Exploring Protein Misfolding in Amyotrophic Lateral Sclerosis: Structural and Functional Insights
Abstract
1. Introduction
2. Genetic Mutations in ALS
| Protein | Mutation | Induction of Further Protein Misfolding | Reference |
|---|---|---|---|
| SOD1 | A4V | Accumulation of ubiquitins into inclusions Disruption of Golgi trafficking Endoplasmic reticulum stress | [14,19,20] |
| W32S | Prion-like seeding | [21] | |
| G37R | N/A | [22] | |
| L38V | N/A | [14] | |
| H46R | Prion-like seeding | [23] | |
| G85R | Prion-like seeding Disruption of Golgi trafficking | [20,24] | |
| D90A | N/A | [25] | |
| G93A | Disruption of autophagy Disruption of axonal transport Disruption of Golgi trafficking Accumulation of ubiquitins into inclusions Induction of Golgi fragmentation Prion-like seeding | [26,27,28] | |
| G93C | N/A | [14] | |
| G127X | Prion-like seeding | [24] | |
| TDP-43 | A90V | Prion-like seeding | [29] |
| D169G | N/A | [30] | |
| K263E | N/A | [31] | |
| G290A | Affects the proteasome | [32] | |
| G294A | Prion-like seeding | [33] | |
| G294V | Affects the proteasome | [32] | |
| G295C | N/A | [34] | |
| G298S | N/A | [31,35] | |
| A315T | Errors in protein synthesis Affects the proteasome | [32,36] | |
| A315E | Prion-like seeding | [37] | |
| A321G | N/A | [38] | |
| Q331K | N/A | [39] | |
| M337V | Accumulation of ubiquitins into inclusions Affects the proteasome | [19,32] | |
| Q343R | Errors in protein synthesis | [40] | |
| N345K | N/A | [39] | |
| G348C | Stress induction | [32,41] | |
| N352S | Affects the proteasome | [42] | |
| G358C | N/A | [34] | |
| R361S | N/A | [39] | |
| S379C | N/A | [34] | |
| A382T | Affects the proteasome | [32] | |
| N390D | N/A | [39] | |
| FUS | G156E | Prion-like seeding | [43] |
| R495X | Accumulation of ubiquitins into inclusions | [19,44] | |
| R514G | N/A | [45] | |
| R521C | Errors in protein synthesis SMN incorporation | [46] | |
| R521H | Errors in protein synthesis Inclusion of SMN | [46] | |
| R521G | Errors in protein synthesis | [47] | |
| R522G | N/A | [47] | |
| R524S | N/A | [44] | |
| P525L | SMN incorporation Errors in protein synthesis Disruption of autophagy | [17,46,48] | |
| P525R | Errors in protein synthesis | [49] | |
| C9orf72 (gene) | CpG methylation | Errors in protein synthesis Disruption of nucleocytoplasmic transport | [50,51] |
3. Errors in the Protein Synthesis Processes
4. Errors in Protein Trafficking
5. Dysfunction of the Folding and Chaperone Machinery
6. Seeding and Cross-Seeding Mechanisms
7. Aging
8. Other Factors
8.1. pH Changes
8.2. Environmental Factors
9. Structural Aspects of ALS-Related Proteins
Post-Translational Modifications
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, R.; Black, K.A.; Stollar, E.J. Uncovering protein function: From classification to complexes. Essays Biochem. 2022, 66, 255–285. [Google Scholar] [CrossRef]
- Krokidis, M.G.; Dimitrakopoulos, G.N.; Vrahatis, A.G.; Exarchos, T.P.; Vlamos, P. Challenges and limitations in computational prediction of protein misfolding in neurodegenerative diseases. Front. Comput. Neurosci. 2024, 17, 1323182. [Google Scholar] [CrossRef]
- Ajmal, M.R. Protein Misfolding and Aggregation in Proteinopathies: Causes, Mechanism and Cellular Response. Diseases 2023, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Chaturvedi, M.; Fatima, S.; Priya, S. Environmental factors modulating protein conformations and their role in protein aggregation diseases. Toxicology 2022, 465, 153049. [Google Scholar] [PubMed]
- Khan, A.N.; Khan, R.H. Protein misfolding and related human diseases: A comprehensive review of toxicity, proteins involved, and current therapeutic strategies. Int. J. Biol. Macromol. 2022, 223 Pt A, 143–160. [Google Scholar]
- Koszła, O.; Sołek, P. Misfolding and aggregation in neurodegenerative diseases: Protein quality control machinery as potential therapeutic clearance pathways. Cell Commun. Signal. 2024, 22, 421. [Google Scholar]
- Kundu, D.; Prerna, K.; Chaurasia, R.; Bharty, M.K.; Dubey, V.K. Advances in protein misfolding, amyloidosis and its correlation with human diseases. 3 Biotech 2020, 10, 193. [Google Scholar]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar]
- Gomez-Gutierrez, R.; Morales, R. The prion-like phenomenon in Alzheimer’s disease: Evidence of pathology transmission in humans. PLoS Pathog. 2020, 16, e1009004. [Google Scholar] [CrossRef]
- Bianchi, E.; Pupillo, E.; De Feudis, A.; Enia, G.; Vitelli, E.; Beghi, E. Trends in survival of ALS from a population-based registry. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 344–352. [Google Scholar] [CrossRef]
