Abstract
Background/Objectives: Major depressive disorder (MDD) is a major global health concern that is intimately linked to neuroinflammation, oxidative stress, mitochondrial dysfunction, and complicated metabolic abnormalities. Traditional antidepressants frequently fall short, highlighting the urgent need for new, safer, and more acceptable therapeutic techniques. Phytochemicals, i.e., natural antidepressants derived from plants, are emerging as powerful plant-based therapies capable of targeting many pathogenic pathways at the same time. Summary: This narrative review synthesizes evidence from preclinical and clinical studies on the efficacy of phytochemicals such as curcumin, polyphenols, flavonoids, and alkaloids in lowering depressed symptoms. Consistent data show that these substances have neuroprotective, anti-inflammatory, and antioxidant properties, altering neuroimmune interactions, reducing oxidative damage, and improving mitochondrial resilience. Particularly, polyphenols and flavonoids have great therapeutic potential because of their capacity to penetrate the blood–brain barrier, inhibit cytokine activity, and encourage neuroplasticity mediated by brain-derived neurotrophic factor (BDNF). Despite promising results, the heterogeneity in study designs, phytochemical formulations, and patient demographics highlights the importance of thorough, standardized clinical studies. Conclusions: This review identifies phytochemicals as compelling adjuvant or independent therapies in depression treatment, providing multimodal mechanisms and enhanced tolerability. Additional research into improved dosage, pharmacokinetics, long-term safety, and integrative therapy approaches is essential. Using phytotherapeutics could considerably improve holistic and customized depression care, encouraging new research routes in integrative neuroscience and clinical psychiatry.
1. Introduction
Major depressive disorder (MDD) is a common and debilitating mental condition characterized by persistent low mood, anhedonia, cognitive dysfunction, and severe impairments in occupational, social, and interpersonal functioning [1]. Aside from its impact on quality of life, MDD remains the leading psychiatric contributor to global suicide mortality, with core symptoms including diminished self-worth, excessive guilt, psychomotor changes, and sleep disturbances—factors that collectively contribute to increased all-cause mortality rates [2]. Patients with MDD often show persistent negative cognitive biases, social withdrawal, and impaired emotional regulation [3]. Epidemiological data reveal an increasing global burden, presently impacting over 350 million individuals, with World Health Organization forecasts indicating that MDD will emerge as the foremost cause of disability worldwide by 2030 [4,5]. Vulnerable populations, such as pregnant women, the elderly, and children, have disproportionately high incidence rates, emphasizing the role of psychological stressors, genetic predisposition, and environmental adversity in its etiology [6,7,8,9]. Chronic stress, in particular, has been linked to the pathophysiology of MDD because of its ability to disrupt neuroplastic processes, resulting in neuronal atrophy, synaptic loss, and volumetric changes in key brain regions such as the hippocampus and prefrontal cortex, all of which contribute to the disorder’s affective, cognitive, and behavioral symptoms [10,11,12]. Figure 1 summarizes the primary neurobiological mechanisms involved in MDD pathogenesis. The purpose of this state-of-the-art critical narrative review is to integrate disparate mechanistic, pre-clinical, and clinical data into a coherent conceptual model of phytochemical action in depression rather than to catalogue every published study.
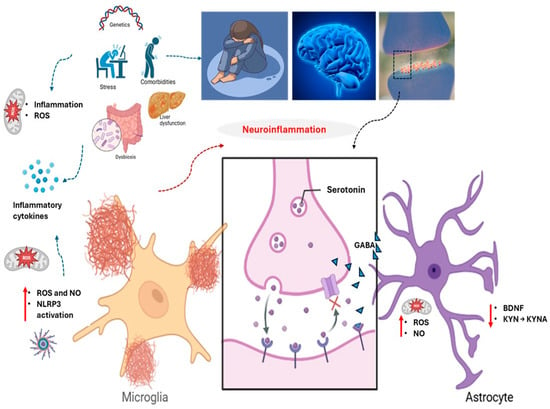
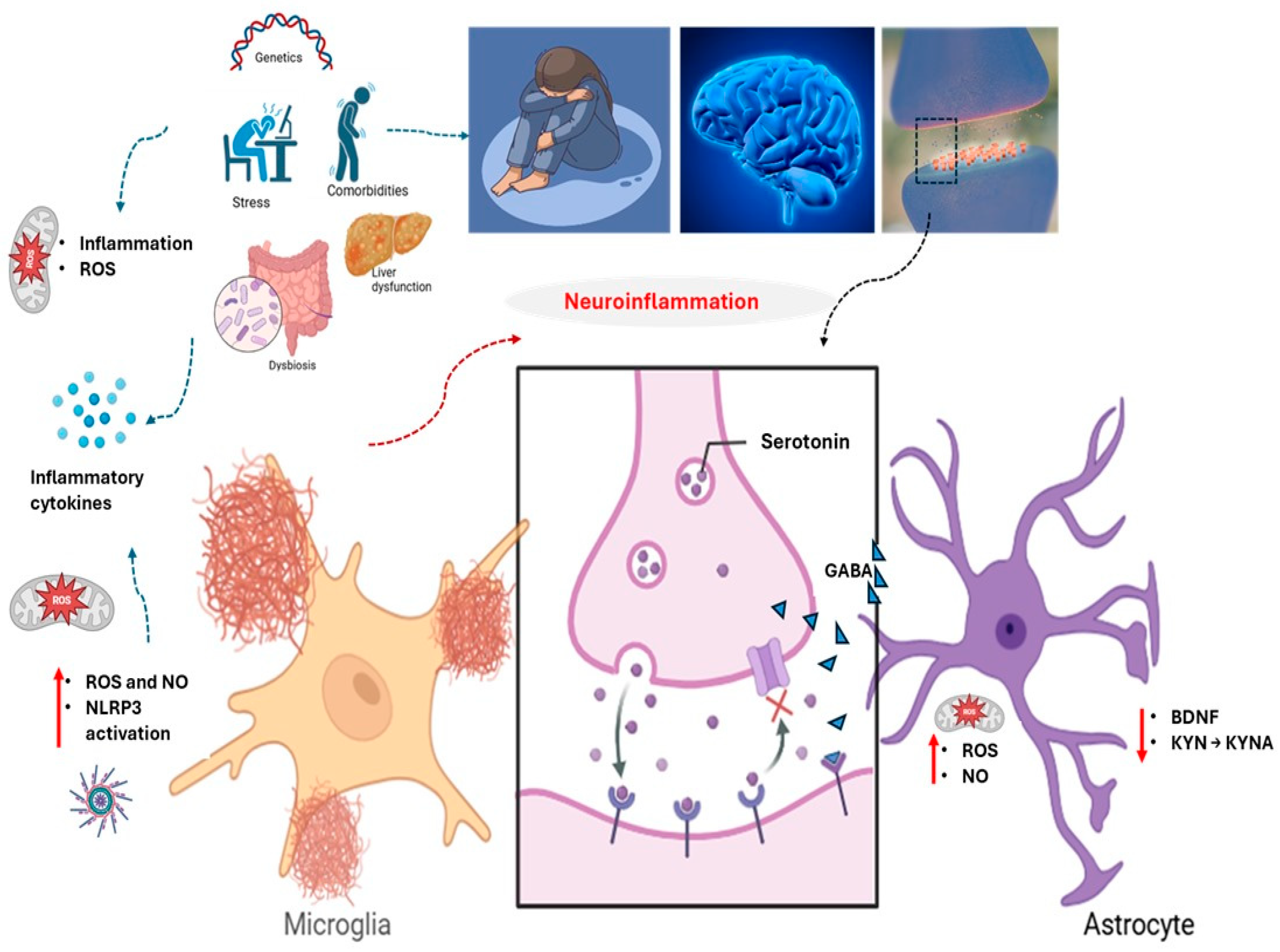
Figure 1.
Neurobiological contributors to the onset and progression of major depressive disorder (MDD). Multiple interacting systems—including genetic predisposition, chronic psychosocial stress, systemic comorbidities, and immune dysregulation—converge to disrupt neuroimmune homeostasis. Central to this pathophysiology are oxidative stress, liver dysfunction, intestinal dysbiosis, and neuroinflammation, driven by activated microglia and astrocytes. These cells increase reactive nitrogen and oxygen species (NO and ROS), impair mitochondrial function, and alter kynurenine (KYN) pathway dynamics, reducing kynurenic acid (KYNA) and promoting neurotoxicity. Dysregulated serotonin and gamma-aminobutyric acid (GABA) signaling, alongside diminished brain-derived neurotrophic factor (BDNF), further impair synaptic plasticity and emotional regulation. ↑: increase; ↓: decrease.
Emerging evidence underscores the critical role of neuroinflammatory processes in the pathogenesis of MDD, positioning central nervous system (CNS) inflammation as a key mechanistic driver in a subset of patients [13]. A growing body of research implicates dysregulation within the kynurenine pathway of tryptophan metabolism—a principal biosynthetic route for serotonin—in contributing to both decreased serotonergic tone and increased production of neurotoxic metabolites [14]. These metabolic changes not only disrupt neurotransmitter homeostasis, but they also increase susceptibility to mood dysregulation and cognitive impairment, which are hallmarks of depressive pathology [15,16,17,18]. Furthermore, metabolomic profiling investigations in MDD patients regularly reveal abnormalities in a variety of circulating metabolites, including tryptophan, tyrosine, methionine, valine, phenylalanine, pyruvate, kynurenic acid, and deoxycholic acid [19]. These metabolic disruptions are closely associated with impaired neuroprotective mechanisms, increased oxidative burden, dysregulated apoptotic signaling, and chronic low-grade inflammation [20,21,22]. Collectively, these pathophysiological alterations foster a neurobiological environment conducive to the initiation and persistence of depressive symptoms, particularly through the destabilization of neuronal resilience and synaptic integrity [23].
While traditional pharmacological treatments, particularly tricyclic antidepressants (TCAs) such as imipramine, amitriptyline, clomipramine, desipramine, and doxepin, remain essential in the treatment of MDD, their therapeutic efficacy is primarily mediated by modulation of monoaminergic neurotransmission, specifically by increasing synaptic concentrations of serotonin and norepinephrine [4]. Despite their clinical value, emerging evidence suggests that while antidepressants are considered first-line therapies, they are not the only effective modality for managing MDD [2]. Notably, in pediatric and adolescent populations, non-pharmacological strategies such as psychological support, scheduled physical activity, and dietary optimization have shown clinically relevant preventative and therapeutic outcomes [24]. Nonetheless, a large proportion of people show partial or complete resistance to these therapies, emphasizing the importance of complementary or alternative therapy approaches [25]. Phytotherapeutics have recently rekindled scientific attention due to their historical use, biological plausibility, cost effectiveness, and acceptable side-effect profiles. Medicinal plants have historically provided bioactive chemicals with neuroprotective, anti-inflammatory, and neuromodulatory effects [26]. These natural compounds persist in guiding the discovery and development of innovative pharmacological medicines, presenting a potentially beneficial adjunctive strategy for persons with treatment-resistant or recurrent depression [27,28].
Phytochemicals are naturally occurring bioactive molecules present in a variety of plants and food sources that influence their pigmentation, fragrance, and flavor profiles [29]. These chemicals, which include alkaloids, flavonoids, steroids, coumarins, and terpenoids like eucalyptol, are increasingly recognized for their therapeutic effects, particularly in situations characterized by oxidative stress and chronic inflammation [30]. Aside from their well-established antioxidant and anti-inflammatory properties, phytochemicals also have vasoprotective and cardiometabolic effects, making them prospective agents in the prevention and treatment of complex, multifactorial illnesses [31]. Phytocompounds have received a lot of attention in neuropsychiatry because of their possible role in altering important neurobiological processes involved in neurodegeneration and mood disorders. Compounds derived from plants such as Aizoaceae, Acorus, Korthalsella, Astragalus membranaceus, Sophora flavescens, and Ononis spinosa have long been employed in traditional medicine systems for their psychotropic and neuromodulatory effects [32,33,34]. Preclinical research and ongoing clinical trials indicate that several of these botanicals have antidepressant-like properties via processes involving monoaminergic regulation, neurotrophic signaling, and neuroinflammatory cascade reduction [35]. As a result, the incorporation of phytocompounds into therapeutic frameworks for MDD is increasingly being investigated both as standalone treatments and as adjuncts to traditional pharmacotherapy [36]. Their ability to address many pathogenic domains at once makes them especially useful in the context of MDD, which has complex and heterogeneous neuronal bases [37].
It is well established that neurodevelopment intricately shapes emotional regulation and cognitive function, both of which are highly susceptible to disruption by psychiatric and neurological disorders [38]. In recent decades, the global burden of neuropsychiatric conditions—particularly those with complex, multifactorial etiologies such as MDD—has escalated significantly, placing substantial pressure on healthcare systems and highlighting the urgent need for innovative, accessible, and well-tolerated therapeutic strategies [39,40,41]. Although conventional antidepressants remain central to current treatment paradigms, their limitations—namely delayed onset of action, treatment resistance, and undesirable side-effect profiles—have driven the search for alternative and adjunctive interventions that can engage broader neurobiological targets with improved safety margins [42,43].
In this regard, phytocompounds have emerged as intriguing candidates due to their pleiotropic modes of action, which include anti-inflammatory, antioxidant, and neuromodulatory capabilities [44]. Recent research has highlighted the intricate connection between phytochemicals, neuroinflammation, and mitochondrial health [22,45]. Yet, no previous analysis has definitively identified the clinical trial landscape assessing the effectiveness of general phytocompounds in MDD despite growing preclinical evidence and sporadic clinical research suggesting their potential usefulness. Notably, recent reviews contend that not all trials have yielded favorable outcomes, reinforcing the need for further research to clarify underlying mechanisms, optimize dosage regimens, assess long-term safety, and determine the comparative effectiveness of medicinal plants in the treatment of depression [46]. Addressing this gap, this comprehensive narrative review seeks to illuminate the diverse roles that phytotherapeutics may play in depression management. We hope these insights will not only guide future investigations on optimal usage and mechanisms of action but also encourage the meaningful integration of plant-based interventions into established, evidence-based psychiatric care.
We compiled an expert-guided evidence map: senior authors snowballed references from seminal phytochemical-depression papers and maintained weekly PubMed, Scopus, and Web of Science keyword alerts (“phytochemical* AND depression OR MDD”) through 28 February 2025. Records were double-screened and retained only when they advanced mechanistic understanding or held translational relevance, deliberately privileging depth over exhaustive coverage. This theory-driven selection underpins the narrative synthesis while avoiding the rigidities of systematic-review protocols. Our theory-driven, expert-guided evidence map inevitably introduces selection bias; some pertinent studies may therefore be absent. This trade-off was accepted to prioritize analytic depth over exhaustive coverage. To improve clarity and avoid redundancy, overlapping descriptions of key mechanisms such as oxidative stress, mitochondrial dysfunction, and neuroinflammation were streamlined. These interconnected processes are herein discussed in focused sections with minimal repetition, ensuring conceptual continuity without diluting analytical depth.
2. Neuroinflammation: A Core Driver of Severe Depression
Emerging data suggest that neuroinflammation plays a critical role in the pathophysiology of MDD, particularly in its more severe and treatment-resistant forms [47]. With immune-mediated processes significantly influencing brain function, inflammation in the CNS is now understood to be a primary cause of depressive illness rather than a supporting actor [48]. This section investigates how neuroinflammatory processes, which are characterized by immunological activation, cytokine dysregulation, and metabolic imbalance, interact with brain areas involved in mood regulation. Novel therapeutic approaches that target these inflammatory pathways, such as phytochemical therapies, are becoming more popular as promising adjuncts in the treatment of depression as knowledge grows.
Neuroinflammation, driven by immune activity in the CNS, is intimately associated with depression through elevated levels of pro-inflammatory cytokines and enhanced microglial activation in the prefrontal cortex, anterior cingulate cortex, and insula [49,50,51,52,53,54]. Among the key cytokines, interleukin (IL)-1β can interfere with neurotransmitter systems and trigger more inflammatory substances, while IL-6 often increases in people with depression, affecting the normal function of the hypothalamic–pituitary–adrenal axis [55,56]. Tumor necrosis factor-α (TNF-α), known for its potent pro-inflammatory role, influences synaptic transmission and neural plasticity [57]. Interferon-gamma (IFN-γ) boosts inflammation in the brain, while IL-2, IL-8, and IL-18 create a harmful inflammatory situation [58,59]. In the prefrontal cortex, microglia adopt a pro-inflammatory phenotype, producing high levels of cytokines that may impair neuronal health [60]. When these cellular sentinels remain on high alert, they release toxic mediators that compromise neuronal functioning and hamper communication between circuits [60]. Similar inflammatory activity in the anterior cingulate cortex affects emotional processing, and overactive microglia in the insula intensify negative emotional states [61]. The hippocampus and amygdala also display maladaptive immune reactivity, undermining neuroplasticity and fueling the progression of depressive symptoms [62].
Approximately 30% of persons with MDD have substantial neuroinflammation in the CNS, which is associated with increased symptom intensity and resistance to standard treatments [47,63]. Neuroinflammation is a key factor in the pathogenesis of MDD, with activation of the NLRP3 inflammasome leading to elevated pro-inflammatory cytokines like IL-1β and IL-18, which are linked to depressive symptoms [64,65,66]. Activation of pattern recognition receptors, such as Toll-like receptor 4 (TLR4) on microglia and astrocytes, can induce NF-κB-driven cytokine production when damage or pathogens are detected [67]. Peripheral immune cells invade the CNS via a weakened blood–brain barrier, exacerbating local inflammation and neurotoxicity [68]. Chronic inflammation exacerbates oxidative and nitrosative stress, damages mitochondria, and enhances glutamate excitotoxicity through reduced astrocytic reuptake, resulting in neuronal injury [69]. Dysfunctional mitochondria produce pro-inflammatory chemicals, which promote inflammation [70]. Chronic stress-induced cortisol elevations activate microglia, whereas gut–brain axis abnormalities allow bacterial endotoxins to worsen systemic inflammation [71]. Last but not least, inflammatory kynurenine pathway activation lowers serotonin synthesis, which feeds a vicious cycle of depression [72].
