Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora
Abstract
1. Introduction
2. Neuroinflammation: A Core Driver of Severe Depression
3. Oxidative Stress: A Silent Driver of Depression
4. Depression’s Mitochondrial Roots and Repair Strategies
5. Nutrition, Inflammation, and Depressive Spirals
6. Rethinking Depression Care: Drugs and Natural Options
7. Plant-Based Therapies: Natural Allies Against Depression
8. From Curcumin to Cocoa: Plant-Based Mood Solutions
9. Discussion
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| DNA | Deoxyribonucleic acid |
| HAM-D | Hamilton depression rating scale |
| IL | Interleukin |
| MMA | Methylmalonic acid |
| MDD | Major depressive disorder |
| mtDNA | Mitochondrial deoxyribonucleic acid |
| NF-κB | Nuclear factor kappa B |
| ROS | Reactive oxygen species |
| SSRI | Selective serotonin reuptake inhibitor |
| TCA | Tricyclic antidepressant |
References
- Manosso, L.M.; Arent, C.O.; Borba, L.A.; Abelaira, H.M.; Réus, G.Z. Natural Phytochemicals for the Treatment of Major Depressive Disorder: A Mini-Review of Pre- and Clinical Studies. CNS Neurol. Disord. Drug Targets 2023, 22, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Suzuki, K.; Kavalali, E.T.; Monteggia, L.M. Ketamine: Mechanisms and Relevance to Treatment of Depression. Annu. Rev. Med. 2024, 75, 129–143. [Google Scholar] [CrossRef]
- Schoeller, F.; Jain, A.; Adrien, V.; Maes, P.; Reggente, N. Aesthetic chills mitigate maladaptive cognition in depression. BMC Psychiatry 2024, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Poluektova, E.U.; Zorkina, Y.A.; Kovtun, A.S.; Danilenko, V.N. Human Gut Microbiota for Diagnosis and Treatment of Depression. Int. J. Mol. Sci. 2024, 25, 5782. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhang, Y.; Wang, S.; Yan, Y.; Liu, M.; Tian, M.; Tian, W. Global, regional, and national temporal trend in burden of major depressive disorder from 1990 to 2019: An analysis of the global burden of disease study. Psychiatry Res. 2024, 337, 115958. [Google Scholar] [CrossRef]
- Bagby, S.P.; Martin, D.; Chung, S.T.; Rajapakse, N. From the outside in: Biological mechanisms linking social and environmental exposures to chronic disease and to health disparities. Am. J. Public Health 2019, 109, S56–S63. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Tantardini, M.; De Simone, S.; Ramella-Cravaro, V.; Oliver, D.; Kingdon, J.; Kotlicka-Antczak, M.; Valmaggia, L.; Lee, J.; Millan, M. Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur. Psychiatry 2017, 40, 65–75. [Google Scholar] [CrossRef]
- Lesch, K.-P. When the serotonin transporter gene meets adversity: The contribution of animal models to understanding epigenetic mechanisms in affective disorders and resilience. In Molecular and Functional Models in Neuropsychiatry; Springer: Berlin/Heidelberg, Germany, 2011; pp. 251–280. [Google Scholar]
- Simons, R.L.; Lei, M.K.; Stewart, E.A.; Beach, S.R.; Brody, G.H.; Philibert, R.A.; Gibbons, F.X. Social adversity, genetic variation, street code, and aggression: A genetically informed model of violent behavior. Youth Violence Juv. Justice 2012, 10, 3–24. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Pase, M.P.; Scholey, A.B.; Pipingas, A.; Kras, M.; Nolidin, K.; Gibbs, A.; Wesnes, K.; Stough, C. Cocoa polyphenols enhance positive mood states but not cognitive performance: A randomized, placebo-controlled trial. J. Psychopharmacol. 2013, 27, 451–458. [Google Scholar] [CrossRef]
- Jia, S.; Hou, Y.; Wang, D.; Zhao, X. Flavonoids for depression and anxiety: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 8839–8849. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, T.; Jiang, P.; Dang, R. The interaction between autophagy and neuroinflammation in major depressive disorder: From pathophysiology to therapeutic implications. Pharmacol. Res. 2021, 168, 105586. [Google Scholar] [CrossRef]
- Bertollo, A.G.; Mingoti, M.E.D.; Ignácio, Z.M. Neurobiological mechanisms in the kynurenine pathway and major depressive disorder. Rev. Neurosci. 2025, 36, 169–187. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, X.; Ma, Y.; Yang, Y.; Pan, C.W.; Zhu, X.; Ke, C. Metabolomics on depression: A comparison of clinical and animal research. J. Affect. Disord. 2024, 349, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals 2025, 18, 133. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15, 124. [Google Scholar] [CrossRef]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.; Beier, K.; Mardis, T.; Munoz, B.; Zaidi, A. The Neurochemistry of Depression: The Good, The Bad and The Ugly. Mo. Med. 2024, 121, 68–75. [Google Scholar] [PubMed]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural antioxidant anthocyanins—A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef]
- Jin, W.; Xu, X.; Chen, X.; Qi, W.; Lu, J.; Yan, X.; Zhao, D.; Cong, D.; Li, X.; Sun, L. Protective effect of pig brain polypeptides against corticosterone-induced oxidative stress, inflammatory response, and apoptosis in PC12 cells. Biomed. Pharmacother. 2019, 115, 108890. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2018, 2018, 1972714. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neural correlates and molecular mechanisms of memory and learning. Int. J. Mol. Sci. 2024, 25, 2724. [Google Scholar] [CrossRef]
- Yoder, R.; Michaud, A.; Feagans, A.; Hinton-Froese, K.E.; Meyer, A.; Powers, V.A.; Stalnaker, L.; Hord, M.K. Family-Based Treatment for Anxiety, Depression, and ADHD for a Parent and Child. Int. J. Environ. Res. Public Health 2024, 21, 504. [Google Scholar] [CrossRef]
- Moreira, J.; Machado, M.; Dias-Teixeira, M.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. The neuroprotective effect of traditional Chinese medicinal plants—A critical review. Acta Pharm. Sin. B 2023, 13, 3208–3237. [Google Scholar] [CrossRef]
- Ajao, A.A.-n.; Sabiu, S.; Balogun, F.O.; Adekomi, D.A.; Saheed, S.A. The Ambit of Phytotherapy in Psychotic Care. In Psychosis-Biopsychosocial and Relational Perspectives; IntechOpen: London, UK, 2018. [Google Scholar]
- Rajpal, V.R.; Koul, H.K.; Raina, S.N.; Kumar, H.M.S.; Qazi, G.N. Phytochemicals for Human Health: The Emerging Trends and Prospects. Curr. Top. Med. Chem. 2024, 24, v–vi. [Google Scholar] [CrossRef]
- Kumar, H.M.S.; Rajpal, V.R.; Koul, H.K.; Raina, S.N.; Qazi, G.N. Phytochemicals for Human Health: The Emerging Trends and Prospects, Part-3. Curr. Top. Med. Chem. 2024, 24, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Bai, W. Bioactive phytochemicals. Crit. Rev. Food Sci. Nutr. 2019, 59, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Fais, A.; Era, B. Phytochemical Composition and Biological Activity. Plants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Cao, G.; Zhao, D.; Li, G.; Zhang, H.; Yan, M. Ethnic, Botanic, Phytochemistry and Pharmacology of the. Molecules 2023, 28, 7117. [Google Scholar] [CrossRef]
- Reddy, K.; Stafford, G.I.; Makunga, N.P. Skeletons in the closet? Using a bibliometric lens to visualise phytochemical and pharmacological activities linked to. Front. Plant Sci. 2024, 15, 1268101. [Google Scholar] [CrossRef]
- Gong, G.; Ganesan, K.; Wang, Y.; Zhang, Z.; Liu, Y.; Wang, J.; Yang, F.; Zheng, Y. Ononin ameliorates depression-like behaviors by regulating BDNF-TrkB-CREB signaling in vitro and in vivo. J. Ethnopharmacol. 2024, 320, 117375. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. From Lab to Life: Exploring Cutting-Edge Models for Neurological and Psychiatric Disorders. Biomedicines 2024, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Seung, H.-B.; Kwon, H.-J.; Kwon, C.-Y.; Kim, S.-H. Neuroendocrine Biomarkers of Herbal Medicine for Major Depressive Disorder: A Systematic Review and Meta-Analysis. Pharmaceuticals 2023, 16, 1176. [Google Scholar] [CrossRef]
- Jaberi, K.R.; Alamdari-Palangi, V.; Savardashtaki, A.; Vatankhah, P.; Jamialahmadi, T.; Tajbakhsh, A.; Sahebkar, A. Modulatory Effects of Phytochemicals on Gut-Brain Axis: Therapeutic Implication. Curr. Dev. Nutr. 2024, 8, 103785. [Google Scholar] [CrossRef] [PubMed]
- Liloia, D.; Zamfira, D.A.; Tanaka, M.; Manuello, J.; Crocetta, A.; Keller, R.; Cozzolino, M.; Duca, S.; Cauda, F.; Costa, T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci. Biobehav. Rev. 2024, 164, 105791. [Google Scholar] [CrossRef]
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef]
- Kakarla, R.; Karuturi, P.; Siakabinga, Q.; Kasi Viswanath, M.; Dumala, N.; Guntupalli, C.; Nalluri, B.N.; Venkateswarlu, K.; Prasanna, V.S.; Gutti, G.; et al. Current understanding and future directions of cruciferous vegetables and their phytochemicals to combat neurological diseases. Phytother. Res. 2024, 38, 1381–1399. [Google Scholar] [CrossRef]