- Jankovska, N.; Matej, R. Molecular Pathology of ALS: What We Currently Know and What Important Information Is Still Missing. Diagnostics 2021, 11, 1365. [Google Scholar] [CrossRef]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar]
- Reaume, A.G.; Elliott, J.L.; Hoffman, E.K.; Kowall, N.W.; Ferrante, R.J.; Siwek, D.F.; Wilcox, H.M.; Flood, D.G.; Beal, M.F.; Brown, R.H.; et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996, 13, 43–47. [Google Scholar] [PubMed]
- Brasil, A.A.; de Carvalho, M.D.C.; Gerhardt, E.; Queiroz, D.D.; Pereira, M.D.; Outeiro, T.F.; Eleutherio, E.C.A. Characterization of the activity, aggregation, and toxicity of heterodimers of WT and ALS-associated mutant Sod1. Proc. Natl. Acad. Sci. USA 2019, 116, 25991–26000. [Google Scholar] [CrossRef]
- de Boer, E.M.J.; Orie, V.K.; Williams, T.; Baker, M.R.; De Oliveira, H.M.; Polvikoski, T.; Silsby, M.; Menon, P.; van den Bos, M.; Halliday, G.M.; et al. TDP-43 proteinopathies: A new wave of neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 2020, 92, 86–95. [Google Scholar]
- Assoni, A.F.; Foijer, F.; Zatz, M. Amyotrophic Lateral Sclerosis, FUS and Protein Synthesis Defects. Stem Cell Rev. Rep. 2023, 19, 625–638. [Google Scholar] [PubMed]
- Marrone, L.; Drexler, H.C.A.; Wang, J.; Tripathi, P.; Distler, T.; Heisterkamp, P.; Anderson, E.N.; Kour, S.; Moraiti, A.; Maharana, S.; et al. FUS pathology in ALS is linked to alterations in multiple ALS-associated proteins and rescued by drugs stimulating autophagy. Acta Neuropathol. 2019, 138, 67–84. [Google Scholar]
- Niu, C.; Zhang, J.; Gao, F.; Yang, L.; Jia, M.; Zhu, H.; Gong, W. FUS-NLS/Transportin 1 complex structure provides insights into the nuclear targeting mechanism of FUS and the implications in ALS. PLoS ONE 2012, 7, e47056. [Google Scholar] [CrossRef]
- Farrawell, N.E.; McAlary, L.; Lum, J.S.; Chisholm, C.G.; Warraich, S.T.; Blair, I.P.; Vine, K.L.; Saunders, D.N.; Yerbury, J.J. Ubiquitin Homeostasis Is Disrupted in TDP-43 and FUS Cell Models of ALS. iScience 2020, 23, 101700. [Google Scholar]
- Atkin, J.D.; Farg, M.A.; Soo, K.Y.; Walker, A.K.; Halloran, M.; Turner, B.J.; Nagley, P.; Horne, M.K. Mutant SOD1 inhibits ER-Golgi transport in amyotrophic lateral sclerosis. J. Neurochem. 2014, 129, 190–204. [Google Scholar] [CrossRef]
- Crown, A.; McAlary, L.; Fagerli, E.; Brown, H.; Yerbury, J.J.; Galaleldeen, A.; Cashman, N.R.; Borchelt, D.R.; Ayers, J.I. Tryptophan residue 32 in human Cu-Zn superoxide dismutase modulates prion-like propagation and strain selection. PLoS ONE 2020, 15, e0227655. [Google Scholar]
- Münch, C.; Bertolotti, A. Exposure of hydrophobic surfaces initiates aggregation of diverse ALS-causing superoxide dismutase-1 mutants. J. Mol. Biol. 2010, 399, 512–525. [Google Scholar] [PubMed]
- Münch, C.; O’Brien, J.; Bertolotti, A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 3548–3553. [Google Scholar] [CrossRef]
- Grad, L.I.; Guest, W.C.; Yanai, A.; Pokrishevsky, E.; O’Neill, M.A.; Gibbs, E.; Semenchenko, V.; Yousefi, M.; Wishart, D.S.; Plotkin, S.S.; et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16398–16403. [Google Scholar] [CrossRef]
- Lang, L.; Zetterström, P.; Brännström, T.; Marklund, S.L.; Danielsson, J.; Oliveberg, M. SOD1 aggregation in ALS mice shows simplistic test tube behavior. Proc. Natl. Acad. Sci. USA 2015, 112, 9878–9883. [Google Scholar] [CrossRef] [PubMed]
- van Dis, V.; Kuijpers, M.; Haasdijk, E.D.; Teuling, E.; Oakes, S.A.; Hoogenraad, C.C.; Jaarsma, D. Golgi fragmentation precedes neuromuscular denervation and is associated with endosome abnormalities in SOD1-ALS mouse motor neurons. Acta Neuropathol. Commun. 2014, 2, 38. [Google Scholar]
- Bruijn, L.I.; Becher, M.W.; Lee, M.K.; Anderson, K.L.; Jenkins, N.A.; Copeland, N.G.; Sisodia, S.S.; Rothstein, J.D.; Borchelt, D.R.; Price, D.L.; et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 1997, 18, 327–338. [Google Scholar] [CrossRef]
- Stevens, J.C.; Chia, R.; Hendriks, W.T.; Bros-Facer, V.; van Minnen, J.; Martin, J.E.; Jackson, G.S.; Greensmith, L.; Schiavo, G.; Fisher, E.M. Modification of superoxide dismutase 1 (SOD1) properties by a GFP tag–implications for research into amyotrophic lateral sclerosis (ALS). PLoS ONE 2010, 5, e9541. [Google Scholar] [CrossRef]
- Winton, M.J.; Igaz, L.M.; Wong, M.M.; Kwong, L.K.; Trojanowski, J.Q.; Lee, V.M.Y. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J. Biol. Chem. 2008, 283, 13302–13309. [Google Scholar] [CrossRef]