Inflammatory cascades, which are characterized by sustained activation of cytokines and immune responses, are not limited to depression; they are also found in various chronic infections, neurodegenerative disorders such as multiple sclerosis, and perinatal depression, demonstrating their widespread influence across pathologies [73,74,75,76,77,78,79]. Chronic infections continue to excite immune cells, resulting in elevated amounts of pro-inflammatory cytokines, which contribute to mood and cognitive impairment [80]. Analogously, autoimmune-mediated inflammation is a feature of neurodegenerative diseases like multiple sclerosis, in which protracted immune responses harm brain tissue, aggravating symptoms of depression [75]. In prenatal depression, immunological activation triggered by hormonal and physiological changes greatly increases inflammation, affecting both maternal mood and child outcomes [81]. Furthermore, immunological dysregulation frequently interacts with metabolic changes such as neuroendocrine dysfunction, gut microbiota dysbiosis, and mitochondrial abnormalities, all of which exacerbate depressive states [82,83,84,85]. Neuroendocrine disruptions, such as cortisol imbalance due to hypothalamic–pituitary–adrenal axis dysfunction, exacerbate inflammation and depressive symptoms [86]. Gut dysbiosis promotes systemic inflammation by increasing intestinal permeability and endotoxin exposure, whereas mitochondrial dysfunction impairs cellular energy metabolism and increases oxidative stress [87]. These interconnected inflammatory and metabolic abnormalities highlight the complicated, diverse pathophysiology of depression.
Given the importance of neuroinflammation in the pathogenesis of severe and treatment-resistant depression, identifying effective therapeutic strategies that target inflammation is critical [47,88]. Notably, accumulating evidence suggests that phytochemical therapies, such as polyphenols, may provide neuroprotective advantages by inhibiting inflammatory pathways and lowering neuronal death, making them a promising adjunct method for treating MD [73,74]. Natural substances can protect neurons by modulating inflammation, inhibiting NF-κB activation, and reducing oxidative stress. Furthermore, polyphenols may repair dysregulated neurotransmission and increase mitochondrial function, addressing the basic metabolic abnormalities seen in depressed states [89]. Because neuroinflammation is so tightly linked to metabolic dysfunction, immunological dysregulation, and neuronal degeneration, therapies that target numerous inflammatory and apoptotic pathways at the same time may produce better therapeutic results [90]. Future research should emphasize investigating the entire therapeutic potential of phytochemicals and comparable molecules as supplemental treatments, which could improve outcomes for people suffering from severe, inflammation-related depression.
3. Oxidative Stress: A Silent Driver of Depression
Amid the complex web of MDD pathology, oxidative stress has emerged as a subtle yet powerful contributor deserving closer scrutiny [90]. Characterized by an imbalance between reactive oxygen species (ROS) and antioxidant defenses, oxidative stress quietly undermines neuronal integrity and brain function [91]. Intriguingly, mounting evidence suggests that this biochemical imbalance not only parallels depressive symptoms but may actively drive their persistence and severity [92]. From antioxidant depletion to mitochondrial dysfunction, oxidative stress orchestrates a cascade of cellular disruptions [93]. In this section, we explore the biochemical undercurrents of oxidative stress and its far-reaching implications in the development and progression of depression.
Oxidative stress occurs when ROS and other free radicals exceed the body’s capacity to neutralize them through antioxidant defenses, leading to cellular harm via the degradation of essential biomolecules such as proteins, lipids, and nucleic acids [94,95]. In individuals with depression, this imbalance is particularly pronounced due to significantly reduced levels of key antioxidants, including zinc, vitamin E, and coenzyme Q10, amplifying cellular vulnerability and intensifying neurodegenerative processes [91,96]. These antioxidant deficits facilitate oxidative damage to neuronal membranes, impair mitochondrial function, and disrupt neurotransmission, further exacerbating depressive symptoms [95]. Zinc deficiency, for example, diminishes neuroplasticity and impairs glutamatergic signaling, whereas insufficient vitamin E reduces neuronal protection against lipid peroxidation [97]. Likewise, low levels of coenzyme Q10 impair mitochondrial respiration, increasing ROS production and energy deficits in neurons [98]. Collectively, this heightened oxidative state promotes neuronal apoptosis and inflammation, reinforcing a destructive cycle that sustains and deepens depressive pathology [89]. Thus, addressing oxidative imbalances through antioxidant supplementation or dietary interventions could potentially mitigate cellular damage and improve therapeutic outcomes in MDD.
Alterations in tryptophan metabolism significantly contribute to oxidative stress in MDD, primarily by shifting this amino acid toward the kynurenine pathway rather than serotonin synthesis. When inflammation activates enzymes such as indoleamine 2,3-dioxygenase (IDO), tryptophan is increasingly converted into kynurenine metabolites, which not only reduce serotonin availability but also generate neurotoxic byproducts like quinolinic acid [19,99,100]. These toxic intermediates promote neuronal damage through enhanced generation of ROS, exacerbating oxidative stress and accelerating cellular injury within vulnerable brain regions [91]. Furthermore, reduced tryptophan availability itself can intensify the formation of free radicals, damaging essential biomolecules including deoxyribonucleic acid (DNA), lipids, and proteins [93]. This cascade creates a destructive cycle of oxidative damage, inflammation, and impaired neurotransmission, significantly correlating with increased severity of depressive symptoms [91]. The resulting neuronal dysfunction, loss of synaptic plasticity, and impaired mitochondrial performance contribute to the pathophysiology of depression [101]. Therapeutic interventions targeting the kynurenine pathway or enhancing tryptophan availability thus hold considerable potential for reducing oxidative damage and alleviating depressive symptoms.
Mitochondrial dysfunction—including structural alterations and disrupted energy metabolism—significantly escalates ROS production, directly promoting neuroinflammation and cognitive decline in depressive disorders [102,103,104,105]. Accumulation of dysfunctional mitochondria further exacerbates neuronal damage, perpetuating chronic inflammatory cycles within the brain [106,107]. Specifically, impaired mitophagy hampers the removal of damaged mitochondria, allowing toxic debris to accumulate and activate inflammatory complexes, notably the NLRP3 inflammasome, leading to increased pro-inflammatory cytokine production [108]. Disrupted mitochondrial calcium buffering elevates intracellular calcium, triggering oxidative damage, microglial activation, and amplified inflammatory signaling [109]. Moreover, mitochondrial energy deficits dysregulate AMP-activated protein kinase (AMPK) signaling, causing aberrant mammalian target of rapamycin (mTOR) activation that sustains inflammatory responses [110]. Increased ROS production triggers transcription factors such as NF-κB, leading to ongoing release of inflammatory cytokines (IL-6 and TNF-α) [111]. Reduced mitochondrial health also lowers NAD+ levels, which weakens protective sirtuin signaling, while imbalanced iron levels encourage ferroptosis, worsening oxidative damage [90,112]. Consequently, impaired neurogenesis and reduced synaptic plasticity intensify cognitive deficits, reinforcing a destructive feedback loop of mitochondrial-driven inflammation in depression [107].
Obesity-induced inflammation significantly heightens oxidative stress by increasing adipokine secretion—such as IL-6, IL-1, and tumor necrosis factor-alpha—while simultaneously impairing insulin regulation, thus elevating risks for type 2 diabetes and depression [113,114,115]. Chronic obesity also fosters leptin resistance, amplifying microglial activation and further promoting inflammatory cascades within the brain [116]. Moreover, lifestyle factors, particularly high-fat diets and sedentary behaviors, escalate ROS production through enhanced mitochondrial β-oxidation and activation of nuclear factor kappa B (NF-κB) signaling, reinforcing inflammation and neuronal stress pathways [117,118,119,120,121]. Dietary saturated fats directly stimulate inflammasome complexes like NLRP3, causing excessive IL-1β release and perpetuating neuroinflammation [122]. Additionally, obesity-related hyperglycemia accelerates formation of advanced glycation end-products (AGEs), activating inflammatory responses via AGE receptors on microglia [123]. High-fat diets exacerbate gut microbiota disruption, increasing endotoxin leakage into circulation and systemic inflammation while simultaneously lowering BDNF levels, impairing neuronal resilience [124]. These interconnected metabolic, inflammatory, and behavioral mechanisms underscore the critical role of dietary antioxidants and regular physical exercise in mitigating inflammation-induced depressive pathology.
Oxidative stress stands as a potent yet often overlooked force driving the progression and persistence of MDD [95]. Rather than being a mere consequence of other pathological changes, it plays an active role in linking mitochondrial dysfunction, immune activation, metabolic disturbances, and adverse lifestyle factors [125]. Through sustained production of ROS and insufficient antioxidant defenses, oxidative stress damages cellular structures, fuels neuroinflammation, and disrupts key neural circuits involved in mood regulation [95]. Its impact extends from molecular disruption to large-scale cognitive decline and emotional dysregulation [126]. Addressing this underlying imbalance—via antioxidant therapies, metabolic support, and targeted lifestyle changes—offers a promising direction for enhancing treatment response and improving long-term outcomes in individuals with depression.
4. Depression’s Mitochondrial Roots and Repair Strategies
Far beyond their role as mere energy suppliers, mitochondria are now recognized as pivotal regulators of brain health and emotional resilience [127]. In the context of MDD, mitochondrial dysfunction has garnered growing attention as a hidden yet potent driver of neurobiological disruption [112]. From impaired energy metabolism to excessive ROS production, mitochondrial abnormalities appear to fuel the progression of depressive symptoms [89]. This section delves into the emerging landscape of mitochondrial pathology in depression—alongside innovative repair strategies—highlighting a compelling shift in how we understand and potentially treat this multifaceted disorder.
Mitochondria also play a pivotal role in regulating neuroinflammation, positioning them as key contributors to both the onset and persistence of MDD [85]. In individuals with MDD, mitochondrial abnormalities—such as structural degradation, disrupted mitochondrial DNA (mtDNA), and impaired electron transport chain (ETC) function—lead to excessive production of ROS, destabilizing neurotransmitter systems and exacerbating depressive symptoms [103,128,129,130]. These disturbances are intensified by additional mitochondrial impairments [131]. Oxidative damage to mtDNA impairs the synthesis of ETC components, while dysregulated biogenesis reduces the formation of healthy mitochondria [132]. Imbalances in fusion and fission dynamics further fragment the mitochondrial network, diminishing metabolic capacity and increasing susceptibility to cell death [133]. Chronic stress-related calcium overload, inhibition of ETC complexes, and glucocorticoid-induced suppression of mitochondrial maintenance genes all contribute to dysfunction [134]. Moreover, a disrupted NAD+/NADH ratio and persistent exposure to pro-inflammatory cytokines impair mitochondrial respiration, DNA repair, and anti-inflammatory signaling [135]. Together, these interconnected mechanisms establish a vicious cycle of mitochondrial breakdown, oxidative stress, and neuroinflammation that drives the neurobiological deterioration seen in MDD.
While psychosocial stressors undoubtedly contribute to the onset of MDD, a growing body of evidence highlights the role of biochemical markers—particularly those tied to mitochondrial health—in its pathophysiology [102]. One such marker, methylmalonic acid (MMA), is elevated in cases of vitamin B12 deficiency and has been shown to impair succinate dehydrogenase activity, thereby increasing ROS production and promoting neuronal injury [136,137,138,139,140]. Additional biomarkers further implicate mitochondrial dysfunction in MDD. Elevated homocysteine, another consequence of B-vitamin deficiency, induces oxidative stress and compromises mitochondrial DNA repair [141]. Increased brain lactate and pyruvate levels suggest a shift from oxidative phosphorylation to inefficient anaerobic metabolism [142]. Accumulation of acylcarnitines reflects disrupted fatty acid oxidation, while elevated ammonia impairs mitochondrial respiration and contributes to neurotoxicity [143]. Depletion of glutathione (GSH), the mitochondria’s frontline antioxidant, leaves cells vulnerable to ROS, and heightened levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG) indicate mtDNA damage [144]. Deficiencies in coenzyme Q10 and imbalances in the NAD+/NADH ratio further hinder ATP production and stress resilience [145]. Together, these markers underscore the biochemical complexity and mitochondrial vulnerability underlying depressive disorders. These observations resonate with broader neurodegenerative research frameworks, which emphasize the integration of imaging biomarkers, inflammatory profiles, and metabolic signatures to characterize disease subtypes and guide personalized intervention strategies [146].
Recent studies underscore several promising therapeutic strategies aimed at correcting mitochondrial dysfunction in MDD [147]. Among them, mitochondrial transplantation—either by replacing damaged mitochondria or introducing healthy mitochondrial DNA and related proteins—has emerged as a novel approach to restoring cellular energy balance and reversing neuronal damage [148]. Similarly, inhibiting the renin–angiotensin system with angiotensin receptor blockers has shown potential in reducing neuroinflammation and oxidative stress, both key drivers of depressive pathology [149,150]. Beyond these, mitochondria-targeted antioxidants like MitoQ and SkQ1 directly neutralize ROS within mitochondria, improving membrane potential and behavioral outcomes [151]. Supplementation with NAD+ precursors (e.g., nicotinamide riboside) enhances mitochondrial biogenesis and DNA repair via sirtuin activation [152]. Coenzyme Q10 boosts ATP production, while PGC-1α activators such as resveratrol and AMPK activators promote mitochondrial renewal [153,154]. Other emerging strategies include inhibition of the mitochondrial permeability transition pore (mPTP), ketogenic diets to optimize mitochondrial fuel use, sirtuin activators to dampen inflammation, and L-carnitine derivatives that support fatty acid oxidation [155,156,157,158]. Gene therapies targeting mtDNA mutations offer future avenues for correcting mitochondrial defects at their source [159].
Ultimately, restoring mitochondrial health offers a compelling path forward in the treatment of MDD. By integrating phytochemicals and other multimodal strategies—ranging from targeted antioxidants to metabolic and genetic interventions—it may be possible to reduce oxidative damage, enhance energy metabolism, and reinforce neuronal resilience [102]. These approaches hold promise not only for symptom relief but also for addressing the cellular dysfunctions at the core of depression’s pathology. Importantly, mitochondrial fragility does not occur in isolation. Caloric excess, insulin resistance, and adipose-derived cytokines impose a chronic oxidative–inflammatory load that further erodes mitochondrial ATP output and calcium buffering in limbic neurons. In turn, energy-starved mitochondria dysregulate appetite and glucose homeostasis via hypothalamic circuits, creating a vicious cycle in which metabolic inflammation and mitochondrial dysfunction co-amplify depressive pathology. This bidirectional loop sets the stage for examining obesity-linked inflammation in greater detail.
5. Nutrition, Inflammation, and Depressive Spirals
While nutrition and lifestyle factors clearly shape metabolic risk and modulate inflammation relevant to MDD, a detailed discussion lies beyond the core focus of this review. We briefly highlight key findings to underscore how diet quality, physical activity, and sleep patterns influence neuroimmune health. These modifiable behaviors may complement pharmacological and phytochemical strategies, but a full exploration of their therapeutic applications warrants separate, dedicated analysis. Nutrition lies at the crossroads of physical and mental health, yet its role in MDD is often underestimated [160]. Increasingly, research points to a vicious cycle in which poor dietary habits not only reflect depressive states but actively intensify them through metabolic disruption and chronic inflammation [161]. As nutrient deficiencies and unhealthy food choices fuel neurobiological stress, depressive symptoms may deepen, further eroding motivation for self-care [162]. This section explores how diet, lifestyle, and inflammation intertwine in self-perpetuating spirals—offering both a cautionary tale and a hopeful lens on how strategic nutritional interventions may help break the cycle.
Recent lines of evidence highlight that dietary behaviors, modulated by psychological factors, significantly shape obesity risk and depressive symptoms by influencing nutrient availability and metabolic function [163,164,165]. In particular, inadequate consumption of vitamin B12, zinc, magnesium, folic acid, and vitamin B6 has been associated with elevated susceptibility to MDD [15]. Moreover, diets dominated by high-fat convenience foods often coincide with lower intake of fruits, vegetables, lean proteins, and whole grains, fueling systemic inflammation and oxidative stress linked to depression pathogenesis [166,167,168]. These findings underscore the multifaceted interplay among dietary patterns, nutritional deficiencies, and mood disorders, suggesting that suboptimal eating habits may potentiate neurobiological pathways underlying depression [169]. By integrating these insights into clinical practice, researchers and clinicians can more effectively target modifiable lifestyle factors in conjunction with pharmacotherapy or psychotherapy [170]. Ultimately, developing evidence-based nutritional interventions, particularly those emphasizing nutrient-rich whole foods, could mitigate depressive symptom severity while reducing broader metabolic risk [171]. This comprehensive approach holds promise for reducing disease burden and improving patient outcomes in MDD.
