Engagement of CD300c by a Novel Monoclonal Antibody Ameliorates Behavioral Deficits in a 5xFAD Mouse Model of Alzheimer’s Disease
Abstract
:1. Background
2. Methods
2.1. Screening and Characterization of Monoclonal Antibodies
2.2. Macrophage Differentiation
2.3. Scheme of Administration Using the 5xFAD Model
2.4. Multiplex Cytokine/Chemokines ELISA Kit Assay
2.5. Behavior Test
2.6. Immunohistochemistry
3. Results
3.1. Binding Affinity and Specificity of CB201 Against CD300c
3.2. Effects of CB201 on Macrophage Differentiation
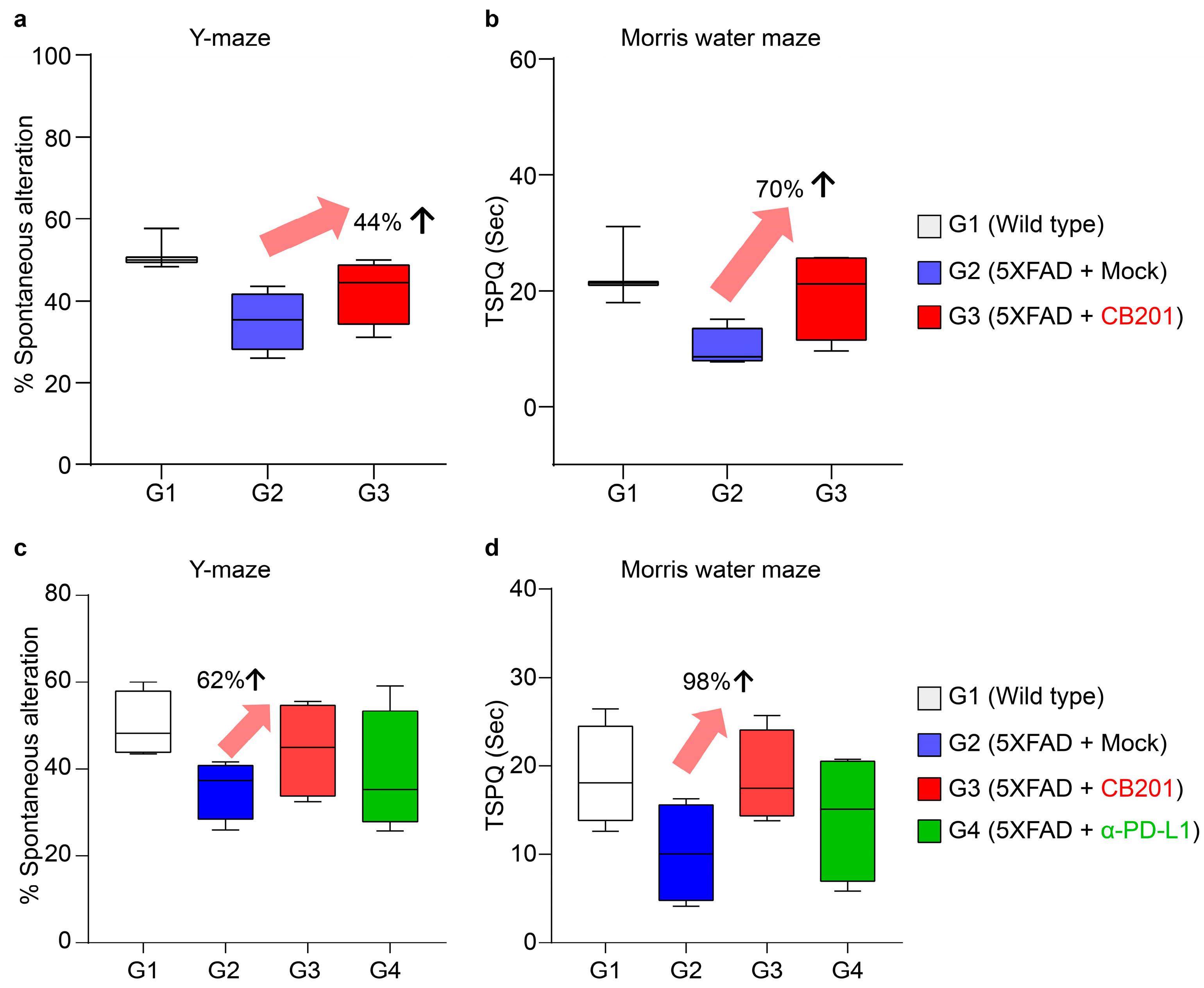
3.3. Behavioral Improvement in 5xFAD Mice
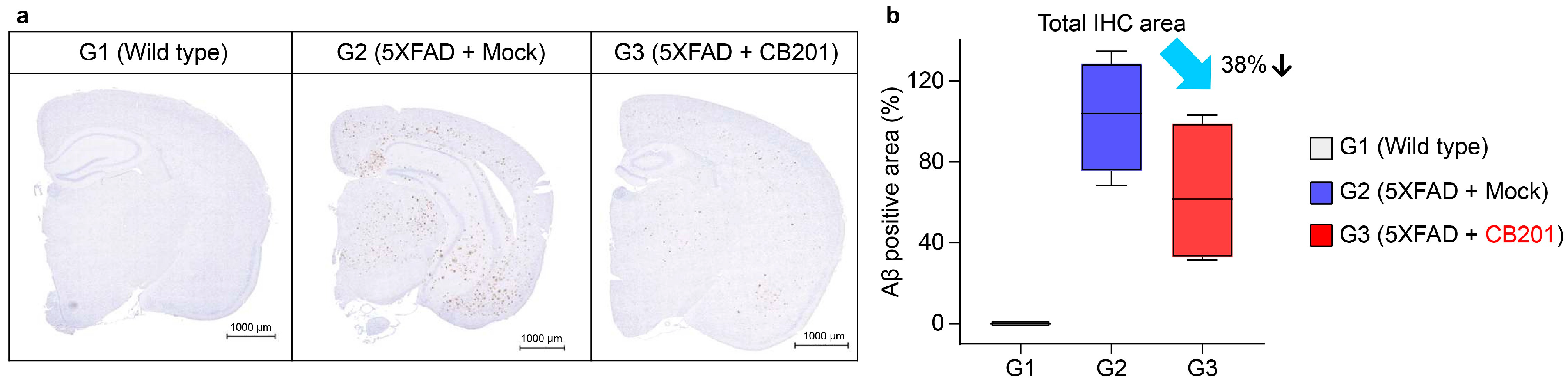
3.4. Reduction in Aβ Deposition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | amyloid β |
| ELISA | enzyme-linked immunosorbent assay |
| FACS | Fluorescence-activated cell sorter |
| FcR | Fc receptor |
| HRP | horseradish peroxidase |
| IL | interleukin |
| KD | dissociation constants |
| Koff | dissociation rate |
| Kon | association rate |
| MDMs | monocyte-derived macrophages |
| MIG | expression of IL-17F, IFN-γ |
| MWM | Morris water maze |
| SPR | surface plasmon resonance |
| TNF | tumor necrosis factor |
References
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Shakir, M.N.; Dugger, B.N. Advances in deep neuropathological phenotyping of Alzheimer disease: Past, present, and future. J. Neuropathol. Exp. Neurol. 2022, 81, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Silva, V.; Monteiro, C.; Silvestre, S. Role of aducanumab in the treatment of Alzheimer’s disease: Challenges and opportunities. Clin. Interv. Aging. 2022, 17, 797–810. [Google Scholar] [CrossRef]
- Vitek, G.E.; Decourt, B.; Sabbagh, M.N. Lecanemab (BAN2401): An anti-beta-amyloid monoclonal antibody for the treatment of Alzheimer disease. Expert Opin. Investig. Drugs 2023, 32, 89–94. [Google Scholar] [CrossRef]
- Park, J.; Simpson, C.; Patel, K. Lecanemab: A humanized monoclonal antibody for the treatment of early Alzheimer disease. Ann. Pharmacother. 2024, 58, 1045–1053. [Google Scholar] [CrossRef]
- Chaintreuil, P.; Kerreneur, E.; Bourgoin, M.; Savy, C.; Favreau, C.; Robert, G.; Jacquel, A.; Auberger, P. The generation, activation, and polarization of monocyte-derived macrophages in human malignancies. Front. Immunol. 2023, 14, 1178337. [Google Scholar] [CrossRef]
- Simard, A.R.; Soulet, D.; Gowing, G.; Julien, J.P.; Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 2006, 49, 489–502. [Google Scholar] [CrossRef]