- McDonald, K.K.; Aulas, A.; Destroismaisons, L.; Pickles, S.; Beleac, E.; Camu, W.; Rouleau, G.A.; Vande Velde, C. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum. Mol. Genet. 2011, 20, 1400–1410. [Google Scholar]
- Austin, J.A.; Wright, G.S.; Watanabe, S.; Grossmann, J.G.; Antonyuk, S.V.; Yamanaka, K.; Hasnain, S.S. Disease causing mutants of TDP-43 nucleic acid binding domains are resistant to aggregation and have increased stability and half-life. Proc. Natl. Acad. Sci. USA 2014, 111, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Riemenschneider, H.; Guo, Q.; Bader, J.; Frottin, F.; Farny, D.; Kleinberger, G.; Haass, C.; Mann, M.; Hartl, F.U.; Baumeister, W.; et al. Gel-like inclusions of C-terminal fragments of TDP-43 sequester stalled proteasomes in neurons. EMBO Rep. 2022, 23, e53890. [Google Scholar] [CrossRef]
- Zeineddine, R.; Whiten, D.R.; Farrawell, N.E.; McAlary, L.; Hanspal, M.A.; Kumita, J.R.; Wilson, M.R.; Yerbury, J.J. Flow cytometric measurement of the cellular propagation of TDP-43 aggregation. Prion 2017, 11, 195–204. [Google Scholar] [CrossRef]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Araki, W.; Minegishi, S.; Motoki, K.; Kume, H.; Hohjoh, H.; Araki, Y.M.; Tamaoka, A. Disease-associated mutations of TDP-43 promote turnover of the protein through the proteasomal pathway. Mol. Neurobiol. 2014, 50, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Gitcho, M.A.; Baloh, R.H.; Chakraverty, S.; Mayo, K.; Norton, J.B.; Levitch, D.; Hatanpaa, K.J.; White, C.L., III; Bigio, E.H.; Caselli, R.; et al. TDP-43 A315T mutation in familial motor neuron disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2008, 63, 535–538. [Google Scholar]
- Liu, X.; Lao, Z.; Li, X.; Dong, X.; Wei, G. ALS-associated A315E and A315pT variants exhibit distinct mechanisms in inducing irreversible aggregation of TDP-43 312–317 peptides. Phys. Chem. Chem. Phys. 2022, 24, 16263–16273. [Google Scholar] [CrossRef]
- Conicella, A.E.; Zerze, G.H.; Mittal, J.; Fawzi, N.L. ALS mutations disrupt phase separation mediated by α-helical structure in the TDP-43 low-complexity C-terminal domain. Structure 2016, 24, 1537–1549. [Google Scholar] [CrossRef]
- Johnson, B.S.; Snead, D.; Lee, J.J.; McCaffery, J.M.; Shorter, J.; Gitler, A.D. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J. Biol. Chem. 2009, 284, 20329–20339. [Google Scholar] [CrossRef]
- Liu-Yesucevitz, L.; Lin, A.Y.; Ebata, A.; Boon, J.Y.; Reid, W.; Xu, Y.F.; Kobrin, K.; Murphy, G.J.; Petrucelli, L.; Wolozin, B. ALS-linked mutations enlarge TDP-43-enriched neuronal RNA granules in the dendritic arbor. J. Neurosci. 2014, 34, 4167–4174. [Google Scholar] [CrossRef]
- Dewey, C.M.; Cenik, B.; Sephton, C.F.; Dries, D.R.; Mayer, P., III; Good, S.K.; Johnson, B.A.; Herz, J.; Yu, G. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol. Cell. Biol. 2011, 31, 1098–1110. [Google Scholar] [CrossRef]
- Blitterswijk, M.; Rademakers, R.; Berg, L.H. Clinical variability and additional mutations in amyotrophic lateral sclerosis patients with p. N352 S mutations in TARDBP. Neuropathol. Appl. Neurobiol. 2014, 40, 356–358. [Google Scholar] [CrossRef]
- Nomura, T.; Watanabe, S.; Kaneko, K.; Yamanaka, K.; Nukina, N.; Furukawa, Y. Intranuclear aggregation of mutant FUS/TLS as a molecular pathomechanism of amyotrophic lateral sclerosis. J. Biol. Chem. 2014, 289, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Mannen, T.; Çağatay, T.; Fujiwara, A.; Matsumura, H.; Niesman, A.B.; Brautigam, C.A.; Chook, Y.M.; Yoshizawa, T. Mechanism of karyopherin-β2 binding and nuclear import of ALS variants FUS (P525L) and FUS (R495X). Sci. Rep. 2021, 11, 3754. [Google Scholar] [CrossRef] [PubMed]
- Shum, C.; Hedges, E.C.; Allison, J.; Lee, Y.B.; Arias, N.; Cocks, G.; Chandran, S.; Ruepp, M.D.; Shaw, C.E.; Nishimura, A.L. Mutations in FUS lead to synaptic dysregulation in ALS-iPSC derived neurons. Stem Cell Rep. 2024, 19, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Groen, E.J.; Fumoto, K.; Blokhuis, A.M.; Engelen-Lee, J.; Zhou, Y.; van den Heuvel, D.M.; Koppers, M.; van Diggelen, F.; van Heest, J.; Demmers, J.A.; et al. ALS-associated mutations in FUS disrupt the axonal distribution and function of SMN. Hum. Mol. Genet. 2013, 22, 3690–3704. [Google Scholar] [CrossRef]
- van Blitterswijk, M.; Wang, E.T.; Friedman, B.A.; Keagle, P.J.; Lowe, P.; Leclerc, A.L.; van den Berg, L.H.; Housman, D.E.; Veldink, J.H.; Landers, J.E. Characterization of FUS mutations in amyotrophic lateral sclerosis using RNA-Seq. PLoS ONE 2013, 8, e60788. [Google Scholar] [CrossRef]
