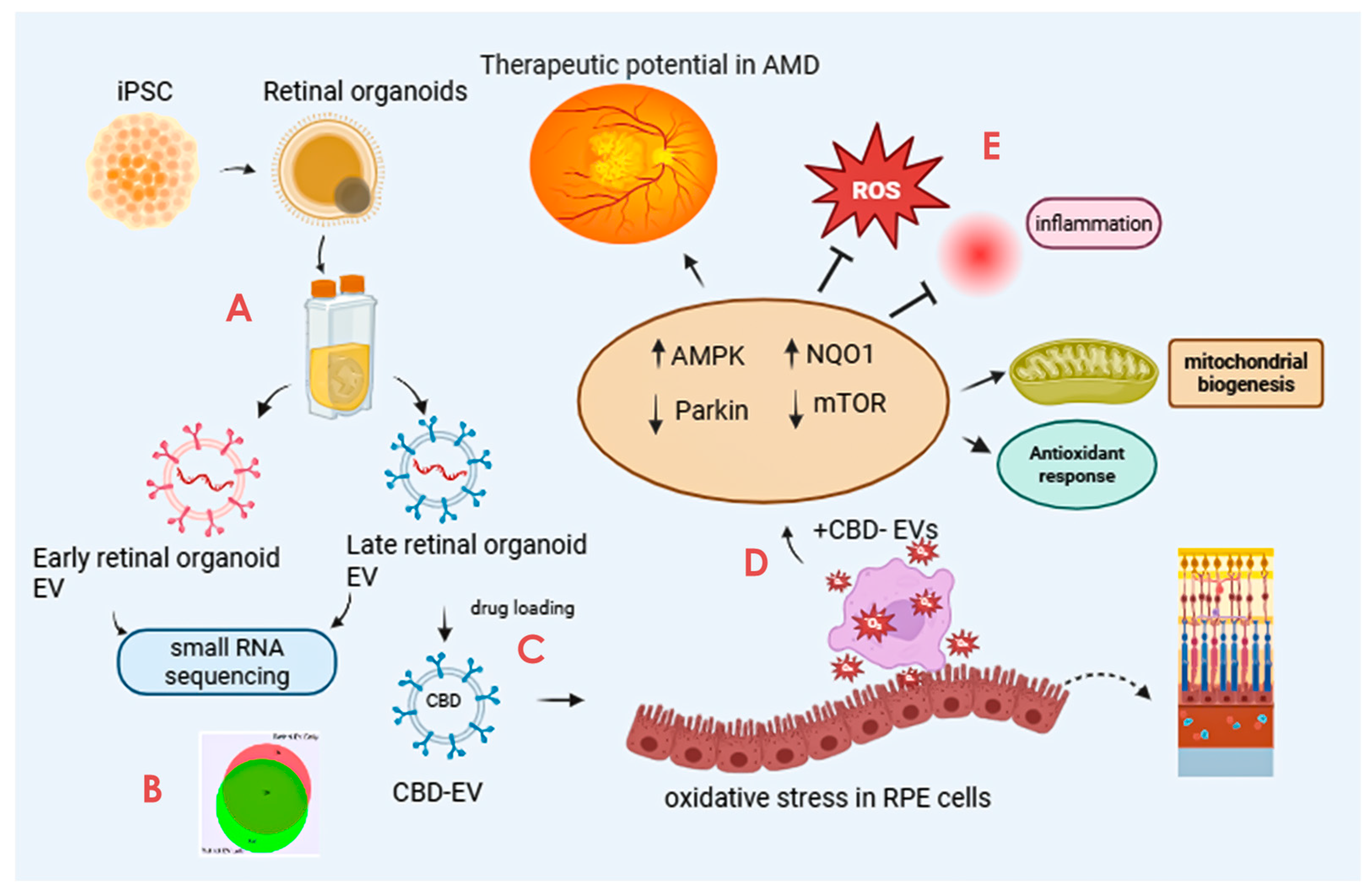
Cannabidiol-Loaded Retinal Organoid-Derived Extracellular Vesicles Protect Oxidatively Stressed ARPE-19 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of EVs from Retinal Organoids
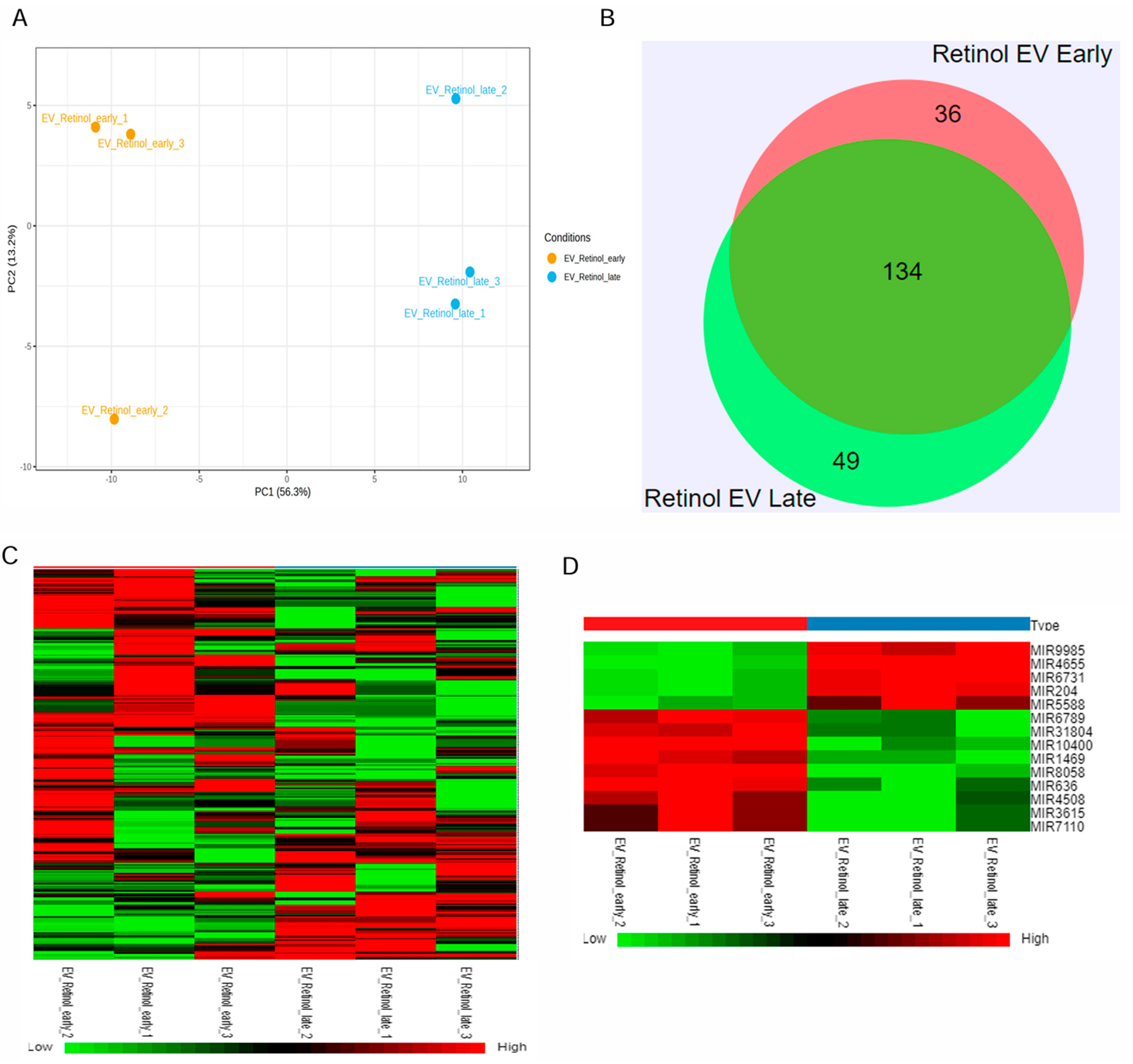
2.2. Small RNA Profiling of EVs
2.3. RNA-Seq Data Analysis
2.4. Preparation of CBD Encapsulation into EVs
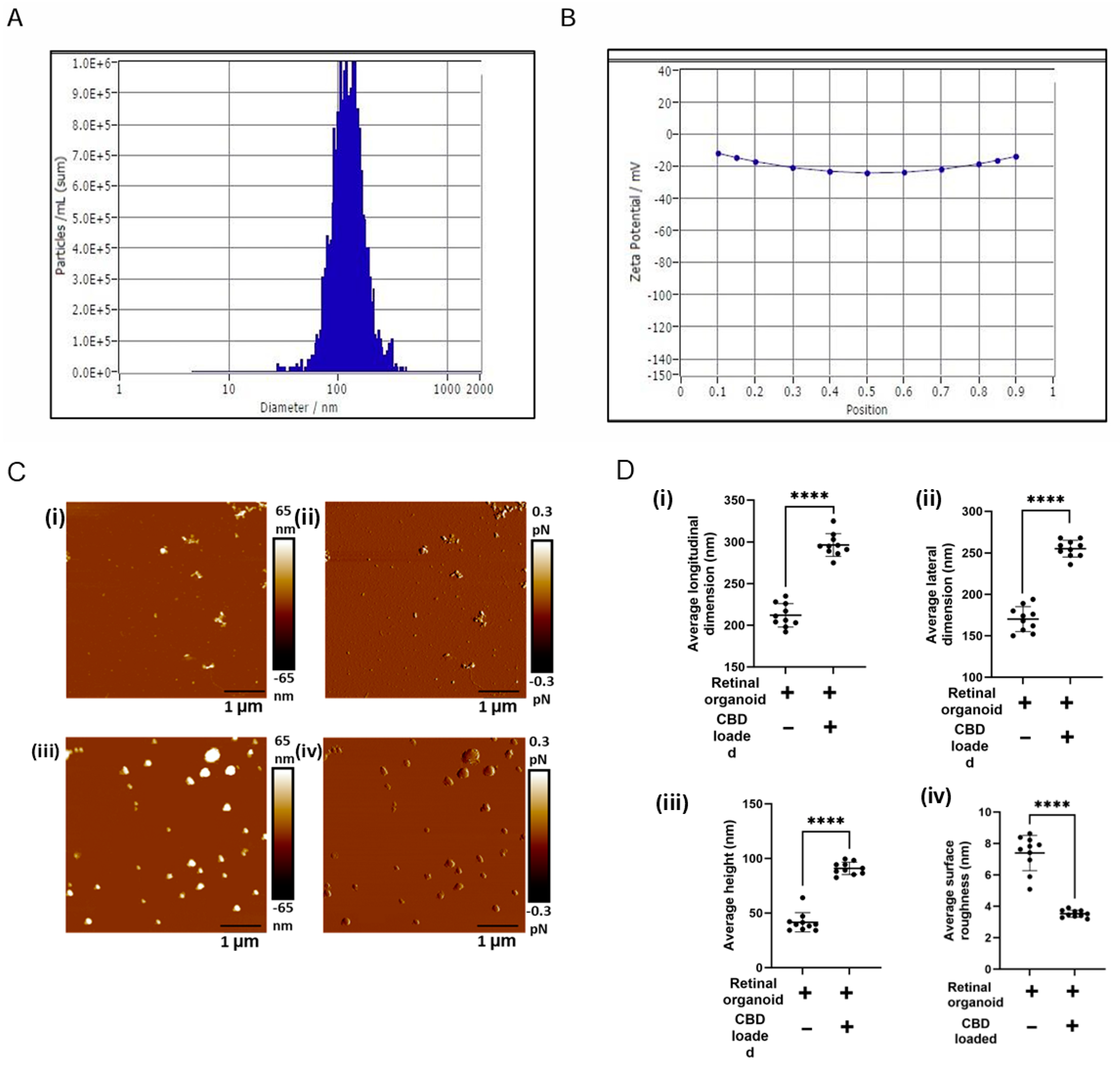
2.5. Characterization of CBD-Loaded EVs by Nanoparticle Tracking Analysis
2.6. Drug Loading and Entrapment Efficiency of CBD-EVs
2.7. Atomic Force Microscopy
2.8. ARPE-19 Cell Culture, Viability, and Cytotoxicity Assay
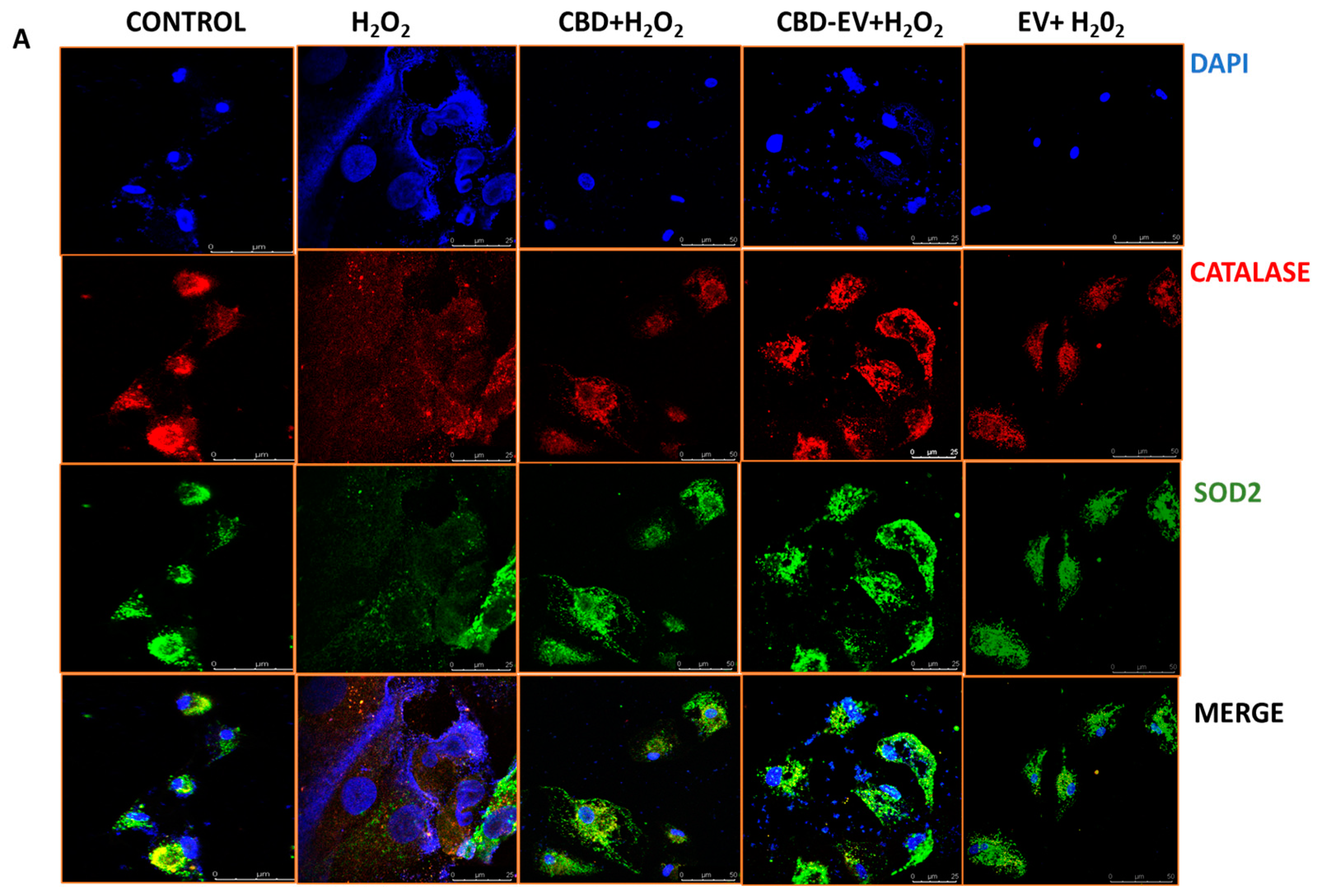
2.9. Immunofluorescence and Imaging
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
3.1. Cargo Characterization Revealed Distinct DEGs in Late and Early Retinal Organoid EVs
3.2. CBD-EVs Structural Properties Supports for Medical Application Use
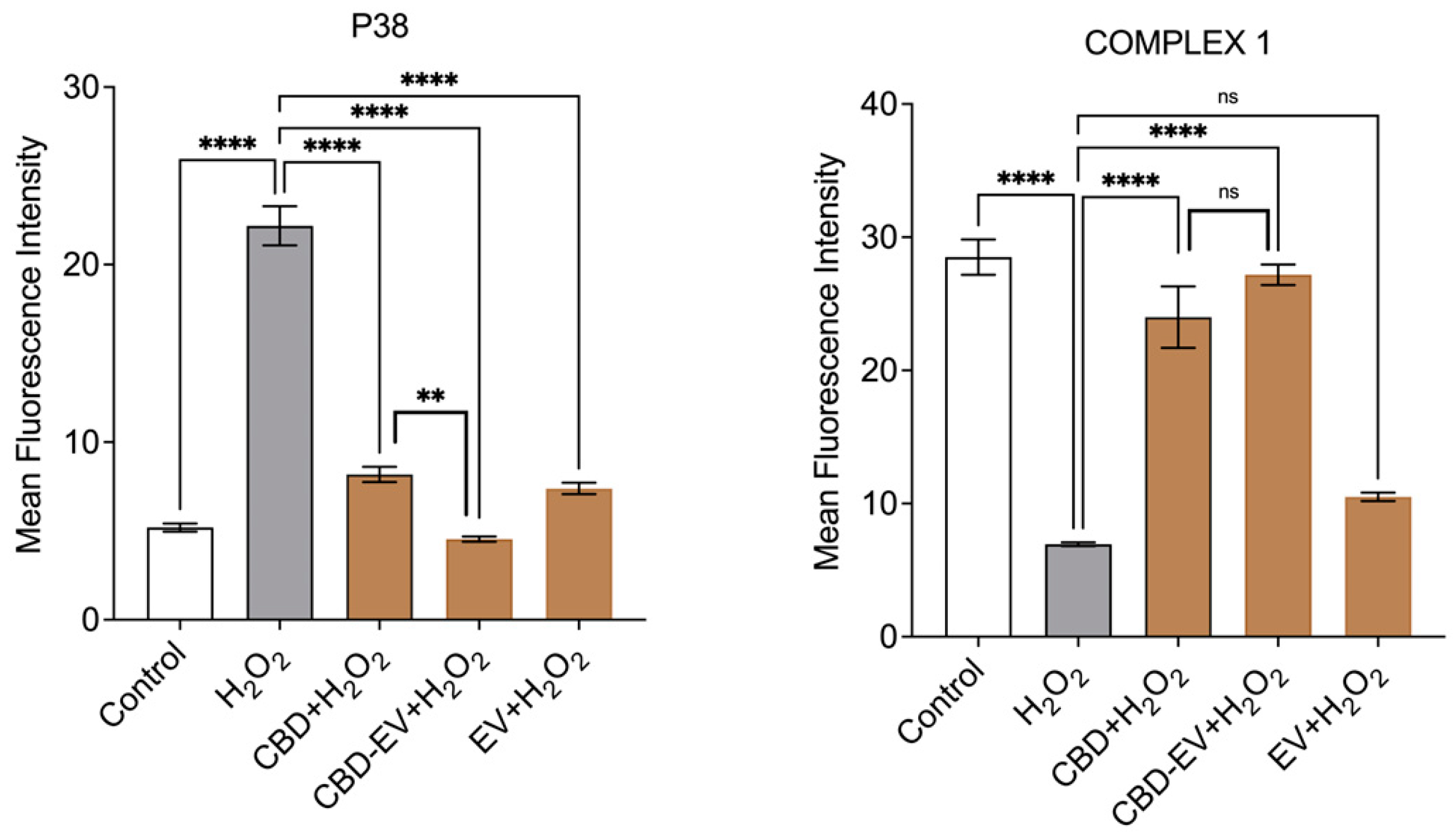
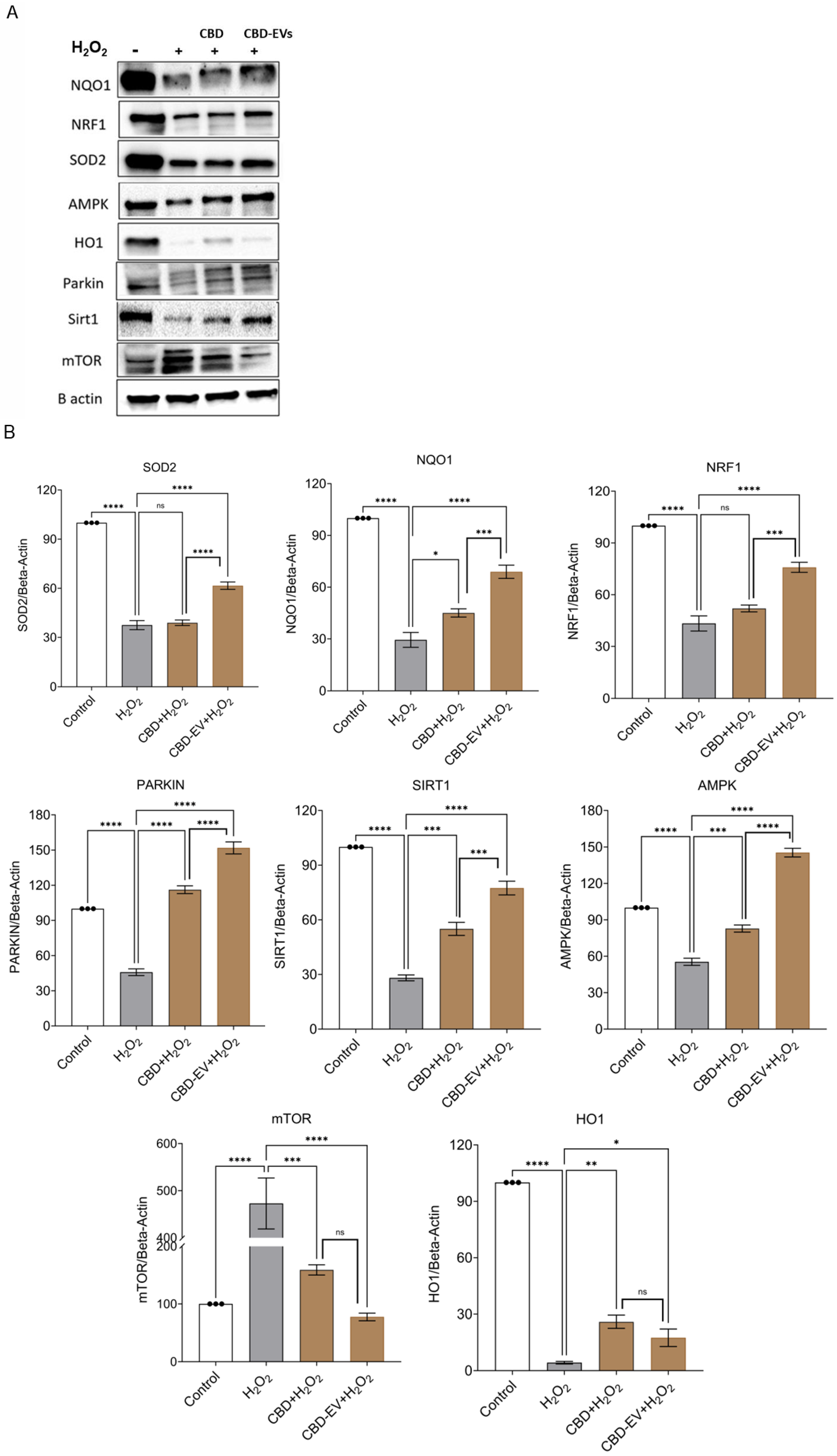
3.3. CBD-EVs Activate AMPK Signaling and Mitigate Apoptosis in Mitochondria-Induced Oxidative ARPE-19 Cells
3.4. CBD-EVs Provide AMPK-Dependent Protective Effects in H2O2-Treated ARPE Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| EV | Extracellular vesicle |
| miRNAs | Micro RNAs |
| RPE | Retinal pigment epithelium |
| SASP | Senescence-associated secretory phenotype |
| CLX | CBD-loaded-exosome delivery platform |
| PTX | Paclitaxel |
| CCM | Culture-conditioned medium |
| PBS | Phosphate-buffered saline |
| PDA | Photodiode array |
| CBD | Cannabidiol |
| hiPSC | Human induced pluripotent stem cell |
| AMPK | Adenosine monophosphate kinase pathway |
| ROS | Reactive oxygen species |
| NMDA | N-methyl-D-aspartate |
| hUCMSC | Human umbilical cord mesenchymal stem cell |
| DRG | Dorsal root ganglion |
| NTA | Nanoparticle tracking analysis |
| AFM | Atomic force microscope |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium-bromide |
| DCFDA | Dichlorofluorescein diacetate |
| MISEV | Minimal information for studies of extracellular vesicles |
| ISEV | International society for extracellular vesicles |
| MAPK | Mitogen-activated protein kinase |
| PIPN | Paclitaxel-induced neuropathic pain |
| DRG | Dorsal root ganglion |
References
- Kozhevnikova, O.S.; Telegina, D.V.; Devyatkin, V.A.; Kolosova, N.G. Involvement of the autophagic pathway in the progression of AMD-like retinopathy in senescence-accelerated OXYS rats. Biogerontology 2018, 19, 223–235. [Google Scholar] [PubMed]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the epidemiology of age-related macular degeneration. Asia-Pac. J. Ophthalmol. 2017, 6, 493–497. [Google Scholar]
