF | Heart failure |
| HMT | Histone methyltransferase |
| KDM | Lysine demethylase |
| LOX | Lysyl oxidase |
| MI | Myocardial infarction |
| MMP | Matrix metalloproteinase |
| MRA | Mineralocorticoid receptor antagonist |
| PDGF | Platelet-derived growth factor |
| RAAS | Renin-angiotensin-aldosterone system |
| ROS | Reactive oxygen species |
| SAM | S-adenosylmethionine |
| scRNA-seq | Single-cell RNA sequencing |
| TF | Transcription factor |
| TGF-β | Transforming growth factor beta |
References
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From Mechanisms to Medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A.; Mensah, G.A.; Abate, Y.H.; Abbasian, M.; Abd-Allah, F.; Abdollahi, A.; Abdollahi, M.; et al. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef]
- Zhao, X.; Kwan, J.Y.Y.; Yip, K.; Liu, P.P.; Liu, F.-F. Targeting Metabolic Dysregulation for Fibrosis Therapy. Nat. Rev. Drug Discov. 2020, 19, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, Definitions, and Functions in Health and Disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Lurje, I.; Gaisa, N.T.; Weiskirchen, R.; Tacke, F. Mechanisms of Organ Fibrosis: Emerging Concepts and Implications for Novel Treatment Strategies. Mol. Asp. Med. 2023, 92, 101191. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Isoyama, M.S.; Nitta-Komatsubara, M.Y. Acute and Chronic Adaptation to Hemodynamic Overload and Ischemia in the Aged Heart. Heart Fail. Rev. 2002, 7, 63–69. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Hinderer, S.; Schenke-Layland, K. Cardiac Fibrosis—A Short Review of Causes and Therapeutic Strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhong, X.; Huang, G.N. Heart Regeneration from the Whole-Organism Perspective to Single-Cell Resolution. NPJ Regen. Med. 2024, 9, 34. [Google Scholar] [CrossRef]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef]
- Kolwicz, S.C.; Purohit, S.; Tian, R. Cardiac Metabolism and Its Interactions with Contraction, Growth, and Survival of Cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Regulation of the Inflammatory Response in Cardiac Repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yabluchanskiy, A.; Lindsey, M.L. Neutrophil Roles in Left Ventricular Remodeling Following Myocardial Infarction. Fibrogenesis Tissue Repair 2013, 6, 11. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.L.; Libby, P.; Weissleder, R.; Pittet, M.J. The Healing Myocardium Sequentially Mobilizes Two Monocyte Subsets with Divergent and Complementary Functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Humeres, C.; Frangogiannis, N.G. Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC Basic Transl. Sci. 2019, 4, 449–467. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- González-Santamaría, J.; Villalba, M.; Busnadiego, O.; López-Olañeta, M.M.; Sandoval, P.; Snabel, J.; López-Cabrera, M.; Erler, J.T.; Hanemaaijer, R.; Lara-Pezzi, E.; et al. Matrix Cross-Linking Lysyl Oxidases Are Induced in Response to Myocardial Infarction and Promote Cardiac Dysfunction. Cardiovasc. Res. 2016, 109, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Martínez-González, J. The Role of Lysyl Oxidase Enzymes in Cardiac Function and Remodeling. Cells 2019, 8, 1483. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Khalil, H.; Kanisicak, O.; Boyer, J.G.; Vagnozzi, R.J.; Maliken, B.D.; Sargent, M.A.; Prasad, V.; Valiente-Alandi, I.; Blaxall, B.C.; et al. Specialized Fibroblast Differentiated States Underlie Scar Formation in the Infarcted Mouse Heart. J. Clin. Investig. 2018, 128, 2127–2143. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Korantzopoulos, P.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 26, 209. [Google Scholar] [CrossRef]