- Chandel, P.; Thapa, K.; Kanojia, N.; Rani, L.; Singh, T.G.; Rohilla, P. Exploring Therapeutic Potential of Phytoconstituents as a Gut Microbiota Modulator in the Management of Neurological and Psychological Disorders. Neuroscience 2024, 551, 69–78. [Google Scholar] [CrossRef]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13, 167. [Google Scholar] [CrossRef]
- Tanaka, M. Beyond the boundaries: Transitioning from categorical to dimensional paradigms in mental health diagnostics. Adv. Clin. Exp. Med. 2024, 33, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.L.d.S.; Martins, V.G.d.Q.A.; Silva, A.P.d.; Rocha, H.A.O.; Rachetti, V.d.P.S.; Scortecci, K.C. Phenolic acids as antidepressant agents. Nutrients 2022, 14, 4309. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.H.; Biswas, P.; Hossain, M.S.; Islam, R.; Hannan, M.A.; Uddin, M.J.; Rhim, H. Potential therapeutic role of phytochemicals to mitigate mitochondrial dysfunctions in Alzheimer’s disease. Antioxidants 2020, 10, 23. [Google Scholar] [CrossRef]
- Fazilat, S.; Tahmasbi, F.; Mirzaei, M.R.; Sanaie, S.; Yousefi, Z.; Asnaashari, S.; Yaqoubi, S.; Mohammadi, A.B.; Araj-khodaei, M. A systematic review on the use of phytotherapy in managing clinical depression. BioImpacts 2024, 15, 30532. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: New approaches to hippocampal neurogenesis. J. Neuroinflammation 2023, 20, 283. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef]
- Fornari Laurindo, L.; Aparecido Dias, J.; Cressoni Araújo, A.; Torres Pomini, K.; Machado Galhardi, C.; Rucco Penteado Detregiachi, C.; Santos de Argollo Haber, L.; Donizeti Roque, D.; Dib Bechara, M.; Vialogo Marques de Castro, M.; et al. Immunological dimensions of neuroinflammation and microglial activation: Exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front. Immunol. 2023, 14, 1305933. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Simili, O.A.G.; Araújo, A.C.; Guiguer, E.L.; Direito, R.; Valenti, V.E.; de Oliveira, V.; de Oliveira, J.S.; Yanaguizawa Junior, J.L.; Dias, J.A.; et al. Melatonin from Plants: Going Beyond Traditional Central Nervous System Targeting-A Comprehensive Review of Its Unusual Health Benefits. Biology 2025, 14, 143. [Google Scholar] [CrossRef]
- Tanaka, M.; Tuka, B.; Vécsei, L. Navigating the Neurobiology of Migraine: From pathways to potential therapies. Cells 2024, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.R.; Musella, A.; De Vito, F.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Stampanoni Bassi, M.; Buttari, F.; Mandolesi, G. Tumor necrosis factor and interleukin-1β modulate synaptic plasticity during neuroinflammation. Neural Plast. 2018, 2018, 8430123. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.V.; Prehar, S.; Kennedy, A.R.; Little, R.A.; Hopkins, S.J. Interleukin-6 is an afferent signal to the hypothalamo-pituitary-adrenal axis during local inflammation in mice. Endocrinology 2003, 144, 1894–1906. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Zhang, L.; Cheng, J.-K.; Ji, R.-R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef]
- De Simoni, M.; Terreni, L.; Chiesa, R.; Mangiarotti, F.; Forloni, G. Interferon-γ potentiates interleukin (IL)-6 and tumor necrosis factor-α but not IL-1β induced by endotoxin in the brain. Endocrinology 1997, 138, 5220–5226. [Google Scholar] [CrossRef]
- Chaves, F.M.; Mansano, N.S.; Frazão, R.; Donato Jr, J. Tumor necrosis factor α and interleukin-1β acutely inhibit AgRP neurons in the arcuate nucleus of the hypothalamus. Int. J. Mol. Sci. 2020, 21, 8928. [Google Scholar] [CrossRef]
- Li, B.; Yang, W.; Ge, T.; Wang, Y.; Cui, R. Stress induced microglial activation contributes to depression. Pharmacol. Res. 2022, 179, 106145. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gao, Z.; Hu, H. Microglia in depression: Current perspectives. Sci. China Life Sci. 2021, 64, 911–925. [Google Scholar] [CrossRef]
- Turkin, A.; Tuchina, O.; Klempin, F. Microglia function on precursor cells in the adult hippocampus and their responsiveness to serotonin signaling. Front. Cell Dev. Biol. 2021, 9, 665739. [Google Scholar] [CrossRef]
- Zanella, I. Neuroinflammation: From Molecular Basis to Therapy. Int. J. Mol. Sci. 2024, 25, 5973. [Google Scholar] [CrossRef]
- Poletti, S.; Mazza, M.G.; Benedetti, F. Inflammatory mediators in major depression and bipolar disorder. Transl. Psychiatry 2024, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.Y.; Guo, Y.X.; Lian, W.W.; Yan, Y.; Ma, B.Z.; Cheng, Y.C.; Xu, J.K.; He, J.; Zhang, W.K. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol. Res. 2023, 187, 106625. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Li, W.; Chen, P.; Wang, L.; Bao, X.; Huang, R.; Liu, G.; Chen, X. Microglial NLRP3 inflammasome-mediated neuroinflammation and therapeutic strategies in depression. Neural Regen. Res. 2024, 19, 1890–1898. [Google Scholar] [CrossRef]
- Bearoff, F.; Dhavale, D.; Kotzbauer, P.; Kortagere, S. Aggregated Alpha-Synuclein Activates Pro-Inflammatory NFKB Signaling Pathways Through TLR-Dependent and Independent Mechanisms in Peripheral Monocytic Cells. FASEB J. 2022, 36, 1–10. [Google Scholar] [CrossRef]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood–brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Wang, X.; Antony, V.; Wang, Y.; Wu, G.; Liang, G. Pattern recognition receptor-mediated inflammation in diabetic vascular complications. Med. Res. Rev. 2020, 40, 2466–2484. [Google Scholar] [CrossRef]
- Barrera, M.-J.; Aguilera, S.; Castro, I.; Carvajal, P.; Jara, D.; Molina, C.; González, S.; González, M.-J. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: Potential role in Sjögren’s syndrome. Autoimmun. Rev. 2021, 20, 102867. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, H.; Zhang, T.; Wu, J.; Tang, H.; Liang, X.; Ren, D.; Huang, J.; Li, W. Mitochondria of intestinal epithelial cells in depression: Are they at a crossroads of gut-brain communication? Neurosci. Biobehav. Rev. 2023, 153, 105403. [Google Scholar] [CrossRef]
- Sipahi, H.; Mat, A.F.; Ozhan, Y.; Aydin, A. The interrelation between oxidative stress, depression and inflammation through the kynurenine pathway. Curr. Top. Med. Chem. 2023, 23, 415–425. [Google Scholar] [CrossRef]
- Wakasugi, D.; Kondo, S.; Ferdousi, F.; Mizuno, S.; Yada, A.; Tominaga, K.; Takahashi, S.; Isoda, H. A rare olive compound oleacein functions as a TrkB agonist and mitigates neuroinflammation both in vitro and in vivo. Cell Commun. Signal 2024, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y. Progress of plant polyphenol extracts in treating depression by anti-neuroinflammatory mechanism: A review. Medicine 2024, 103, e37151. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Dolcetti, E.; Rizzo, F.R.; Fresegna, D.; Musella, A.; Gentile, A.; De Vito, F.; Caioli, S.; Guadalupi, L.; Bullitta, S.; et al. Corrigendum: Inflammation-Associated Synaptic Alterations as Shared Threads in Depression and Multiple Sclerosis. Front. Cell Neurosci. 2020, 14, 647259. [Google Scholar] [CrossRef]
- Sha, Q.; Escobar Galvis, M.L.; Madaj, Z.B.; Keaton, S.A.; Smart, L.; Edgerly, Y.M.; Anis, E.; Leach, R.; Osborne, L.M.; Achtyes, E.; et al. Dysregulated placental expression of kynurenine pathway enzymes is associated with inflammation and depression in pregnancy. Brain Behav. Immun. 2024, 119, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Achtyes, E.; Keaton, S.A.; Smart, L.; Burmeister, A.R.; Heilman, P.L.; Krzyzanowski, S.; Nagalla, M.; Guillemin, G.J.; Escobar Galvis, M.L.; Lim, C.K.; et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 2020, 83, 239–247. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J. Neural Transm. 2024, 131, 1367–1387. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. A decade of dedication: Pioneering perspectives on neurological diseases and mental illnesses. Biomedicines 2024, 12, 1083. [Google Scholar] [CrossRef]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016, 58, 1–8. [Google Scholar] [CrossRef]
- Gustafsson, H.C.; Sullivan, E.L.; Nousen, E.K.; Sullivan, C.A.; Huang, E.; Rincon, M.; Nigg, J.T.; Loftis, J.M. Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain Behav. Immun. 2018, 73, 470–481. [Google Scholar] [CrossRef]
- Pontillo, G.; Cepas, M.B.; Broeders, T.A.A.; Koubiyr, I.; Schoonheim, M.M. Network Analysis in Multiple Sclerosis and Related Disorders. Neuroimaging Clin. N. Am. 2024, 34, 375–384. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Z.; Wang, J.; Gao, M.; Zhang, Y.; Yang, C.; Zhang, A.; Li, G.; Li, X.; Liu, S.; et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat. Commun. 2024, 15, 3003. [Google Scholar] [CrossRef] [PubMed]