- Wei, Q.; Hu, W.; Lou, Q.; Yu, J. NAD+ Inhibits the Metabolic Reprogramming of RPE Cells in Early Age-related Macular Degeneration by Upregulating Mitophagy. Discov. Med. 2019, 27, 189–196. [Google Scholar]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-related macular degeneration: New paradigms for treatment and management of AMD. Oxid. Med. Cell. Longev. 2018, 2018, 8374647. [Google Scholar]
- Klettner, A.; Kauppinen, A.; Blasiak, J.; Roider, J.; Salminen, A.; Kaarniranta, K. Cellular and molecular mechanisms of age-related macular degeneration: From impaired autophagy to neovascularization. Int. J. Biochem. Cell Biol. 2013, 45, 1457–1467. [Google Scholar] [PubMed]
- Blasiak, J.; Petrovski, G.; Veréb, Z.; Facskó, A.; Kaarniranta, K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. BioMed Res. Int. 2014, 2014, 768026. [Google Scholar]
- Pool, F.M.; Kiel, C.; Serrano, L.; Luthert, P.J. Repository of proposed pathways and protein–protein interaction networks in age-related macular degeneration. Npj Aging Mech. Dis. 2020, 6, 2. [Google Scholar] [PubMed]
- Islam, F.M.A.; Chong, E.W.; Hodge, A.M.; Guymer, R.H.; Aung, K.Z.; Makeyeva, G.A.; Baird, P.N.; Hopper, J.L.; English, D.R.; Giles, G.G. Dietary patterns and their associations with age-related macular degeneration: The Melbourne collaborative cohort study. Ophthalmology 2014, 121, 1428–1434.e2. [Google Scholar]
- Uchiki, T.; Weikel, K.A.; Jiao, W.; Shang, F.; Caceres, A.; Pawlak, D.; Handa, J.T.; Brownlee, M.; Nagaraj, R.; Taylor, A. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics). Aging Cell 2012, 11, 1–13. [Google Scholar]
- Kaarniranta, K.; Salminen, A.; Haapasalo, A.; Soininen, H.; Hiltunen, M. Age-related macular degeneration (AMD): Alzheimer’s disease in the eye? J. Alzheimer’s Dis. 2011, 24, 615–631. [Google Scholar]
- Zhang, C.; Miyagishima, K.J.; Dong, L.; Rising, A.; Nimmagadda, M.; Liang, G.; Sharma, R.; Dejene, R.; Wang, Y.; Abu-Asab, M. Regulation of phagolysosomal activity by miR-204 critically influences structure and function of retinal pigment epithelium/retina. Hum. Mol. Genet. 2019, 28, 3355–3368. [Google Scholar]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [PubMed]
- Szatmári-Tóth, M.; Ilmarinen, T.; Mikhailova, A.; Skottman, H.; Kauppinen, A.; Kaarniranta, K.; Kristóf, E.; Lytvynchuk, L.; Veréb, Z.; Fésüs, L. Human embryonic stem cell-derived retinal pigment epithelium-role in dead cell clearance and inflammation. Int. J. Mol. Sci. 2019, 20, 926. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-M.; Huang, D.-Y.; Sekar, P.; Hsu, S.-H.; Lin, W.-W. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 40. [Google Scholar]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar]
