Urothelial Carcinoma Arising on a Functional Kidney Graft
Abstract
1. Introduction
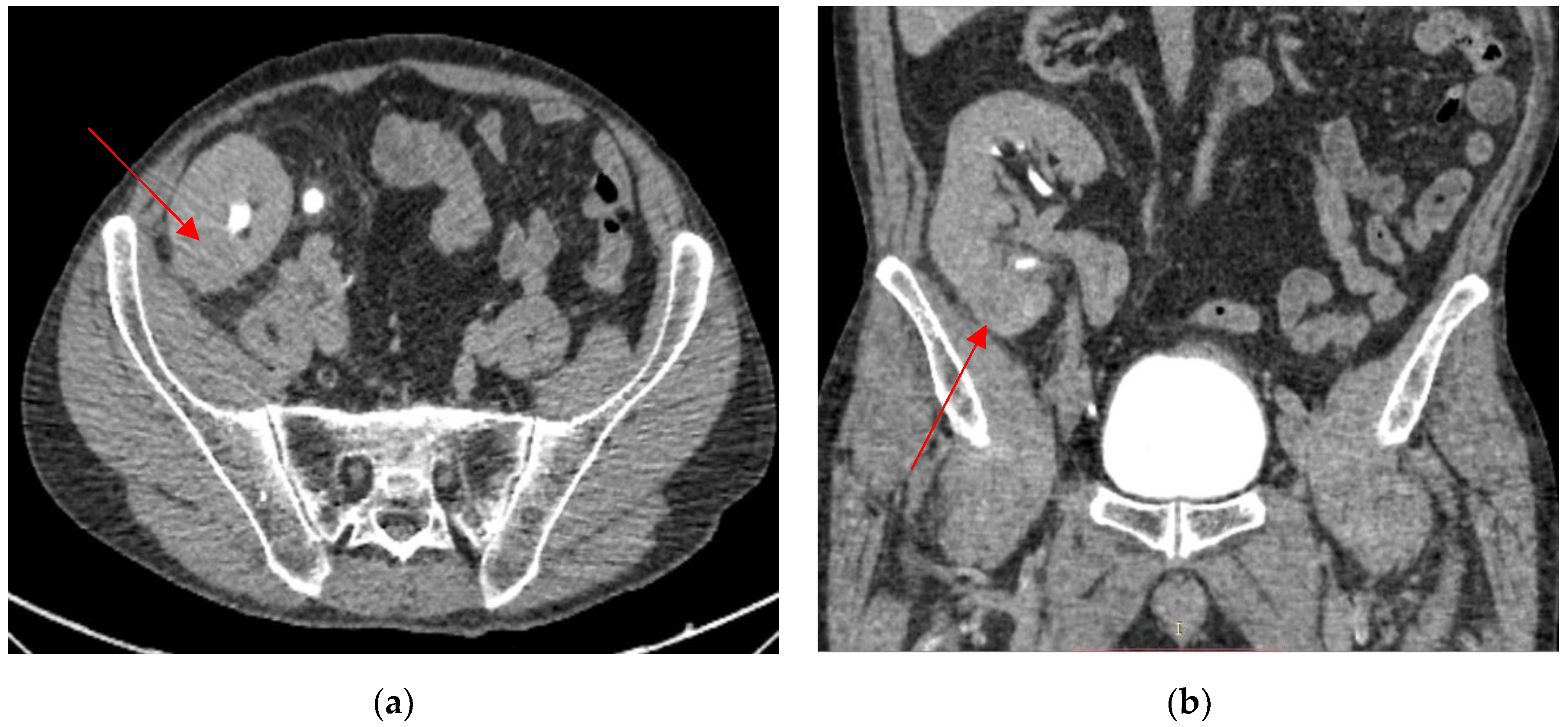
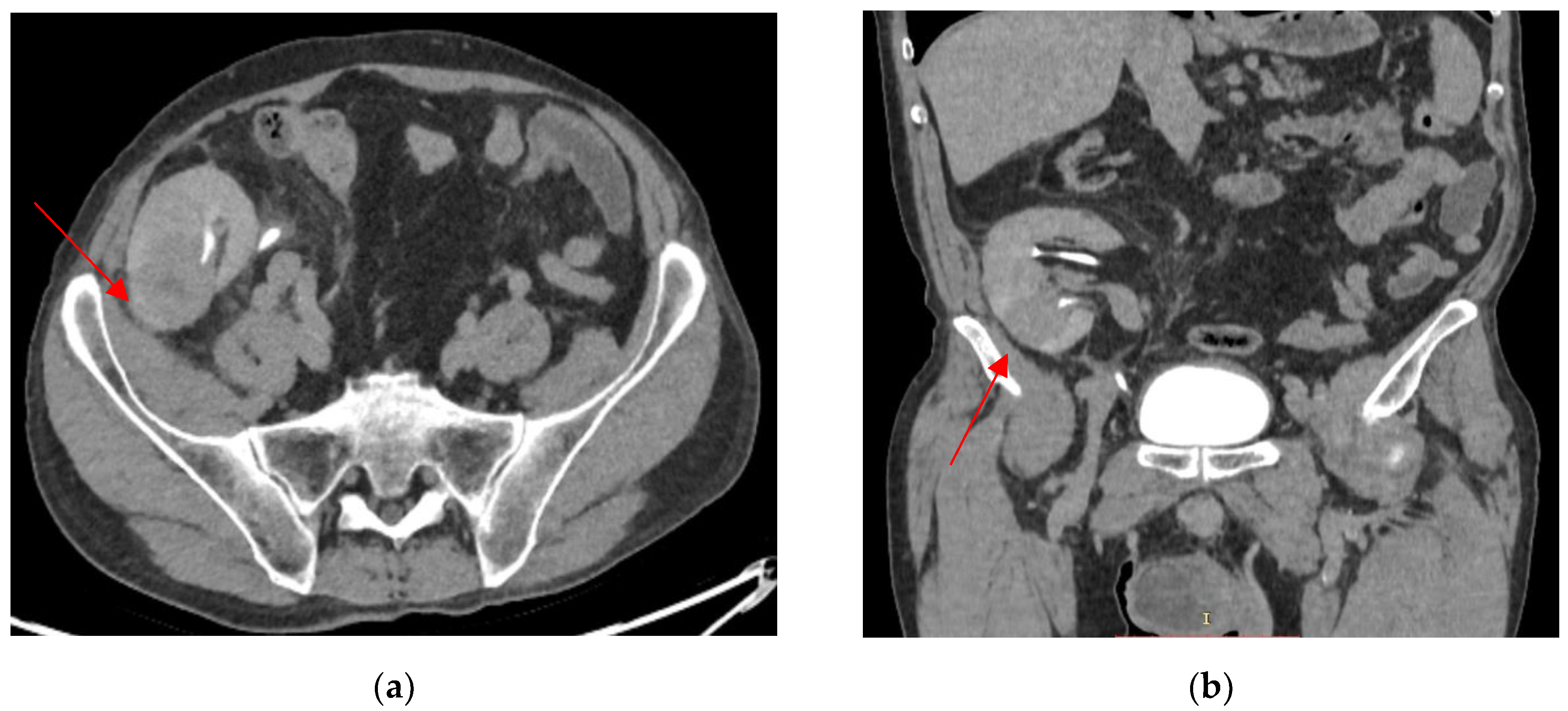
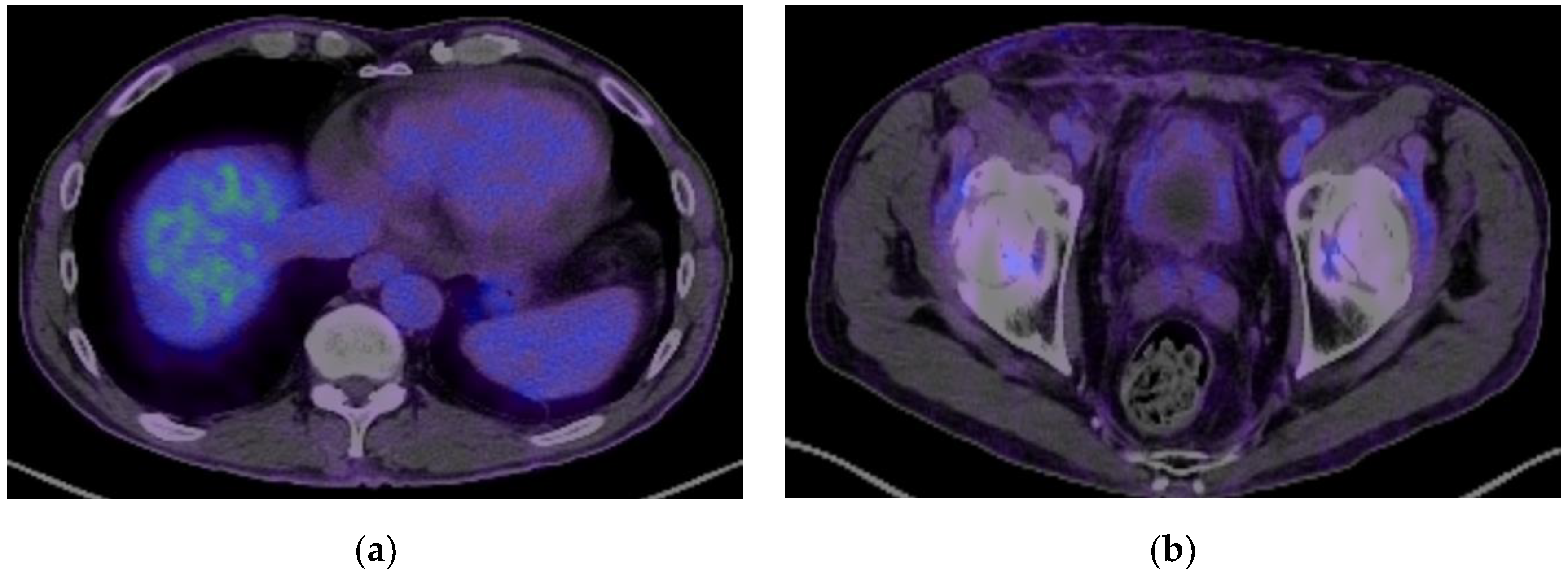
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oëlle, J.; Ortier, L.N.; Armen, A.-C.; Uniz, M.; Artinez, M.; Chmeiser, E.H.S.; Olker, V.; Rlt, M.A.; Ieler, H.A.B.; Epierreux, I.F.D.; et al. Urothelial Carcinoma Associated with the Use of a Chinese Herb (Aristolochia Fangchi). N. Engl. J. Med. 2000, 342, 1686–1692. [Google Scholar]
- Green, D.A.; Rink, M.; Xylinas, E.; Matin, S.F.; Stenzl, A.; Roupret, M.; Karakiewicz, P.I.; Scherr, D.S.; Shariat, S.F. Urothelial Carcinoma of the Bladder and the Upper Tract: Disparate Twins. J. Urol. 2013, 189, 1214–1221. [Google Scholar] [CrossRef]
- Tsaur, I.; Karalis, A.; Blaheta, R.; Juengel, E.; Vallo, S.; Scheuermann, E.H.; Kachel, H.G.; Waaga-Gasser, A.M.; Chandraker, A.; Obermüller, N.; et al. Transitional Cell Carcinoma of the Native Urinary Tract after Kidney Transplantation: Recommendations Following a Long-Term Retrospective Analysis. Am. J. Med. Sci. 2011, 341, 478–483. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Li, Y.; Lu, M.; Zhang, H.; Qiu, M.; Zhao, L.; Zhang, S.; Huang, Y.; Hou, X.; et al. Bilateral Nephroureterectomy Versus Unilateral Nephroureterectomy for Treating De Novo Upper Tract Urothelial Carcinoma After Renal Transplantation: A Comparison of Surgical and Oncological Outcomes. Clin. Med. Insights Oncol. 2021, 15, 11795549211035541. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Chen, Y.J.; Tseng, W.C.; Lin, M.W.; Chen, T.J.; Chu, S.Y.; Hwang, C.Y.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; et al. Malignancies after Renal Transplantation in Taiwan: A Nationwide Population-Based Study. Nephrol. Dial. Transplant. 2012, 27, 833–839. [Google Scholar] [CrossRef]
- Liang, J.A.; Sun, L.M.; Yeh, J.J.; Sung, F.C.; Chang, S.N.; Kao, C.H. The Association between Malignancy and End-Stage Renal Disease in Taiwan. Jpn. J. Clin. Oncol. 2011, 41, 752–757. [Google Scholar] [CrossRef]
- Leon, G.; Szabla, N.; Boissier, R.; Gigante, M.; Caillet, K.; Verhoest, G.; Tillou, X. Kidney Graft Urothelial Carcinoma: Results From a Multicentric Retrospective National Study. Urology 2020, 135, 101–105. [Google Scholar] [CrossRef]