Obesity is increasingly recognized as a critical comorbidity in depression, given that excess adiposity promotes a pro-inflammatory milieu and metabolic dysregulation, potentially exacerbating psychiatric symptomatology [83,84]. In parallel, emerging data indicate that individuals with severe depressive disorders often exhibit behaviors that intensify these physiological burdens, such as smoking, physical inactivity, and insufficient sleep [172,173]. These lifestyle factors further elevate systemic inflammation and oxidative stress, reinforcing a feedback loop that intensifies both mood disturbances and metabolic risk [47]. Notably, recent studies underscore that even modest reductions in body mass index or targeted interventions to improve sleep quality can substantially attenuate neuroinflammatory processes linked to depression [174]. By highlighting these interdependent mechanisms, the present review affirms the need for comprehensive management approaches that address not only psychological distress but also modifiable health behaviors [170]. Tailored interventions that incorporate nutritional counseling, physical activity regimens, and sleep hygiene may thus hold therapeutic promise, mitigating the dual burden of metabolic and mental health complications [175,176]. Advancing this integrative perspective could ultimately refine treatment algorithms and inform future research directions in translational psychiatric care.
Emerging data consistently underscore the potential for lifestyle modifications to alleviate mood-related symptomatology and address comorbid metabolic disorders in individuals with depression [170]. Notably, adopting healthier diets characterized by nutrient-dense foods, minimized refined sugars, and balanced macronutrient intake appears to diminish systemic inflammation and improve metabolic regulation [177]. When combined with regular physical activity, such dietary patterns may enhance neuroplasticity through increased neurotrophic factor release, supporting neuronal resilience [178]. Stress management techniques, including mindfulness-based practices and cognitive–behavioral strategies, further mitigate hypothalamic–pituitary–adrenal axis dysregulation, thereby moderating pro-inflammatory responses and bolstering mental health [165,179,180]. Although these findings remain preliminary, they collectively suggest that targeted lifestyle programs can serve as essential components of comprehensive treatment plans [181]. Moreover, recent randomized controlled trials affirm that multifaceted approaches integrating nutritional counseling, behavioral interventions, and exercise regimens yield meaningful improvements in depressive symptoms and metabolic risk profiles [182]. This perspective highlights the need to broaden therapeutic frameworks beyond pharmacological paradigms to address the complex interplay among diet, behavior, and psychological well-being in depression [183].
Collectively, these findings illuminate how poor dietary habits can instigate a vicious cycle in which chronic inflammation exacerbates depressive pathology, further eroding motivation for healthy behavior [161]. By perpetuating neurobiological stress, this spiral intensifies symptom severity and increases metabolic risk, reinforcing suboptimal eating patterns [161]. Adopting balanced, nutrient-rich diets, regular physical activity, and evidence-based stress management may disrupt this harmful feedback loop, thereby enhancing neurotransmitter balance, mitigating systemic inflammation, and restoring metabolic homeostasis [170]. Although rigorous trials remain necessary to pinpoint optimal protocols, strategic nutritional interventions hold considerable promise for curtailing the depressive spiral and advancing patient-centered treatment paradigms in MDD [184].
6. Rethinking Depression Care: Drugs and Natural Options
As our understanding of MDD deepens, so too does the landscape of its treatment—now evolving beyond traditional pharmacotherapy toward more integrative, personalized care [185]. While established medications like SSRIs and SNRIs remain central, their side-effect profiles and limitations prompt a reexamination of what comprehensive care can look like [186]. Intriguingly, nature itself may offer untapped solutions [187]. From time-honored botanicals to novel phytochemicals, natural compounds are gaining recognition as adjunctive or alternative treatments with promising efficacy and improved tolerability [188]. This section explores the growing synergy between conventional drugs and natural options in the quest for more holistic depression care.
Pharmacological and non-pharmacological therapies for MDD are increasingly being delivered through multidisciplinary care teams, reflecting a shift toward more holistic, patient-centered treatment models [189,190]. These teams typically include psychiatrists—who oversee diagnosis, medication management, and complex case coordination—and clinical psychologists or psychotherapists, who deliver evidence-based therapies such as CBT, IPT, and mindfulness-based interventions [191,192]. Nutritionists or dietitians also play a key role by addressing nutritional deficiencies and promoting anti-inflammatory dietary patterns that support mitochondrial and mental health [191,193]. Exercise physiologists or physical therapists prescribe tailored physical activity regimens to reduce depressive symptoms and improve neurobiological function [191,192]. Primary care physicians manage medical comorbidities that often worsen mood disorders, while pharmacists ensure medication safety and adherence [194]. Complementary and integrative health practitioners may introduce acupuncture, yoga, or nutraceutical support to reduce oxidative stress [195]. Social workers and case managers address psychosocial barriers and improve continuity of care, and peer support specialists offer lived-experience insights that build trust and engagement [196]. This interdisciplinary approach is especially effective in treatment-resistant or complex cases, offering a comprehensive strategy that integrates biological, psychological, and social dimensions of depression [197,198,199].
Among pharmacological options for MDD, selective serotonin reuptake inhibitors (SSRIs) remain the preferred first-line treatment due to their efficacy and relative tolerability. However, alternative classes such as serotonin–norepinephrine reuptake inhibitors (SNRIs), TCAs, monoamine oxidase inhibitors (MAOIs), and N-methyl-D-aspartate (NMDA) antagonists provide additional therapeutic pathways, especially in treatment-resistant cases [10,200,201,202]. Despite their benefits, these agents are associated with a range of adverse effects [203]. SSRIs can cause gastrointestinal disturbances, insomnia, sexual dysfunction, and in some cases, QTc prolongation—particularly with citalopram [204]. SNRIs like venlafaxine and duloxetine may induce hypertension, hepatotoxicity, and withdrawal symptoms [205]. TCAs carry anticholinergic burdens (e.g., dry mouth, constipation) and significant cardiovascular risks, including arrhythmias [206]. MAOIs, though effective, are limited by dietary restrictions and the risk of hypertensive crises or serotonin syndrome [207]. Atypical antidepressants such as bupropion and mirtazapine offer varied mechanisms but introduce risks like insomnia, weight gain, or seizure potential [208]. Novel agents, including NMDA antagonists like esketamine, present dissociative and cardiovascular side effects [209]. Given these diverse profiles, careful, individualized treatment planning is essential [208,210,211,212,213]. The limitations of conventional antidepressants, such as the risk of hyponatremia in older adults and individuals taking diuretics, underscore the need for safer and more tolerable treatment options [214,215].
These safety concerns have prompted increasing interest in alternative and complementary strategies that can enhance efficacy while reducing adverse effects [216]. Herbal medicines are gaining recognition as potential adjuncts in the treatment of MDD [217]. These natural compounds may exert neuroprotective, anti-inflammatory, and mood-regulating effects while offering better tolerability and fewer side effects compared to standard medications [218,219,220]. By improving safety profiles and supporting long-term adherence, herbal therapies may help address some of the limitations associated with conventional pharmacological approaches [221]. Their integration into clinical practice could also support a more holistic and individualized model of care [222]. As research continues to explore these compounds’ mechanisms and clinical potential, combining pharmacological and natural interventions may represent a promising path toward more comprehensive and patient-centered depression management.
7. Plant-Based Therapies: Natural Allies Against Depression
As science turns its gaze toward nature’s pharmacopoeia, plant-based therapies are emerging as exciting frontiers in the treatment of MDD [223]. Rich in bioactive compounds, plants offer a diverse array of phytochemicals capable of modulating the same neurobiological pathways targeted by conventional antidepressants—often with fewer side effects [224]. From polyphenols and flavonoids to carotenoids and alkaloids, these natural agents show potential to combat oxidative stress, inflammation, and neurodegeneration [225]. This section delves into the growing body of evidence supporting phytotherapies as integrative, multifaceted allies in the fight against depression—opening the door to greener, gentler innovations in mental health care.
Phytochemicals, as bioactive compounds naturally derived from plants, have attracted increasing interest for their ability to modulate key neurobiological pathways involved in MDD [37,226]. Among them, polyphenols are particularly noteworthy for their ability to cross the blood–brain barrier and exert neuroprotective, antioxidant, and anti-inflammatory effects, thereby attenuating cytokine activity and reducing neuronal apoptosis [74,227]. Within this class, flavonoids such as luteolin effectively scavenge free radicals and suppress neuroinflammation [227,228,229], while carotenoids offer similar antidepressant benefits through antioxidant and anti-inflammatory mechanisms [230,231,232,233]. These effects align with the growing evidence that enhancing BDNF signaling improves synaptic plasticity and emotional regulation. Compounds like curcumin and resveratrol have demonstrated the ability to upregulate BDNF in animal models, contributing to mood stabilization and resilience [234,235,236]. Additional polyphenols—including quercetin, EGCG, fisetin, apigenin, and baicalein—further reinforce these effects by modulating inflammatory signaling, reducing oxidative stress, and promoting mitochondrial function [237,238,239,240]. Collectively, these natural agents represent promising adjunctive therapies for improving clinical outcomes in MDD through multimodal neuroprotective actions.
Additional phytochemicals found in medicinal plants such as Panax ginseng, Mitragyna speciosa, Astragalus membranaceus, and species within the Acorus genus have shown antidepressant-like effects primarily through their anti-inflammatory and antioxidant properties [32,34,241,242,243]. These effects are echoed in other plant-derived compounds that act on convergent molecular pathways [130]. Withanolides from Withania somnifera modulate the HPA axis, reduce pro-inflammatory cytokines like IL-6 and TNF-α, and elevate BDNF levels, enhancing stress resilience [244]. Hericenones and erinacines from Hericium erinaceus stimulate nerve growth factor synthesis, reduce oxidative stress, and improve monoaminergic balance [245]. Ginsenosides from Panax ginseng and bacopasides from Bacopa monnieri both boost mitochondrial efficiency, suppress neuroinflammation, and promote neuroplasticity [130,246]. Other promising agents include honokiol from Magnolia officinalis, paeoniflorin from Paeonia lactiflora, and berberine from Berberis species, all of which influence oxidative, inflammatory, and neurotransmitter systems [247,248,249]. Rhynchophylline and salidroside further contribute to this phytochemical network by protecting neurons and regulating mood circuits [250]. Collectively, these compounds offer diverse mechanisms for mitigating the multifaceted pathways underlying mood disorders by reducing inflammation, alleviating oxidative stress, fine-tuning neurotransmitter balance, and fostering neuroplasticity, thereby highlighting their integrative therapeutic potential.
Although plant-based interventions often exhibit fewer side effects than conventional antidepressants, their clinical application still requires deeper investigation to refine optimal dosing, clarify underlying mechanisms, and ensure long-term safety [251,252]. Nonetheless, mounting evidence supports the potential of phytotherapeutics as effective, integrative strategies for addressing the complex and multifactorial nature of MDD [1]. By targeting neuroinflammation, oxidative stress, mitochondrial dysfunction, and neurotransmitter imbalances, these compounds offer a multi-pronged approach to mood regulation [253]. Their ability to enhance neurotrophic factors such as BDNF further strengthens their therapeutic value [254]. As the field of psychoneuropharmacology evolves, incorporating plant-derived compounds into treatment frameworks may improve outcomes for patients who are unresponsive or intolerant to traditional therapies [255]. Collectively, these findings highlight the critical need for continued clinical research on phytomedicines in mood disorder management [256]. Figure 2 illustrates select phytochemicals and their modulatory effects on brain metabolism and neural function, underscoring their relevance as natural allies in depression care.
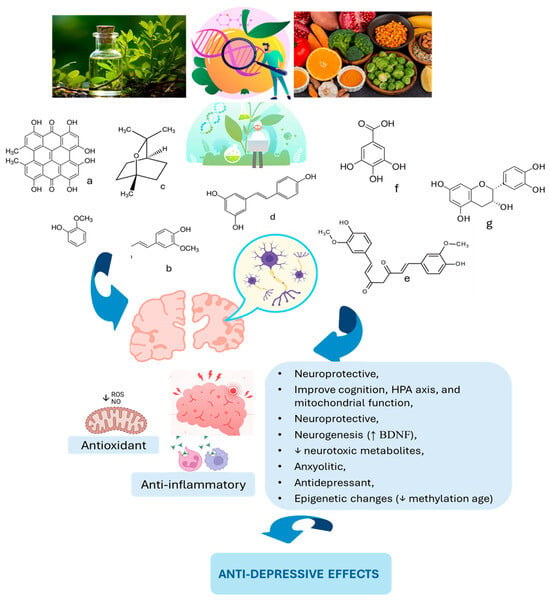
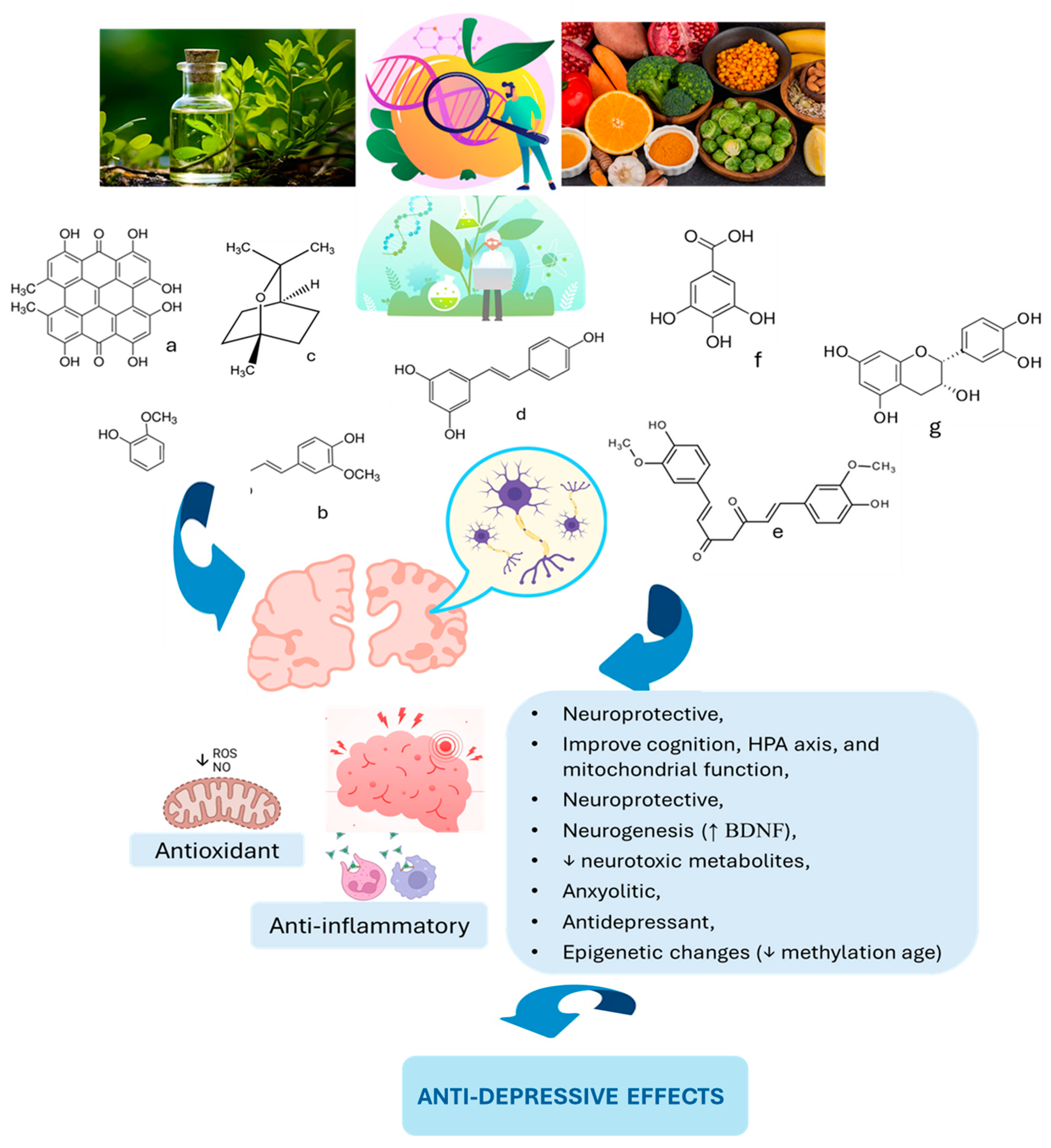
Figure 2.
Phytochemicals and the role in brain health and reduction in depression. HPA: hypothalamic–pituitary–adrenal; BDNF: brain-derived neurotrophic factor; a: hypericin; b: coumarin; c: eucalyptol; d: resveratrol; e: curcumin; f: gallic acid; g: epicatechin. ↑: increase; ↓: decrease.
8. From Curcumin to Cocoa: Plant-Based Mood Solutions
The following interventional studies reveal a promising though heterogeneous body of evidence regarding the therapeutic potential of phytocompounds in MDD. These trials examined a wide range of phytochemicals, including curcumin, quercetin, polyphenols, anthocyanins, isoflavones, proanthocyanidins, and flavonoid-rich extracts, often administered in monotherapy or adjunctive formats. Although sample sizes, duration, and design quality varied, most studies used validated depression scales and biochemical endpoints, supporting a neurobiological rationale for clinical translation.
In a well-structured randomized controlled trial, Soltani et al. [25] evaluated the impact of nanocurcumin supplementation in individuals with coronary slow flow phenomenon (CSFP). The intervention led to improvements in depressive symptoms, physical and psychological health-related quality of life, and cardiometabolic parameters, with biochemical assays confirming anti-inflammatory and antioxidant effects [257]. In another study examining quercetin effects in patients who have experienced myocardial infarction, the authors did failed to not find significant antidepressant effects [258].
Methodological strengths such as stratified randomization and biomarker analysis were evident in the study by Hajiluian et al. [34], which demonstrated reduced depressive symptoms in individuals with multiple sclerosis following polyphenol-rich interventions. However, its narrow population limits generalizability to broader MDD cohorts [259]. In a similarly well-structured clinical trial, Choi et al. [27] examined the effects of flavonoid-enriched orange juice in healthy adults. Improvements in depressive symptoms were paralleled by increased serum BDNF and reduced zonulin levels, indicating neuromodulatory and gut–brain axis effects. Despite robust biochemical correlates, the sample size of 40 reduced statistical power [27].