- Celkova, L.; Doyle, S.L.; Campbell, M. NLRP3 inflammasome and pathobiology in AMD. J. Clin. Med. 2015, 4, 172–192. [Google Scholar] [CrossRef]
- Piippo, N.; Korkmaz, A.; Hytti, M.; Kinnunen, K.; Salminen, A.; Atalay, M.; Kaarniranta, K.; Kauppinen, A. Decline in cellular clearance systems induces inflammasome signaling in human ARPE-19 cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 3038–3046. [Google Scholar]
- Wang, Y.; Hanus, J.W.; Abu-Asab, M.S.; Shen, D.; Ogilvy, A.; Ou, J.; Chu, X.K.; Shi, G.; Li, W.; Wang, S. NLRP3 upregulation in retinal pigment epithelium in age-related macular degeneration. Int. J. Mol. Sci. 2016, 17, 73. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar]
- Nishimura, Y.; Hara, H.; Kondo, M.; Hong, S.; Matsugi, T. Oxidative stress in retinal diseases. Oxid. Med. Cell. Longev. 2017, 2017, 4076518. [Google Scholar]
- Hampson, A.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−) Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [PubMed]
- Marsicano, G.; Moosmann, B.; Hermann, H.; Lutz, B.; Behl, C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. J. Neurochem. 2002, 80, 448–456. [Google Scholar] [PubMed]
- Buckley, N.E.; McCoy, K.L.; Mezey, É.; Bonner, T.; Zimmer, A.; Felder, C.C.; Glass, M.; Zimmer, A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur. J. Pharmacol. 2000, 396, 141–149. [Google Scholar]
- Malfait, A.; Gallily, R.; Sumariwalla, P.; Malik, A.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [PubMed]
- Shohami, E.; Gallily, R.; Mechoulam, R.; Bass, R.; Ben-Hur, T. Cytokine production in the brain following closed head injury: Dexanabinol (HU-211) is a novel TNF-α inhibitor and an effective neuroprotectant. J. Neuroimmunol. 1997, 72, 169–177. [Google Scholar]
- Braida, D.; Pegorini, S.; Arcidiacono, M.V.; Consalez, G.G.; Croci, L.; Sala, M. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci. Lett. 2003, 346, 61–64. [Google Scholar] [PubMed]
- El-Remessy, A.B.; Khalil, I.E.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.-J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G.I. Neuroprotective effect of (−) Δ9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am. J. Pathol. 2003, 163, 1997–2008. [Google Scholar]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar]
- Patel, N.; Kommineni, N.; Surapaneni, S.K.; Kalvala, A.; Yaun, X.; Gebeyehu, A.; Arthur, P.; Duke, L.C.; York, S.B.; Bagde, A.; et al. Cannabidiol loaded extracellular vesicles sensitize triple-negative breast cancer to doxorubicin in both in-vitro and in vivo models. Int. J. Pharm. 2021, 607, 120943. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, 34562. [Google Scholar]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [PubMed]