- Hall, C.; Gehmlich, K.; Denning, C.; Pavlovic, D. Complex Relationship between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J. Am. Heart Assoc. 2021, 10, e019338. [Google Scholar] [CrossRef] [PubMed]
- Ivey, M.J.; Tallquist, M.D. Defining the Cardiac Fibroblast. Circ. J. 2016, 80, 2269–2276. [Google Scholar] [CrossRef]
- Gladka, M.M.; Molenaar, B.; De Ruiter, H.; Van Der Elst, S.; Tsui, H.; Versteeg, D.; Lacraz, G.P.A.; Huibers, M.M.H.; Van Oudenaarden, A.; Van Rooij, E. Single-Cell Sequencing of the Healthy and Diseased Heart Reveals Cytoskeleton-Associated Protein 4 as a New Modulator of Fibroblasts Activation. Circulation 2018, 138, 166–180. [Google Scholar] [CrossRef]
- Ruiz-Villalba, A.; Romero, J.P.; Hernández, S.C.; Vilas-Zornoza, A.; Fortelny, N.; Castro-Labrador, L.; San Martin-Uriz, P.; Lorenzo-Vivas, E.; García-Olloqui, P.; Palacio, M.; et al. Single-Cell RNA Sequencing Analysis Reveals a Crucial Role for CTHRC1 (Collagen Triple Helix Repeat Containing 1) Cardiac Fibroblasts After Myocardial Infarction. Circulation 2020, 142, 1831–1847. [Google Scholar] [CrossRef]
- Forte, E.; Skelly, D.A.; Chen, M.; Daigle, S.; Morelli, K.A.; Hon, O.; Philip, V.M.; Costa, M.W.; Rosenthal, N.A.; Furtado, M.B. Dynamic Interstitial Cell Response during Myocardial Infarction Predicts Resilience to Rupture in Genetically Diverse Mice. Cell Rep. 2020, 30, 3149–3163.e6. [Google Scholar] [CrossRef]
- Buechler, M.B.; Pradhan, R.N.; Krishnamurty, A.T.; Cox, C.; Calviello, A.K.; Wang, A.W.; Yang, Y.A.; Tam, L.; Caothien, R.; Roose-Girma, M.; et al. Cross-Tissue Organization of the Fibroblast Lineage. Nature 2021, 593, 575–579. [Google Scholar] [CrossRef]
- Koenig, A.L.; Shchukina, I.; Amrute, J.; Andhey, P.S.; Zaitsev, K.; Lai, L.; Bajpai, G.; Bredemeyer, A.; Smith, G.; Jones, C.; et al. Single-Cell Transcriptomics Reveals Cell-Type-Specific Diversification in Human Heart Failure. Nat. Cardiovasc. Res. 2022, 1, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, M.; Papangeli, I.; Simonson, B.; Akkad, A.D.; Hill, M.C.; Arduini, A.; Fleming, S.J.; Melanson, M.; Hayat, S.; Kost-Alimova, M.; et al. Single-Nucleus Profiling of Human Dilated and Hypertrophic Cardiomyopathy. Nature 2022, 608, 174–180. [Google Scholar] [CrossRef]
- Kuppe, C.; Ramirez Flores, R.O.; Li, Z.; Hayat, S.; Levinson, R.T.; Liao, X.; Hannani, M.T.; Tanevski, J.; Wünnemann, F.; Nagai, J.S.; et al. Spatial Multi-Omic Map of Human Myocardial Infarction. Nature 2022, 608, 766–777. [Google Scholar] [CrossRef]
- Amrute, J.M.; Lai, L.; Ma, P.; Koenig, A.L.; Kamimoto, K.; Bredemeyer, A.; Shankar, T.S.; Kuppe, C.; Kadyrov, F.F.; Schulte, L.J.; et al. Defining Cardiac Functional Recovery in End-Stage Heart Failure at Single-Cell Resolution. Nat. Cardiovasc. Res. 2023, 2, 399–416. [Google Scholar] [CrossRef]