Maeda-Yamamoto et al. [35] assessed the impact of anthocyanin-rich Solanum tuberosum L. on stress and mesenchymal stem cell proliferation. Although qualitative outcomes were positive, the trial was limited by its very small sample (n = 15) and short duration (8 weeks), which precluded long-term conclusions. Similarly, Barfoot et al. [36] conducted a randomized study during the COVID-19 pandemic, which introduced significant confounding related to global psychological stress. While randomization via software strengthened validity, high dropout rates impaired data reliability.
Parilli-Moser et al. [37] explored the effects of botanical cognitive enhancers on depressive symptoms. Although standardized cognitive assessments were used, low group sizes and a lack of blinding limited interpretive strength and COVID-19-related disruptions likely impacted outcome validity. In contrast, the RISTOMED study by Bourdel-Marchasson et al. [38] assessed a dietary protocol rich in antioxidants and polyphenols. After 2 months, reductions in depressive symptoms were observed, but these were found to be independent of inflammatory biomarker changes, suggesting mechanisms beyond systemic inflammation.
Kontogianni et al. [260] investigated high versus low polyphenol diets in the PPhIT trial, noting improved psychological well-being in the high-polyphenol group using standardized lifestyle and mood assessments. Park, Choi, and Lee [260] similarly conducted a placebo-controlled flavonoid study using orange juice and observed improvements in depression alongside increased serum serotonin, BDNF, and reduced CRP—though the small sample again limits external validity.
Smetanka et al. [260] focused on the potential for pycnogenol to mitigate SSRI-induced sexual dysfunction in patients taking escitalopram. However, the open-label design and simultaneous pharmacologic interventions created confounding that weakens causal inference. In the curcumin trial by Kanchanatawan et al. [40], methodological strengths included matched placebo capsules and double-blinding, although prior treatments continued during the trial, potentially masking true curcumin effects.
The study by Esmaily et al. [41] observed only marginal reductions in anxiety and depressive symptoms in response to saffron extract, with effects limited to a single outcome measure—suggesting potential underpowering or scale insensitivity. In a postmenopausal population, Terauchi et al. [33] demonstrated that grape seed proanthocyanidins reduced depressive symptoms in a dose-dependent manner, using validated questionnaires. However, the short duration (8 weeks) and modest sample size limit generalizability.
Equol and resveratrol were evaluated in a longitudinal 12-week study on menopausal women aged 50–55, where supplementation improved mood as assessed by the Hamilton depression rating scale (HAM-D) [261]. Hirose et al. [262] explored low-dose isoflavone aglycone for postmenopausal symptoms, including depression and anxiety, using HADS and AIS. Despite positive outcomes, lack of adverse event documentation and small sample size limit clinical confidence.
Cognitive-affective benefits of blueberry-derived flavonoids were tested in a crossover design by Khalid et al. [262], involving both children and young adults. Stratified analysis revealed reductions in depressogenic cognitive patterns two hours after consumption, supporting the acute neuromodulatory potential of these compounds.
Lastly, Sathyapalan et al. [262] evaluated chocolate rich in cocoa liquor and polyphenols versus low-polyphenol control chocolate in patients with chronic fatigue syndrome. Participants in the high-cocoa group reported improvements in depressive and fatigue symptoms, supporting flavonoid efficacy in neuropsychological syndromes. Pase et al. [11] reported mood-enhancing effects of cocoa polyphenols but no cognitive improvements after 30 days of supplementation.
Overall, these reviewed studies underscore the emerging promise of phytocompounds as adjunctive or standalone interventions for MDD (Table 1). While most trials reported clinically meaningful reductions in depressive symptoms, variability in methodological rigor, sample size, and treatment duration limited the generalizability of these findings. Notably, studies incorporating double-blind, placebo-controlled designs and objective biomarkers (e.g., inflammatory mediators neurotrophic factors) provided stronger evidence for neuromodulatory and neuroprotective mechanisms. Nevertheless, several investigations reported inconclusive or modest effects, indicating the necessity of improved trial designs and larger, more diverse samples. Given the heterogeneous nature of depression, a multi-targeted approach—such as that offered by phytochemicals—holds particular relevance. Future research efforts should focus on optimizing dosage regimens, exploring synergistic effects with standard antidepressants, and elucidating long-term safety profiles to solidify phytocompounds’ place in evidence-based psychiatric care.

Table 1.
Summary of Interventional Studies Evaluating the Therapeutic Effects of Phytocompounds in Major Depressive Disorder (MDD). This table presents a structured synthesis of the 19 clinical trials included in the systematic review, selected according to PRISMA guidelines. Each study is characterized by its design, geographic location, sample demographics, intervention type, control conditions, outcome measures, and reported adverse events. The interventions include a range of phytocompounds—such as curcumin, flavonoids, anthocyanins, and isoflavones—administered in monotherapy or adjunctive formats. Outcomes were assessed via validated psychometric scales and, where applicable, supported by biochemical and neurotrophic markers. This compilation provides a comparative overview of study quality, efficacy, and safety in the clinical application of phytochemicals for MDD.
9. Discussion
MDD remains a complex, multifaceted psychiatric condition that resists simple explanations or treatments [275]. The primary goal of this review was to explore how phytochemicals—naturally occurring plant-derived compounds—may offer a biologically plausible and clinically relevant approach to modulating the intricate neurobiological disruptions observed in MDD [1]. Central to the disorder’s pathophysiology are sustained neuroinflammation, oxidative stress, mitochondrial dysfunction, and metabolic disturbances, which interact to compromise neuronal integrity, synaptic plasticity, and emotional regulation [276]. While monoaminergic deficits have long dominated depression theories, current evidence indicates these downstream effects may be fueled by deeper cellular and immune dysregulation [277]. Phytocompounds such as polyphenols, flavonoids, and alkaloids appear to influence these upstream processes by attenuating pro-inflammatory cytokines, scavenging ROS, restoring mitochondrial dynamics, and modulating neurotrophic signaling [136]. In doing so, they challenge conventional paradigms and suggest a more integrative neuropsychiatric model—one that views depression through the lens of systemic inflammation and neuroenergetic compromise rather than solely neurotransmitter depletion [278].
A central finding of this review is the growing body of evidence supporting the antidepressant potential of phytochemicals, particularly polyphenols, flavonoids, and alkaloid-rich plant extracts. Compounds such as curcumin, resveratrol, quercetin, and luteolin consistently demonstrate antidepressant-like effects across preclinical models and emerging clinical studies. These benefits are mediated through a combination of neuroprotective, anti-inflammatory, and antioxidant mechanisms. Notably, curcumin has been shown to downregulate pro-inflammatory cytokines (e.g., IL-1β and TNF-α), reduce lipid peroxidation, and enhance BDNF expression—key pathways implicated in MDD. Similarly, flavonoids such as quercetin and luteolin attenuate microglial activation, restore mitochondrial function, and improve synaptic plasticity. These multi-target effects distinguish phytochemicals from traditional antidepressants, which primarily modulate monoamine reuptake and often require several weeks to achieve symptom relief. Moreover, phytochemicals tend to have more favorable safety profiles, presenting a lower risk of side effects such as sexual dysfunction, weight gain, or hyponatremia. For individuals with treatment-resistant depression or intolerance to conventional therapies, these natural agents may offer a promising adjunct or alternative approach. Their ability to modulate upstream drivers of MDD rather than solely downstream neurotransmitter imbalances reflects a potentially paradigm-shifting advance in how we conceptualize and manage depression.
The therapeutic potential of phytochemicals in MDD is rooted in their ability to engage a broad array of molecular targets implicated in the neurobiology of depression [279]. One of the most prominent mechanisms is the upregulation of BDNF, a key molecule in synaptic plasticity, neurogenesis, and neuronal survival [226]. Phytocompounds such as curcumin, resveratrol, and apigenin have been shown to elevate BDNF expression in preclinical models, countering the synaptic deficits commonly observed in depression [280]. Another critical mechanism involves the inhibition of the NLRP3 inflammasome, a key driver of neuroinflammation [276]. Polyphenols like quercetin and baicalein suppress this pathway, leading to decreased release of pro-inflammatory cytokines such as IL-1β and IL-18 [281]. These compounds also reduce oxidative stress by enhancing endogenous antioxidant defenses (e.g., upregulation of Nrf2 and glutathione pathways), protecting neurons from ROS-induced apoptosis [282]. Importantly, many of these phytochemicals—especially flavonoids and terpenoids—demonstrate the capacity to cross the blood–brain barrier due to their lipophilic structures and low molecular weight [283]. Once in the CNS, they can stabilize mitochondrial function, modulate calcium homeostasis, and maintain membrane potential, all of which are disrupted in MDD [284]. These mechanistic actions align closely with contemporary neurobiological models of depression, which emphasize the roles of neuroinflammation, mitochondrial dysfunction, and impaired neuroplasticity over simplistic monoamine depletion theories [89]. By targeting upstream cellular stressors and restoring homeostatic balance across neuroimmune and neurometabolic pathways, phytochemicals represent a compelling therapeutic modality that resonates with the current shift toward systems-based approaches in psychiatric research. Complementing this systems-level approach, recent insights into quinoline-based drug design suggest that subtle structural modifications, such as halogenation or esterification, can meaningfully modulate excitotoxicity and immunoactivity signaling relevant to depression pathophysiology [285].
The incorporation of phytochemicals into clinical treatment strategies for MDD presents several promising implications for practice [1]. Unlike conventional antidepressants, which often carry a high burden of side effects—such as sexual dysfunction, weight gain, insomnia, or gastrointestinal disturbances—phytochemicals typically exhibit favorable safety and tolerability profiles [256]. This reduced side-effect burden may significantly enhance patient adherence, particularly among individuals who are medication-sensitive, elderly, or managing comorbid conditions [224]. Furthermore, many phytocompounds, such as curcumin, resveratrol, and quercetin, are available in standardized formulations and have shown efficacy when used alongside standard antidepressant regimens, supporting their role as adjunctive therapies [286]. Their natural origin also makes them appealing to patients seeking holistic or integrative approaches to mental health [287]. To maximize the benefits of phytochemical interventions, multidisciplinary care teams—comprising psychiatrists, psychologists, nutritionists, pharmacists, and exercise specialists—are essential [288]. These teams can tailor phytochemical strategies to the individual’s biological and psychosocial profile, monitor for herb–drug interactions, and integrate them into broader lifestyle and behavioral interventions [289]. This integrative model not only broadens therapeutic options but aligns with growing interest in personalized medicine, where treatment is adapted to the individual’s unique physiological and lifestyle context [290].
Despite encouraging findings, several challenges and limitations temper the immediate clinical translation of phytochemicals for depression [252]. A primary concern lies in the methodological variability across studies [256]. Many preclinical and clinical trials differ widely in terms of dosage, treatment duration, and formulation, making it difficult to establish standardized protocols [1]. Sample sizes are often small, with limited representation across age groups, genders, and ethnic backgrounds, reducing the generalizability of results [291]. Moreover, key pharmacokinetic parameters—such as bioavailability, half-life, and tissue distribution—remain poorly characterized for many phytochemicals [292]. This complicates efforts to optimize dosage and timing for maximum therapeutic effect [293]. Additionally, the long-term safety of chronic phytochemical use is not well understood, particularly when used in combination with conventional antidepressants, raising concerns about potential herb–drug interactions [294]. These knowledge gaps highlight the need for rigorously designed, large-scale randomized, controlled trials that incorporate standardized extract preparations, dose-ranging studies, and biomarker-based outcome measures [295]. Future research should also emphasize population diversity and assess pharmacogenomic factors that may influence individual responses [296]. Addressing these limitations is essential for moving phytochemicals from promising adjuncts to reliable, evidence-based components of depression treatment protocols.
Future research on phytochemicals in the treatment of MDD should prioritize rigorously designed clinical trials and translational studies that bridge the gap between laboratory findings and real-world clinical application [279]. Large-scale, placebo-controlled trials are essential to validate the antidepressant efficacy of individual phytochemicals, establish optimal dosing strategies, and assess long-term safety [297]. In addition to studying single compounds, future investigations should explore the therapeutic potential of phytochemical combinations [252]. Many plant-derived compounds act on overlapping molecular targets, and their synergistic effects on neuroinflammation, oxidative stress, and mitochondrial dysfunction may offer enhanced efficacy compared to isolated agents [298]. Combinatorial approaches could mirror the polypharmacological nature of depression, targeting its multifactorial pathophysiology more effectively [225]. Moreover, research should move toward precision medicine models that account for individual differences in genetic makeup, metabolic profiles, gut microbiota composition, and inflammatory status [299]. Stratifying participants based on these biomarkers could identify responders and non-responders to specific phytochemicals, allowing for more personalized treatment [300]. Integrating omics technologies—such as metabolomics, transcriptomics, and pharmacogenomics—into trial designs will enhance mechanistic understanding and treatment customization [301]. Finally, future studies should include robust secondary endpoints that assess cognitive function, quality of life, and functional recovery, not just symptom reduction [199]. By embracing these strategies, the field can move toward the development of targeted, effective, and biologically grounded phytochemical interventions that expand the therapeutic landscape for depression.
Effective management of MDD increasingly requires a multidisciplinary approach that integrates biological, psychological, and lifestyle-based strategies [302]. Collaborative care teams—comprising psychiatrists, psychologists, nutritionists, pharmacists, and exercise specialists—are well positioned to deliver comprehensive, personalized treatment plans [303]. Phytochemical supplementation can be particularly valuable when embedded within this broader therapeutic framework [304]. Nutritionists can guide dietary modifications that reinforce the anti-inflammatory and antioxidant actions of phytocompounds, while exercise specialists can prescribe physical activity regimens that synergize with phytochemicals to enhance mitochondrial health and neuroplasticity [305]. Psychologists can provide cognitive–behavioral or mindfulness-based therapies that further modulate stress-related pathways implicated in MDD [306]. The literature from integrated care models demonstrates that combining pharmacological and non-pharmacological interventions—such as in collaborative care or lifestyle psychiatry frameworks—improves clinical outcomes, enhances patient satisfaction, and reduces relapse rates [307]. For example, interventions that pair dietary polyphenols with structured aerobic exercise and therapy have shown additive effects on depressive symptom reduction and cognitive function [308]. This integrated model not only reflects the multifactorial nature of depression but also offers a pragmatic pathway to improve treatment adherence, reduce polypharmacy, and promote long-term mental wellness [309]. Phytochemicals, when coordinated with other interventions, may serve as powerful components of such a multidimensional care strategy.
This review illuminates the multifaceted nature of MDD and the potential utility of phytocompounds in its management. Recent evidence reveals that MDD involves not only monoaminergic dysregulation but also neuroinflammatory processes, oxidative stress, and mitochondrial dysfunction, implicating diverse pathophysiological pathways [223]. By evaluating interventional studies centered on phytochemicals—including polyphenols, flavonoids, and alkaloids—this review highlights their potential neuroprotective, anti-inflammatory, and antioxidant properties [22]. These effects align with emerging translational research suggesting that multi-targeted strategies may offer enhanced therapeutic outcomes in complex mood disorders [79,310]. Nevertheless, the heterogeneity of study designs, phytocompound formulations, and patient populations underscores the need for more robust trials with standardized protocols [311,312,313]. This discussion aims to synthesize the mechanistic underpinnings and clinical implications of phytocompound use in MDD, situating these findings within broader neuropsychiatric research and outlining key directions for future investigation. Taken together, these insights reaffirm the value of phytochemicals as complementary or alternative therapies in MDD, particularly given their favorable safety profiles, multimodal mechanisms of action, and promise for treatment-resistant populations. Continued clinical exploration of these natural interventions may significantly enhance holistic, personalized approaches to depression care and expand the future of integrative psychiatric treatment.
10. Conclusions
Emerging data suggest that specific plant-derived compounds can influence the neuro–immune interface and mitochondrial health in major depressive disorder, yet these signals remain preliminary. Wide variation in extract standardization, dosing protocols, study designs, and participant profiles hampers direct comparison, and most trials enroll modest, geographically limited samples. To translate early promise into clinical practice, large phase-III studies with rigorous pharmacokinetic assessment, active comparators, and extended safety follow-up are essential. Nonetheless, the literature reviewed here highlights the distinctive neuroprotective, anti-inflammatory, and antioxidant capacities of phytochemicals—attributes that align well with the complex biology of depression, extending therapeutic thinking beyond monoamine modulation alone. Although several investigations report meaningful symptom relief with minimal adverse effects, methodological shortcomings—small cohorts, heterogeneous interventions, and inconsistent endpoints—preclude definitive guidance. Future research must emphasize harmonized formulations, adequately powered populations, and mechanistic biomarkers to clarify durability, optimal dosing, and synergy with standard antidepressants. Collectively, the current evidence positions phytocompounds as promising adjuncts, warranting continued, methodologically robust exploration to fully harness their translational potential.
Author Contributions
Conceptualization, S.M.B., M.T. and L.F.L.; methodology, A.C.F.G.; investigation, F.F.F., V.E.V., L.P.A., C.R.P.D. and F.C.C. and L.F.L.; writing—original draft preparation, S.M.B., L.F.L., L.P.A. and M.T.; writing—review and editing, S.M.B., F.F.F., V.E.V., C.M.G., E.d.S.B.M.P. and R.S.A.H.; visualization, S.M.B., M.T. and F.F.F.; supervision, S.M.B. and M.T.; project administration, S.M.B.; funding acquisition, S.M.B. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the HUN-REN Hungarian Research Network to M.T.