- Molina Galindo, L.S.; Gonzalez-Escamilla, G.; Fleischer, V.; Grotegerd, D.; Meinert, S.; Ciolac, D.; Person, M.; Stein, F.; Brosch, K.; Nenadić, I.; et al. Concurrent inflammation-related brain reorganization in multiple sclerosis and depression. Brain Behav. Immun. 2024, 119, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int. J. Mol. Med. 2024, 53, 47. [Google Scholar] [CrossRef]
- Cui, J.-J.; Huang, Z.-Y.; Xie, Y.-H.; Wu, J.-B.; Xu, G.-H.; Li, C.-F.; Zhang, M.-M.; Yi, L.-T. Gut microbiota mediated inflammation, neuroendocrine and neurotrophic functions involved in the antidepressant-like effects of diosgenin in chronic restraint stress. J. Affect. Disord. 2023, 321, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Reiche, E.M.V.; Murru, A.; Carvalho, A.F.; Maes, M.; Berk, M.; Puri, B.K. Multiple immune-inflammatory and oxidative and nitrosative stress pathways explain the frequent presence of depression in multiple sclerosis. Mol. Neurobiol. 2018, 55, 6282–6306. [Google Scholar] [CrossRef]
- Adzic, M.; Brkic, Z.; Mitic, M.; Francija, E.; Jovicic, M.J.; Radulovic, J.; Maric, N.P. Therapeutic strategies for treatment of inflammation-related depression. Curr. Neuropharmacol. 2018, 16, 176–209. [Google Scholar] [CrossRef]
- Khan, M.; Baussan, Y.; Hebert-Chatelain, E. Connecting dots between mitochondrial dysfunction and depression. Biomolecules 2023, 13, 695. [Google Scholar] [CrossRef]
- Casaril, A.M.; Dantzer, R.; Bas-Orth, C. Neuronal mitochondrial dysfunction and bioenergetic failure in inflammation-associated depression. Front. Neurosci. 2021, 15, 725547. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative stress in depression: The link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Int. J. Mol. Sci. 2024, 25, 3360. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: Treatment implications. Curr. Pharm. Des. 2014, 20, 3812–3847. [Google Scholar] [CrossRef]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef]
- Ilavská, L.; Morvová, M.; Paduchová, Z.; Muchová, J.; Garaiova, I.; Ďuračková, Z.; Šikurová, L.; Trebatická, J. The kynurenine and serotonin pathway, neopterin and biopterin in depressed children and adolescents: An impact of omega-3 fatty acids, and association with markers related to depressive disorder. A randomized, blinded, prospective study. Front. Psychiatry 2024, 15, 1347178. [Google Scholar] [CrossRef]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knock-out Mice: A Novel Model for Experience-Based Depression and Post-Traumatic Stress Disorder. Front. Biosci. 2025, 30, 25706. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Larrea, A.; Sánchez-Sánchez, L.; Diez-Martin, E.; Elexpe, A.; Torrecilla, M.; Astigarraga, E.; Barreda-Gómez, G. Mitochondrial Metabolism in Major Depressive Disorder: From Early Diagnosis to Emerging Treatment Options. J. Clin. Med. 2024, 13, 1727. [Google Scholar] [CrossRef]
- Chen, H.; Lu, M.; Lyu, Q.; Shi, L.; Zhou, C.; Li, M.; Feng, S.; Liang, X.; Zhou, X.; Ren, L. Mitochondrial dynamics dysfunction: Unraveling the hidden link to depression. Biomed. Pharmacother. 2024, 175, 116656. [Google Scholar] [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. The impact of C-3 side chain modifications on Kynurenic Acid: A behavioral analysis of its analogs in the Motor Domain. Int. J. Mol. Sci. 2024, 25, 3394. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in cognitive impairment and mood disorders for experimental, clinical and translational neuropsychiatry. Biomedicines 2024, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, H.; Tan, Y.; Yu, X.D.; Xiao, C.; Li, Y.; Reilly, J.; He, Z.; Shu, X. Protection of p-Coumaric acid against chronic stress-induced neurobehavioral deficits in mice via activating the PKA-CREB-BDNF pathway. Physiol. Behav. 2024, 273, 114415. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cao, H.; Zuo, C.; Gu, Z.; Huang, Y.; Miao, J.; Fu, Y.; Guo, Y.; Jiang, Y.; Wang, F. Mitochondrial dysfunction: A fatal blow in depression. Biomed. Pharmacother. 2023, 167, 115652. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, S.; Liu, J.; Wu, X.; Zhou, S.; Dai, K.; Kou, Y. Mitophagy Contributes to the Pathogenesis of Inflammatory Diseases. Inflammation 2018, 41, 1590–1600. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Han, S.; Zhang, M.; Jeong, Y.Y.; Margolis, D.J.; Cai, Q. The role of mitophagy in the regulation of mitochondrial energetic status in neurons. Autophagy 2021, 17, 4182–4201. [Google Scholar] [CrossRef]
- Visentin, A.P.V.; Colombo, R.; Scotton, E.; Fracasso, D.S.; da Rosa, A.R.; Branco, C.S.; Salvador, M. Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxid. Med. Cell Longev. 2020, 2020, 2972968. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front. Endocrinol. 2023, 14, 1134025. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Moreno, J.J.; Bodega, P.; de Cos-Gandoy, A.; de Miguel, M.; Santos-Beneit, G.; Fernández-Alvira, J.M.; Fernández-Jiménez, R.; Martínez-Gómez, J.; et al. Metabolic syndrome, adiposity, diet, and emotional eating are associated with oxidative stress in adolescents. Front. Nutr. 2023, 10, 1216445. [Google Scholar] [CrossRef]
- Heiss, C.N.; Mannerås-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Håkansson Gladh, A.; Seeley, R.J.; Drucker, D.J.; Bäckhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021, 35, 109163. [Google Scholar] [CrossRef]
- Li, H.; Song, L.; Cen, M.; Fu, X.; Gao, X.; Zuo, Q.; Wu, J. Oxidative balance scores and depressive symptoms: Mediating effects of oxidative stress and inflammatory factors. J. Affect. Disord. 2023, 334, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16, 2721. [Google Scholar] [CrossRef]
- Pagotto, G.L.O.; Santos, L.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.; Goulart, R.A.; Catharin, V.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef]
- Takeda, L.N.; Omine, A.; Laurindo, L.F.; Araújo, A.C.; Machado, N.M.; Dias, J.A.; Kavalakatt, J.; Banerjee, S.; de Alvares Goulart, R.; Atanasov, A.G.; et al. Brazil nut (Bertholletia excelsa Bonpl.) in health and disease: A narrative review. Food Chem. 2025, 477, 143425. [Google Scholar] [CrossRef]
- Corraliza-Gómez, M.; Gallardo, A.B.; Díaz-Marrero, A.R.; de la Rosa, J.M.; D’Croz, L.; Darias, J.; Arranz, E.; Cózar-Castellano, I.; Ganfornina, M.D.; Cueto, M. Modulation of Glial Responses by Furanocembranolides: Leptolide Diminishes Microglial Inflammation in Vitro and Ameliorates Gliosis In Vivo in a Mouse Model of Obesity and Insulin Resistance. Mar. Drugs 2020, 18. [Google Scholar] [CrossRef]
- Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Szalai, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: Effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1212–1226. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, Y.; Qin, Y.; Zhou, Y.; Tang, R.; Wang, Q.; Li, X.; Wang, H.; Weston-Green, K.; Huang, X.F.; et al. Alterations to the microbiota-colon-brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J. Nutr. Biochem. 2019, 65, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Tobe, E.H. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr. Dis. Treat. 2013, 9, 567–573. [Google Scholar] [CrossRef]
- Maurya, P.K.; Noto, C.; Rizzo, L.B.; Rios, A.C.; Nunes, S.O.; Barbosa, D.S.; Sethi, S.; Zeni, M.; Mansur, R.B.; Maes, M.; et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 134–144. [Google Scholar] [CrossRef]
- Allen, J.; Romay-Tallon, R.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Mitochondria and Mood: Mitochondrial Dysfunction as a Key Player in the Manifestation of Depression. Front. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, L.; Chen, Y.; Zhuo, Y.; Wan, S.; Guo, R. Association between mitochondrial DNA levels and depression: A systematic review and meta-analysis. BMC Psychiatry 2023, 23, 866. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, L.; Sheng, H. Mitochondria in depression: The dysfunction of mitochondrial energy metabolism and quality control systems. CNS Neurosci. Ther. 2024, 30, e14576. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Rezin, G.T.; Amboni, G.; Zugno, A.I.; Quevedo, J.; Streck, E.L. Mitochondrial dysfunction and psychiatric disorders. Neurochem. Res. 2009, 34, 1021–1029. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial dysfunction in aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Mason, B.L.; Diaz, A.P.; Jha, M.K.; Soares, J.C.; Trivedi, M.H.; Quevedo, J. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: Does inflammation play a role? Mol. Psychiatry 2022, 27, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Lin, G.; Su, L.; Xue, L.; Duan, K.; Chen, L.; Ni, J. The association between methylmalonic acid, a biomarker of mitochondrial dysfunction, and cause-specific mortality in Alzheimer’s disease and Parkinson’s disease. Heliyon 2024, 10, e29357. [Google Scholar] [CrossRef]