- Fu, M.; Hua, X.; Shu, S.; Xu, X.; Zhang, H.; Peng, Z.; Mo, H.; Liu, Y.; Chen, X.; Yang, Y.; et al. Single-Cell RNA Sequencing in Donor and End-Stage Heart Failure Patients Identifies NLRP3 as a Therapeutic Target for Arrhythmogenic Right Ventricular Cardiomyopathy. BMC Med. 2024, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Alvaro, L.P.; Garitano, N.; Esser-Skala, W.; Sayers, J.; del Valle, C.; Alameda-Serrano, D.; Mendieta-Esteban, J.; Calleja-Cervantes, M.E.; Goñi-Salaverri, A.; Zazpe, J.; et al. Identification of Epigenetic Regulators of Fibrotic Transformation in Cardiac Fibroblasts through Bulk and Single-Cell CRISPR Screens. bioRxiv 2025. bioRxiv:645873. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The Molecular Hallmarks of Epigenetic Control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Kaluscha, S.; Domcke, S.; Wirbelauer, C.; Stadler, M.B.; Durdu, S.; Burger, L.; Schübeler, D. Evidence That Direct Inhibition of Transcription Factor Binding Is the Prevailing Mode of Gene and Repeat Repression by DNA Methylation. Nat. Genet. 2022, 54, 1895–1906. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational Modifications of Histones: Mechanisms, Biological Functions, and Therapeutic Targets. MedComm 2023, 4, e292. [Google Scholar] [CrossRef]
- Greer, E.L.; Shi, Y. Histone Methylation: A Dynamic Mark in Health, Disease and Inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef]
- Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone Acetyltransferases. Annu. Rev. Biochem. 2001, 70, 81–120. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Picaud, S.; Mangos, M.; Keates, T.; Lambert, J.P.; Barsyte-Lovejoy, D.; Felletar, I.; Volkmer, R.; Müller, S.; Pawson, T.; et al. Histone Recognition and Large-Scale Structural Analysis of the Human Bromodomain Family. Cell 2012, 149, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Eustermann, S.; Patel, A.B.; Hopfner, K.-P.; He, Y.; Korber, P. Energy-Driven Genome Regulation by ATP-Dependent Chromatin Remodellers. Nat. Rev. Mol. Cell Biol. 2024, 25, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Westerman, K.; Sebastiani, P.; Jacques, P.; Liu, S.; DeMeo, D.; Ordovás, J.M. DNA Methylation Modules Associate with Incident Cardiovascular Disease and Cumulative Risk Factor Exposure. Clin. Epigenetics 2019, 11, 142. [Google Scholar] [CrossRef]
- Aguet, F.; Brown, A.A.; Castel, S.E.; Davis, J.R.; He, Y.; Jo, B.; Mohammadi, P.; Park, Y.S.; Parsana, P.; Segrè, A.V.; et al. Genetic Effects on Gene Expression across Human Tissues. Nature 2017, 550, 204–213. [Google Scholar] [CrossRef]
- Luo, X.; Hu, Y.; Shen, J.; Liu, X.; Wang, T.; Li, L.; Li, J. Integrative Analysis of DNA Methylation and Gene Expression Reveals Key Molecular Signatures in Acute Myocardial Infarction. Clin. Epigenetics 2022, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Moran, B.; Collier, P.; Moravec, C.S.; Phelan, D.; Donnellan, E.; Russell-Hallinan, A.; O’Connor, D.P.; Gallagher, W.M.; Gallagher, J.; et al. Targeted DNA Methylation Profiling of Human Cardiac Tissue Reveals Novel Epigenetic Traits and Gene Deregulation Across Different Heart Failure Patient Subtypes. Circ. Heart Fail. 2019, 12, e005765. [Google Scholar] [CrossRef]
- Madsen, A.; Höppner, G.; Krause, J.; Hirt, M.N.; Laufer, S.D.; Schweizer, M.; Tan, W.L.W.; Mosqueira, D.; Anene-Nzelu, C.G.; Lim, I.; et al. An Important Role for DNMT3A-Mediated DNA Methylation in Cardiomyocyte Metabolism and Contractility. Circulation 2020, 142, 1562–1578. [Google Scholar] [CrossRef]
- Papait, R.; Serio, S.; Pagiatakis, C.; Rusconi, F.; Carullo, P.; Mazzola, M.; Salvarani, N.; Miragoli, M.; Condorelli, G. Histone Methyltransferase G9a Is Required for Cardiomyocyte Homeostasis and Hypertrophy. Circulation 2017, 136, 1233–1246. [Google Scholar] [CrossRef]
- Yu, L.; Yang, G.; Weng, X.; Liang, P.; Li, L.; Li, J.; Fan, Z.; Tian, W.; Wu, X.; Xu, H.; et al. Histone Methyltransferase SET1 Mediates Angiotensin II–Induced Endothelin-1 Transcription and Cardiac Hypertrophy in Mice. Arter. Thromb. Vasc. Biol. 2015, 35, 1207–1217. [Google Scholar] [CrossRef]