- Lehmkuhl, E.M.; Zarnescu, D.C. Lost in Translation: Evidence for Protein Synthesis Deficits in ALS/FTD and Related Neurodegenerative Diseases. Adv. Neurobiol. 2018, 20, 283–301. [Google Scholar]
- Kuang, L.; Kamelgarn, M.; Arenas, A.; Gal, J.; Taylor, D.; Gong, W.; Brown, M.; St Clair, D.; Kasarskis, E.J.; Zhu, H. Clinical and experimental studies of a novel P525R FUS mutation in amyotrophic lateral sclerosis. Neurol. Genet. 2017, 3, e172. [Google Scholar] [CrossRef]
- Xi, Z.; Zinman, L.; Moreno, D.; Schymick, J.; Liang, Y.; Sato, C.; Zheng, Y.; Ghani, M.; Dib, S.; Keith, J.; et al. Hypermethylation of the CpG island near the G4C2 repeat in ALS with a C9orf72 expansion. Am. J. Hum. Genet. 2013, 92, 981–989. [Google Scholar] [CrossRef]
- Liu, F.; Morderer, D.; Wren, M.C.; Vettleson-Trutza, S.A.; Wang, Y.; Rabichow, B.E.; Salemi, M.R.; Phinney, B.S.; Oskarsson, B.; Dickson, D.W.; et al. Proximity proteomics of C9orf72 dipeptide repeat proteins identifies molecular chaperones as modifiers of poly-GA aggregation. Acta Neuropathol. Commun. 2022, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.K.; Gregory, J.M.; Chandran, S.; Turner, B.J. Could an Impairment in Local Translation of mRNAs in Glia be Contributing to Pathogenesis in ALS? Front. Mol. Neurosci. 2019, 12, 124. [Google Scholar] [CrossRef]
- Kamelgarn, M.; Chen, J.; Kuang, L.; Jin, H.; Kasarskis, E.J.; Zhu, H. ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay. Proc. Natl. Acad. Sci. USA 2018, 115, E11904–E11913. [Google Scholar] [CrossRef]
- Murakami, T.; Qamar, S.; Lin, J.Q.; Schierle, G.S.; Rees, E.; Miyashita, A.; Costa, A.R.; Dodd, R.B.; Chan, F.T.; Michel, C.H.; et al. ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron 2015, 88, 678–690. [Google Scholar] [CrossRef]
- Yasuda, K.; Zhang, H.; Loiselle, D.; Haystead, T.; Macara, I.G.; Mili, S. The RNA-binding protein Fus directs translation of localized mRNAs in APC-RNP granules. J. Cell Biol. 2013, 203, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Jha, N.N.; Awano, T.; Caine, C.; Gollapalli, K.; Welby, E.; Kim, S.S.; Fuentes-Moliz, A.; Wang, X.; Feng, Z.; et al. A spinal muscular atrophy modifier implicates the SMN protein in SNARE complex assembly at neuromuscular synapses. Neuron 2023, 111, 1423–1439.e4. [Google Scholar] [CrossRef] [PubMed]
- Altman, T.; Ionescu, A.; Ibraheem, A.; Priesmann, D.; Gradus-Pery, T.; Farberov, L.; Alexandra, G.; Shelestovich, N.; Dafinca, R.; Shomron, N.; et al. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat. Commun. 2021, 12, 6914. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.C.; Wang, Y.D.; Scarborough, E.A.; Moore, J.; Diaz, Z.; MacLea, K.S.; Freibaum, B.; Li, S.; Molliex, A.; et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 2013, 495, 467–473. [Google Scholar] [CrossRef]
- Deshaies, J.E.; Shkreta, L.; Moszczynski, A.J.; Sidibé, H.; Semmler, S.; Fouillen, A.; Bennett, E.R.; Bekenstein, U.; Destroismaisons, L.; Toutant, J.; et al. TDP-43 regulates the alternative splicing of hnRNP A1 to yield an aggregation-prone variant in amyotrophic lateral sclerosis. Brain 2018, 141, 1320–1333. [Google Scholar] [CrossRef]
- Burk, K.; Pasterkamp, R.J. Disrupted neuronal trafficking in amyotrophic lateral sclerosis. Acta Neuropathol. 2019, 137, 859–877. [Google Scholar] [CrossRef]
- Sundaramoorthy, V.; Walker, A.K.; Tan, V.; Fifita, J.A.; Mccann, E.P.; Williams, K.L.; Blair, I.P.; Guillemin, G.J.; Farg, M.A.; Atkin, J.D. Defects in optineurin- and myosin VI-mediated cellular trafficking in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2017, 26, 3452. [Google Scholar] [CrossRef]
- Zhang, K.; Daigle, J.G.; Cunningham, K.M.; Coyne, A.N.; Ruan, K.; Grima, J.C.; Bowen, K.E.; Wadhwa, H.; Yang, P.; Rigo, F.; et al. Stress Granule Assembly Disrupts Nucleocytoplasmic Transport. Cell 2018, 173, 958–971.e17. [Google Scholar] [CrossRef] [PubMed]
- Riboldi, G.M.; Faravelli, I.; Rinchetti, P.; Lotti, F. SMN post-translational modifications in spinal muscular atrophy. Front. Cell. Neurosci. 2023, 17, 1092488. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Zhang, Y.; Umoh, M.E.; Vaughan, S.W.; Lorenzini, I.; Liu, F.; Sayegh, M.; Donlin-Asp, P.G.; Chen, Y.H.; Duong, D.M.; et al. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat. Neurosci. 2018, 21, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Woerner, A.C.; Frottin, F.; Hornburg, D.; Feng, L.R.; Meissner, F.; Patra, M.; Tatzelt, J.; Mann, M.; Winklhofer, K.F.; Hartl, F.U.; et al. Cytoplasmic protein aggregates interfere with nucleocytoplasmic transport of protein and RNA. Science 2016, 351, 173–176. [Google Scholar] [CrossRef]