- Guo, J.; Wang, S.; Wan, X.; Liu, X.; Wang, Z.; Liang, C.; Zhang, Z.; Wang, Y.; Yan, M.; Wu, P.; et al. Mitochondria-derived methylmalonic acid aggravates ischemia-reperfusion injury by activating reactive oxygen species-dependent ferroptosis. Cell Commun. Signal 2024, 22, 53. [Google Scholar] [CrossRef]
- Cao, B.; Xiao, Y.; Liu, D. Associations of methylmalonic acid and depressive symptoms with mortality: A population-based study. Transl. Psychiatry 2024, 14, 297. [Google Scholar] [CrossRef]
- Huang, Q.N.; Watanabe, F.; Koseki, K.; He, R.E.; Lee, H.L.; Chiu, T.H.T. Effect of roasted purple laver (nori) on vitamin B(12) nutritional status of vegetarians: A dose-response trial. Eur. J. Nutr. 2024, 63, 3269–3279. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Z.; Zhao, Y.; Sai, N.; Wang, X.; Liu, H.; Huang, G.; Zhang, X. Homocysteine induces mitochondrial dysfunction involving the crosstalk between oxidative stress and mitochondrial pSTAT3 in rat ischemic brain. Sci. Rep. 2017, 7, 6932. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- de Moraes, M.S.; Guerreiro, G.; Sitta, A.; de Moura Coelho, D.; Manfredini, V.; Wajner, M.; Vargas, C.R. Oxidative damage in mitochondrial fatty acids oxidation disorders patients and the in vitro effect of l-carnitine on DNA damage induced by the accumulated metabolites. Arch. Biochem. Biophys. 2020, 679, 108206. [Google Scholar] [CrossRef] [PubMed]
- Butkowski, E.G.; Al-Aubaidy, H.A.; Jelinek, H.F. Interaction of homocysteine, glutathione and 8-hydroxy-2′-deoxyguanosine in metabolic syndrome progression. Clin. Biochem. 2017, 50, 116–120. [Google Scholar] [CrossRef]
- Zhao, X.; Abulikemu, A.; Lv, S.; Qi, Y.; Duan, J.; Zhang, J.; Chen, R.; Guo, C.; Li, Y.; Sun, Z. Oxidative stress- and mitochondrial dysfunction-mediated cytotoxicity by silica nanoparticle in lung epithelial cells from metabolomic perspective. Chemosphere 2021, 275, 129969. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S.; Liloia, D. Navigating Neurodegeneration: Integrating Biomarkers, Neuroinflammation, and Imaging in Parkinson’s, Alzheimer’s, and Motor Neuron Disorders. Biomedicines 2025, 13, 1045. [Google Scholar] [CrossRef]
- Park, A.; Oh, M.; Lee, S.J.; Oh, K.J.; Lee, E.W.; Lee, S.C.; Bae, K.H.; Han, B.S.; Kim, W.K. Mitochondrial Transplantation as a Novel Therapeutic Strategy for Mitochondrial Diseases. Int. J. Mol. Sci. 2021, 22, 4793. [Google Scholar] [CrossRef]
- Riou, A.; Broeglin, A.; Grimm, A. Mitochondrial transplantation in brain disorders: Achievements, methods, and challenges. Neurosci. Biobehav. Rev. 2025, 169, 105971. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Hamad, R.S.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E. Role of brain renin-angiotensin system in depression: A new perspective. CNS Neurosci. Ther. 2024, 30, e14525. [Google Scholar] [CrossRef]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective Effects of Melatonin and Mitochondria-targeted Antioxidants Against Oxidative Stress: A Review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef]
- Koh, J.H.; Kim, J.Y. Role of PGC-1α in the Mitochondrial NAD(+) Pool in Metabolic Diseases. Int. J. Mol. Sci. 2021, 22, 4558. [Google Scholar] [CrossRef]
- Hidalgo-Gutiérrez, A.; González-García, P.; Díaz-Casado, M.E.; Barriocanal-Casado, E.; López-Herrador, S.; Quinzii, C.M.; López, L.C. Metabolic Targets of Coenzyme Q10 in Mitochondria. Antioxidants 2021, 10, 520. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Briston, T.; Selwood, D.L.; Szabadkai, G.; Duchen, M.R. Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol. Sci. 2019, 40, 50–70. [Google Scholar] [CrossRef]
- Tao, J.; Chen, H.; Wang, Y.J.; Qiu, J.X.; Meng, Q.Q.; Zou, R.J.; Li, L.; Huang, J.G.; Zhao, Z.K.; Huang, Y.L.; et al. Ketogenic Diet Suppressed T-Regulatory Cells and Promoted Cardiac Fibrosis via Reducing Mitochondria-Associated Membranes and Inhibiting Mitochondrial Function. Oxid. Med. Cell Longev. 2021, 2021, 5512322. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Tian, Y.; Shi, J. Sirtuin 3 and mitochondrial permeability transition pore (mPTP): A systematic review. Mitochondrion 2022, 64, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, H.; Yang, X.X.; Pang, L.; Liu, J.; Ng, K.T.P.; Yeung, O.W.H.; Lam, Y.F.; Zhang, W.Y.; Lo, C.M.; et al. Inhibition of Carnitine Palmitoyltransferase 1A Aggravates Fatty Liver Graft Injury via Promoting Mitochondrial Permeability Transition. Transplantation 2021, 105, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Kalani, K.; Yan, S.F.; Yan, S.S. Mitochondrial permeability transition pore: A potential drug target for neurodegeneration. Drug Discov. Today 2018, 23, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Khiroya, K.; Sekyere, E.; McEwen, B.; Bayes, J. Nutritional considerations in major depressive disorder: Current evidence and functional testing for clinical practice. Nutr. Res. Rev. 2023, 38, 25–36. [Google Scholar] [CrossRef]
- Bernier, V.; Debarge, M.H.; Hein, M.; Ammendola, S.; Mungo, A.; Loas, G. Major Depressive Disorder, Inflammation, and Nutrition: A Tricky Pattern? Nutrients 2023, 15, 3438. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Mora, F.; Rodríguez-Jiménez, R.; Fernandez-Rojo, S.; et al. Nutrition, Epigenetics, and Major Depressive Disorder: Understanding the Connection. Front. Nutr. 2022, 9, 867150. [Google Scholar] [CrossRef]
- Muha, J.; Schumacher, A.; Campisi, S.C.; Korczak, D.J. Depression and emotional eating in children and adolescents: A systematic review and meta-analysis. Appetite 2024, 200, 107511. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, G.N.; Sanlier, N. The relationship between nutrition and depression in the life process: A mini-review. Exp. Gerontol. 2023, 172, 112072. [Google Scholar] [CrossRef]
- Chrzastek, Z.; Guligowska, A.; Sobczuk, P.; Kostka, T. Dietary factors, risk of developing depression, and severity of its symptoms in older adults-A narrative review of current knowledge. Nutrition 2023, 106, 111892. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’Neil, A.; Jacka, F.N.; Marx, W. Ultra-Processed Food Consumption and Mental Health: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2568. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mardi, P.; Hejrani, B.; Mahdavi, F.S.; Ghoreshi, B.; Gohari, K.; Heidari-Beni, M.; Qorbani, M. Association between junk food consumption and mental health problems in adults: A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 438. [Google Scholar] [CrossRef]
- Malmir, H.; Mahdavi, F.S.; Ejtahed, H.S.; Kazemian, E.; Chaharrahi, A.; Mohammadian Khonsari, N.; Mahdavi-Gorabi, A.; Qorbani, M. Junk food consumption and psychological distress in children and adolescents: A systematic review and meta-analysis. Nutr. Neurosci. 2023, 26, 807–827. [Google Scholar] [CrossRef]
- Aly, J.; Engmann, O. The Way to a Human’s Brain Goes Through Their Stomach: Dietary Factors in Major Depressive Disorder. Front. Neurosci. 2020, 14, 582853. [Google Scholar] [CrossRef]
- Kunugi, H. Depression and lifestyle: Focusing on nutrition, exercise, and their possible relevance to molecular mechanisms. Psychiatry Clin. Neurosci. 2023, 77, 420–433. [Google Scholar] [CrossRef]
- Swainson, J.; Reeson, M.; Malik, U.; Stefanuk, I.; Cummins, M.; Sivapalan, S. Diet and depression: A systematic review of whole dietary interventions as treatment in patients with depression. J. Affect. Disord. 2023, 327, 270–278. [Google Scholar] [CrossRef]
- Selvaraj, R.; Selvamani, T.Y.; Zahra, A.; Malla, J.; Dhanoa, R.K.; Venugopal, S.; Shoukrie, S.I.; Hamouda, R.K.; Hamid, P. Association Between Dietary Habits and Depression: A Systematic Review. Cureus 2022, 14, e32359. [Google Scholar] [CrossRef]
- Gorbachev, D.; Markina, E.; Chigareva, O.; Gradinar, A.; Borisova, N.; Syunyakov, T. Dietary Patterns as Modifiable Risk Factors for Depression: A Narrative Review. Psychiatr. Danub. 2023, 35, 423–431. [Google Scholar] [PubMed]
- Zhang, Q.; Yi, J.; Wu, Y. Oxidative stress and inflammation mediate the association between elevated oxidative balance scores and improved sleep quality: Evidence from NHANES. Front. Nutr. 2024, 11, 1469779. [Google Scholar] [CrossRef]
- Wilson, D.; Driller, M.; Winwood, P.; Clissold, T.; Johnston, B.; Gill, N. The Effectiveness of a Combined Healthy Eating, Physical Activity, and Sleep Hygiene Lifestyle Intervention on Health and Fitness of Overweight Airline Pilots: A Controlled Trial. Nutrients 2022, 14, 1988. [Google Scholar] [CrossRef]