- Leng, Y.; Wu, Y.; Lei, S.; Zhou, B.; Qiu, Z.; Wang, K.; Xia, Z. Inhibition of HDAC6 Activity Alleviates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats: Potential Role of Peroxiredoxin 1 Acetylation and Redox Regulation. Oxid. Med. Cell Longev. 2018, 2018, 9494052. [Google Scholar] [CrossRef] [PubMed]
- Bochaton, T.; Crola-Da-Silva, C.; Pillot, B.; Villedieu, C.; Ferreras, L.; Alam, M.R.; Thibault, H.; Strina, M.; Gharib, A.; Ovize, M.; et al. Inhibition of Myocardial Reperfusion Injury by Ischemic Postconditioning Requires Sirtuin 3-Mediated Deacetylation of Cyclophilin D. J. Mol. Cell. Cardiol. 2015, 84, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, X.-F.; Wang, N.-Y.; Wang, X.-M.; Liang, S.-T.; Zheng, W.; Lu, Y.-B.; Zhao, X.; Hao, D.-L.; Zhang, Z.-Q.; et al. SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation 2017, 136, 2051–2067. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Bindu, S.; Pillai, V.B.; Samant, S.; Pan, Y.; Huang, J.-Y.; Gupta, M.; Nagalingam, R.S.; Wolfgeher, D.; Verdin, E.; et al. SIRT3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3β. Mol. Cell. Biol. 2016, 36, 678–692. [Google Scholar] [CrossRef]
- Luo, Y.-X.; Tang, X.; An, X.-Z.; Xie, X.-M.; Chen, X.-F.; Zhao, X.; Hao, D.-L.; Chen, H.-Z.; Liu, D.-P. Sirt4 Accelerates Ang II-Induced Pathological Cardiac Hypertrophy by Inhibiting Manganese Superoxide Dismutase Activity. Eur. Heart J. 2016, 38, 1389–1398. [Google Scholar] [CrossRef]
- Sapountzi, V.; Logan, I.R.; Robson, C.N. Cellular Functions of TIP60. Int. J. Biochem. Cell Biol. 2006, 38, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.; Merlo, X.; Lough, J. Characterization and Expression of the Mouse Tat Interactive Protein 60 KD (TIP60) Gene. Gene 2002, 289, 169–176. [Google Scholar] [CrossRef]
- Wang, X.; Wan, T.C.; Lauth, A.; Purdy, A.L.; Kulik, K.R.; Patterson, M.; Lough, J.W.; Auchampach, J.A. Conditional Depletion of the Acetyltransferase Tip60 Protects against the Damaging Effects of Myocardial Infarction. J. Mol. Cell. Cardiol. 2022, 163, 9–19. [Google Scholar] [CrossRef]
- Wang, X.; Lupton, C.; Lauth, A.; Wan, T.C.; Foster, P.; Patterson, M.; Auchampach, J.A.; Lough, J.W. Evidence That the Acetyltransferase Tip60 Induces the DNA Damage Response and Cell-Cycle Arrest in Neonatal Cardiomyocytes. J. Mol. Cell. Cardiol. 2021, 155, 88–98. [Google Scholar] [CrossRef]
- Fisher, J.B.; Kim, M.-S.; Blinka, S.; Ge, Z.-D.; Wan, T.; Duris, C.; Christian, D.; Twaroski, K.; North, P.; Auchampach, J.; et al. Stress-Induced Cell-Cycle Activation in Tip60 Haploinsufficient Adult Cardiomyocytes. PLoS ONE 2012, 7, e31569. [Google Scholar] [CrossRef]
- Fisher, J.B.; Horst, A.; Wan, T.; Kim, M.-S.; Auchampach, J.; Lough, J. Depletion of Tip60 from In Vivo Cardiomyocytes Increases Myocyte Density, Followed by Cardiac Dysfunction, Myocyte Fallout and Lethality. PLoS ONE 2016, 11, e0164855. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M.; Howard, S.; Villa del Campo, C.; Bollini, S.; Dubé, K.N.; Masters, M.; Barnette, D.N.; Rohling, M.; Sun, X.; Hankins, L.E.; et al. BRG1-SWI/SNF-Dependent Regulation of the Wt1 Transcriptional Landscape Mediates Epicardial Activity during Heart Development and Disease. Nat. Commun. 2017, 8, 16034. [Google Scholar] [CrossRef] [PubMed]
- Hota, S.K.; Bruneau, B.G. ATP-Dependent Chromatin Remodeling during Mammalian Development. Development 2016, 143, 2882–2897. [Google Scholar] [CrossRef]