Acknowledgments
Images were built with biorender.com, Free.Pick.com, Canva Free, and pixabay.com.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| DNA | Deoxyribonucleic acid |
| HAM-D | Hamilton depression rating scale |
| IL | Interleukin |
| MMA | Methylmalonic acid |
| MDD | Major depressive disorder |
| mtDNA | Mitochondrial deoxyribonucleic acid |
| NF-κB | Nuclear factor kappa B |
| ROS | Reactive oxygen species |
| SSRI | Selective serotonin reuptake inhibitor |
| TCA | Tricyclic antidepressant |
References
- Manosso, L.M.; Arent, C.O.; Borba, L.A.; Abelaira, H.M.; Réus, G.Z. Natural Phytochemicals for the Treatment of Major Depressive Disorder: A Mini-Review of Pre- and Clinical Studies. CNS Neurol. Disord. Drug Targets 2023, 22, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Suzuki, K.; Kavalali, E.T.; Monteggia, L.M. Ketamine: Mechanisms and Relevance to Treatment of Depression. Annu. Rev. Med. 2024, 75, 129–143. [Google Scholar] [CrossRef]
- Schoeller, F.; Jain, A.; Adrien, V.; Maes, P.; Reggente, N. Aesthetic chills mitigate maladaptive cognition in depression. BMC Psychiatry 2024, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Poluektova, E.U.; Zorkina, Y.A.; Kovtun, A.S.; Danilenko, V.N. Human Gut Microbiota for Diagnosis and Treatment of Depression. Int. J. Mol. Sci. 2024, 25, 5782. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhang, Y.; Wang, S.; Yan, Y.; Liu, M.; Tian, M.; Tian, W. Global, regional, and national temporal trend in burden of major depressive disorder from 1990 to 2019: An analysis of the global burden of disease study. Psychiatry Res. 2024, 337, 115958. [Google Scholar] [CrossRef]
- Bagby, S.P.; Martin, D.; Chung, S.T.; Rajapakse, N. From the outside in: Biological mechanisms linking social and environmental exposures to chronic disease and to health disparities. Am. J. Public Health 2019, 109, S56–S63. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Tantardini, M.; De Simone, S.; Ramella-Cravaro, V.; Oliver, D.; Kingdon, J.; Kotlicka-Antczak, M.; Valmaggia, L.; Lee, J.; Millan, M. Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur. Psychiatry 2017, 40, 65–75. [Google Scholar] [CrossRef]
- Lesch, K.-P. When the serotonin transporter gene meets adversity: The contribution of animal models to understanding epigenetic mechanisms in affective disorders and resilience. In Molecular and Functional Models in Neuropsychiatry; Springer: Berlin/Heidelberg, Germany, 2011; pp. 251–280. [Google Scholar]
- Simons, R.L.; Lei, M.K.; Stewart, E.A.; Beach, S.R.; Brody, G.H.; Philibert, R.A.; Gibbons, F.X. Social adversity, genetic variation, street code, and aggression: A genetically informed model of violent behavior. Youth Violence Juv. Justice 2012, 10, 3–24. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Pase, M.P.; Scholey, A.B.; Pipingas, A.; Kras, M.; Nolidin, K.; Gibbs, A.; Wesnes, K.; Stough, C. Cocoa polyphenols enhance positive mood states but not cognitive performance: A randomized, placebo-controlled trial. J. Psychopharmacol. 2013, 27, 451–458. [Google Scholar] [CrossRef]
- Jia, S.; Hou, Y.; Wang, D.; Zhao, X. Flavonoids for depression and anxiety: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 8839–8849. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, T.; Jiang, P.; Dang, R. The interaction between autophagy and neuroinflammation in major depressive disorder: From pathophysiology to therapeutic implications. Pharmacol. Res. 2021, 168, 105586. [Google Scholar] [CrossRef]
- Bertollo, A.G.; Mingoti, M.E.D.; Ignácio, Z.M. Neurobiological mechanisms in the kynurenine pathway and major depressive disorder. Rev. Neurosci. 2025, 36, 169–187. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, X.; Ma, Y.; Yang, Y.; Pan, C.W.; Zhu, X.; Ke, C. Metabolomics on depression: A comparison of clinical and animal research. J. Affect. Disord. 2024, 349, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals 2025, 18, 133. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15, 124. [Google Scholar] [CrossRef]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.; Beier, K.; Mardis, T.; Munoz, B.; Zaidi, A. The Neurochemistry of Depression: The Good, The Bad and The Ugly. Mo. Med. 2024, 121, 68–75. [Google Scholar] [PubMed]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural antioxidant anthocyanins—A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef]
- Jin, W.; Xu, X.; Chen, X.; Qi, W.; Lu, J.; Yan, X.; Zhao, D.; Cong, D.; Li, X.; Sun, L. Protective effect of pig brain polypeptides against corticosterone-induced oxidative stress, inflammatory response, and apoptosis in PC12 cells. Biomed. Pharmacother. 2019, 115, 108890. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2018, 2018, 1972714. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neural correlates and molecular mechanisms of memory and learning. Int. J. Mol. Sci. 2024, 25, 2724. [Google Scholar] [CrossRef]
- Yoder, R.; Michaud, A.; Feagans, A.; Hinton-Froese, K.E.; Meyer, A.; Powers, V.A.; Stalnaker, L.; Hord, M.K. Family-Based Treatment for Anxiety, Depression, and ADHD for a Parent and Child. Int. J. Environ. Res. Public Health 2024, 21, 504. [Google Scholar] [CrossRef]
- Moreira, J.; Machado, M.; Dias-Teixeira, M.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. The neuroprotective effect of traditional Chinese medicinal plants—A critical review. Acta Pharm. Sin. B 2023, 13, 3208–3237. [Google Scholar] [CrossRef]
- Ajao, A.A.-n.; Sabiu, S.; Balogun, F.O.; Adekomi, D.A.; Saheed, S.A. The Ambit of Phytotherapy in Psychotic Care. In Psychosis-Biopsychosocial and Relational Perspectives; IntechOpen: London, UK, 2018. [Google Scholar]
- Rajpal, V.R.; Koul, H.K.; Raina, S.N.; Kumar, H.M.S.; Qazi, G.N. Phytochemicals for Human Health: The Emerging Trends and Prospects. Curr. Top. Med. Chem. 2024, 24, v–vi. [Google Scholar] [CrossRef]
- Kumar, H.M.S.; Rajpal, V.R.; Koul, H.K.; Raina, S.N.; Qazi, G.N. Phytochemicals for Human Health: The Emerging Trends and Prospects, Part-3. Curr. Top. Med. Chem. 2024, 24, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Bai, W. Bioactive phytochemicals. Crit. Rev. Food Sci. Nutr. 2019, 59, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Fais, A.; Era, B. Phytochemical Composition and Biological Activity. Plants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Cao, G.; Zhao, D.; Li, G.; Zhang, H.; Yan, M. Ethnic, Botanic, Phytochemistry and Pharmacology of the. Molecules 2023, 28, 7117. [Google Scholar] [CrossRef]
- Reddy, K.; Stafford, G.I.; Makunga, N.P. Skeletons in the closet? Using a bibliometric lens to visualise phytochemical and pharmacological activities linked to. Front. Plant Sci. 2024, 15, 1268101. [Google Scholar] [CrossRef]
- Gong, G.; Ganesan, K.; Wang, Y.; Zhang, Z.; Liu, Y.; Wang, J.; Yang, F.; Zheng, Y. Ononin ameliorates depression-like behaviors by regulating BDNF-TrkB-CREB signaling in vitro and in vivo. J. Ethnopharmacol. 2024, 320, 117375. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. From Lab to Life: Exploring Cutting-Edge Models for Neurological and Psychiatric Disorders. Biomedicines 2024, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Seung, H.-B.; Kwon, H.-J.; Kwon, C.-Y.; Kim, S.-H. Neuroendocrine Biomarkers of Herbal Medicine for Major Depressive Disorder: A Systematic Review and Meta-Analysis. Pharmaceuticals 2023, 16, 1176. [Google Scholar] [CrossRef]
- Jaberi, K.R.; Alamdari-Palangi, V.; Savardashtaki, A.; Vatankhah, P.; Jamialahmadi, T.; Tajbakhsh, A.; Sahebkar, A. Modulatory Effects of Phytochemicals on Gut-Brain Axis: Therapeutic Implication. Curr. Dev. Nutr. 2024, 8, 103785. [Google Scholar] [CrossRef] [PubMed]
- Liloia, D.; Zamfira, D.A.; Tanaka, M.; Manuello, J.; Crocetta, A.; Keller, R.; Cozzolino, M.; Duca, S.; Cauda, F.; Costa, T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci. Biobehav. Rev. 2024, 164, 105791. [Google Scholar] [CrossRef]
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef]
- Kakarla, R.; Karuturi, P.; Siakabinga, Q.; Kasi Viswanath, M.; Dumala, N.; Guntupalli, C.; Nalluri, B.N.; Venkateswarlu, K.; Prasanna, V.S.; Gutti, G.; et al. Current understanding and future directions of cruciferous vegetables and their phytochemicals to combat neurological diseases. Phytother. Res. 2024, 38, 1381–1399. [Google Scholar] [CrossRef]
- Chandel, P.; Thapa, K.; Kanojia, N.; Rani, L.; Singh, T.G.; Rohilla, P. Exploring Therapeutic Potential of Phytoconstituents as a Gut Microbiota Modulator in the Management of Neurological and Psychological Disorders. Neuroscience 2024, 551, 69–78. [Google Scholar] [CrossRef]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13, 167. [Google Scholar] [CrossRef]
- Tanaka, M. Beyond the boundaries: Transitioning from categorical to dimensional paradigms in mental health diagnostics. Adv. Clin. Exp. Med. 2024, 33, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.L.d.S.; Martins, V.G.d.Q.A.; Silva, A.P.d.; Rocha, H.A.O.; Rachetti, V.d.P.S.; Scortecci, K.C. Phenolic acids as antidepressant agents. Nutrients 2022, 14, 4309. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.H.; Biswas, P.; Hossain, M.S.; Islam, R.; Hannan, M.A.; Uddin, M.J.; Rhim, H. Potential therapeutic role of phytochemicals to mitigate mitochondrial dysfunctions in Alzheimer’s disease. Antioxidants 2020, 10, 23. [Google Scholar] [CrossRef]
- Fazilat, S.; Tahmasbi, F.; Mirzaei, M.R.; Sanaie, S.; Yousefi, Z.; Asnaashari, S.; Yaqoubi, S.; Mohammadi, A.B.; Araj-khodaei, M. A systematic review on the use of phytotherapy in managing clinical depression. BioImpacts 2024, 15, 30532. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: New approaches to hippocampal neurogenesis. J. Neuroinflammation 2023, 20, 283. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef]
- Fornari Laurindo, L.; Aparecido Dias, J.; Cressoni Araújo, A.; Torres Pomini, K.; Machado Galhardi, C.; Rucco Penteado Detregiachi, C.; Santos de Argollo Haber, L.; Donizeti Roque, D.; Dib Bechara, M.; Vialogo Marques de Castro, M.; et al. Immunological dimensions of neuroinflammation and microglial activation: Exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front. Immunol. 2023, 14, 1305933. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Simili, O.A.G.; Araújo, A.C.; Guiguer, E.L.; Direito, R.; Valenti, V.E.; de Oliveira, V.; de Oliveira, J.S.; Yanaguizawa Junior, J.L.; Dias, J.A.; et al. Melatonin from Plants: Going Beyond Traditional Central Nervous System Targeting-A Comprehensive Review of Its Unusual Health Benefits. Biology 2025, 14, 143. [Google Scholar] [CrossRef]
- Tanaka, M.; Tuka, B.; Vécsei, L. Navigating the Neurobiology of Migraine: From pathways to potential therapies. Cells 2024, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.R.; Musella, A.; De Vito, F.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Stampanoni Bassi, M.; Buttari, F.; Mandolesi, G. Tumor necrosis factor and interleukin-1β modulate synaptic plasticity during neuroinflammation. Neural Plast. 2018, 2018, 8430123. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.V.; Prehar, S.; Kennedy, A.R.; Little, R.A.; Hopkins, S.J. Interleukin-6 is an afferent signal to the hypothalamo-pituitary-adrenal axis during local inflammation in mice. Endocrinology 2003, 144, 1894–1906. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Zhang, L.; Cheng, J.-K.; Ji, R.-R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef]
- De Simoni, M.; Terreni, L.; Chiesa, R.; Mangiarotti, F.; Forloni, G. Interferon-γ potentiates interleukin (IL)-6 and tumor necrosis factor-α but not IL-1β induced by endotoxin in the brain. Endocrinology 1997, 138, 5220–5226. [Google Scholar] [CrossRef]
- Chaves, F.M.; Mansano, N.S.; Frazão, R.; Donato Jr, J. Tumor necrosis factor α and interleukin-1β acutely inhibit AgRP neurons in the arcuate nucleus of the hypothalamus. Int. J. Mol. Sci. 2020, 21, 8928. [Google Scholar] [CrossRef]
- Li, B.; Yang, W.; Ge, T.; Wang, Y.; Cui, R. Stress induced microglial activation contributes to depression. Pharmacol. Res. 2022, 179, 106145. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gao, Z.; Hu, H. Microglia in depression: Current perspectives. Sci. China Life Sci. 2021, 64, 911–925. [Google Scholar] [CrossRef]
- Turkin, A.; Tuchina, O.; Klempin, F. Microglia function on precursor cells in the adult hippocampus and their responsiveness to serotonin signaling. Front. Cell Dev. Biol. 2021, 9, 665739. [Google Scholar] [CrossRef]
- Zanella, I. Neuroinflammation: From Molecular Basis to Therapy. Int. J. Mol. Sci. 2024, 25, 5973. [Google Scholar] [CrossRef]
- Poletti, S.; Mazza, M.G.; Benedetti, F. Inflammatory mediators in major depression and bipolar disorder. Transl. Psychiatry 2024, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.Y.; Guo, Y.X.; Lian, W.W.; Yan, Y.; Ma, B.Z.; Cheng, Y.C.; Xu, J.K.; He, J.; Zhang, W.K. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol. Res. 2023, 187, 106625. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Li, W.; Chen, P.; Wang, L.; Bao, X.; Huang, R.; Liu, G.; Chen, X. Microglial NLRP3 inflammasome-mediated neuroinflammation and therapeutic strategies in depression. Neural Regen. Res. 2024, 19, 1890–1898. [Google Scholar] [CrossRef]
- Bearoff, F.; Dhavale, D.; Kotzbauer, P.; Kortagere, S. Aggregated Alpha-Synuclein Activates Pro-Inflammatory NFKB Signaling Pathways Through TLR-Dependent and Independent Mechanisms in Peripheral Monocytic Cells. FASEB J. 2022, 36, 1–10. [Google Scholar] [CrossRef]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood–brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Wang, X.; Antony, V.; Wang, Y.; Wu, G.; Liang, G. Pattern recognition receptor-mediated inflammation in diabetic vascular complications. Med. Res. Rev. 2020, 40, 2466–2484. [Google Scholar] [CrossRef]
- Barrera, M.-J.; Aguilera, S.; Castro, I.; Carvajal, P.; Jara, D.; Molina, C.; González, S.; González, M.-J. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: Potential role in Sjögren’s syndrome. Autoimmun. Rev. 2021, 20, 102867. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, H.; Zhang, T.; Wu, J.; Tang, H.; Liang, X.; Ren, D.; Huang, J.; Li, W. Mitochondria of intestinal epithelial cells in depression: Are they at a crossroads of gut-brain communication? Neurosci. Biobehav. Rev. 2023, 153, 105403. [Google Scholar] [CrossRef]
- Sipahi, H.; Mat, A.F.; Ozhan, Y.; Aydin, A. The interrelation between oxidative stress, depression and inflammation through the kynurenine pathway. Curr. Top. Med. Chem. 2023, 23, 415–425. [Google Scholar] [CrossRef]
- Wakasugi, D.; Kondo, S.; Ferdousi, F.; Mizuno, S.; Yada, A.; Tominaga, K.; Takahashi, S.; Isoda, H. A rare olive compound oleacein functions as a TrkB agonist and mitigates neuroinflammation both in vitro and in vivo. Cell Commun. Signal 2024, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y. Progress of plant polyphenol extracts in treating depression by anti-neuroinflammatory mechanism: A review. Medicine 2024, 103, e37151. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Dolcetti, E.; Rizzo, F.R.; Fresegna, D.; Musella, A.; Gentile, A.; De Vito, F.; Caioli, S.; Guadalupi, L.; Bullitta, S.; et al. Corrigendum: Inflammation-Associated Synaptic Alterations as Shared Threads in Depression and Multiple Sclerosis. Front. Cell Neurosci. 2020, 14, 647259. [Google Scholar] [CrossRef]
- Sha, Q.; Escobar Galvis, M.L.; Madaj, Z.B.; Keaton, S.A.; Smart, L.; Edgerly, Y.M.; Anis, E.; Leach, R.; Osborne, L.M.; Achtyes, E.; et al. Dysregulated placental expression of kynurenine pathway enzymes is associated with inflammation and depression in pregnancy. Brain Behav. Immun. 2024, 119, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Achtyes, E.; Keaton, S.A.; Smart, L.; Burmeister, A.R.; Heilman, P.L.; Krzyzanowski, S.; Nagalla, M.; Guillemin, G.J.; Escobar Galvis, M.L.; Lim, C.K.; et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 2020, 83, 239–247. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J. Neural Transm. 2024, 131, 1367–1387. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. A decade of dedication: Pioneering perspectives on neurological diseases and mental illnesses. Biomedicines 2024, 12, 1083. [Google Scholar] [CrossRef]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016, 58, 1–8. [Google Scholar] [CrossRef]