- Zhang, K.; Donnelly, C.J.; Haeusler, A.R.; Grima, J.C.; Machamer, J.B.; Steinwald, P.; Daley, E.L.; Miller, S.J.; Cunningham, K.M.; Vidensky, S.; et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 2015, 525, 56–61. [Google Scholar] [CrossRef]
- Alaei, L.; Ashengroph, M.; Moosavi-Movahedi, A.A. The concept of protein folding/unfolding and its impacts on human health. Adv. Protein Chem. Struct. Biol. 2021, 126, 227–278. [Google Scholar]
- Tang, J.; Kang, Y.; Zhou, Y.; Li, X.; Lan, J.; Wu, L.; Feng, X.; Peng, Y. ALS-causing SOD1 mutants regulate occludin phosphorylation/ubiquitination and endocytic trafficking via the ITCH/Eps15/Rab5 axis. Neurobiol. Dis. 2021, 153, 105315. [Google Scholar] [CrossRef]
- Claes, F.; Rudyak, S.; Laird, A.S.; Louros, N.; Beerten, J.; Debulpaep, M.; Michiels, E.; van der Kant, R.; Van Durme, J.; De Baets, G.; et al. Exposure of a cryptic Hsp70 binding site determines the cytotoxicity of the ALS-associated SOD1-mutant A4V. Protein Eng. Des. Sel. 2019, 32, 443–457. [Google Scholar] [CrossRef]
- Filareti, M.; Luotti, S.; Pasetto, L.; Pignataro, M.; Paolella, K.; Messina, P.; Pupillo, E.; Filosto, M.; Lunetta, C.; Mandrioli, J.; et al. Decreased Levels of Foldase and Chaperone Proteins Are Associated with an Early-Onset Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2017, 10, 99. [Google Scholar] [CrossRef]
- Bharathi, V.; Bajpai, A.; Parappuram, I.T.; Patel, B.K. Elevated constitutive expression of Hsp40 chaperone Sis1 reduces TDP-43 aggregation-induced oxidative stress in Ire1 pathway dependent-manner in yeast TDP-43 proteinopathy model of amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2022, 595, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Rozales, K.; Younis, A.; Saida, N.; Meller, A.; Goldman, H.; Kellerman, L.; Heinrich, R.; Berlin, S.; Shalgi, R. Differential roles for DNAJ isoforms in HTT-polyQ and FUS aggregation modulation revealed by chaperone screens. Nat. Commun. 2022, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Subedi, S.; Sasidharan, S.; Nag, N.; Saudagar, P.; Tripathi, T. Amyloid Cross-Seeding: Mechanism, Implication, and Inhibition. Molecules 2022, 27, 1776. [Google Scholar] [CrossRef] [PubMed]
- Grad, L.I.; Yerbury, J.J.; Turner, B.J.; Guest, W.C.; Pokrishevsky, E.; O’Neill, M.A.; Yanai, A.; Silverman, J.M.; Zeineddine, R.; Corcoran, L.; et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 3620–3625. [Google Scholar] [CrossRef]
- Wang, L.Q.; Ma, Y.; Zhang, M.Y.; Yuan, H.Y.; Li, X.N.; Xia, W.; Zhao, K.; Huang, X.; Chen, J.; Li, D.; et al. Amyloid fibril structures and ferroptosis activation induced by ALS-causing SOD1 mutations. Sci. Adv. 2024, 10, eado8499. [Google Scholar] [CrossRef]
- Laferrière, F.; Maniecka, Z.; Pérez-Berlanga, M.; Hruska-Plochan, M.; Gilhespy, L.; Hock, E.M.; Wagner, U.; Afroz, T.; Boersema, P.J.; Barmettler, G.; et al. TDP-43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates. Nat. Neurosci. 2019, 22, 65–77. [Google Scholar] [CrossRef]
- Nonaka, T.; Masuda-Suzukake, M.; Arai, T.; Hasegawa, Y.; Akatsu, H.; Obi, T.; Yoshida, M.; Murayama, S.; Mann, D.M.; Akiyama, H.; et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013, 4, 124–134. [Google Scholar] [CrossRef]
- Porta, S.; Xu, Y.; Restrepo, C.R.; Kwong, L.K.; Zhang, B.; Brown, H.J.; Lee, E.B.; Trojanowski, J.Q.; Lee, V.M. Patient-derived frontotemporal lobar degeneration brain extracts induce formation and spreading of TDP-43 pathology in vivo. Nat. Commun. 2018, 9, 4220. [Google Scholar] [CrossRef]
- Smethurst, P.; Newcombe, J.; Troakes, C.; Simone, R.; Chen, Y.R.; Patani, R.; Sidle, K. In vitro prion-like behaviour of TDP-43 in ALS. Neurobiol. Dis. 2016, 96, 236–247. [Google Scholar] [CrossRef]
- Feiler, M.S.; Strobel, B.; Freischmidt, A.; Helferich, A.M.; Kappel, J.; Brewer, B.M.; Li, D.; Thal, D.R.; Walther, P.; Ludolph, A.C.; et al. TDP-43 is intercellularly transmitted across axon terminals. J. Cell Biol. 2015, 211, 897–911. [Google Scholar] [CrossRef]
- Bolognesi, B.; Faure, A.J.; Seuma, M.; Schmiedel, J.M.; Tartaglia, G.G.; Lehner, B. The mutational landscape of a prion-like domain. Nat. Commun. 2019, 10, 4162. [Google Scholar] [CrossRef] [PubMed]