- Bolognese, M.A.; Franco, C.B.; Ferrari, A.; Bennemann, R.M.; Lopes, S.M.A.; Bertolini, S.; Júnior, N.N.; Branco, B.H.M. Group Nutrition Counseling or Individualized Prescription for Women With Obesity? A Clinical Trial. Front. Public Health 2020, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.; Wani, S.U.D.; Krishna, K.L.; Kinattingal, N.; Roohi, T.F. A review on linking stress, depression, and insulin resistance via low-grade chronic inflammation. Biochem. Biophys. Rep. 2023, 36, 101571. [Google Scholar] [CrossRef]
- Pickersgill, J.W.; Turco, C.V.; Ramdeo, K.; Rehsi, R.S.; Foglia, S.D.; Nelson, A.J. The Combined Influences of Exercise, Diet and Sleep on Neuroplasticity. Front. Psychol. 2022, 13, 831819. [Google Scholar] [CrossRef] [PubMed]
- Magzal, F.; Turroni, S.; Fabbrini, M.; Barone, M.; Vitman Schorr, A.; Ofran, A.; Tamir, S. A personalized diet intervention improves depression symptoms and changes microbiota and metabolite profiles among community-dwelling older adults. Front. Nutr. 2023, 10, 1234549. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, W.; Zhu, X.; Hu, Z.; Li, S.; Zheng, B.; Xu, H.; Long, W.; Xiong, X. Association between dietary intake and symptoms of depression and anxiety in pregnant women: Evidence from a community-based observational study. Food Sci. Nutr. 2023, 11, 7555–7564. [Google Scholar] [CrossRef]
- Serrano Ripoll, M.J.; Oliván-Blázquez, B.; Vicens-Pons, E.; Roca, M.; Gili, M.; Leiva, A.; García-Campayo, J.; Demarzo, M.P.; García-Toro, M. Lifestyle change recommendations in major depression: Do they work? J. Affect. Disord. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Ip, A.K.; Ho, F.Y.; Yeung, W.F.; Chung, K.F.; Ng, C.H.; Oliver, G.; Sarris, J. Effects of a group-based lifestyle medicine for depression: A pilot randomized controlled trial. PLoS ONE 2021, 16, e0258059. [Google Scholar] [CrossRef]
- Sarris, J.; O’Neil, A.; Coulson, C.E.; Schweitzer, I.; Berk, M. Lifestyle medicine for depression. BMC Psychiatry 2014, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Dale, E.; Bang-Andersen, B.; Sánchez, C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem. Pharmacol. 2015, 95, 81–97. [Google Scholar] [CrossRef]
- Fagiolini, A.; Florea, I.; Loft, H.; Christensen, M.C. Effectiveness of Vortioxetine on Emotional Blunting in Patients with Major Depressive Disorder with inadequate response to SSRI/SNRI treatment. J. Affect. Disord. 2021, 283, 472–479. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, D.; Wang, J.; Zhou, D.; Liu, A.; Sun, Y.; Yuan, Y.; Li, J.; Guo, W. The pharmacological mechanism of chaihu-jia-longgu-muli-tang for treating depression: Integrated meta-analysis and network pharmacology analysis. Front. Pharmacol. 2023, 14, 1257617. [Google Scholar] [CrossRef] [PubMed]
- Zhichao, H.; Ching, L.W.; Huijuan, L.; Liang, Y.; Zhiyu, W.; Weiyang, H.; Zhaoxiang, B.; Linda, Z.L.D. A network meta-analysis on the effectiveness and safety of acupuncture in treating patients with major depressive disorder. Sci. Rep. 2021, 11, 10384. [Google Scholar] [CrossRef]
- Qaseem, A.; Owens, D.K.; Etxeandia-Ikobaltzeta, I.; Tufte, J.E.; Cross, J.T.; Wilt, T.J.; Physicians, C.G.C.f.t.A.C.o. Nonpharmacologic and Pharmacologic Treatments of Adults in the Acute Phase of Major Depressive Disorder: A Living Clinical Guideline From the American College of Physicians (Version 1, Update Alert 2). Ann. Intern. Med. 2024, 177, 8. [Google Scholar] [CrossRef]
- Direito, R.; Barbalho, S.M.; Sepodes, B.; Figueira, M.E. Plant-Derived Bioactive Compounds: Exploring Neuroprotective, Metabolic, and Hepatoprotective Effects for Health Promotion and Disease Prevention. Pharmaceutics 2024, 16, 577. [Google Scholar] [CrossRef]
- Marx, W.; Manger, S.H.; Blencowe, M.; Murray, G.; Ho, F.Y.; Lawn, S.; Blumenthal, J.A.; Schuch, F.; Stubbs, B.; Ruusunen, A.; et al. Clinical guidelines for the use of lifestyle-based mental health care in major depressive disorder: World Federation of Societies for Biological Psychiatry (WFSBP) and Australasian Society of Lifestyle Medicine (ASLM) taskforce. World J. Biol. Psychiatry 2023, 24, 333–386. [Google Scholar] [CrossRef]
- Smith, R.D.; Dang, W.; Shen, S.; Hung, S.C.; Lam, I.H.; Kwok, J.Y.Y.; Choi, E.P.H.; Fong, D.Y.T.; Ali, S.; Wilson, C.A.; et al. Comparative effectiveness of interventions for the prevention and treatment of perinatal depression: A systematic review and network meta-analysis. Asian J. Psychiatr. 2025, 103, 104316. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant depression: Definition, prevalence, detection, management, and investigational interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Filteau, M.J.; Martin, L.; Patry, S.; Carvalho, A.; Cha, D.S.; Barakat, M.; Miguelez, M. Treatment-resistant depression: Definitions, review of the evidence, and algorithmic approach. J. Affect. Disord. 2014, 156, 1–7. [Google Scholar] [CrossRef]
- De Raedt, R.; Vanderhasselt, M.A.; Baeken, C. Neurostimulation as an intervention for treatment resistant depression: From research on mechanisms towards targeted neurocognitive strategies. Clin. Psychol. Rev. 2015, 41, 61–69. [Google Scholar] [CrossRef]
- Amasi-Hartoonian, N.; Pariante, C.M.; Cattaneo, A.; Sforzini, L. Understanding treatment-resistant depression using “omics” techniques: A systematic review. J. Affect. Disord. 2022, 318, 423–455. [Google Scholar] [CrossRef]
- Bayes, A.; Parker, G. How to choose an antidepressant medication. Acta Psychiatr. Scand. 2019, 139, 280–291. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef]
- Arakawa, R.; Stenkrona, P.; Takano, A.; Svensson, J.; Andersson, M.; Nag, S.; Asami, Y.; Hirano, Y.; Halldin, C.; Lundberg, J. Venlafaxine ER Blocks the Norepinephrine Transporter in the Brain of Patients with Major Depressive Disorder: A PET Study Using [18F]FMeNER-D2. Int. J. Neuropsychopharmacol. 2019, 22, 278–285. [Google Scholar] [CrossRef]
- Settle, E.C., Jr. Antidepressant drugs: Disturbing and potentially dangerous adverse effects. J. Clin. Psychiatry 1998, 59 (Suppl. 16), 25–30, discussion 40–22. [Google Scholar]
- Teply, R.M.; Packard, K.A.; White, N.D.; Hilleman, D.E.; DiNicolantonio, J.J. Treatment of Depression in Patients with Concomitant Cardiac Disease. Prog. Cardiovasc. Dis. 2016, 58, 514–528. [Google Scholar] [CrossRef]
- Wang, S.M.; Han, C.; Bahk, W.M.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, B. Adverse effects of antidepressant drugs: Part 1: Monoamine oxidase inhibitors and tricyclics. Drugs 1981, 21, 201–219. [Google Scholar] [CrossRef]
- Feighner, J.P. Mechanism of action of antidepressant medications. J. Clin. Psychiatry 1999, 60 (Suppl. 4), 4–11, discussion 12–13. [Google Scholar]
- Zhou, S.; Li, P.; Lv, X.; Lai, X.; Liu, Z.; Zhou, J.; Liu, F.; Tao, Y.; Zhang, M.; Yu, X.; et al. Adverse effects of 21 antidepressants on sleep during acute-phase treatment in major depressive disorder: A systemic review and dose-effect network meta-analysis. Sleep 2023, 46, zsad177. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Lees, K.R. Clinical experience with excitatory amino acid antagonist drugs. Stroke 1995, 26, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Okuya, M.; Hatano, M.; Matsuda, Y.; Iwata, N. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: A systematic review and network meta-analysis. Mol. Psychiatry 2023, 28, 402–409. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.; Walęcka, M.; Jernajczyk, W. Effects of Antidepressants on Sleep. Curr. Psychiatry Rep. 2017, 19, 63. [Google Scholar] [CrossRef]
- Beach, S.R.; Kostis, W.J.; Celano, C.M.; Januzzi, J.L.; Ruskin, J.N.; Noseworthy, P.A.; Huffman, J.C. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J. Clin. Psychiatry 2014, 75, e441–e449. [Google Scholar] [CrossRef] [PubMed]
- Gheysens, T.; Van Den Eede, F.; De Picker, L. The risk of antidepressant-induced hyponatremia: A meta-analysis of antidepressant classes and compounds. Eur. Psychiatry 2024, 67, e20. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W. Reminiscence therapy-based care program alleviates anxiety and depression, as well as improves the quality of life in recurrent gastric cancer patients. Front. Psychol. 2023, 14, 1133470. [Google Scholar] [CrossRef]
- Yeung, W.F.; Chung, K.F.; Ng, K.Y.; Yu, Y.M.; Ziea, E.T.; Ng, B.F. A systematic review on the efficacy, safety and types of Chinese herbal medicine for depression. J. Psychiatr. Res. 2014, 57, 165–175. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother. Res. 2018, 32, 1147–1162. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; Antonioli, L.; Blandizzi, C.; Calderone, V. Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 2876. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15, 229. [Google Scholar] [CrossRef]