- Gustafsson, H.C.; Sullivan, E.L.; Nousen, E.K.; Sullivan, C.A.; Huang, E.; Rincon, M.; Nigg, J.T.; Loftis, J.M. Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain Behav. Immun. 2018, 73, 470–481. [Google Scholar] [CrossRef]
- Pontillo, G.; Cepas, M.B.; Broeders, T.A.A.; Koubiyr, I.; Schoonheim, M.M. Network Analysis in Multiple Sclerosis and Related Disorders. Neuroimaging Clin. N. Am. 2024, 34, 375–384. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Z.; Wang, J.; Gao, M.; Zhang, Y.; Yang, C.; Zhang, A.; Li, G.; Li, X.; Liu, S.; et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat. Commun. 2024, 15, 3003. [Google Scholar] [CrossRef] [PubMed]
- Molina Galindo, L.S.; Gonzalez-Escamilla, G.; Fleischer, V.; Grotegerd, D.; Meinert, S.; Ciolac, D.; Person, M.; Stein, F.; Brosch, K.; Nenadić, I.; et al. Concurrent inflammation-related brain reorganization in multiple sclerosis and depression. Brain Behav. Immun. 2024, 119, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int. J. Mol. Med. 2024, 53, 47. [Google Scholar] [CrossRef]
- Cui, J.-J.; Huang, Z.-Y.; Xie, Y.-H.; Wu, J.-B.; Xu, G.-H.; Li, C.-F.; Zhang, M.-M.; Yi, L.-T. Gut microbiota mediated inflammation, neuroendocrine and neurotrophic functions involved in the antidepressant-like effects of diosgenin in chronic restraint stress. J. Affect. Disord. 2023, 321, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Reiche, E.M.V.; Murru, A.; Carvalho, A.F.; Maes, M.; Berk, M.; Puri, B.K. Multiple immune-inflammatory and oxidative and nitrosative stress pathways explain the frequent presence of depression in multiple sclerosis. Mol. Neurobiol. 2018, 55, 6282–6306. [Google Scholar] [CrossRef]
- Adzic, M.; Brkic, Z.; Mitic, M.; Francija, E.; Jovicic, M.J.; Radulovic, J.; Maric, N.P. Therapeutic strategies for treatment of inflammation-related depression. Curr. Neuropharmacol. 2018, 16, 176–209. [Google Scholar] [CrossRef]
- Khan, M.; Baussan, Y.; Hebert-Chatelain, E. Connecting dots between mitochondrial dysfunction and depression. Biomolecules 2023, 13, 695. [Google Scholar] [CrossRef]
- Casaril, A.M.; Dantzer, R.; Bas-Orth, C. Neuronal mitochondrial dysfunction and bioenergetic failure in inflammation-associated depression. Front. Neurosci. 2021, 15, 725547. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative stress in depression: The link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Int. J. Mol. Sci. 2024, 25, 3360. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: Treatment implications. Curr. Pharm. Des. 2014, 20, 3812–3847. [Google Scholar] [CrossRef]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef]
- Ilavská, L.; Morvová, M.; Paduchová, Z.; Muchová, J.; Garaiova, I.; Ďuračková, Z.; Šikurová, L.; Trebatická, J. The kynurenine and serotonin pathway, neopterin and biopterin in depressed children and adolescents: An impact of omega-3 fatty acids, and association with markers related to depressive disorder. A randomized, blinded, prospective study. Front. Psychiatry 2024, 15, 1347178. [Google Scholar] [CrossRef]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knock-out Mice: A Novel Model for Experience-Based Depression and Post-Traumatic Stress Disorder. Front. Biosci. 2025, 30, 25706. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Larrea, A.; Sánchez-Sánchez, L.; Diez-Martin, E.; Elexpe, A.; Torrecilla, M.; Astigarraga, E.; Barreda-Gómez, G. Mitochondrial Metabolism in Major Depressive Disorder: From Early Diagnosis to Emerging Treatment Options. J. Clin. Med. 2024, 13, 1727. [Google Scholar] [CrossRef]
- Chen, H.; Lu, M.; Lyu, Q.; Shi, L.; Zhou, C.; Li, M.; Feng, S.; Liang, X.; Zhou, X.; Ren, L. Mitochondrial dynamics dysfunction: Unraveling the hidden link to depression. Biomed. Pharmacother. 2024, 175, 116656. [Google Scholar] [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. The impact of C-3 side chain modifications on Kynurenic Acid: A behavioral analysis of its analogs in the Motor Domain. Int. J. Mol. Sci. 2024, 25, 3394. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in cognitive impairment and mood disorders for experimental, clinical and translational neuropsychiatry. Biomedicines 2024, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, H.; Tan, Y.; Yu, X.D.; Xiao, C.; Li, Y.; Reilly, J.; He, Z.; Shu, X. Protection of p-Coumaric acid against chronic stress-induced neurobehavioral deficits in mice via activating the PKA-CREB-BDNF pathway. Physiol. Behav. 2024, 273, 114415. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cao, H.; Zuo, C.; Gu, Z.; Huang, Y.; Miao, J.; Fu, Y.; Guo, Y.; Jiang, Y.; Wang, F. Mitochondrial dysfunction: A fatal blow in depression. Biomed. Pharmacother. 2023, 167, 115652. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, S.; Liu, J.; Wu, X.; Zhou, S.; Dai, K.; Kou, Y. Mitophagy Contributes to the Pathogenesis of Inflammatory Diseases. Inflammation 2018, 41, 1590–1600. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Han, S.; Zhang, M.; Jeong, Y.Y.; Margolis, D.J.; Cai, Q. The role of mitophagy in the regulation of mitochondrial energetic status in neurons. Autophagy 2021, 17, 4182–4201. [Google Scholar] [CrossRef]
- Visentin, A.P.V.; Colombo, R.; Scotton, E.; Fracasso, D.S.; da Rosa, A.R.; Branco, C.S.; Salvador, M. Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxid. Med. Cell Longev. 2020, 2020, 2972968. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front. Endocrinol. 2023, 14, 1134025. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Moreno, J.J.; Bodega, P.; de Cos-Gandoy, A.; de Miguel, M.; Santos-Beneit, G.; Fernández-Alvira, J.M.; Fernández-Jiménez, R.; Martínez-Gómez, J.; et al. Metabolic syndrome, adiposity, diet, and emotional eating are associated with oxidative stress in adolescents. Front. Nutr. 2023, 10, 1216445. [Google Scholar] [CrossRef]
- Heiss, C.N.; Mannerås-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Håkansson Gladh, A.; Seeley, R.J.; Drucker, D.J.; Bäckhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021, 35, 109163. [Google Scholar] [CrossRef]
- Li, H.; Song, L.; Cen, M.; Fu, X.; Gao, X.; Zuo, Q.; Wu, J. Oxidative balance scores and depressive symptoms: Mediating effects of oxidative stress and inflammatory factors. J. Affect. Disord. 2023, 334, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16, 2721. [Google Scholar] [CrossRef]
- Pagotto, G.L.O.; Santos, L.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.; Goulart, R.A.; Catharin, V.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef]
- Takeda, L.N.; Omine, A.; Laurindo, L.F.; Araújo, A.C.; Machado, N.M.; Dias, J.A.; Kavalakatt, J.; Banerjee, S.; de Alvares Goulart, R.; Atanasov, A.G.; et al. Brazil nut (Bertholletia excelsa Bonpl.) in health and disease: A narrative review. Food Chem. 2025, 477, 143425. [Google Scholar] [CrossRef]
- Corraliza-Gómez, M.; Gallardo, A.B.; Díaz-Marrero, A.R.; de la Rosa, J.M.; D’Croz, L.; Darias, J.; Arranz, E.; Cózar-Castellano, I.; Ganfornina, M.D.; Cueto, M. Modulation of Glial Responses by Furanocembranolides: Leptolide Diminishes Microglial Inflammation in Vitro and Ameliorates Gliosis In Vivo in a Mouse Model of Obesity and Insulin Resistance. Mar. Drugs 2020, 18. [Google Scholar] [CrossRef]
- Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Szalai, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: Effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1212–1226. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, Y.; Qin, Y.; Zhou, Y.; Tang, R.; Wang, Q.; Li, X.; Wang, H.; Weston-Green, K.; Huang, X.F.; et al. Alterations to the microbiota-colon-brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J. Nutr. Biochem. 2019, 65, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Tobe, E.H. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr. Dis. Treat. 2013, 9, 567–573. [Google Scholar] [CrossRef]
- Maurya, P.K.; Noto, C.; Rizzo, L.B.; Rios, A.C.; Nunes, S.O.; Barbosa, D.S.; Sethi, S.; Zeni, M.; Mansur, R.B.; Maes, M.; et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 134–144. [Google Scholar] [CrossRef]
- Allen, J.; Romay-Tallon, R.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Mitochondria and Mood: Mitochondrial Dysfunction as a Key Player in the Manifestation of Depression. Front. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, L.; Chen, Y.; Zhuo, Y.; Wan, S.; Guo, R. Association between mitochondrial DNA levels and depression: A systematic review and meta-analysis. BMC Psychiatry 2023, 23, 866. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, L.; Sheng, H. Mitochondria in depression: The dysfunction of mitochondrial energy metabolism and quality control systems. CNS Neurosci. Ther. 2024, 30, e14576. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Rezin, G.T.; Amboni, G.; Zugno, A.I.; Quevedo, J.; Streck, E.L. Mitochondrial dysfunction and psychiatric disorders. Neurochem. Res. 2009, 34, 1021–1029. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial dysfunction in aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Mason, B.L.; Diaz, A.P.; Jha, M.K.; Soares, J.C.; Trivedi, M.H.; Quevedo, J. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: Does inflammation play a role? Mol. Psychiatry 2022, 27, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Lin, G.; Su, L.; Xue, L.; Duan, K.; Chen, L.; Ni, J. The association between methylmalonic acid, a biomarker of mitochondrial dysfunction, and cause-specific mortality in Alzheimer’s disease and Parkinson’s disease. Heliyon 2024, 10, e29357. [Google Scholar] [CrossRef]
- Guo, J.; Wang, S.; Wan, X.; Liu, X.; Wang, Z.; Liang, C.; Zhang, Z.; Wang, Y.; Yan, M.; Wu, P.; et al. Mitochondria-derived methylmalonic acid aggravates ischemia-reperfusion injury by activating reactive oxygen species-dependent ferroptosis. Cell Commun. Signal 2024, 22, 53. [Google Scholar] [CrossRef]
- Cao, B.; Xiao, Y.; Liu, D. Associations of methylmalonic acid and depressive symptoms with mortality: A population-based study. Transl. Psychiatry 2024, 14, 297. [Google Scholar] [CrossRef]
- Huang, Q.N.; Watanabe, F.; Koseki, K.; He, R.E.; Lee, H.L.; Chiu, T.H.T. Effect of roasted purple laver (nori) on vitamin B(12) nutritional status of vegetarians: A dose-response trial. Eur. J. Nutr. 2024, 63, 3269–3279. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Z.; Zhao, Y.; Sai, N.; Wang, X.; Liu, H.; Huang, G.; Zhang, X. Homocysteine induces mitochondrial dysfunction involving the crosstalk between oxidative stress and mitochondrial pSTAT3 in rat ischemic brain. Sci. Rep. 2017, 7, 6932. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- de Moraes, M.S.; Guerreiro, G.; Sitta, A.; de Moura Coelho, D.; Manfredini, V.; Wajner, M.; Vargas, C.R. Oxidative damage in mitochondrial fatty acids oxidation disorders patients and the in vitro effect of l-carnitine on DNA damage induced by the accumulated metabolites. Arch. Biochem. Biophys. 2020, 679, 108206. [Google Scholar] [CrossRef] [PubMed]
- Butkowski, E.G.; Al-Aubaidy, H.A.; Jelinek, H.F. Interaction of homocysteine, glutathione and 8-hydroxy-2′-deoxyguanosine in metabolic syndrome progression. Clin. Biochem. 2017, 50, 116–120. [Google Scholar] [CrossRef]
- Zhao, X.; Abulikemu, A.; Lv, S.; Qi, Y.; Duan, J.; Zhang, J.; Chen, R.; Guo, C.; Li, Y.; Sun, Z. Oxidative stress- and mitochondrial dysfunction-mediated cytotoxicity by silica nanoparticle in lung epithelial cells from metabolomic perspective. Chemosphere 2021, 275, 129969. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S.; Liloia, D. Navigating Neurodegeneration: Integrating Biomarkers, Neuroinflammation, and Imaging in Parkinson’s, Alzheimer’s, and Motor Neuron Disorders. Biomedicines 2025, 13, 1045. [Google Scholar] [CrossRef]
- Park, A.; Oh, M.; Lee, S.J.; Oh, K.J.; Lee, E.W.; Lee, S.C.; Bae, K.H.; Han, B.S.; Kim, W.K. Mitochondrial Transplantation as a Novel Therapeutic Strategy for Mitochondrial Diseases. Int. J. Mol. Sci. 2021, 22, 4793. [Google Scholar] [CrossRef]
- Riou, A.; Broeglin, A.; Grimm, A. Mitochondrial transplantation in brain disorders: Achievements, methods, and challenges. Neurosci. Biobehav. Rev. 2025, 169, 105971. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Hamad, R.S.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E. Role of brain renin-angiotensin system in depression: A new perspective. CNS Neurosci. Ther. 2024, 30, e14525. [Google Scholar] [CrossRef]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective Effects of Melatonin and Mitochondria-targeted Antioxidants Against Oxidative Stress: A Review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef]
- Koh, J.H.; Kim, J.Y. Role of PGC-1α in the Mitochondrial NAD(+) Pool in Metabolic Diseases. Int. J. Mol. Sci. 2021, 22, 4558. [Google Scholar] [CrossRef]
- Hidalgo-Gutiérrez, A.; González-García, P.; Díaz-Casado, M.E.; Barriocanal-Casado, E.; López-Herrador, S.; Quinzii, C.M.; López, L.C. Metabolic Targets of Coenzyme Q10 in Mitochondria. Antioxidants 2021, 10, 520. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Briston, T.; Selwood, D.L.; Szabadkai, G.; Duchen, M.R. Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol. Sci. 2019, 40, 50–70. [Google Scholar] [CrossRef]
- Tao, J.; Chen, H.; Wang, Y.J.; Qiu, J.X.; Meng, Q.Q.; Zou, R.J.; Li, L.; Huang, J.G.; Zhao, Z.K.; Huang, Y.L.; et al. Ketogenic Diet Suppressed T-Regulatory Cells and Promoted Cardiac Fibrosis via Reducing Mitochondria-Associated Membranes and Inhibiting Mitochondrial Function. Oxid. Med. Cell Longev. 2021, 2021, 5512322. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Tian, Y.; Shi, J. Sirtuin 3 and mitochondrial permeability transition pore (mPTP): A systematic review. Mitochondrion 2022, 64, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, H.; Yang, X.X.; Pang, L.; Liu, J.; Ng, K.T.P.; Yeung, O.W.H.; Lam, Y.F.; Zhang, W.Y.; Lo, C.M.; et al. Inhibition of Carnitine Palmitoyltransferase 1A Aggravates Fatty Liver Graft Injury via Promoting Mitochondrial Permeability Transition. Transplantation 2021, 105, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Kalani, K.; Yan, S.F.; Yan, S.S. Mitochondrial permeability transition pore: A potential drug target for neurodegeneration. Drug Discov. Today 2018, 23, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Khiroya, K.; Sekyere, E.; McEwen, B.; Bayes, J. Nutritional considerations in major depressive disorder: Current evidence and functional testing for clinical practice. Nutr. Res. Rev. 2023, 38, 25–36. [Google Scholar] [CrossRef]
- Bernier, V.; Debarge, M.H.; Hein, M.; Ammendola, S.; Mungo, A.; Loas, G. Major Depressive Disorder, Inflammation, and Nutrition: A Tricky Pattern? Nutrients 2023, 15, 3438. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Mora, F.; Rodríguez-Jiménez, R.; Fernandez-Rojo, S.; et al. Nutrition, Epigenetics, and Major Depressive Disorder: Understanding the Connection. Front. Nutr. 2022, 9, 867150. [Google Scholar] [CrossRef]
- Muha, J.; Schumacher, A.; Campisi, S.C.; Korczak, D.J. Depression and emotional eating in children and adolescents: A systematic review and meta-analysis. Appetite 2024, 200, 107511. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, G.N.; Sanlier, N. The relationship between nutrition and depression in the life process: A mini-review. Exp. Gerontol. 2023, 172, 112072. [Google Scholar] [CrossRef]
- Chrzastek, Z.; Guligowska, A.; Sobczuk, P.; Kostka, T. Dietary factors, risk of developing depression, and severity of its symptoms in older adults-A narrative review of current knowledge. Nutrition 2023, 106, 111892. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’Neil, A.; Jacka, F.N.; Marx, W. Ultra-Processed Food Consumption and Mental Health: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2568. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mardi, P.; Hejrani, B.; Mahdavi, F.S.; Ghoreshi, B.; Gohari, K.; Heidari-Beni, M.; Qorbani, M. Association between junk food consumption and mental health problems in adults: A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 438. [Google Scholar] [CrossRef]