- Guenther, E.L.; Cao, Q.; Trinh, H.; Lu, J.; Sawaya, M.R.; Cascio, D.; Boyer, D.R.; Rodriguez, J.A.; Hughes, M.P.; Eisenberg, D.S. Atomic structures of TDP-43 LCD segments and insights into reversible or pathogenic aggregation. Nat. Struct. Mol. Biol. 2018, 25, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Guenther, E.L.; Ge, P.; Trinh, H.; Sawaya, M.R.; Cascio, D.; Boyer, D.R.; Gonen, T.; Zhou, Z.H.; Eisenberg, D.S. Atomic-level evidence for packing and positional amyloid polymorphism by segment from TDP-43 RRM2. Nat. Struct. Mol. Biol. 2018, 25, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Tavella, D.; Zitzewitz, J.A.; Massi, F. Characterization of TDP-43 RRM2 Partially Folded States and Their Significance to ALS Pathogenesis. Biophys. J. 2018, 115, 1673–1680. [Google Scholar] [CrossRef]
- Owen, I.; Rhoads, S.; Yee, D.; Wyne, H.; Gery, K.; Hannula, I.; Sundrum, M.; Shewmaker, F. The prion-like domain of Fused in Sarcoma is phosphorylated by multiple kinases affecting liquid- and solid-phase transitions. Mol. Biol. Cell 2020, 31, 2522–2536. [Google Scholar] [CrossRef]
- Kato, M.; Han, T.W.; Xie, S.; Shi, K.; Du, X.; Wu, L.C.; Mirzaei, H.; Goldsmith, E.J.; Longgood, J.; Pei, J.; et al. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149, 753–767. [Google Scholar] [CrossRef]
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef]
- Cicardi, M.E.; Marrone, L.; Azzouz, M.; Trotti, D. Proteostatic imbalance and protein spreading in amyotrophic lateral sclerosis. EMBO J. 2021, 40, e106389. [Google Scholar] [CrossRef]
- Jinwal, U.K.; O’Leary, J.C., 3rd; Borysov, S.I.; Jones, J.R.; Li, Q.; Koren, J., 3rd; Abisambra, J.F.; Vestal, G.D.; Lawson, L.Y.; Johnson, A.G.; et al. Hsc70 rapidly engages tau after microtubule destabilization. J. Biol. Chem. 2010, 285, 16798–16805. [Google Scholar] [CrossRef]
- Russo, A.; Scardigli, R.; La Regina, F.; Murray, M.E.; Romano, N.; Dickson, D.W.; Wolozin, B.; Cattaneo, A.; Ceci, M. Increased cytoplasmic TDP-43 reduces global protein synthesis by interacting with RACK1 on polyribosomes. Hum. Mol. Genet. 2017, 26, 1407–1418. [Google Scholar] [CrossRef]
- Pinarbasi, E.S.; Cağatay, T.; Fung, H.Y.J.; Li, Y.C.; Chook, Y.M.; Thomas, P.J. Active nuclear import and passive nuclear export are the primary determinants of TDP-43 localization. Sci. Rep. 2018, 8, 7083. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kaneko, K.; Watanabe, S.; Yamanaka, K.; Nukina, N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J. Biol. Chem. 2011, 286, 18664–18672. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Huang, E.J. Mechanisms of FUS mutations in familial amyotrophic lateral sclerosis. Brain Res. 2016, 1647, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Quan, J.; Nowakowski, D.W.; Hancock, M.L.; Shi, J.; Tcherkezian, J.; Young-Pearse, T.L.; Flanagan, J.G. APC is an RNA-binding protein, and its interactome provides a link to neural development and microtubule assembly. Cell 2014, 158, 368–382. [Google Scholar] [CrossRef]
- Wang, S.; Sun, S. Translation dysregulation in neurodegenerative diseases: A focus on ALS. Mol. Neurodegener. 2023, 18, 58. [Google Scholar] [CrossRef]
- Jagaraj, C.J.; Shadfar, S.; Kashani, S.A.; Saravanabavan, S.; Farzana, F.; Atkin, J.D. Molecular hallmarks of ageing in amyotrophic lateral sclerosis. Cell. Mol. Life Sci. 2024, 81, 111. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular rejuvenation: Molecular mechanisms and potential therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 116. [Google Scholar] [CrossRef]
- Xu, S.; Dantuma, N.P. Stress granules: Friend or foe in neurodegenerative disorders? Neural Regen. Res. 2024, 19, 403–404. [Google Scholar] [CrossRef]
- Carey, J.L.; Guo, L. Liquid-Liquid Phase Separation of TDP-43 and FUS in Physiology and Pathology of Neurodegenerative Diseases. Front. Mol. Biosci. 2022, 9, 826719. [Google Scholar] [CrossRef]
- Cao, X.; Jin, X.; Liu, B. The involvement of stress granules in aging and aging-associated diseases. Aging Cell 2020, 19, e13136. [Google Scholar] [CrossRef]
- Nassif, M.; Woehlbier, U.; Manque, P.A. The Enigmatic Role of C9ORF72 in Autophagy. Front. Neurosci. 2017, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Pandey, U.B. Autophagy Dysregulation in ALS: When Protein Aggregates Get Out of Hand. Front. Mol. Neurosci. 2017, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, H.; Murano, T.; Miyakawa, T. The gene expression patterns as surrogate indices of pH in the brain. Front. Psychiatry 2023, 14, 1151480. [Google Scholar] [CrossRef] [PubMed]
- Storozhuk, M.; Cherninskyi, A.; Maximyuk, O.; Isaev, D.; Krishtal, O. Acid-Sensing Ion Channels: Focus on Physiological and Some Pathological Roles in the Brain. Curr. Neuropharmacol. 2021, 19, 1570–1589. [Google Scholar]