- Baruch, K.; Deczkowska, A.; Rosenzweig, N.; Tsitsou-Kampeli, A.; Sharif, A.M.; Matcovitch-Natan, O.; Kertser, A.; David, E.; Amit, I.; Schwartz, M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 2016, 22, 135–137. [Google Scholar] [CrossRef]
- Dvir-Szternfeld, R.; Castellani, G.; Arad, M.; Cahalon, L.; Colaiuta, S.P.; Keren-Shaul, H.; Croese, T.; Burgaletto, C.; Baruch, K.; Ulland, T.; et al. Alzheimer’s disease modification mediated by bone marrow-derived macrophages via a TREM2-independent pathway in mouse model of amyloidosis. Nat. Aging 2022, 2, 60–73. [Google Scholar] [CrossRef]
- Zhong, M.Z.; Peng, T.; Duarte, M.L.; Wang, M.; Cai, D. Updates on mouse models of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Borrego, F. The CD300 molecules: An emerging family of regulators of the immune system. Blood 2013, 121, 1951–1960. [Google Scholar] [CrossRef]
- Clark, G.J.; Ju, X.; Azlan, M.; Tate, C.; Ding, Y.; Hart, D.N.J. The CD300 molecules regulate monocyte and dendritic cell functions. Immunobiology 2009, 214, 730–736. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, H.; Lim, C.K.; Kim, J.D.; Heo, J.S.; Jung, J.; Kim, C.; Chon, H.J.; Jeon, J.W. Engagement of CD300c by a novel monoclonal antibody induces the differentiation of monocytes to M1 macrophages. Immunobiology 2024, 229, 152780. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Chung, Y.C.; Jin, B.K. Interleukin-4 and interleukin-13 exacerbate neurotoxicity of prothrombin Kringle-2 in cortex in vivo via oxidative stress. Int. J. Mol. Sci. 2019, 20, 1927. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, N.; Dvir-Szternfeld, R.; Tsitsou-Kampeli, A.; Keren-Shaul, H.; Ben-Yehuda, H.; Weill-Raynal, P.; Cahalon, L.; Kertser, A.; Baruch, K.; Amit, I.; et al. PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat. Commun. 2019, 10, 465. [Google Scholar] [CrossRef]
- Tsai, K.J.; Tsai, Y.C.; Shen, C.K.J. G-CSF rescues the memory impairment of animal models of Alzheimer’s disease. J. Exp. Med. 2007, 204, 1273–1280. [Google Scholar] [CrossRef]
- Potter, H.; Woodcock, J.H.; Boyd, T.D.; Coughlan, C.M.; O’Shaughnessy, J.R.; Borges, M.T.; Thaker, A.A.; Raj, B.A.; Adamszuk, K.; Scott, D.; et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer’s disease. Alzheimer’s Dement. 2021, 7, e12158. [Google Scholar] [CrossRef]