- Arthur, P.; Kandoi, S.; Sun, L.; Kalvala, A.; Kutlehria, S.; Bhattacharya, S.; Kulkarni, T.; Nimma, R.; Li, Y.; Lamba, D.A. Biophysical, molecular and proteomic profiling of human retinal organoid-derived exosomes. Pharm. Res. 2023, 40, 801–816. [Google Scholar] [PubMed]
- Nathani, A.; Sun, L.; Khan, I.; Aare, M.; Bagde, A.; Li, Y.; Singh, M. Combined Role of Interleukin-15 Stimulated Natural Killer Cell-Derived Extracellular Vesicles and Carboplatin in Osimertinib-Resistant H1975 Lung Cancer Cells with EGFR Mutations. Pharmaceutics 2024, 16, 83. [Google Scholar] [CrossRef]
- Newsfile Corp. Innocan Announces the Execution of a Research & License Agreement with Ramot, the Technology Transfer Company of the Tel Aviv University for Cannabinoids Loaded Exosome Delivery Platform (Clx). 2021. Available online: https://www.newsfilecorp.com/release/107099/Innocan-Announces-the-Execution-of-a-Research-License-Agreement-with-Ramot-the-Technology-Transfer-Company-of-the-Tel-Aviv-University-for-Cannabinoids-Loaded-Exosome-Delivery-Platform-CLX#:~:text=Herzliya%2C%20Israel%20and%20Calgary%2C%20Alberta--%20%28Newsfile%20Corp.%20-,Aviv%20University%20%28TAU%29%2C%20as%20of%20December%206%2C%202021 (accessed on 31 March 2025).
- Kalvala, A.K.; Bagde, A.; Arthur, P.; Kulkarni, T.; Bhattacharya, S.; Surapaneni, S.; Patel, N.K.; Nimma, R.; Gebeyehu, A.; Kommineni, N. Cannabidiol-loaded extracellular vesicles from human umbilical cord mesenchymal stem cells alleviate paclitaxel-induced peripheral neuropathy. Pharmaceutics 2023, 15, 554. [Google Scholar] [PubMed]
- Muok, L.; Sun, L.; Esmonde, C.; Worden, H.; Vied, C.; Duke, L.; Ma, S.; Zeng, O.; Driscoll, T.; Jung, S. Extracellular vesicle biogenesis of three-dimensional human pluripotent stem cells in a novel Vertical-Wheel bioreactor. J. Extracell. Biol. 2024, 3, e133. [Google Scholar]
- Jeske, R.; Liu, C.; Duke, L.; Castro, M.L.C.; Muok, L.; Arthur, P.; Singh, M.; Jung, S.; Sun, L.; Li, Y. Upscaling human mesenchymal stromal cell production in a novel vertical-wheel bioreactor enhances extracellular vesicle secretion and cargo profile. Bioact. Mater. 2023, 25, 732–747. [Google Scholar]
- Kandoi, S.; Martinez, C.; Chen, K.X.; Mehine, M.; Reddy, L.V.K.; Mansfield, B.C.; Duncan, J.L.; Lamba, D.A. Disease modeling and pharmacological rescue of autosomal dominant Retinitis Pigmentosa associated with RHO copy number variation. Elife 2024, 12, RP90575. [Google Scholar]