- Nishikito, D.F.; Borges, A.C.A.; Laurindo, L.F.; Otoboni, A.; Direito, R.; Goulart, R.A.; Nicolau, C.C.T.; Fiorini, A.M.R.; Sinatora, R.V.; Barbalho, S.M. Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds. Pharmaceutics 2023, 15, 159. [Google Scholar] [CrossRef]
- Liu, L.Y.; Feng, B.; Chen, J.; Tan, Q.R.; Chen, Z.X.; Chen, W.S.; Wang, P.R.; Zhang, Z.J. Herbal medicine for hospitalized patients with severe depressive episode: A retrospective controlled study. J. Affect. Disord. 2015, 170, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.Y.; Yang, Z.; Huang, X.J.; Li, J.; Bao, W.Y.; Hurilebagen; Wulanqiqige; Wuyunsiriguleng; Cui, J.W.; Ma, L.Q.; et al. Mongolian Medicine Areca Thirteen Pill (GY-13) Improved Depressive Syndrome via upregulating cAMP/PKA/CREB/BDNF signaling pathway. J. Ethnopharmacol. 2022, 293, 115310. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Garg, M.; Prajapati, P.; Singh, P.K.; Chopra, R.; Kumari, A.; Mittal, A. Adaptogenic property of Asparagus racemosus: Future trends and prospects. Heliyon 2023, 9, e14932. [Google Scholar] [CrossRef]
- Mu, D.; Ma, Q. A Review of Antidepressant Effects and Mechanisms of Three Common Herbal Medicines: Panax ginseng, Bupleurum chinense, and Gastrodia elata. CNS Neurol. Disord. Drug Targets 2023, 22, 1164–1175. [Google Scholar] [CrossRef]
- Mokhtari, T. Targeting autophagy and neuroinflammation pathways with plant-derived natural compounds as potential antidepressant agents. Phytother. Res. 2022, 36, 3470–3489. [Google Scholar] [CrossRef]
- Yoon, S.; Iqbal, H.; Kim, S.M.; Jin, M. Phytochemicals That Act on Synaptic Plasticity as Potential Prophylaxis against Stress-Induced Depressive Disorder. Biomol. Ther. 2023, 31, 148–160. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Xi, Y.; Zhou, H.; Tang, Z.; Xiong, L.; Qin, D. Polyphenols: Natural Food-Grade Biomolecules for the Treatment of Nervous System Diseases from a Multi-Target Perspective. Pharmaceuticals 2024, 17, 775. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Li, Z.; Li, T.; Zhang, R.; Zhao, Y.; Cheng, T.; Zong, Z.; Ma, Y.; Zhang, D.; et al. Flavonoid 4,4’-dimethoxychalcone selectively eliminates senescent cells via activating ferritinophagy. Redox Biol. 2024, 69, 103017. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Y.; Su, Y.; Guan, W.; Wang, Y.; Han, J.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Luteolin: A promising multifunctional natural flavonoid for human diseases. Phytother. Res. 2024, 38, 3417–3443. [Google Scholar] [CrossRef]
- Rasmus, P.; Kozłowska, E. Antioxidant and Anti-Inflammatory Effects of Carotenoids in Mood Disorders: An Overview. Antioxidants 2023, 12, 676. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E.; et al. AdipoRon’s Impact on Alzheimer’s Disease-A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 484. [Google Scholar] [CrossRef]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Buchaim, R.L.; Goulart, R.A.; Rubira, C.J.; Barbalho, S.M. The Role of Resveratrol in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J. Med. Food 2022, 25, 797–806. [Google Scholar] [CrossRef]
- Costa, R.O.; Martins, L.F.; Tahiri, E.; Duarte, C.B. Brain-derived neurotrophic factor-induced regulation of RNA metabolism in neuronal development and synaptic plasticity. Wiley Interdiscip. Rev. RNA 2022, 13, e1713. [Google Scholar] [CrossRef]
- Gadad, B.S.; Vargas-Medrano, J.; Ramos, E.I.; Najera, K.; Fagan, M.; Forero, A.; Thompson, P.M. Altered levels of interleukins and neurotrophic growth factors in mood disorders and suicidality: An analysis from periphery to central nervous system. Transl. Psychiatry 2021, 11, 341. [Google Scholar] [CrossRef]
- Afzal, A.; Batool, Z.; Sadir, S.; Liaquat, L.; Shahzad, S.; Tabassum, S.; Ahmad, S.; Kamil, N.; Perveen, T.; Haider, S. Therapeutic Potential of Curcumin in Reversing the Depression and Associated Pseudodementia via Modulating Stress Hormone, Hippocampal Neurotransmitters, and BDNF Levels in Rats. Neurochem. Res. 2021, 46, 3273–3285. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Li, H.; Hao, B.; Cui, D.; Shang, R.; Lv, Y.; Liu, Y.; Pu, W.; Zhang, H.; et al. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. Int. J. Mol. Sci. 2023, 24, 5542. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Li, Y.; Kou, J.; Huang, F.; Liu, B.; Liu, K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur. J. Pharmacol. 2014, 745, 59–68. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Samanta, S.; Dash, R.; Karpiński, T.M.; Habibi, E.; Sadiq, A.; Ahmadi, A.; Bunagu, S. The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: Focus on the role of oxidative stress. Front. Pharmacol. 2022, 13, 1015835. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef]
- Idayu, N.F.; Hidayat, M.T.; Moklas, M.A.; Sharida, F.; Raudzah, A.R.; Shamima, A.R.; Apryani, E. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine 2011, 18, 402–407. [Google Scholar] [CrossRef]
- Ahmad, I.; Prabowo, W.C.; Arifuddin, M.; Fadraersada, J.; Indriyanti, N.; Herman, H.; Purwoko, R.Y.; Nainu, F.; Rahmadi, A.; Paramita, S.; et al. Species as Pharmacological Agents: From Abuse to Promising Pharmaceutical Products. Life 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fei, S.; Olatunji, O.J. LC/ESI/TOF-MS Characterization, Anxiolytic and Antidepressant-like Effects of. Molecules 2022, 27, 193. [Google Scholar] [CrossRef] [PubMed]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress- Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr. Neuropharmacol. 2021, 19, 1468–1495. [Google Scholar] [CrossRef]
- Szućko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and Neuroprotective Effects of Hericium erinaceus. Int. J. Mol. Sci. 2023, 24, 15960. [Google Scholar] [CrossRef]
- de Oliveira Zanuso, B.; de Oliveira Dos Santos, A.R.; Miola, V.F.B.; Guissoni Campos, L.M.; Spilla, C.S.G.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022, 161, 111731. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Peng, S.; Li, X.; Yao, J.; Xu, J.; Fang, J. Honokiol alleviates oxidative stress-induced neurotoxicity via activation of Nrf2. ACS Chem. Neurosci. 2018, 9, 3108–3116. [Google Scholar] [CrossRef]
- Wang, D.; Cao, L.; Zhou, X.; Wang, G.; Ma, Y.; Hao, X.; Fan, H. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. J. Hazard. Mater. 2022, 437, 129381. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, N. Berberine: Pathways to protect neurons. Phytother. Res. 2018, 32, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, Z.; Zhu, L.; Zhou, J.; Zhuo, L.; Zhang, J.; Zhang, X.; Liu, W.; Han, L.; Liao, W. Rhynchophylline alleviates neuroinflammation and regulates metabolic disorders in a mouse model of Parkinson’s disease. Food Funct. 2023, 14, 3208–3219. [Google Scholar] [CrossRef]
- Fathinezhad, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Depression and Treatment with Effective Herbs. Curr. Pharm. Des. 2019, 25, 738–745. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Therapeutic Effects of Phytochemicals and Medicinal Herbs on Depression. Biomed. Res. Int. 2017, 2017, 6596241. [Google Scholar] [CrossRef]
- Kou, Y.; Li, Z.; Yang, T.; Shen, X.; Wang, X.; Li, H.; Zhou, K.; Li, L.; Xia, Z.; Zheng, X. Therapeutic potential of plant iridoids in depression: A review. Pharm. Biol. 2022, 60, 2167–2181. [Google Scholar] [CrossRef]
- Xiong, Z.; Jiang, B.; Wu, P.-F.; Tian, J.; Shi, L.-L.; Gu, J.; Hu, Z.-L.; Fu, H.; Wang, F.; Chen, J.-G. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol. Pharm. Bull. 2011, 34, 253–259. [Google Scholar] [CrossRef]
- Gupta, J.; Gupta, R.; Varshney, K.K. Emerging mechanisms and potential antidepressant action of medicinal plants. Int. J. Pharm. Sci. Res. 2020, 11, 1–3. [Google Scholar]
- Picheta, N.; Piekarz, J.; Daniłowska, K.; Mazur, K.; Piecewicz-Szczęsna, H.; Smoleń, A. Phytochemicals in the treatment of patients with depression: A systemic review. Front. Psychiatry 2024, 15, 1509109. [Google Scholar] [CrossRef]