- Malmir, H.; Mahdavi, F.S.; Ejtahed, H.S.; Kazemian, E.; Chaharrahi, A.; Mohammadian Khonsari, N.; Mahdavi-Gorabi, A.; Qorbani, M. Junk food consumption and psychological distress in children and adolescents: A systematic review and meta-analysis. Nutr. Neurosci. 2023, 26, 807–827. [Google Scholar] [CrossRef]
- Aly, J.; Engmann, O. The Way to a Human’s Brain Goes Through Their Stomach: Dietary Factors in Major Depressive Disorder. Front. Neurosci. 2020, 14, 582853. [Google Scholar] [CrossRef]
- Kunugi, H. Depression and lifestyle: Focusing on nutrition, exercise, and their possible relevance to molecular mechanisms. Psychiatry Clin. Neurosci. 2023, 77, 420–433. [Google Scholar] [CrossRef]
- Swainson, J.; Reeson, M.; Malik, U.; Stefanuk, I.; Cummins, M.; Sivapalan, S. Diet and depression: A systematic review of whole dietary interventions as treatment in patients with depression. J. Affect. Disord. 2023, 327, 270–278. [Google Scholar] [CrossRef]
- Selvaraj, R.; Selvamani, T.Y.; Zahra, A.; Malla, J.; Dhanoa, R.K.; Venugopal, S.; Shoukrie, S.I.; Hamouda, R.K.; Hamid, P. Association Between Dietary Habits and Depression: A Systematic Review. Cureus 2022, 14, e32359. [Google Scholar] [CrossRef]
- Gorbachev, D.; Markina, E.; Chigareva, O.; Gradinar, A.; Borisova, N.; Syunyakov, T. Dietary Patterns as Modifiable Risk Factors for Depression: A Narrative Review. Psychiatr. Danub. 2023, 35, 423–431. [Google Scholar] [PubMed]
- Zhang, Q.; Yi, J.; Wu, Y. Oxidative stress and inflammation mediate the association between elevated oxidative balance scores and improved sleep quality: Evidence from NHANES. Front. Nutr. 2024, 11, 1469779. [Google Scholar] [CrossRef]
- Wilson, D.; Driller, M.; Winwood, P.; Clissold, T.; Johnston, B.; Gill, N. The Effectiveness of a Combined Healthy Eating, Physical Activity, and Sleep Hygiene Lifestyle Intervention on Health and Fitness of Overweight Airline Pilots: A Controlled Trial. Nutrients 2022, 14, 1988. [Google Scholar] [CrossRef]
- Bolognese, M.A.; Franco, C.B.; Ferrari, A.; Bennemann, R.M.; Lopes, S.M.A.; Bertolini, S.; Júnior, N.N.; Branco, B.H.M. Group Nutrition Counseling or Individualized Prescription for Women With Obesity? A Clinical Trial. Front. Public Health 2020, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.; Wani, S.U.D.; Krishna, K.L.; Kinattingal, N.; Roohi, T.F. A review on linking stress, depression, and insulin resistance via low-grade chronic inflammation. Biochem. Biophys. Rep. 2023, 36, 101571. [Google Scholar] [CrossRef]
- Pickersgill, J.W.; Turco, C.V.; Ramdeo, K.; Rehsi, R.S.; Foglia, S.D.; Nelson, A.J. The Combined Influences of Exercise, Diet and Sleep on Neuroplasticity. Front. Psychol. 2022, 13, 831819. [Google Scholar] [CrossRef] [PubMed]
- Magzal, F.; Turroni, S.; Fabbrini, M.; Barone, M.; Vitman Schorr, A.; Ofran, A.; Tamir, S. A personalized diet intervention improves depression symptoms and changes microbiota and metabolite profiles among community-dwelling older adults. Front. Nutr. 2023, 10, 1234549. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, W.; Zhu, X.; Hu, Z.; Li, S.; Zheng, B.; Xu, H.; Long, W.; Xiong, X. Association between dietary intake and symptoms of depression and anxiety in pregnant women: Evidence from a community-based observational study. Food Sci. Nutr. 2023, 11, 7555–7564. [Google Scholar] [CrossRef]
- Serrano Ripoll, M.J.; Oliván-Blázquez, B.; Vicens-Pons, E.; Roca, M.; Gili, M.; Leiva, A.; García-Campayo, J.; Demarzo, M.P.; García-Toro, M. Lifestyle change recommendations in major depression: Do they work? J. Affect. Disord. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Ip, A.K.; Ho, F.Y.; Yeung, W.F.; Chung, K.F.; Ng, C.H.; Oliver, G.; Sarris, J. Effects of a group-based lifestyle medicine for depression: A pilot randomized controlled trial. PLoS ONE 2021, 16, e0258059. [Google Scholar] [CrossRef]
- Sarris, J.; O’Neil, A.; Coulson, C.E.; Schweitzer, I.; Berk, M. Lifestyle medicine for depression. BMC Psychiatry 2014, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Dale, E.; Bang-Andersen, B.; Sánchez, C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem. Pharmacol. 2015, 95, 81–97. [Google Scholar] [CrossRef]
- Fagiolini, A.; Florea, I.; Loft, H.; Christensen, M.C. Effectiveness of Vortioxetine on Emotional Blunting in Patients with Major Depressive Disorder with inadequate response to SSRI/SNRI treatment. J. Affect. Disord. 2021, 283, 472–479. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, D.; Wang, J.; Zhou, D.; Liu, A.; Sun, Y.; Yuan, Y.; Li, J.; Guo, W. The pharmacological mechanism of chaihu-jia-longgu-muli-tang for treating depression: Integrated meta-analysis and network pharmacology analysis. Front. Pharmacol. 2023, 14, 1257617. [Google Scholar] [CrossRef] [PubMed]
- Zhichao, H.; Ching, L.W.; Huijuan, L.; Liang, Y.; Zhiyu, W.; Weiyang, H.; Zhaoxiang, B.; Linda, Z.L.D. A network meta-analysis on the effectiveness and safety of acupuncture in treating patients with major depressive disorder. Sci. Rep. 2021, 11, 10384. [Google Scholar] [CrossRef]
- Qaseem, A.; Owens, D.K.; Etxeandia-Ikobaltzeta, I.; Tufte, J.E.; Cross, J.T.; Wilt, T.J.; Physicians, C.G.C.f.t.A.C.o. Nonpharmacologic and Pharmacologic Treatments of Adults in the Acute Phase of Major Depressive Disorder: A Living Clinical Guideline From the American College of Physicians (Version 1, Update Alert 2). Ann. Intern. Med. 2024, 177, 8. [Google Scholar] [CrossRef]
- Direito, R.; Barbalho, S.M.; Sepodes, B.; Figueira, M.E. Plant-Derived Bioactive Compounds: Exploring Neuroprotective, Metabolic, and Hepatoprotective Effects for Health Promotion and Disease Prevention. Pharmaceutics 2024, 16, 577. [Google Scholar] [CrossRef]
- Marx, W.; Manger, S.H.; Blencowe, M.; Murray, G.; Ho, F.Y.; Lawn, S.; Blumenthal, J.A.; Schuch, F.; Stubbs, B.; Ruusunen, A.; et al. Clinical guidelines for the use of lifestyle-based mental health care in major depressive disorder: World Federation of Societies for Biological Psychiatry (WFSBP) and Australasian Society of Lifestyle Medicine (ASLM) taskforce. World J. Biol. Psychiatry 2023, 24, 333–386. [Google Scholar] [CrossRef]
- Smith, R.D.; Dang, W.; Shen, S.; Hung, S.C.; Lam, I.H.; Kwok, J.Y.Y.; Choi, E.P.H.; Fong, D.Y.T.; Ali, S.; Wilson, C.A.; et al. Comparative effectiveness of interventions for the prevention and treatment of perinatal depression: A systematic review and network meta-analysis. Asian J. Psychiatr. 2025, 103, 104316. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant depression: Definition, prevalence, detection, management, and investigational interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Filteau, M.J.; Martin, L.; Patry, S.; Carvalho, A.; Cha, D.S.; Barakat, M.; Miguelez, M. Treatment-resistant depression: Definitions, review of the evidence, and algorithmic approach. J. Affect. Disord. 2014, 156, 1–7. [Google Scholar] [CrossRef]
- De Raedt, R.; Vanderhasselt, M.A.; Baeken, C. Neurostimulation as an intervention for treatment resistant depression: From research on mechanisms towards targeted neurocognitive strategies. Clin. Psychol. Rev. 2015, 41, 61–69. [Google Scholar] [CrossRef]
- Amasi-Hartoonian, N.; Pariante, C.M.; Cattaneo, A.; Sforzini, L. Understanding treatment-resistant depression using “omics” techniques: A systematic review. J. Affect. Disord. 2022, 318, 423–455. [Google Scholar] [CrossRef]
- Bayes, A.; Parker, G. How to choose an antidepressant medication. Acta Psychiatr. Scand. 2019, 139, 280–291. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef]
- Arakawa, R.; Stenkrona, P.; Takano, A.; Svensson, J.; Andersson, M.; Nag, S.; Asami, Y.; Hirano, Y.; Halldin, C.; Lundberg, J. Venlafaxine ER Blocks the Norepinephrine Transporter in the Brain of Patients with Major Depressive Disorder: A PET Study Using [18F]FMeNER-D2. Int. J. Neuropsychopharmacol. 2019, 22, 278–285. [Google Scholar] [CrossRef]
- Settle, E.C., Jr. Antidepressant drugs: Disturbing and potentially dangerous adverse effects. J. Clin. Psychiatry 1998, 59 (Suppl. 16), 25–30, discussion 40–22. [Google Scholar]
- Teply, R.M.; Packard, K.A.; White, N.D.; Hilleman, D.E.; DiNicolantonio, J.J. Treatment of Depression in Patients with Concomitant Cardiac Disease. Prog. Cardiovasc. Dis. 2016, 58, 514–528. [Google Scholar] [CrossRef]
- Wang, S.M.; Han, C.; Bahk, W.M.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, B. Adverse effects of antidepressant drugs: Part 1: Monoamine oxidase inhibitors and tricyclics. Drugs 1981, 21, 201–219. [Google Scholar] [CrossRef]
- Feighner, J.P. Mechanism of action of antidepressant medications. J. Clin. Psychiatry 1999, 60 (Suppl. 4), 4–11, discussion 12–13. [Google Scholar]
- Zhou, S.; Li, P.; Lv, X.; Lai, X.; Liu, Z.; Zhou, J.; Liu, F.; Tao, Y.; Zhang, M.; Yu, X.; et al. Adverse effects of 21 antidepressants on sleep during acute-phase treatment in major depressive disorder: A systemic review and dose-effect network meta-analysis. Sleep 2023, 46, zsad177. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Lees, K.R. Clinical experience with excitatory amino acid antagonist drugs. Stroke 1995, 26, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Okuya, M.; Hatano, M.; Matsuda, Y.; Iwata, N. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: A systematic review and network meta-analysis. Mol. Psychiatry 2023, 28, 402–409. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.; Walęcka, M.; Jernajczyk, W. Effects of Antidepressants on Sleep. Curr. Psychiatry Rep. 2017, 19, 63. [Google Scholar] [CrossRef]
- Beach, S.R.; Kostis, W.J.; Celano, C.M.; Januzzi, J.L.; Ruskin, J.N.; Noseworthy, P.A.; Huffman, J.C. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J. Clin. Psychiatry 2014, 75, e441–e449. [Google Scholar] [CrossRef] [PubMed]
- Gheysens, T.; Van Den Eede, F.; De Picker, L. The risk of antidepressant-induced hyponatremia: A meta-analysis of antidepressant classes and compounds. Eur. Psychiatry 2024, 67, e20. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W. Reminiscence therapy-based care program alleviates anxiety and depression, as well as improves the quality of life in recurrent gastric cancer patients. Front. Psychol. 2023, 14, 1133470. [Google Scholar] [CrossRef]
- Yeung, W.F.; Chung, K.F.; Ng, K.Y.; Yu, Y.M.; Ziea, E.T.; Ng, B.F. A systematic review on the efficacy, safety and types of Chinese herbal medicine for depression. J. Psychiatr. Res. 2014, 57, 165–175. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother. Res. 2018, 32, 1147–1162. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; Antonioli, L.; Blandizzi, C.; Calderone, V. Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 2876. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15, 229. [Google Scholar] [CrossRef]
- Nishikito, D.F.; Borges, A.C.A.; Laurindo, L.F.; Otoboni, A.; Direito, R.; Goulart, R.A.; Nicolau, C.C.T.; Fiorini, A.M.R.; Sinatora, R.V.; Barbalho, S.M. Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds. Pharmaceutics 2023, 15, 159. [Google Scholar] [CrossRef]
- Liu, L.Y.; Feng, B.; Chen, J.; Tan, Q.R.; Chen, Z.X.; Chen, W.S.; Wang, P.R.; Zhang, Z.J. Herbal medicine for hospitalized patients with severe depressive episode: A retrospective controlled study. J. Affect. Disord. 2015, 170, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.Y.; Yang, Z.; Huang, X.J.; Li, J.; Bao, W.Y.; Hurilebagen; Wulanqiqige; Wuyunsiriguleng; Cui, J.W.; Ma, L.Q.; et al. Mongolian Medicine Areca Thirteen Pill (GY-13) Improved Depressive Syndrome via upregulating cAMP/PKA/CREB/BDNF signaling pathway. J. Ethnopharmacol. 2022, 293, 115310. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Garg, M.; Prajapati, P.; Singh, P.K.; Chopra, R.; Kumari, A.; Mittal, A. Adaptogenic property of Asparagus racemosus: Future trends and prospects. Heliyon 2023, 9, e14932. [Google Scholar] [CrossRef]
- Mu, D.; Ma, Q. A Review of Antidepressant Effects and Mechanisms of Three Common Herbal Medicines: Panax ginseng, Bupleurum chinense, and Gastrodia elata. CNS Neurol. Disord. Drug Targets 2023, 22, 1164–1175. [Google Scholar] [CrossRef]
- Mokhtari, T. Targeting autophagy and neuroinflammation pathways with plant-derived natural compounds as potential antidepressant agents. Phytother. Res. 2022, 36, 3470–3489. [Google Scholar] [CrossRef]
- Yoon, S.; Iqbal, H.; Kim, S.M.; Jin, M. Phytochemicals That Act on Synaptic Plasticity as Potential Prophylaxis against Stress-Induced Depressive Disorder. Biomol. Ther. 2023, 31, 148–160. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Xi, Y.; Zhou, H.; Tang, Z.; Xiong, L.; Qin, D. Polyphenols: Natural Food-Grade Biomolecules for the Treatment of Nervous System Diseases from a Multi-Target Perspective. Pharmaceuticals 2024, 17, 775. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Li, Z.; Li, T.; Zhang, R.; Zhao, Y.; Cheng, T.; Zong, Z.; Ma, Y.; Zhang, D.; et al. Flavonoid 4,4’-dimethoxychalcone selectively eliminates senescent cells via activating ferritinophagy. Redox Biol. 2024, 69, 103017. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Y.; Su, Y.; Guan, W.; Wang, Y.; Han, J.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Luteolin: A promising multifunctional natural flavonoid for human diseases. Phytother. Res. 2024, 38, 3417–3443. [Google Scholar] [CrossRef]
- Rasmus, P.; Kozłowska, E. Antioxidant and Anti-Inflammatory Effects of Carotenoids in Mood Disorders: An Overview. Antioxidants 2023, 12, 676. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E.; et al. AdipoRon’s Impact on Alzheimer’s Disease-A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 484. [Google Scholar] [CrossRef]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Buchaim, R.L.; Goulart, R.A.; Rubira, C.J.; Barbalho, S.M. The Role of Resveratrol in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J. Med. Food 2022, 25, 797–806. [Google Scholar] [CrossRef]
- Costa, R.O.; Martins, L.F.; Tahiri, E.; Duarte, C.B. Brain-derived neurotrophic factor-induced regulation of RNA metabolism in neuronal development and synaptic plasticity. Wiley Interdiscip. Rev. RNA 2022, 13, e1713. [Google Scholar] [CrossRef]
- Gadad, B.S.; Vargas-Medrano, J.; Ramos, E.I.; Najera, K.; Fagan, M.; Forero, A.; Thompson, P.M. Altered levels of interleukins and neurotrophic growth factors in mood disorders and suicidality: An analysis from periphery to central nervous system. Transl. Psychiatry 2021, 11, 341. [Google Scholar] [CrossRef]
- Afzal, A.; Batool, Z.; Sadir, S.; Liaquat, L.; Shahzad, S.; Tabassum, S.; Ahmad, S.; Kamil, N.; Perveen, T.; Haider, S. Therapeutic Potential of Curcumin in Reversing the Depression and Associated Pseudodementia via Modulating Stress Hormone, Hippocampal Neurotransmitters, and BDNF Levels in Rats. Neurochem. Res. 2021, 46, 3273–3285. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Li, H.; Hao, B.; Cui, D.; Shang, R.; Lv, Y.; Liu, Y.; Pu, W.; Zhang, H.; et al. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. Int. J. Mol. Sci. 2023, 24, 5542. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Li, Y.; Kou, J.; Huang, F.; Liu, B.; Liu, K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur. J. Pharmacol. 2014, 745, 59–68. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Samanta, S.; Dash, R.; Karpiński, T.M.; Habibi, E.; Sadiq, A.; Ahmadi, A.; Bunagu, S. The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: Focus on the role of oxidative stress. Front. Pharmacol. 2022, 13, 1015835. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef]
- Idayu, N.F.; Hidayat, M.T.; Moklas, M.A.; Sharida, F.; Raudzah, A.R.; Shamima, A.R.; Apryani, E. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine 2011, 18, 402–407. [Google Scholar] [CrossRef]
- Ahmad, I.; Prabowo, W.C.; Arifuddin, M.; Fadraersada, J.; Indriyanti, N.; Herman, H.; Purwoko, R.Y.; Nainu, F.; Rahmadi, A.; Paramita, S.; et al. Species as Pharmacological Agents: From Abuse to Promising Pharmaceutical Products. Life 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fei, S.; Olatunji, O.J. LC/ESI/TOF-MS Characterization, Anxiolytic and Antidepressant-like Effects of. Molecules 2022, 27, 193. [Google Scholar] [CrossRef] [PubMed]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress- Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr. Neuropharmacol. 2021, 19, 1468–1495. [Google Scholar] [CrossRef]