- Al Ojaimi, Y.; Slek, C.; Osman, S.; Alarcan, H.; Marouillat, S.; Corcia, P.; Vourc’h, P.; Lanznaster, D.; Blasco, H. The effect of pH alterations on TDP-43 in a cellular model of amyotrophic lateral sclerosis. Biochem. Biophys. Rep. 2024, 38, 101664. [Google Scholar] [CrossRef]
- Bozzoni, V.; Pansarasa, O.; Diamanti, L.; Nosari, G.; Cereda, C.; Ceroni, M. Amyotrophic lateral sclerosis and environmental factors. Funct. Neurol. 2016, 31, 7–19. [Google Scholar] [CrossRef]
- Michaelson, N.; Facciponte, D.; Bradley, W.; Stommel, E. Cytokine expression levels in ALS: A potential link between inflammation and BMAA-triggered protein misfolding. Cytokine Growth Factor Rev. 2017, 37, 81–88. [Google Scholar] [CrossRef]
- Demircan, N.; Ozgur, R.; Turkan, I.; Uzilday, B. Heavy metal toxicity leads to accumulation of insoluble proteins and induces endoplasmic reticulum stress-specific unfolded protein response in Arabidopsis thaliana. Environ. Sci. Pollut. Res. Int. 2024, 31, 53206–53218. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Farace, C.; Fiorito, G.; Pisano, A.; Etzi, F.; Sabalic, A.; Fenu, G.; Asara, Y.; Solinas, G.; Madeddu, R. Human tissue lead (Pb) levels and amyotrophic lateral sclerosis: A systematic review and meta-analysis of case-control studies. Neurol. Sci. 2022, 43, 5851–5859. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Boca, M.; Girotto, S.; Martinelli, M.; Valentine, J.S.; Vieru, M. SOD1 and Amyotrophic Lateral Sclerosis: Mutations and Oligomerization. PLoS ONE 2008, 3, e1677. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Woo, T.G.; Ahn, J.; Lee, D.; Kwon, Y.; Park, B.J.; Ha, N.C. Structural analysis of the overoxidized Cu/Zn-superoxide dismutase in ROS-induced ALS filament formation. Commun. Biol. 2022, 5, 1085. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Kurnik, M.; Danielsson, J.; Oliveberg, M. Fibrillation precursor of superoxide dismutase 1 revealed by gradual tuning of the protein-folding equilibrium. Proc. Natl Acad. Sci. USA 2012, 109, 17868–17873. [Google Scholar] [CrossRef]
- Dang, M.; Wu, L.; Zhang, X. Structural insights and milestones in TDP-43 research: A comprehensive review of its pathological and therapeutic advances. Int. J. Biol. Macromol. 2025, 306 Pt 3, 141677. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Arai, T.; Nonaka, T.; Kametani, F.; Yoshida, M.; Hashizume, Y.; Beach, T.G.; Buratti, E.; Baralle, F.; Morita, M.; et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann. Neurol. 2008, 64, 60–70. [Google Scholar] [CrossRef]
- Lye, Y.S.; Chen, Y.R. TAR DNA-binding protein 43 oligomers in physiology and pathology. IUBMB Life 2022, 74, 794–811. [Google Scholar] [CrossRef]
- Ishiguro, A.; Lu, J.; Ozawa, D.; Nagai, Y.; Ishihama, A. ALS-linked FUS mutations dysregulate G-quadruplex-dependent liquid–liquid phase separation and liquid-to-solid transition. J. Biol. Chem. 2021, 297, 101284. [Google Scholar] [CrossRef]
- Huang, M.; Liu, Y.U.; Yao, X.; Qin, D.; Su, H. Variability in SOD1-associated amyotrophic lateral sclerosis: Geographic patterns, clinical heterogeneity, molecular alterations, and therapeutic implications. Transl. Neurodegener. 2024, 13, 28. [Google Scholar] [CrossRef]
- Tsekrekou, M.; Giannakou, M.; Papanikolopoulou, K.; Skretas, G. Protein aggregation and therapeutic strategies in SOD1-and TDP-43-linked ALS. Front. Mol. Biosci. 2024, 11, 1383453. [Google Scholar] [CrossRef]
- Prasad, A.; Sivalingam, V. The amyloidogenicity of a C-terminal region of TDP-43 implicated in Amyotrophic Lateral Sclerosis can be affected by anions, acetylation, and homodimerization. Biochimie 2018, 150, 76–87. [Google Scholar] [CrossRef]
- Arenas, A.; Chen, J.; Kuang, L.; Barnett, K.R.; Kasarskis, E.J.; Gal, J.; Zhu, H. Lysine Acetylation Regulates the RNA Binding, Subcellular Localization and Inclusion Formation of FUS. Hum. Mol. Genet. 2020, 29, 2684–2697. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Wiedau, M. Therapeutic approaches targeting protein aggregation in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2020, 13, 98. [Google Scholar] [CrossRef] [PubMed]

| Protein | Process Affected | Mechanism | References |
|---|---|---|---|
| SOD1 | Mutations in the gene sequence | Collapse of the homodimeric complex, resulting in aggregation | [88] |
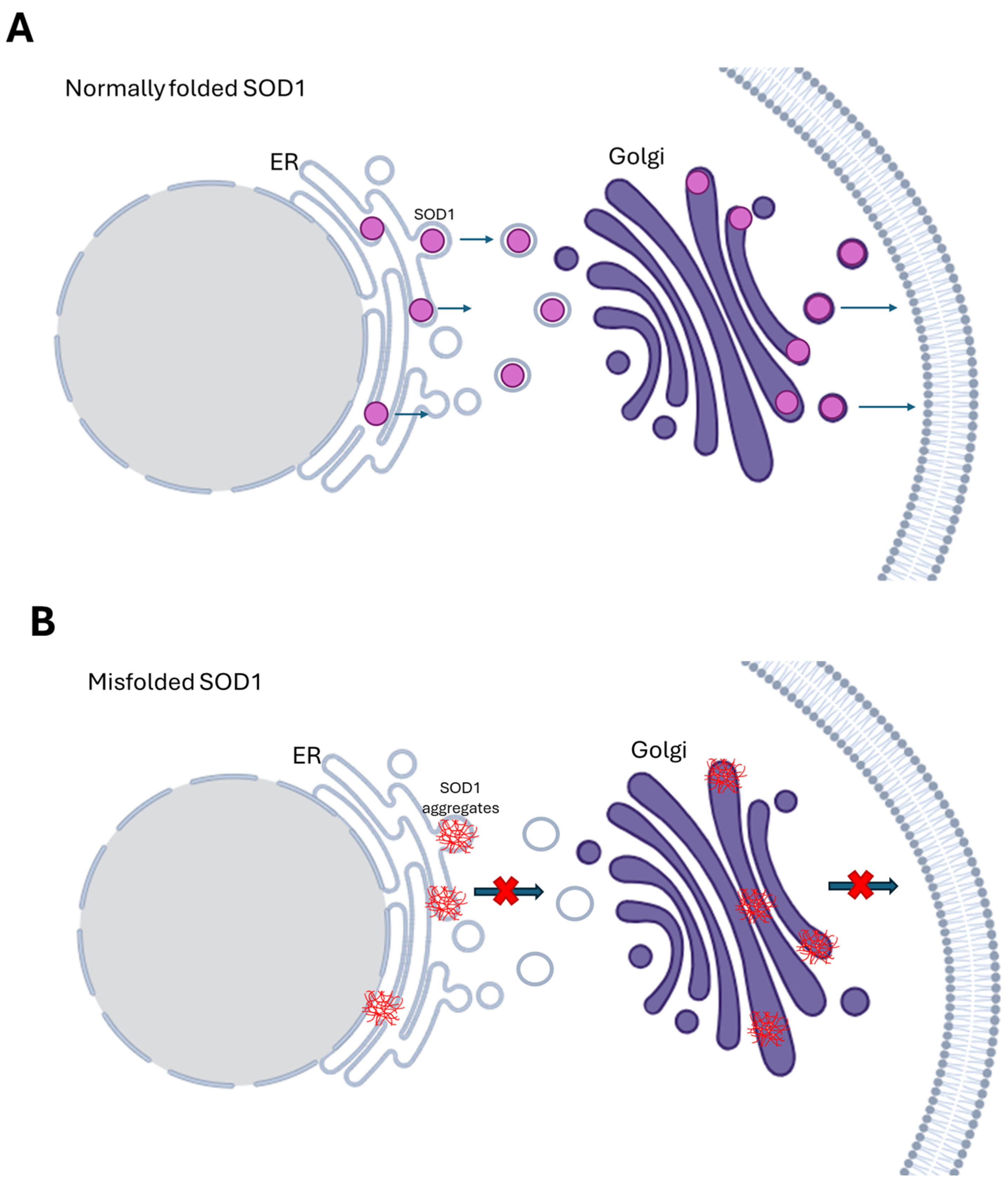
| Errors in the protein trafficking | ALS-associated mutations SOD1A4V, SOD1G85R, and SOD1G93A disrupt the secretory pathway | [20] | |