- Soltani, M.; Hosseinzadeh-Attar, M.J.; Rezaei, M.; Alipoor, E.; Vasheghani-Farahani, A.; Yaseri, M.; Rezayat, S.M. Effect of nano-curcumin supplementation on cardiometabolic risk factors, physical and psychological quality of life, and depression in patients with coronary slow flow phenomenon: A randomized double-blind clinical trial. Trials 2024, 25, 515. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Vafa, M.; Ebrahimkhani, A.; Găman, M.A.; Sezavar Seyedi Jandaghi, S.H. Effects of quercetin supplementation on endothelial dysfunction biomarkers and depression in post-myocardial infarction patients: A double-blind, placebo-controlled, randomized clinical trial. Clin. Nutr. ESPEN 2023, 56, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hajiluian, G.; Karegar, S.J.; Shidfar, F.; Aryaeian, N.; Salehi, M.; Lotfi, T.; Farhangnia, P.; Heshmati, J.; Delbandi, A.A. The effects of Ellagic acid supplementation on neurotrophic, inflammation, and oxidative stress factors, and indoleamine 2, 3-dioxygenase gene expression in multiple sclerosis patients with mild to moderate depressive symptoms: A randomized, triple-blind, placebo-controlled trial. Phytomedicine 2023, 121, 155094. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Vijayakumar, A.; Rooney, C.; Noad, R.L.; Appleton, K.M.; McCarthy, D.; Donnelly, M.; Young, I.S.; McKinley, M.C.; McKeown, P.P.; et al. A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients 2020, 12, 2445. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef]
- Hirose, A.; Terauchi, M.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Low-dose isoflavone aglycone alleviates psychological symptoms of menopause in Japanese women: A randomized, double-blind, placebo-controlled study. Arch. Gynecol. Obstet. 2016, 293, 609–615. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.H.; Park, M.; Lee, H.J. Effects of Flavonoid-Rich Orange Juice Intervention on Major Depressive Disorder in Young Adults: A Randomized Controlled Trial. Nutrients 2022, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Yamamoto, M.; Honmou, O.; Sasaki, M.; Haseda, A.; Kagami-Katsuyama, H.; Shoji, T.; Namioka, A.; Namioka, T.; Magota, H.; Oka, S.; et al. The Impact of Purple-Flesh Potato (Solanum tuberosum L.) cv. “Shadow Queen” on Minor Health Complaints in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 2446. [Google Scholar] [CrossRef]
- Barfoot, K.L.; Forster, R.; Lamport, D.J. Mental Health in New Mothers: A Randomised Controlled Study into the Effects of Dietary Flavonoids on Mood and Perceived Quality of Life. Nutrients 2021, 13, 2383. [Google Scholar] [CrossRef]
- Parilli-Moser, I.; Domínguez-López, I.; Trius-Soler, M.; Castellví, M.; Bosch, B.; Castro-Barquero, S.; Estruch, R.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M. Consumption of peanut products improves memory and stress response in healthy adults from the ARISTOTLE study: A 6-month randomized controlled trial. Clin. Nutr. 2021, 40, 5556–5567. [Google Scholar] [CrossRef] [PubMed]
- Bourdel-Marchasson, I.; Ostan, R.; Regueme, S.C.; Pinto, A.; Pryen, F.; Charrouf, Z.; d’Alessio, P.; Baudron, C.R.; Guerville, F.; Durrieu, J.; et al. Quality of Life: Psychological Symptoms-Effects of a 2-Month Healthy Diet and Nutraceutical Intervention; A Randomized, Open-Label Intervention Trial (RISTOMED). Nutrients 2020, 12, 800. [Google Scholar] [CrossRef]
- Park, M.; Choi, J.; Lee, H.J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef]
- Smetanka, A.; Stara, V.; Farsky, I.; Tonhajzerova, I.; Ondrejka, I. Pycnogenol supplementation as an adjunct treatment for antidepressant-induced sexual dysfunction. Physiol. Int. 2019, 106, 59–69. [Google Scholar] [CrossRef]
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox. Res. 2018, 33, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Barfoot, K.L.; May, G.; Lamport, D.J.; Reynolds, S.A.; Williams, C.M. Effects of Acute Blueberry Flavonoids on Mood in Children and Young Adults. Nutrients 2017, 9, 158. [Google Scholar] [CrossRef]
- Esmaily, H.; Sahebkar, A.; Iranshahi, M.; Ganjali, S.; Mohammadi, A.; Ferns, G.; Ghayour-Mobarhan, M. An investigation of the effects of curcumin on anxiety and depression in obese individuals: A randomized controlled trial. Chin. J. Integr. Med. 2015, 21, 332–338. [Google Scholar] [CrossRef]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Beckett, S.; Rigby, A.S.; Mellor, D.D.; Atkin, S.L. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr. J. 2010, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Malau, I.A.; Chang, J.P.-C.; Lin, Y.-W.; Chang, C.-C.; Chiu, W.-C.; Su, K.-P. Omega-3 Fatty Acids and Neuroinflammation in Depression: Targeting Damage-Associated Molecular Patterns and Neural Biomarkers. Cells 2024, 13, 1791. [Google Scholar] [CrossRef]
- Wang, J.; Behl, T.; Rana, T.; Sehgal, A.; Wal, P.; Saxena, B.; Yadav, S.; Mohan, S.; Anwer, M.K.; Chigurupati, S. Exploring the pathophysiological influence of heme oxygenase-1 on neuroinflammation and depression: A study of phytotherapeutic-based modulation. Phytomedicine 2024, 127, 155466. [Google Scholar] [CrossRef] [PubMed]
- Haase, J.; Brown, E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression—A central role for the serotonin transporter? Pharmacol. Ther. 2015, 147, 1–11. [Google Scholar] [CrossRef]
- Halaris, A. Inflammation and depression but where does the inflammation come from? Curr. Opin. Psychiatry 2019, 32, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, J.; Chung, S.K.; Xu, B. New insights into anti-depression effects of bioactive phytochemicals. Pharmacological Research 2024, 212, 107566. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Cunha, M.P.; Kaster, M.P.; Rodrigues, A.L.S. Natural polyphenols and terpenoids for depression treatment: Current status. Stud. Nat. Prod. Chem. 2018, 55, 181–221. [Google Scholar]
- Shi, X.; Fu, Y.; Zhang, S.; Ding, H.; Chen, J. Baicalin attenuates subarachnoid hemorrhagic brain injury by modulating blood-brain barrier disruption, inflammation, and oxidative damage in mice. Oxidative Med. Cell. Longev. 2017, 2017, 1401790. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Soltani, A.; Khodarahmi, Z.; Alameri, A.A.; Alwan, A.M.; Ramírez-Coronel, A.A.; Obaid, R.F.; Abosaooda, M.; Heidari, M.; Golmohammadi, M. Targeting Nrf2 signaling pathway by quercetin in the prevention and treatment of neurological disorders: An overview and update on new developments. Fundam. Clin. Pharmacol. 2023, 37, 1050–1064. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Grabacka, M.M.; Gawin, M.; Pierzchalska, M. Phytochemical modulators of mitochondria: The search for chemopreventive agents and supportive therapeutics. Pharmaceuticals 2014, 7, 913–942. [Google Scholar] [CrossRef]
- Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals 2025, 18, 607. [Google Scholar] [CrossRef]
- Bozzuto, G.; Calcabrini, A.; Colone, M.; Condello, M.; Dupuis, M.L.; Pellegrini, E.; Stringaro, A. Phytocompounds and nanoformulations for anticancer therapy: A review. Molecules 2024, 29, 3784. [Google Scholar] [CrossRef]
- Aburel, O.M.; Pavel, I.Z.; Dănilă, M.D.; Lelcu, T.; Roi, A.; Lighezan, R.; Muntean, D.M.; Rusu, L.C. Pleiotropic effects of eugenol: The good, the bad, and the unknown. Oxidative Med. Cell. Longev. 2021, 2021, 3165159. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jahan, S.; Imtiyaz, Z.; Alshahrani, S.; Antar Makeen, H.; Mohammed Alshehri, B.; Kumar, A.; Arafah, A.; Rehman, M.U. Neuroprotection: Targeting multiple pathways by naturally occurring phytochemicals. Biomedicines 2020, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.; Renner, B. Herb–drug interaction in inflammatory diseases: Review of phytomedicine and herbal supplements. J. Clin. Med. 2022, 11, 1567. [Google Scholar] [CrossRef]
- Tayeb, B.A.; Kusuma, I.Y.; Osman, A.A.; Minorics, R. Herbal compounds as promising therapeutic agents in precision medicine strategies for cancer: A systematic review. J. Integr. Med. 2024, 22, 137–162. [Google Scholar] [CrossRef]
- Dobrek, L.; Głowacka, K. Depression and its phytopharmacotherapy—A narrative review. Int. J. Mol. Sci. 2023, 24, 4772. [Google Scholar] [CrossRef]
- Singh, S.K.; Rashid, M.; Chaturvedi, S.; Agarwal, A.; Chauhan, D.; Gayen, J.R.; Wahajuddin, M. Preclinical pharmacokinetics, absolute bioavailability and dose proportionality evaluation of bioactive phytochemical Withanone in rats. Bioorganic Chem. 2025, 155, 108128. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M. Phytosomes as innovative delivery systems for phytochemicals: A comprehensive review of literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.; Hamman, J.H. What are the dangers of drug interactions with herbal medicines? Expert Opin. Drug Metab. toxicology 2020, 16, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Holleran, G.; Scaldaferri, F.; Gasbarrini, A.; Currò, D. Herbal medicinal products for inflammatory bowel disease: A focus on those assessed in double-blind randomised controlled trials. Phytother. Res. 2020, 34, 77–93. [Google Scholar] [CrossRef]
- Murphy, E.; McMahon, F.J. Pharmacogenetics of antidepressants, mood stabilizers, and antipsychotics in diverse human populations. Discov. Med. 2013, 16, 113–122. [Google Scholar]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Celenza, G.; Petricca, S. Multi-Target Effects of ß-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants 2022, 11, 1199. [Google Scholar] [CrossRef]