- Szućko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and Neuroprotective Effects of Hericium erinaceus. Int. J. Mol. Sci. 2023, 24, 15960. [Google Scholar] [CrossRef]
- de Oliveira Zanuso, B.; de Oliveira Dos Santos, A.R.; Miola, V.F.B.; Guissoni Campos, L.M.; Spilla, C.S.G.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022, 161, 111731. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Peng, S.; Li, X.; Yao, J.; Xu, J.; Fang, J. Honokiol alleviates oxidative stress-induced neurotoxicity via activation of Nrf2. ACS Chem. Neurosci. 2018, 9, 3108–3116. [Google Scholar] [CrossRef]
- Wang, D.; Cao, L.; Zhou, X.; Wang, G.; Ma, Y.; Hao, X.; Fan, H. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. J. Hazard. Mater. 2022, 437, 129381. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, N. Berberine: Pathways to protect neurons. Phytother. Res. 2018, 32, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, Z.; Zhu, L.; Zhou, J.; Zhuo, L.; Zhang, J.; Zhang, X.; Liu, W.; Han, L.; Liao, W. Rhynchophylline alleviates neuroinflammation and regulates metabolic disorders in a mouse model of Parkinson’s disease. Food Funct. 2023, 14, 3208–3219. [Google Scholar] [CrossRef]
- Fathinezhad, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Depression and Treatment with Effective Herbs. Curr. Pharm. Des. 2019, 25, 738–745. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Therapeutic Effects of Phytochemicals and Medicinal Herbs on Depression. Biomed. Res. Int. 2017, 2017, 6596241. [Google Scholar] [CrossRef]
- Kou, Y.; Li, Z.; Yang, T.; Shen, X.; Wang, X.; Li, H.; Zhou, K.; Li, L.; Xia, Z.; Zheng, X. Therapeutic potential of plant iridoids in depression: A review. Pharm. Biol. 2022, 60, 2167–2181. [Google Scholar] [CrossRef]
- Xiong, Z.; Jiang, B.; Wu, P.-F.; Tian, J.; Shi, L.-L.; Gu, J.; Hu, Z.-L.; Fu, H.; Wang, F.; Chen, J.-G. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol. Pharm. Bull. 2011, 34, 253–259. [Google Scholar] [CrossRef]
- Gupta, J.; Gupta, R.; Varshney, K.K. Emerging mechanisms and potential antidepressant action of medicinal plants. Int. J. Pharm. Sci. Res. 2020, 11, 1–3. [Google Scholar]
- Picheta, N.; Piekarz, J.; Daniłowska, K.; Mazur, K.; Piecewicz-Szczęsna, H.; Smoleń, A. Phytochemicals in the treatment of patients with depression: A systemic review. Front. Psychiatry 2024, 15, 1509109. [Google Scholar] [CrossRef]
- Soltani, M.; Hosseinzadeh-Attar, M.J.; Rezaei, M.; Alipoor, E.; Vasheghani-Farahani, A.; Yaseri, M.; Rezayat, S.M. Effect of nano-curcumin supplementation on cardiometabolic risk factors, physical and psychological quality of life, and depression in patients with coronary slow flow phenomenon: A randomized double-blind clinical trial. Trials 2024, 25, 515. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Vafa, M.; Ebrahimkhani, A.; Găman, M.A.; Sezavar Seyedi Jandaghi, S.H. Effects of quercetin supplementation on endothelial dysfunction biomarkers and depression in post-myocardial infarction patients: A double-blind, placebo-controlled, randomized clinical trial. Clin. Nutr. ESPEN 2023, 56, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hajiluian, G.; Karegar, S.J.; Shidfar, F.; Aryaeian, N.; Salehi, M.; Lotfi, T.; Farhangnia, P.; Heshmati, J.; Delbandi, A.A. The effects of Ellagic acid supplementation on neurotrophic, inflammation, and oxidative stress factors, and indoleamine 2, 3-dioxygenase gene expression in multiple sclerosis patients with mild to moderate depressive symptoms: A randomized, triple-blind, placebo-controlled trial. Phytomedicine 2023, 121, 155094. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Vijayakumar, A.; Rooney, C.; Noad, R.L.; Appleton, K.M.; McCarthy, D.; Donnelly, M.; Young, I.S.; McKinley, M.C.; McKeown, P.P.; et al. A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients 2020, 12, 2445. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef]
- Hirose, A.; Terauchi, M.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Low-dose isoflavone aglycone alleviates psychological symptoms of menopause in Japanese women: A randomized, double-blind, placebo-controlled study. Arch. Gynecol. Obstet. 2016, 293, 609–615. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.H.; Park, M.; Lee, H.J. Effects of Flavonoid-Rich Orange Juice Intervention on Major Depressive Disorder in Young Adults: A Randomized Controlled Trial. Nutrients 2022, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Yamamoto, M.; Honmou, O.; Sasaki, M.; Haseda, A.; Kagami-Katsuyama, H.; Shoji, T.; Namioka, A.; Namioka, T.; Magota, H.; Oka, S.; et al. The Impact of Purple-Flesh Potato (Solanum tuberosum L.) cv. “Shadow Queen” on Minor Health Complaints in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 2446. [Google Scholar] [CrossRef]
- Barfoot, K.L.; Forster, R.; Lamport, D.J. Mental Health in New Mothers: A Randomised Controlled Study into the Effects of Dietary Flavonoids on Mood and Perceived Quality of Life. Nutrients 2021, 13, 2383. [Google Scholar] [CrossRef]
- Parilli-Moser, I.; Domínguez-López, I.; Trius-Soler, M.; Castellví, M.; Bosch, B.; Castro-Barquero, S.; Estruch, R.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M. Consumption of peanut products improves memory and stress response in healthy adults from the ARISTOTLE study: A 6-month randomized controlled trial. Clin. Nutr. 2021, 40, 5556–5567. [Google Scholar] [CrossRef] [PubMed]
- Bourdel-Marchasson, I.; Ostan, R.; Regueme, S.C.; Pinto, A.; Pryen, F.; Charrouf, Z.; d’Alessio, P.; Baudron, C.R.; Guerville, F.; Durrieu, J.; et al. Quality of Life: Psychological Symptoms-Effects of a 2-Month Healthy Diet and Nutraceutical Intervention; A Randomized, Open-Label Intervention Trial (RISTOMED). Nutrients 2020, 12, 800. [Google Scholar] [CrossRef]
- Park, M.; Choi, J.; Lee, H.J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef]
- Smetanka, A.; Stara, V.; Farsky, I.; Tonhajzerova, I.; Ondrejka, I. Pycnogenol supplementation as an adjunct treatment for antidepressant-induced sexual dysfunction. Physiol. Int. 2019, 106, 59–69. [Google Scholar] [CrossRef]
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox. Res. 2018, 33, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Barfoot, K.L.; May, G.; Lamport, D.J.; Reynolds, S.A.; Williams, C.M. Effects of Acute Blueberry Flavonoids on Mood in Children and Young Adults. Nutrients 2017, 9, 158. [Google Scholar] [CrossRef]
- Esmaily, H.; Sahebkar, A.; Iranshahi, M.; Ganjali, S.; Mohammadi, A.; Ferns, G.; Ghayour-Mobarhan, M. An investigation of the effects of curcumin on anxiety and depression in obese individuals: A randomized controlled trial. Chin. J. Integr. Med. 2015, 21, 332–338. [Google Scholar] [CrossRef]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Beckett, S.; Rigby, A.S.; Mellor, D.D.; Atkin, S.L. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr. J. 2010, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Malau, I.A.; Chang, J.P.-C.; Lin, Y.-W.; Chang, C.-C.; Chiu, W.-C.; Su, K.-P. Omega-3 Fatty Acids and Neuroinflammation in Depression: Targeting Damage-Associated Molecular Patterns and Neural Biomarkers. Cells 2024, 13, 1791. [Google Scholar] [CrossRef]
- Wang, J.; Behl, T.; Rana, T.; Sehgal, A.; Wal, P.; Saxena, B.; Yadav, S.; Mohan, S.; Anwer, M.K.; Chigurupati, S. Exploring the pathophysiological influence of heme oxygenase-1 on neuroinflammation and depression: A study of phytotherapeutic-based modulation. Phytomedicine 2024, 127, 155466. [Google Scholar] [CrossRef] [PubMed]
- Haase, J.; Brown, E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression—A central role for the serotonin transporter? Pharmacol. Ther. 2015, 147, 1–11. [Google Scholar] [CrossRef]
- Halaris, A. Inflammation and depression but where does the inflammation come from? Curr. Opin. Psychiatry 2019, 32, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, J.; Chung, S.K.; Xu, B. New insights into anti-depression effects of bioactive phytochemicals. Pharmacological Research 2024, 212, 107566. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Cunha, M.P.; Kaster, M.P.; Rodrigues, A.L.S. Natural polyphenols and terpenoids for depression treatment: Current status. Stud. Nat. Prod. Chem. 2018, 55, 181–221. [Google Scholar]
- Shi, X.; Fu, Y.; Zhang, S.; Ding, H.; Chen, J. Baicalin attenuates subarachnoid hemorrhagic brain injury by modulating blood-brain barrier disruption, inflammation, and oxidative damage in mice. Oxidative Med. Cell. Longev. 2017, 2017, 1401790. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Soltani, A.; Khodarahmi, Z.; Alameri, A.A.; Alwan, A.M.; Ramírez-Coronel, A.A.; Obaid, R.F.; Abosaooda, M.; Heidari, M.; Golmohammadi, M. Targeting Nrf2 signaling pathway by quercetin in the prevention and treatment of neurological disorders: An overview and update on new developments. Fundam. Clin. Pharmacol. 2023, 37, 1050–1064. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Grabacka, M.M.; Gawin, M.; Pierzchalska, M. Phytochemical modulators of mitochondria: The search for chemopreventive agents and supportive therapeutics. Pharmaceuticals 2014, 7, 913–942. [Google Scholar] [CrossRef]
- Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals 2025, 18, 607. [Google Scholar] [CrossRef]
- Bozzuto, G.; Calcabrini, A.; Colone, M.; Condello, M.; Dupuis, M.L.; Pellegrini, E.; Stringaro, A. Phytocompounds and nanoformulations for anticancer therapy: A review. Molecules 2024, 29, 3784. [Google Scholar] [CrossRef]
- Aburel, O.M.; Pavel, I.Z.; Dănilă, M.D.; Lelcu, T.; Roi, A.; Lighezan, R.; Muntean, D.M.; Rusu, L.C. Pleiotropic effects of eugenol: The good, the bad, and the unknown. Oxidative Med. Cell. Longev. 2021, 2021, 3165159. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jahan, S.; Imtiyaz, Z.; Alshahrani, S.; Antar Makeen, H.; Mohammed Alshehri, B.; Kumar, A.; Arafah, A.; Rehman, M.U. Neuroprotection: Targeting multiple pathways by naturally occurring phytochemicals. Biomedicines 2020, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.; Renner, B. Herb–drug interaction in inflammatory diseases: Review of phytomedicine and herbal supplements. J. Clin. Med. 2022, 11, 1567. [Google Scholar] [CrossRef]
- Tayeb, B.A.; Kusuma, I.Y.; Osman, A.A.; Minorics, R. Herbal compounds as promising therapeutic agents in precision medicine strategies for cancer: A systematic review. J. Integr. Med. 2024, 22, 137–162. [Google Scholar] [CrossRef]
- Dobrek, L.; Głowacka, K. Depression and its phytopharmacotherapy—A narrative review. Int. J. Mol. Sci. 2023, 24, 4772. [Google Scholar] [CrossRef]
- Singh, S.K.; Rashid, M.; Chaturvedi, S.; Agarwal, A.; Chauhan, D.; Gayen, J.R.; Wahajuddin, M. Preclinical pharmacokinetics, absolute bioavailability and dose proportionality evaluation of bioactive phytochemical Withanone in rats. Bioorganic Chem. 2025, 155, 108128. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M. Phytosomes as innovative delivery systems for phytochemicals: A comprehensive review of literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.; Hamman, J.H. What are the dangers of drug interactions with herbal medicines? Expert Opin. Drug Metab. toxicology 2020, 16, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Holleran, G.; Scaldaferri, F.; Gasbarrini, A.; Currò, D. Herbal medicinal products for inflammatory bowel disease: A focus on those assessed in double-blind randomised controlled trials. Phytother. Res. 2020, 34, 77–93. [Google Scholar] [CrossRef]
- Murphy, E.; McMahon, F.J. Pharmacogenetics of antidepressants, mood stabilizers, and antipsychotics in diverse human populations. Discov. Med. 2013, 16, 113–122. [Google Scholar]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Celenza, G.; Petricca, S. Multi-Target Effects of ß-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants 2022, 11, 1199. [Google Scholar] [CrossRef]
- Magni, G.; Riboldi, B.; Petroni, K.; Ceruti, S. Flavonoids bridging the gut and the brain: Intestinal metabolic fate, and direct or indirect effects of natural supporters against neuroinflammation and neurodegeneration. Biochem. Pharmacol. 2022, 205, 115257. [Google Scholar] [CrossRef]
- Coen, M.; Want, E.J.; Clayton, T.A.; Rhode, C.M.; Hong, Y.S.; Keun, H.C.; Cantor, G.H.; Metz, A.L.; Robertson, D.G.; Reily, M.D.; et al. Mechanistic aspects and novel biomarkers of responder and non-responder phenotypes in galactosamine-induced hepatitis. J. Proteome Res. 2009, 8, 5175–5187. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Almeida, S.S.; Zizzi, F.B.; Cattaneo, A.; Comandini, A.; Di Dato, G.; Lubrano, E.; Pellicano, C.; Spallone, V.; Tongiani, S.; Torta, R. Management and Treatment of Patients With Major Depressive Disorder and Chronic Diseases: A Multidisciplinary Approach. Front. Psychol. 2020, 11, 542444. [Google Scholar] [CrossRef]
- Pepe, M.; Bartolucci, G.; Marcelli, I.; Pesaresi, F.; Brugnami, A.; Caso, R.; Fischetti, A.; Grisoni, F.; Mazza, M.; Camardese, G.; et al. The Patient’s Perspective on the Effects of Intranasal Esketamine in Treatment-Resistant Depression. Brain Sci. 2023, 13, 1494. [Google Scholar] [CrossRef]
- Stolfi, F.; Abreu, H.; Sinella, R.; Nembrini, S.; Centonze, S.; Landra, V.; Brasso, C.; Cappellano, G.; Rocca, P.; Chiocchetti, A. Omics approaches open new horizons in major depressive disorder: From biomarkers to precision medicine. Front. Psychiatry 2024, 15, 1422939. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, T.; Liu, J.; Gao, C.; Zhang, X. Physical exercise and mitochondrial function: New therapeutic interventions for psychiatric and neurodegenerative disorders. Front. Neurol. 2022, 13, 929781. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Holtzheimer, P.E.; Gao, S.; Kirwin, D.S.; Price, R.B. Leveraging Neuroplasticity to Enhance Adaptive Learning: The Potential for Synergistic Somatic-Behavioral Treatment Combinations to Improve Clinical Outcomes in Depression. Biol. Psychiatry 2019, 85, 454–465. [Google Scholar] [CrossRef]
- Ashdown-Franks, G.; Firth, J.; Carney, R.; Carvalho, A.F.; Hallgren, M.; Koyanagi, A.; Rosenbaum, S.; Schuch, F.B.; Smith, L.; Solmi, M.; et al. Exercise as Medicine for Mental and Substance Use Disorders: A Meta-review of the Benefits for Neuropsychiatric and Cognitive Outcomes. Sports Med. 2020, 50, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, M. Effects of exercise therapy on global cognitive function and, depression in older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 106, 104855. [Google Scholar] [CrossRef]
- Gamage, E.; Orr, R.; Travica, N.; Lane, M.M.; Dissanayaka, T.; Kim, J.H.; Grosso, G.; Godos, J.; Marx, W. Polyphenols as novel interventions for depression: Exploring the efficacy, mechanisms of action, and implications for future research. Neurosci. Biobehav. Rev. 2023, 151, 105225. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L.; Giménez-Llort, L. Emerging translational research in neurological and psychiatric diseases: From in vitro to in vivo models. Int. J. Mol. Sci. 2023, 24, 15739. [Google Scholar] [CrossRef]
- Kessler, R.C.; van Loo, H.M.; Wardenaar, K.J.; Bossarte, R.M.; Brenner, L.; Ebert, D.; De Jonge, P.; Nierenberg, A.; Rosellini, A.; Sampson, N. Using patient self-reports to study heterogeneity of treatment effects in major depressive disorder. Epidemiol. Psychiatr. Sci. 2017, 26, 22–36. [Google Scholar] [CrossRef]
- Malgaroli, M.; Calderon, A.; Bonanno, G.A. Networks of major depressive disorder: A systematic review. Clin. Psychol. Rev. 2021, 85, 102000. [Google Scholar] [CrossRef]
- Mew, E.J.; Monsour, A.; Saeed, L.; Santos, L.; Patel, S.; Courtney, D.B.; Watson, P.N.; Szatmari, P.; Offringa, M.; Monga, S. Systematic scoping review identifies heterogeneity in outcomes measured in adolescent depression clinical trials. J. Clin. Epidemiol. 2020, 126, 71–79. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).