- Welsh, J.A.; Goberdhan, D.C.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.; Erdbrügger, U. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar]
- Han, J.; Wu, J.; Silke, J. An overview of mammalian p38 mitogen-activated protein kinases, central regulators of cell stress and receptor signaling. F1000Res 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Arthur, P.; Kalvala, A.K.; Surapaneni, S.K.; Singh, M.S. Applications of cannabinoids in neuropathic pain: An updated review. Crit. Rev. Ther. Drug Carrier Syst. 2024, 41, 1–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Khanal, D.; Kalionis, B.; Chrzanowski, W. High-fidelity probing of the structure and heterogeneity of extracellular vesicles by resonance-enhanced atomic force microscopy infrared spectroscopy. Nat. Protoc. 2019, 14, 576–593. [Google Scholar] [CrossRef]
- Ruozi, B.; Belletti, D.; Tombesi, A.; Tosi, G.; Bondioli, L.; Forni, F.; Vandelli, M.A. AFM, ESEM, TEM, and CLSM in liposomal characterization: A comparative study. Int. J. Nanomed. 2011, 6, 557–563. [Google Scholar] [CrossRef]
- Yurtsever, A.; Yoshida, T.; Badami Behjat, A.; Araki, Y.; Hanayama, R.; Fukuma, T. Structural and mechanical characteristics of exosomes from osteosarcoma cells explored by 3D-atomic force microscopy. Nanoscale 2021, 13, 6661–6677. [Google Scholar] [CrossRef]
- Palacios-Ferrer, J.L.; García-Ortega, M.B.; Gallardo-Gómez, M.; García, M.; Díaz, C.; Boulaiz, H.; Valdivia, J.; Jurado, J.M.; Almazan-Fernandez, F.M.; Arias-Santiago, S.; et al. Metabolomic profile of cancer stem cell-derived exosomes from patients with malignant melanoma. Mol. Oncol. 2021, 15, 407–428. [Google Scholar] [CrossRef]
- Hardij, J.; Cecchet, F.; Berquand, A.; Gheldof, D.; Chatelain, C.; Mullier, F.; Chatelain, B.; Dogné, J.M. Characterisation of tissue factor-bearing extracellular vesicles with AFM: Comparison of air-tapping-mode AFM and liquid Peak Force AFM. J. Extracell. Vesicles 2013, 2, 21045. [Google Scholar] [CrossRef]
- Gazze, S.A.; Thomas, S.J.; Garcia-Parra, J.; James, D.W.; Rees, P.; Marsh-Durban, V.; Corteling, R.; Gonzalez, D.; Conlan, R.S.; Francis, L.W. High content, quantitative AFM analysis of the scalable biomechanical properties of extracellular vesicles. Nanoscale 2021, 13, 6129–6141. [Google Scholar]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar]
- Oda, S.; Yokoi, T. Recent progress in the use of microRNAs as biomarkers for drug-induced toxicities in contrast to traditional biomarkers: A comparative review. Drug Metab. Pharmacokinet. 2021, 37, 100372. [Google Scholar] [PubMed]
- Maes, O.C.; An, J.; Sarojini, H.; Wang, E. Murine microRNAs implicated in liver functions and aging process. Mech. Ageing Dev. 2008, 129, 534–541. [Google Scholar]