- Craft, J.M.; Watterson, D.M.; Hirsch, E.; Van Eldik, L.J. Interleukin 1 receptor antagonist knockout mice show enhanced microglial activation and neuronal damage induced by intracerebroventricular infusion of human beta-amyloid. J. Neuroinflamm. 2005, 2, 15. [Google Scholar] [CrossRef]
- Price, B.R.; Sudduth, T.L.; Weekman, E.M.; Johnson, S.; Hawthorne, D.; Woolums, A.; Wilcock, D.M. Therapeutic Trem2 activation ameliorates amyloid-beta deposition and improves cognition in the 5XFAD model of amyloid deposition. J. Neuroinflamm. 2020, 17, 238. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S. The IL-17 family in diseases: From bench to bedside. Signal Transduct. Target. Ther. 2023, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Kil Lee, J.; Schuchman, E.H.; Jin, H.K.; Bae, J.-S. Soluble CCL5 Derived from Bone Marrow-Derived Mesenchymal Stem Cells and Activated by Amyloid β Ameliorates Alzheimer’s Disease in Mice by Recruiting Bone Marrow-Induced Microglia Immune Responses. Stem Cell 2012, 30, 1544–1555. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Ceballos-Diaz, C.; Beccard, A.; Janus, C.; Dickson, D.; Todd, E.; Golde; Das, P. IFN-γ Promotes Complement Expression and Attenuates Amyloid Plaque Deposition in Amyloid β Precursor Protein Transgenic Mice. J. Immunol. 2010, 184, 5333–5343. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lim, C.K.; Kim, J.; Kim, J.; Jin, H.K.; Bae, J.-s.; Jeon, J.-W. Engagement of CD300c by a Novel Monoclonal Antibody Ameliorates Behavioral Deficits in a 5xFAD Mouse Model of Alzheimer’s Disease. Biomedicines 2025, 13, 1169. https://doi.org/10.3390/biomedicines13051169
Lee S, Lim CK, Kim J, Kim J, Jin HK, Bae J-s, Jeon J-W. Engagement of CD300c by a Novel Monoclonal Antibody Ameliorates Behavioral Deficits in a 5xFAD Mouse Model of Alzheimer’s Disease. Biomedicines. 2025; 13(5):1169. https://doi.org/10.3390/biomedicines13051169
Chicago/Turabian StyleLee, Suin, Chang Ki Lim, Jongyeob Kim, Joon Kim, Hee Kyung Jin, Jae-sung Bae, and Jae-Won Jeon. 2025. "Engagement of CD300c by a Novel Monoclonal Antibody Ameliorates Behavioral Deficits in a 5xFAD Mouse Model of Alzheimer’s Disease" Biomedicines 13, no. 5: 1169. https://doi.org/10.3390/biomedicines13051169
APA StyleLee, S., Lim, C. K., Kim, J., Kim, J., Jin, H. K., Bae, J.-s., & Jeon, J.-W. (2025). Engagement of CD300c by a Novel Monoclonal Antibody Ameliorates Behavioral Deficits in a 5xFAD Mouse Model of Alzheimer’s Disease. Biomedicines, 13(5), 1169. https://doi.org/10.3390/biomedicines13051169