| SOD1A4V mutation triggers ER stress causing the accumulation of secretory proteins and apoptosis | [20] | ||
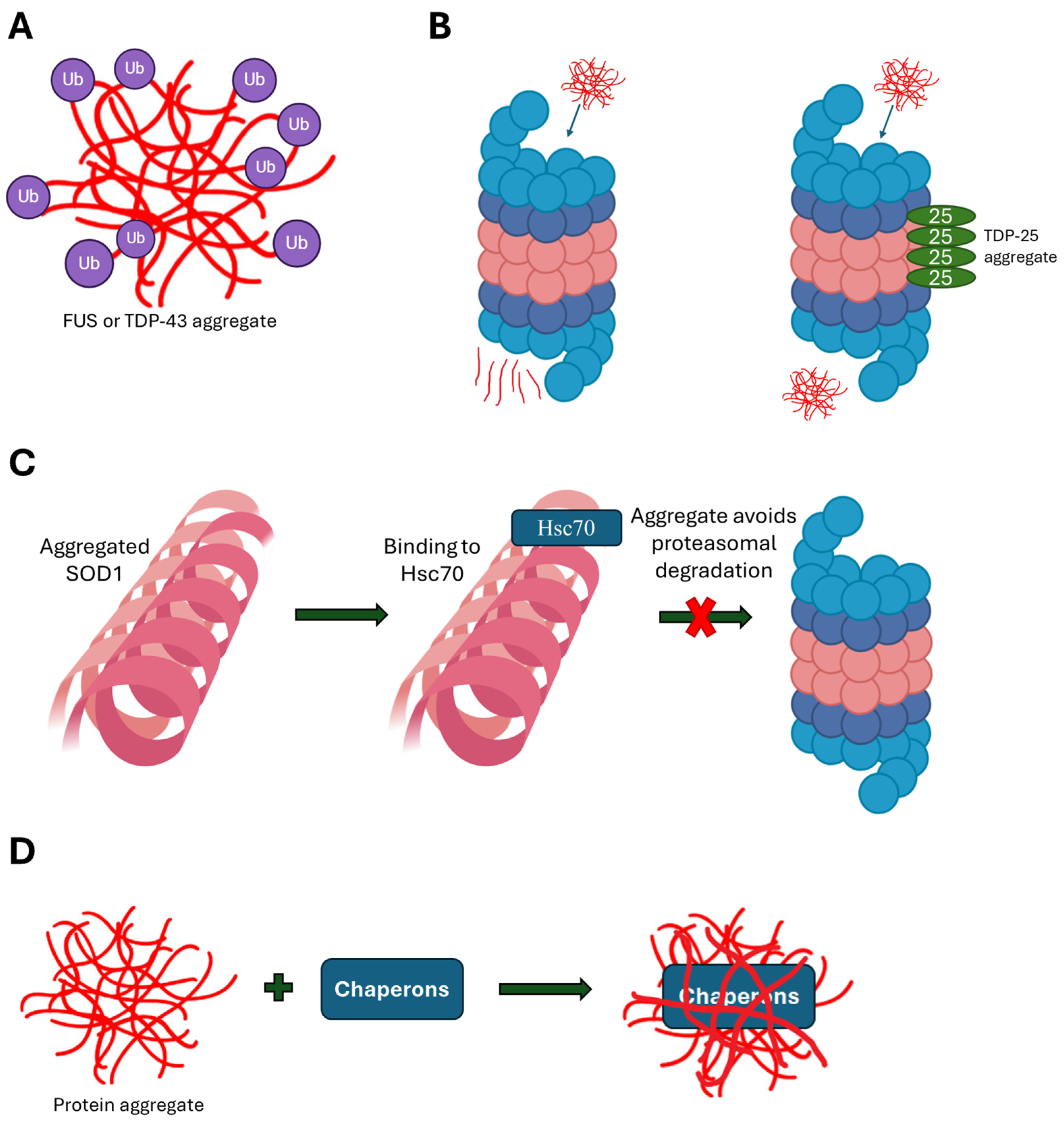
| Dysfunction of the folding and chaperone machinery | SOD1 aggregates incorporate Hsc70, which protects the aggregates from proteasomal degradation | [89] | |
| SOD1 aggregates chaperones, minimizing their availability | [70] | ||
| Seeding and cross-seeding mechanisms | ALS-associated mutant SOD1 causes wild-type SOD1 misfolding | [74] | |
| TDP-43 | Errors in the processes of protein production | TDP-43 binds to the RACK1 of polyribosomes, inhibiting protein synthesis | [90] |
| Axonal TDP-43 condensates inhibit local protein synthesis | [57] | ||
| TDP-43 depletion leads to the upregulation of specific isoforms of hnRNP A1, one of which has been reported to be highly prone to aggregation | [59] | ||
| Errors in the protein trafficking | TDP-43 aggregates in ALS patients incorporate nuclear import and export proteins | [91] | |
| Mutant TDP-43 causes stress granule formation and protein aggregation | [62] | ||
| Mutant TDP-43 disrupts the nuclear membrane and NPC | [64] | ||
| Dysfunction of the folding and chaperone machinery | Mutant TDP-43 tends to aggregate and undergo ubiquitination, leading to a significant reduction in free ubiquitins | [19] | |
| The TDP-43 aggregation-prone prion-like domain binds to proteasome subunits, minimizing their availability | [32] | ||
| TDP-43 aggregates chaperones, minimizing their availability | [70] | ||
| Seeding and cross-seeding mechanisms | TDP-43 aggregates cause the aggregation of other proteins through seeding | [92] | |
| FUS | Mutations in the gene sequence | Mutations affect protein folding, altering the ability of the protein to form solid aggregates | [17] |
| Mutations inhibit the nuclear transport of FUS, resulting in cytoplasmic aggregation | [93] | ||
| Errors in the processes of protein production | FUS inclusions incorporate key translation proteins | [53] | |
| FUS inclusions cause ectopic protein expression | [55] | ||
| FUS inclusions incorporate the APC protein, affecting functions in nerve cells | [94] | ||
| FUS inclusions incorporate the SMN protein that plays a role in protein translation | [46] | ||
| Errors in the protein trafficking | FUS inclusions incorporate the SMN protein that plays a role in mRNA trafficking in the axon | [46] | |
| Mutant FUS causes stress granule formation and protein aggregation | [62] | ||
| Mutant TDP-43 tends to aggregate and undergo ubiquitination, leading to a significant reduction in free ubiquitins | [19] | ||
| Dysfunction of the folding and chaperone machinery | FUS aggregates chaperones, minimizing their availability | [70] | |
| Seeding and cross-seeding mechanisms | The G156E mutation favors the transition from liquid to fibrous solid FUS, and these fibrils seed pure wild-type FUS | [87] | |
| C9ORF72 | Errors in the processes of protein production | Dipeptides produced by C9ORF72 translation inhibit translation by preventing the binding of translation factors to mRNA | [95] |
| Errors in the protein trafficking | Hexanucleotide repeat-containing C9ORF72 RNA species sequester components of the NPC, including RanGAP1 and RNA-binding proteins, disrupting their and other proteins’ nucleocytoplasmic trafficking | [66] | |
| Dysfunction of the folding and chaperone machinery | C9ORF72 aggregates chaperones, minimizing their availability | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivantsik, O.; Exarchos, T.P.; Vrahatis, A.G.; Vlamos, P.; Krokidis, M.G. Exploring Protein Misfolding in Amyotrophic Lateral Sclerosis: Structural and Functional Insights. Biomedicines 2025, 13, 1146. https://doi.org/10.3390/biomedicines13051146
Ivantsik O, Exarchos TP, Vrahatis AG, Vlamos P, Krokidis MG. Exploring Protein Misfolding in Amyotrophic Lateral Sclerosis: Structural and Functional Insights. Biomedicines. 2025; 13(5):1146. https://doi.org/10.3390/biomedicines13051146
Chicago/Turabian StyleIvantsik, Ouliana, Themis P. Exarchos, Aristidis G. Vrahatis, Panagiotis Vlamos, and Marios G. Krokidis. 2025. "Exploring Protein Misfolding in Amyotrophic Lateral Sclerosis: Structural and Functional Insights" Biomedicines 13, no. 5: 1146. https://doi.org/10.3390/biomedicines13051146
APA StyleIvantsik, O., Exarchos, T. P., Vrahatis, A. G., Vlamos, P., & Krokidis, M. G. (2025). Exploring Protein Misfolding in Amyotrophic Lateral Sclerosis: Structural and Functional Insights. Biomedicines, 13(5), 1146. https://doi.org/10.3390/biomedicines13051146