- Drummond, M.J.; McCarthy, J.J.; Sinha, M.; Spratt, H.M.; Volpi, E.; Esser, K.A.; Rasmussen, B.B. Aging and microRNA expression in human skeletal muscle: A microarray and bioinformatics analysis. Physiol. Genom. 2011, 43, 595–603. [Google Scholar]
- Persengiev, S.; Kondova, I.; Otting, N.; Koeppen, A.H.; Bontrop, R.E. Genome-wide analysis of miRNA expression reveals a potential role for miR-144 in brain aging and spinocerebellar ataxia pathogenesis. Neurobiol. Aging 2011, 32, 2316.e17–2316.e27. [Google Scholar] [CrossRef] [PubMed]
- Smith-Vikos, T.; Slack, F.J. MicroRNAs and their roles in aging. J. Cell Sci. 2012, 125, 7–17. [Google Scholar]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar]
- Gutsaeva, D.R.; Thounaojam, M.; Rajpurohit, S.; Powell, F.L.; Martin, P.M.; Goei, S.; Duncan, M.; Bartoli, M. STAT3-mediated activation of miR-21 is involved in down-regulation of TIMP3 and neovascularization in the ischemic retina. Oncotarget 2017, 8, 103568. [Google Scholar] [PubMed]
- Chen, Q.; Qiu, F.; Zhou, K.; Matlock, H.G.; Takahashi, Y.; Rajala, R.V.; Yang, Y.; Moran, E.; Ma, J.-x. Pathogenic role of microRNA-21 in diabetic retinopathy through downregulation of PPARα. Diabetes 2017, 66, 1671–1682. [Google Scholar]
- Xu, S.; Witmer, P.D.; Lumayag, S.; Kovacs, B.; Valle, D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J. Biol. Chem. 2007, 282, 25053–25066. [Google Scholar]
- Busskamp, V.; Krol, J.; Nelidova, D.; Daum, J.; Szikra, T.; Tsuda, B.; Jüttner, J.; Farrow, K.; Scherf, B.G.; Alvarez, C.P.P. miRNAs 182 and 183 are necessary to maintain adult cone photoreceptor outer segments and visual function. Neuron 2014, 83, 586–600. [Google Scholar] [CrossRef]
- La Torre, A.; Georgi, S.; Reh, T.A. Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, E2362–E2370. [Google Scholar] [CrossRef] [PubMed]
- Decembrini, S.; Bressan, D.; Vignali, R.; Pitto, L.; Mariotti, S.; Rainaldi, G.; Wang, X.; Evangelista, M.; Barsacchi, G.; Cremisi, F. MicroRNAs couple cell fate and developmental timing in retina. Proc. Natl. Acad. Sci. USA 2009, 106, 21179–21184. [Google Scholar] [CrossRef]
- Xia, X.; Ahmad, I. let-7 microRNA regulates neurogliogenesis in the mammalian retina through Hmga2. Dev. Biol. 2016, 410, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.L.; Sun, H. Identification of hsa-mir-34a, hsa-mir-124, and hsa-mir-204 as signatures for cataract. J. Cell. Physiol. 2019, 234, 10709–10717. [Google Scholar] [CrossRef] [PubMed]
- Conte, I.; Carrella, S.; Avellino, R.; Karali, M.; Marco-Ferreres, R.; Bovolenta, P.; Banfi, S. miR-204 is required for lens and retinal development via Meis2 targeting. Proc. Natl. Acad. Sci. USA 2010, 107, 15491–15496. [Google Scholar] [CrossRef]