- Han, P.; Hang, C.T.; Yang, J.; Chang, C.-P. Chromatin Remodeling in Cardiovascular Development and Physiology. Circ. Res. 2011, 108, 378–396. [Google Scholar] [CrossRef]
- Rhee, S.; Chung, J.I.; King, D.A.; D’amato, G.; Paik, D.T.; Duan, A.; Chang, A.; Nagelberg, D.; Sharma, B.; Jeong, Y.; et al. Endothelial Deletion of Ino80 Disrupts Coronary Angiogenesis and Causes Congenital Heart Disease. Nat. Commun. 2018, 9, 368. [Google Scholar] [CrossRef]
- Webber, M.; Jackson, S.P.; Moon, J.C.; Captur, G. Myocardial Fibrosis in Heart Failure: Anti-Fibrotic Therapies and the Role of Cardiovascular Magnetic Resonance in Drug Trials. Cardiol. Ther. 2020, 9, 363–376. [Google Scholar] [CrossRef]
- Zhi, H.; Luptak, I.; Alreja, G.; Shi, J.; Guan, J.; Metes-Kosik, N.; Joseph, J. Effects of Direct Renin Inhibition on Myocardial Fibrosis and Cardiac Fibroblast Function. PLoS ONE 2013, 8, e81612. [Google Scholar] [CrossRef] [PubMed]
- Abareshi, A.; Norouzi, F.; Asgharzadeh, F.; Beheshti, F.; Hosseini, M.; Farzadnia, M.; Khazaei, M. Effect of Angiotensin-Converting Enzyme Inhibitor on Cardiac Fibrosis and Oxidative Stress Status in Lipopolysaccharide-Induced Inflammation Model in Rats. Int. J. Prev. Med. 2017, 8, 69. [Google Scholar] [CrossRef]
- Wu, L.; Iwai, M.; Nakagami, H.; Chen, R.; Suzuki, J.; Akishita, M.; de Gasparo, M.; Horiuchi, M. Effect of Angiotensin II Type 1 Receptor Blockade on Cardiac Remodeling in Angiotensin II Type 2 Receptor Null Mice. Arter. Thromb. Vasc. Biol. 2002, 22, 49–54. [Google Scholar] [CrossRef]
- Brilla, C.G.; Matsubara, L.S.; Weber, K.T. Antifibrotic Effects of Spironolactone in Preventing Myocardial Fibrosis in Systemic Arterial Hypertension. Am. J. Cardiol. 1993, 71, A12–A16. [Google Scholar] [CrossRef]
- Chimenti, I.; Pagano, F.; Cavarretta, E.; Angelini, F.; Peruzzi, M.; Barretta, A.; Greco, E.; De Falco, E.; Marullo, A.G.M.; Sciarretta, S.; et al. Β-Blockers Treatment of Cardiac Surgery Patients Enhances Isolation and Improves Phenotype of Cardiosphere-Derived Cells. Sci. Rep. 2016, 6, 36774. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.M.; Reed, C.R.; Gargiulo, K.A.; Rodrigues, A.E.; Galiano, R.D.; Mustoe, T.A.; Hong, S.J. Anti-Fibrotic Effects of Statin Drugs: A Review of Evidence and Mechanisms. Biochem. Pharmacol. 2023, 214, 115644. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.; Hu, K.; Adamek, A.; Wolf, J.; Sallam, A.; KG Maier, S.; Lonning, S.; Ling, H.; Ertl, G.; Bauersachs, J. Transforming Growth Factor Beta Inhibition Increases Mortality and Left Ventricular Dilatation after Myocardial Infarction. Basic Res. Cardiol. 2008, 103, 485–492. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Ding, C.; Wilson, E.; Marcus, G.M.; Olgin, J.E. Pirfenidone Mitigates Left Ventricular Fibrosis and Dysfunction after Myocardial Infarction and Reduces Arrhythmias. Heart Rhythm. 2010, 7, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Rol, N.; De Raaf, M.A.; Sun, X.Q.; Kuiper, V.P.; Da Silva Gonçalves Bos, D.; Happé, C.; Kurakula, K.; Dickhoff, C.; Thuillet, R.; Tu, L.; et al. Nintedanib Improves Cardiac Fibrosis but Leaves Pulmonary Vascular Remodelling Unaltered in Experimental Pulmonary Hypertension. Cardiovasc. Res. 2019, 115, 432–439. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of Heart Failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- Dai, W.; Qiao, X.; Fang, Y.; Guo, R.; Bai, P.; Liu, S.; Li, T.; Jiang, Y.; Wei, S.; Na, Z.; et al. Epigenetics-Targeted Drugs: Current Paradigms and Future Challenges. Signal Transduct. Target. Ther. 2024, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Dasgupta, C.; Chen, M.; Zhang, K.; Buchholz, J.; Xu, Z.; Zhang, L. Inhibition of DNA Methylation Reverses Norepinephrine-Induced Cardiac Hypertrophy in Rats. Cardiovasc. Res. 2014, 101, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Horgan, S.; Neary, R.; Glezeva, N.; Tea, I.; Corrigan, N.; McDonald, K.; Ledwidge, M.; Baugh, J. Epigenetic Therapy for the Treatment of Hypertension-Induced Cardiac Hypertrophy and Fibrosis. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 127–137. [Google Scholar] [CrossRef]
- Nührenberg, T.G.; Hammann, N.; Schnick, T.; Preißl, S.; Witten, A.; Stoll, M.; Gilsbach, R.; Neumann, F.-J.; Hein, L. Cardiac Myocyte De Novo DNA Methyltransferases 3a/3b Are Dispensable for Cardiac Function and Remodeling after Chronic Pressure Overload in Mice. PLoS ONE 2015, 10, e0131019. [Google Scholar] [CrossRef]
- Stenzig, J.; Schneeberger, Y.; Löser, A.; Peters, B.S.; Schaefer, A.; Zhao, R.-R.; Ng, S.L.; Höppner, G.; Geertz, B.; Hirt, M.N.; et al. Pharmacological Inhibition of DNA Methylation Attenuates Pressure Overload-Induced Cardiac Hypertrophy in Rats. J. Mol. Cell. Cardiol. 2018, 120, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Kamimura, N.; Matsuhashi, T.; Nagai, T.; Nishiyama, T.; Endo, J.; Hishiki, T.; Nakanishi, T.; Shimizu, N.; Tanaka, H.; et al. The Histone 3 Lysine 9 Methyltransferase Inhibitor Chaetocin Improves Prognosis in a Rat Model of High Salt Diet-Induced Heart Failure. Sci. Rep. 2017, 7, 39752. [Google Scholar] [CrossRef]
- Sung, P.-H.; Luo, C.-W.; Chiang, J.Y.; Yip, H.-K. The Combination of G9a Histone Methyltransferase Inhibitors with Erythropoietin Protects Heart against Damage from Acute Myocardial Infarction. Am. J. Transl. Res. 2020, 12, 3255–3271. [Google Scholar] [PubMed]
- Zhang, Q.-J.; Tran, T.A.T.; Wang, M.; Ranek, M.J.; Kokkonen-Simon, K.M.; Gao, J.; Luo, X.; Tan, W.; Kyrychenko, V.; Liao, L.; et al. Histone Lysine Dimethyl-Demethylase KDM3A Controls Pathological Cardiac Hypertrophy and Fibrosis. Nat. Commun. 2018, 9, 5230. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Meng, X.; Yang, X.; Liu, X.; Ou-Yang, C.; Liu, C. Curcumin Administration Suppresses Collagen Synthesis in the Hearts of Rats with Experimental Diabetes. Acta Pharmacol. Sin. 2018, 39, 195–204. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Chen, Q.; Hong, T.; Zhong, Z.; He, J.; Ni, C. Curcumin Ameliorates Cardiac Fibrosis by Regulating Macrophage-Fibroblast Crosstalk via IL18-P-SMAD2/3 Signaling Pathway Inhibition. Front. Pharmacol. 2022, 12, 784041. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qiao, Z.; Xu, Y. Protective Effect of Curcumin against Myocardium Injury in Ischemia Reperfusion Rats. Pharm. Biol. 2017, 55, 1144–1148. [Google Scholar] [CrossRef]
- Rai, R.; Verma, S.K.; Kim, D.; Ramirez, V.; Lux, E.; Li, C.; Sahoo, S.; Wilsbacher, L.D.; Vaughan, D.E.; Quaggin, S.E.; et al. A Novel Acetyltransferase P300 Inhibitor Ameliorates Hypertension-Associated Cardio-Renal Fibrosis. Epigenetics 2017, 12, 1004–1013. [Google Scholar] [CrossRef]
- Rai, R.; Sun, T.; Ramirez, V.; Lux, E.; Eren, M.; Vaughan, D.E.; Ghosh, A.K. Acetyltransferase P300 Inhibitor Reverses Hypertension-induced Cardiac Fibrosis. J. Cell. Mol. Med. 2019, 23, 3026–3031. [Google Scholar] [CrossRef]