- Magni, G.; Riboldi, B.; Petroni, K.; Ceruti, S. Flavonoids bridging the gut and the brain: Intestinal metabolic fate, and direct or indirect effects of natural supporters against neuroinflammation and neurodegeneration. Biochem. Pharmacol. 2022, 205, 115257. [Google Scholar] [CrossRef]
- Coen, M.; Want, E.J.; Clayton, T.A.; Rhode, C.M.; Hong, Y.S.; Keun, H.C.; Cantor, G.H.; Metz, A.L.; Robertson, D.G.; Reily, M.D.; et al. Mechanistic aspects and novel biomarkers of responder and non-responder phenotypes in galactosamine-induced hepatitis. J. Proteome Res. 2009, 8, 5175–5187. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Almeida, S.S.; Zizzi, F.B.; Cattaneo, A.; Comandini, A.; Di Dato, G.; Lubrano, E.; Pellicano, C.; Spallone, V.; Tongiani, S.; Torta, R. Management and Treatment of Patients With Major Depressive Disorder and Chronic Diseases: A Multidisciplinary Approach. Front. Psychol. 2020, 11, 542444. [Google Scholar] [CrossRef]
- Pepe, M.; Bartolucci, G.; Marcelli, I.; Pesaresi, F.; Brugnami, A.; Caso, R.; Fischetti, A.; Grisoni, F.; Mazza, M.; Camardese, G.; et al. The Patient’s Perspective on the Effects of Intranasal Esketamine in Treatment-Resistant Depression. Brain Sci. 2023, 13, 1494. [Google Scholar] [CrossRef]
- Stolfi, F.; Abreu, H.; Sinella, R.; Nembrini, S.; Centonze, S.; Landra, V.; Brasso, C.; Cappellano, G.; Rocca, P.; Chiocchetti, A. Omics approaches open new horizons in major depressive disorder: From biomarkers to precision medicine. Front. Psychiatry 2024, 15, 1422939. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, T.; Liu, J.; Gao, C.; Zhang, X. Physical exercise and mitochondrial function: New therapeutic interventions for psychiatric and neurodegenerative disorders. Front. Neurol. 2022, 13, 929781. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Holtzheimer, P.E.; Gao, S.; Kirwin, D.S.; Price, R.B. Leveraging Neuroplasticity to Enhance Adaptive Learning: The Potential for Synergistic Somatic-Behavioral Treatment Combinations to Improve Clinical Outcomes in Depression. Biol. Psychiatry 2019, 85, 454–465. [Google Scholar] [CrossRef]
- Ashdown-Franks, G.; Firth, J.; Carney, R.; Carvalho, A.F.; Hallgren, M.; Koyanagi, A.; Rosenbaum, S.; Schuch, F.B.; Smith, L.; Solmi, M.; et al. Exercise as Medicine for Mental and Substance Use Disorders: A Meta-review of the Benefits for Neuropsychiatric and Cognitive Outcomes. Sports Med. 2020, 50, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, M. Effects of exercise therapy on global cognitive function and, depression in older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 106, 104855. [Google Scholar] [CrossRef]
- Gamage, E.; Orr, R.; Travica, N.; Lane, M.M.; Dissanayaka, T.; Kim, J.H.; Grosso, G.; Godos, J.; Marx, W. Polyphenols as novel interventions for depression: Exploring the efficacy, mechanisms of action, and implications for future research. Neurosci. Biobehav. Rev. 2023, 151, 105225. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L.; Giménez-Llort, L. Emerging translational research in neurological and psychiatric diseases: From in vitro to in vivo models. Int. J. Mol. Sci. 2023, 24, 15739. [Google Scholar] [CrossRef]
- Kessler, R.C.; van Loo, H.M.; Wardenaar, K.J.; Bossarte, R.M.; Brenner, L.; Ebert, D.; De Jonge, P.; Nierenberg, A.; Rosellini, A.; Sampson, N. Using patient self-reports to study heterogeneity of treatment effects in major depressive disorder. Epidemiol. Psychiatr. Sci. 2017, 26, 22–36. [Google Scholar] [CrossRef]
- Malgaroli, M.; Calderon, A.; Bonanno, G.A. Networks of major depressive disorder: A systematic review. Clin. Psychol. Rev. 2021, 85, 102000. [Google Scholar] [CrossRef]
- Mew, E.J.; Monsour, A.; Saeed, L.; Santos, L.; Patel, S.; Courtney, D.B.; Watson, P.N.; Szatmari, P.; Offringa, M.; Monga, S. Systematic scoping review identifies heterogeneity in outcomes measured in adolescent depression clinical trials. J. Clin. Epidemiol. 2020, 126, 71–79. [Google Scholar] [CrossRef] [PubMed]
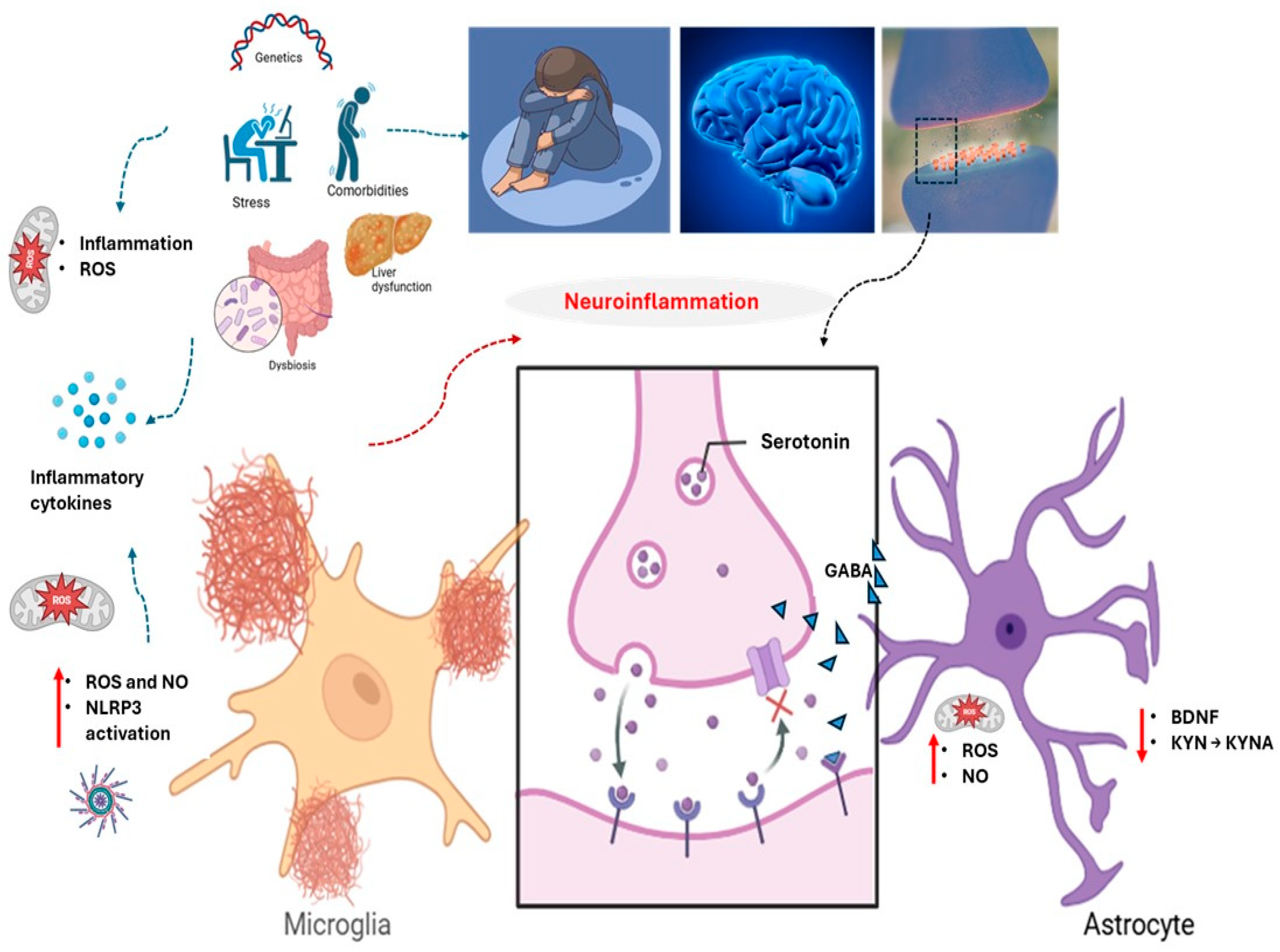
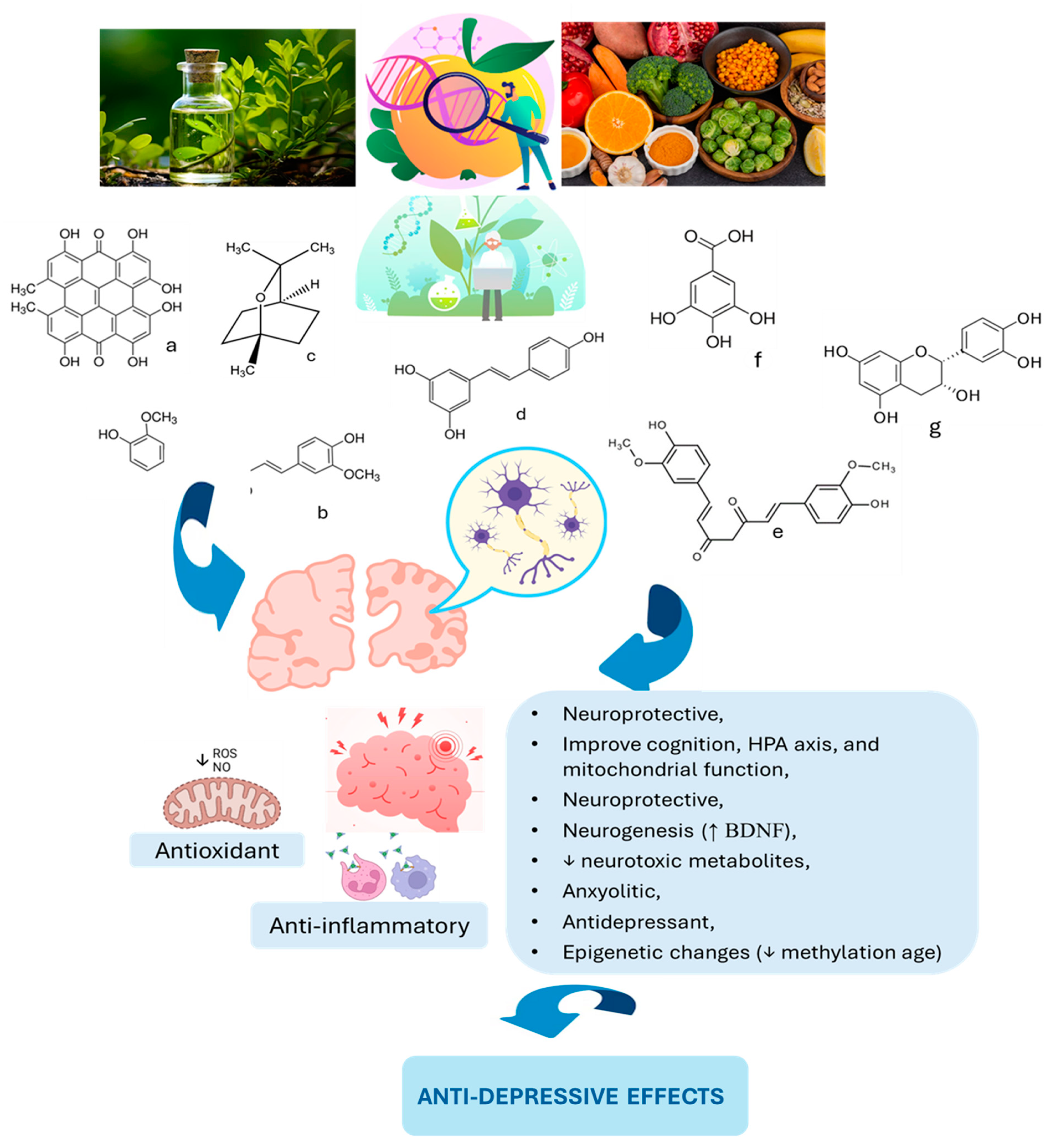
| Study [Ref.] | Country | Population | Intervention | Outcomes | Side Effects |
|---|---|---|---|---|---|
| Soltani et al. [257] | Iran | 42 patients (CSFP) aged 35–70 | Nano-curcumin 80 mg/day, 12 weeks | Improved depression and quality of life | Nausea, headache, and diarrhea |
| Dehghani et al. [258] | Iran | 76 individuals aged 35–65 | Quercetin 500 mg/day, 8 weeks | Marginal depression improvement | Headache, joint pain, and abdominal discomfort |
| Hajiluian et al. [259] | Iran | 50 MS patients with depression, aged 18–55 | Ellagic acid 180 mg/day, 12 weeks | Reduced inflammation and depression | None significant |
| Choi et al. [263] | Republic of Korea | 40 young adults aged 18–29 with MDD | Flavonoid-rich orange juice, 8 weeks | Significant improvement in depressive scores | None significant |
| Maeda-Yamamoto et al. [264] | Japan | 15 older adults aged 50–70 | Anthocyanin-rich potatoes, 8 weeks | Improved psychological stress response | None significant |
| Parilli-Moser et al. [265] | U.K. | 38 new mothers | High-flavonoid diet, 2 weeks | Reduced anxiety and improved social relationships | None significant |
| Barfoot et al. [266] | Spain | 63 young overweight adults | Roasted peanuts and peanut butter, 6–7 months | Significant depression reduction | Digestive symptoms and softer stools |
| Bourdel-Marchasson et al. [267] | Europe | 125 elderly adults aged ~70 | Antioxidant-rich diet, 2 months | Reduced depressive symptoms | None serious |
| Kontogianni et al. [260] | U.K. | 99 mildly hypertensive adults aged 40–65 | High vs. low polyphenol diet, 8 weeks | Improved depressive symptoms | Not reported |
| Park et al. [268] | Republic of Korea | 40 healthy adults (20–30 years old) | Flavonoid-rich (FR) or flavonoid-low (FL) drinks, 190 mL twice daily for 8 week | ↓ Depressive symptoms (CES-D < 20) | None significant |
| Smetanka et al. [269] | Slovakia | 67 adults with MDD, aged 18–65 | Pycnogenol with escitalopram, 12 weeks | No additional benefit compared to escitalopram alone | None significant |
| Kanchanatawan et al. [270] | Multinational | 61 adults aged 18–63 with MDD history | Curcumin 500–1500 mg/day, 12–16 weeks | Improved depression scores (MADRS) | Dizziness, nausea, insomnia, and diarrhea |
| Terauchi et al. [261] | Italy | 60 menopausal women aged 50–55 | Equol and resveratrol, 12 weeks | Reduced depressive symptoms (HAM-D) | Mild diarrhea |
| Khalid et al. [271] | U.K. | Young adults and children | Wild blueberry drink (flavonoids), acute administration | Improved positive affect shortly after consumption | None significant |
| Hirose et al. [262] | Japan | 87 menopausal women aged 40–60 | Isoflavone aglycones, 8 weeks | Improved anxiety and modest depression changes | None significant |
| Sathyapalan et al. [272] | U.K., Iran | 30 obese adults | Curcumin 1 g/day, 30 days | Reduced anxiety and no significant depression improvement | None significant |
| Terauch et al. [273] | Japan | 91 menopausal women (40–60 years old) | GSPE (100 or 200 mg/day) vs. placebo, 8 weeks | ↓ Anxiety (dose-dependent); no effect on depression | None significant |
| Pase et al. [11] | Australia | Adults aged 40–65 | Cocoa polyphenols 250–500 mg/day, 30 days | Improved mood and calmness | None |
| Clinical pilot [274] | U.K. | 10 adults | High-polyphenol chocolate, crossover | Improved anxiety and depression scores | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.d.S.B.M.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13, 1129. https://doi.org/10.3390/biomedicines13051129
Figueiredo Godoy AC, Frota FF, Araújo LP, Valenti VE, Pereira EdSBM, Detregiachi CRP, Galhardi CM, Caracio FC, Haber RSA, Fornari Laurindo L, et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines. 2025; 13(5):1129. https://doi.org/10.3390/biomedicines13051129
Chicago/Turabian StyleFigueiredo Godoy, Ana Clara, Fernanda Fortes Frota, Larissa Parreira Araújo, Vitor E. Valenti, Eliana de Souza Bastos Mazuqueli Pereira, Claudia Rucco P. Detregiachi, Cristiano M. Galhardi, Flávia Cristina Caracio, Rafael S. A. Haber, Lucas Fornari Laurindo, and et al. 2025. "Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora" Biomedicines 13, no. 5: 1129. https://doi.org/10.3390/biomedicines13051129
APA StyleFigueiredo Godoy, A. C., Frota, F. F., Araújo, L. P., Valenti, V. E., Pereira, E. d. S. B. M., Detregiachi, C. R. P., Galhardi, C. M., Caracio, F. C., Haber, R. S. A., Fornari Laurindo, L., Tanaka, M., & Barbalho, S. M. (2025). Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines, 13(5), 1129. https://doi.org/10.3390/biomedicines13051129