- Olena, A.F.; Rao, M.B.; Thatcher, E.J.; Wu, S.-Y.; Patton, J.G. miR-216a regulates snx5, a novel notch signaling pathway component, during zebrafish retinal development. Dev. Biol. 2015, 400, 72–81. [Google Scholar] [CrossRef]
- Zhou, J.; Flores-Bellver, M.; Pan, J.; Benito-Martin, A.; Shi, C.; Onwumere, O.; Mighty, J.; Qian, J.; Zhong, X.; Hogue, T. Human retinal organoids release extracellular vesicles that regulate gene expression in target human retinal progenitor cells. Sci. Rep. 2021, 11, 21128. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Ebeling, M.C.; Kapphahn, R.J.; Terluk, M.R.; Fisher, C.R.; Polanco, J.R.; Roehrich, H.; Leary, M.M.; Geng, Z.; Dutton, J.R. Altered bioenergetics and enhanced resistance to oxidative stress in human retinal pigment epithelial cells from donors with age-related macular degeneration. Redox Biol. 2017, 13, 255–265. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Xu, L.; Ash, J.D. The Role of AMPK Pathway in Neuroprotection. Retinal Degenerative Diseases: Mechanisms and Experimental Therapy; Springer: Berlin, Germany, 2016; pp. 425–430. [Google Scholar]
- Xu, L.; Kong, L.; Wang, J.; Ash, J.D. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2018, 115, 10475–10480. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Chen, K.; Wang, X.; Wang, Z.; Lian, H.; Lin, Y.; Rong, X.; Chu, M.; Lin, J. Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. J. Cell. Mol. Med. 2020, 24, 7515–7530. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Li, J.; Yao, Z.; Liu, J. Cannabidiol induces autophagy to protects neural cells from mitochondrial dysfunction by upregulating SIRT1 to inhibits NF-κB and NOTCH pathways. Front. Cell. Neurosci. 2021, 15, 654340. [Google Scholar] [CrossRef] [PubMed]
- Hasanain, M.; Bhattacharjee, A.; Pandey, P.; Ashraf, R.; Singh, N.; Sharma, S.; Vishwakarma, A.; Datta, D.; Mitra, K.; Sarkar, J. α-Solanine induces ROS-mediated autophagy through activation of endoplasmic reticulum stress and inhibition of Akt/mTOR pathway. Cell Death Dis. 2015, 6, e1860. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Golestaneh, N.; Chu, Y.; Cheng, S.K.; Cao, H.; Poliakov, E.; Berinstein, D.M. Repressed SIRT1/PGC-1α pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. J. Transl. Med. 2016, 14, 344. [Google Scholar] [CrossRef]
- Arbab, A.A.I.; Lu, X.; Abdalla, I.M.; Idris, A.A.; Chen, Z.; Li, M.; Mao, Y.; Xu, T.; Yang, Z. Metformin inhibits lipoteichoic acid–induced oxidative stress and inflammation through AMPK/NRF2/NF-κB signaling pathway in bovine mammary epithelial cells. Front. Vet. Sci. 2021, 8, 661380. [Google Scholar] [CrossRef]