- Wang, X.; Wan, T.C.; Kulik, K.R.; Lauth, A.; Smith, B.C.; Lough, J.W.; Auchampach, J.A. Pharmacological Inhibition of the Acetyltransferase Tip60 Mitigates Myocardial Infarction Injury. Dis. Model. Mech. 2023, 16, dmm049786. [Google Scholar] [CrossRef]
- Yakubu, J.; Pandey, A.V. Innovative Delivery Systems for Curcumin: Exploring Nanosized and Conventional Formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Lv, M.; Li, Y. Nanotechnology-Based Drug Delivery Systems for Curcumin and Its Derivatives in the Treatment of Cardiovascular Diseases. J. Funct. Foods 2024, 122, 106476. [Google Scholar] [CrossRef]
- Freundt, J.K.; Frommeyer, G.; Spieker, T.; Wötzel, F.; Grotthoff, J.S.; Stypmann, J.; Hempel, G.; Schäfers, M.; Jacobs, A.H.; Eckardt, L.; et al. Histone Deacetylase Inhibition by Entinostat for the Prevention of Electrical and Structural Remodeling in Heart Failure. BMC Pharmacol. Toxicol. 2019, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Herr, D.J.; Baarine, M.; Aune, S.E.; Li, X.; Ball, L.E.; Lemasters, J.J.; Beeson, C.C.; Chou, J.C.; Menick, D.R. HDAC1 Localizes to the Mitochondria of Cardiac Myocytes and Contributes to Early Cardiac Reperfusion Injury. J. Mol. Cell. Cardiol. 2018, 114, 309–319. [Google Scholar] [CrossRef]
- Somanna, N.K.; Valente, A.J.; Krenz, M.; McDonald, K.S.; Higashi, Y.; Noda, M.; Chandrasekar, B. Histone Deacetyltransferase Inhibitors Trichostatin A and Mocetinostat Differentially Regulate MMP9, IL-18 and RECK Expression, and Attenuate Angiotensin II-Induced Cardiac Fibroblast Migration and Proliferation. Hypertens. Res. 2016, 39, 709–716. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Wang, L.; Zhao, J.; Zhong, Z.; Wang, Y.; Xu, J. Inhibition of Histone Deacetylases Prevents Cardiac Remodeling After Myocardial Infarction by Restoring Autophagosome Processing in Cardiac Fibroblasts. Cell. Physiol. Biochem. 2018, 49, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, X.; Zhao, Y.; Fast, L.; Zhuang, S.; Liu, P.; Cheng, G.; Zhao, T.C. Inhibition of Histone Deacetylases Preserves Myocardial Performance and Prevents Cardiac Remodeling through Stimulation of Endogenous Angiomyogenesis. J. Pharmacol. Exp. Ther. 2012, 341, 285–293. [Google Scholar] [CrossRef]
- Xie, M.; Kong, Y.; Tan, W.; May, H.; Battiprolu, P.K.; Pedrozo, Z.; Wang, Z.V.; Morales, C.; Luo, X.; Cho, G.; et al. Histone Deacetylase Inhibition Blunts Ischemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy. Circulation 2014, 129, 1139–1151. [Google Scholar] [CrossRef]
- Nagata, S.; Marunouchi, T.; Tanonaka, K. Histone Deacetylase Inhibitor SAHA Treatment Prevents the Development of Heart Failure after Myocardial Infarction via an Induction of Heat-Shock Proteins in Rats. Biol. Pharm. Bull. 2019, 42, 453–461. [Google Scholar] [CrossRef]
- Lee, T.-M.; Lin, M.-S.; Chang, N.-C. Inhibition of Histone Deacetylase on Ventricular Remodeling in Infarcted Rats. Am. J. Physiol. -Heart Circ. Physiol. 2007, 293, H968–H977. [Google Scholar] [CrossRef]
- Wang, K.; Tang, R.; Wang, S.; Wang, W.; Zhang, K.; Li, J.; Li, P.; Tang, Y.-D. SAHA Could Inhibit TGF-Β1/P38 Pathway in MI-Induced Cardiac Fibrosis through DUSP4 Overexpression. Heart Vessel. 2022, 37, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.T.; Orlandin, C.B.; Bonácio, G.F.; Sanchez, E.R.; Durão, M.P.; Lataro, R.M.; Ramalho, L.N.; Silva, C.A.; Amaral, J.H.; Restini, C.B.; et al. Antioxidant Resveratrol Attenuates Cardiac Fibrosis and Reduces Matrix Metalloproteinase-2 Activity in Heart Failure Rats. Hypertension 2019, 74, Abstract P2005. [Google Scholar] [CrossRef]