| EV_Retinal_Early | EV_Retinal_Late |
|---|---|
| has_miR-21 | has_miR-21 |
| has_miR-146b | has_let-7f |
| has_let-7f | has_let-7i |
| has_let-7a | has_miR-9 |
| has_let-7i | has_let-7a |
| has_let-7g | has_miR-1246 |
| has_miR-100 | has_miR-146b |
| has_miR-636 | has_miR-100 |
| has_miR-143 | has_let-7g |
| has_miR-1246 | has_miR-7 |
| has_miR-7 | has_miR-26a |
| has_miR-10400 | has_miR-143 |
| has_miR-9 | has_miR-92a |
| has_miR-92a | has_miR-148a |
| has_miR-26a | has_miR-182 |
| has_let-7b | has_miR-5588 |
| has_miR-146a | has_let-7b |
| has_miR-148a | has_miR-183 |
| has_miR-182 | has_miR-146a |
| has_miR-8058 | has_miR-423 |
| Name | Log2FC | Adj.P. Val |
|---|---|---|
| miR-4655 | 5.26 | 3.8 × 10−4 |
| miR-6731 | 4.36 | 3.8 × 10−4 |
| miR-204 | 4.26 | 3.8 × 10−4 |
| miR-9985 | 3.59 | 8.9 × 10−4 |
| miR-5588 | 1.94 | 3.2 × 10−4 |
| miR-3615 | −2.09 | 4.7 × 10−2 |
| miR-7110 | −2.09 | 4.7 × 10−2 |
| miR-31804 | −2.21 | 1.8 × 10−2 |
| miR-6789 | −2.46 | 1.1 × 10−2 |
| miR-4508 | −3.89 | 1.1 × 10−2 |
| miR-1469 | −4.45 | 8.9 × 10−4 |
| miR-8058 | −5.74 | 3.8 × 10−4 |
| miR-636 | −5.98 | 8.9 × 10−4 |
| miR-10400 | −6.01 | 3.8 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arthur, P.; Kandoi, S.; Kalvala, A.; Boirie, B.; Nathani, A.; Aare, M.; Bhattacharya, S.; Kulkarni, T.; Sun, L.; Lamba, D.A.; et al. Cannabidiol-Loaded Retinal Organoid-Derived Extracellular Vesicles Protect Oxidatively Stressed ARPE-19 Cells. Biomedicines 2025, 13, 1167. https://doi.org/10.3390/biomedicines13051167
Arthur P, Kandoi S, Kalvala A, Boirie B, Nathani A, Aare M, Bhattacharya S, Kulkarni T, Sun L, Lamba DA, et al. Cannabidiol-Loaded Retinal Organoid-Derived Extracellular Vesicles Protect Oxidatively Stressed ARPE-19 Cells. Biomedicines. 2025; 13(5):1167. https://doi.org/10.3390/biomedicines13051167
Chicago/Turabian StyleArthur, Peggy, Sangeetha Kandoi, Anil Kalvala, Breana Boirie, Aakash Nathani, Mounika Aare, Santanu Bhattacharya, Tanmay Kulkarni, Li Sun, Deepak A. Lamba, and et al. 2025. "Cannabidiol-Loaded Retinal Organoid-Derived Extracellular Vesicles Protect Oxidatively Stressed ARPE-19 Cells" Biomedicines 13, no. 5: 1167. https://doi.org/10.3390/biomedicines13051167
APA StyleArthur, P., Kandoi, S., Kalvala, A., Boirie, B., Nathani, A., Aare, M., Bhattacharya, S., Kulkarni, T., Sun, L., Lamba, D. A., Li, Y., & Singh, M. (2025). Cannabidiol-Loaded Retinal Organoid-Derived Extracellular Vesicles Protect Oxidatively Stressed ARPE-19 Cells. Biomedicines, 13(5), 1167. https://doi.org/10.3390/biomedicines13051167