- Chen, Q.; Zeng, Y.; Yang, X.; Wu, Y.; Zhang, S.; Huang, S.; Zhong, Y.; Chen, M. Resveratrol Ameliorates Myocardial Fibrosis by Regulating Sirt1/Smad3 Deacetylation Pathway in Rat Model with Dilated Cardiomyopathy. BMC Cardiovasc. Disord. 2022, 22, 17. [Google Scholar] [CrossRef]
- Nural-Guvener, H.F.; Zakharova, L.; Nimlos, J.; Popovic, S.; Mastroeni, D.; Gaballa, M.A. HDAC Class I Inhibitor, Mocetinostat, Reverses Cardiac Fibrosis in Heart Failure and Diminishes CD90+ Cardiac Myofibroblast Activation. Fibrogenesis Tissue Repair 2014, 7, 10. [Google Scholar] [CrossRef]
- Gallo, P.; Latronico, M.V.G.; Gallo, P.; Grimaldi, S.; Borgia, F.; Todaro, M.; Jones, P.; Gallinari, P.; De Francesco, R.; Ciliberto, G.; et al. Inhibition of Class I Histone Deacetylase with an Apicidin Derivative Prevents Cardiac Hypertrophy and Failure. Cardiovasc. Res. 2008, 80, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Nural-Guvener, H.; Zakharova, L.; Feehery, L.; Sljukic, S.; Gaballa, M. Anti-Fibrotic Effects of Class I HDAC Inhibitor, Mocetinostat Is Associated with IL-6/Stat3 Signaling in Ischemic Heart Failure. Int. J. Mol. Sci. 2015, 16, 11482–11499. [Google Scholar] [CrossRef]
- Milan, M.; Pace, V.; Maiullari, F.; Chirivì, M.; Baci, D.; Maiullari, S.; Madaro, L.; Maccari, S.; Stati, T.; Marano, G.; et al. Givinostat Reduces Adverse Cardiac Remodeling through Regulating Fibroblasts Activation. Cell. Death Dis. 2018, 9, 108. [Google Scholar] [CrossRef]
- Ford, C.M.; Civitarese, R.A.; Bugyei-Twum, A.; Mitchell, M.; Desjardins, J.-F.; Thai, K.; Abadeh, A.; Zhang, Y.; Switzer, J.; Advani, A.; et al. The Sirt1 Activator, SRT1720, Attenuates Cardiac Hypertrophy and Fibrosis in a Rodent Pressure Overload Model. Circulation 2014, 130, A19029. [Google Scholar]
- Bugyei-Twum, A.; Ford, C.; Civitarese, R.; Seegobin, J.; Advani, S.L.; Desjardins, J.-F.; Kabir, G.; Zhang, Y.; Mitchell, M.; Switzer, J.; et al. Sirtuin 1 Activation Attenuates Cardiac Fibrosis in a Rodent Pressure Overload Model by Modifying Smad2/3 Transactivation. Cardiovasc. Res. 2018, 114, 1629–1641. [Google Scholar] [CrossRef]
- Anand, P.; Brown, J.D.; Lin, C.Y.; Qi, J.; Zhang, R.; Artero, P.C.; Alaiti, M.A.; Bullard, J.; Alazem, K.; Margulies, K.B.; et al. BET Bromodomains Mediate Transcriptional Pause Release in Heart Failure. Cell 2013, 154, 569–582. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic Regulation in Cardiovascular Disease: Mechanisms and Advances in Clinical Trials. Signal Transduct. Target. Ther. 2022, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Liu, X.; Qi, Z.; Fang, Z.; Jiang, Y.; Huang, Y.; Wang, Y.; Wu, L.; Yang, Y. An Antioxidant Nanozyme for Targeted Cardiac Fibrosis Therapy Post Myocardial Infarction. J. Nanobiotechnol. 2024, 22, 760. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Zou, W.; Tian, F.; Xie, H.; Liu, A.; Liu, W.; Liu, Y.; Zhou, N.; Cai, X.; Wu, J.; et al. Inhalable Cardiac Targeting Peptide Modified Nanomedicine Prevents Pressure Overload Heart Failure in Male Mice. Nat. Commun. 2024, 15, 6058. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, Y.; Li, S. The Advances in the Development of Epigenetic Modifications Therapeutic Drugs Delivery Systems. Int. J. Nanomed. 2024, 19, 10623–10637. [Google Scholar] [CrossRef]
- Papazoglou, P.; Peng, L.; Sachinidis, A. Epigenetic Mechanisms Involved in the Cardiovascular Toxicity of Anticancer Drugs. Front. Cardiovasc. Med. 2021, 8, 658900. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).