- Rogers, A.; Koo Ng, J.; Glendinning, J.; Rix, D. The Management of Transitional Cell Carcinoma (TCC) in a European Regional Renal Transplant Population. BJU Int. 2012, 110, E34–E40. [Google Scholar] [CrossRef]
- Hickman, L.A.; Sawinski, D.; Guzzo, T.; Locke, J.E. Urologic Malignancies in Kidney Transplantation. Am. J. Transplant. 2018, 18, 13–22. [Google Scholar] [CrossRef]
- Wang, H.H.; Liu, K.L.; Chu, S.H.; Tian, Y.C.; Lai, P.C.; Chiang, Y.J. BK Virus Infection in Association With Posttransplant Urothelial Carcinoma. Transpl. Proc. 2009, 41, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Penn, I. Primary Kidney Tumors before and after Renal Transplantation. Transplantation 1995, 4, 480–485. [Google Scholar] [CrossRef]
- Jue, J.S.; Alameddine, M.; Gonzále, J.; Cianci, G. Risk Factors, Management, and Survival of Bladder Cancer after Kidney Transplantation. Actas Urológicas Españolas (Engl. Ed.) 2021, 45, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.K.; Van Buren, D.H.; MacDonell, R.C.; Delbeke, D. Carcinoma in a Transplanted Kidney Detected with MAG3 Scintigraphy. J. Nucl. Med. 1993, 12, 2185–2187. [Google Scholar]
- Prabharasuth, D.; Moses, K.A.; Bernstein, M.; Dalbagni, G.; Herr, H.W. Management of Bladder Cancer after Renal Transplantation. Urology 2013, 81, 813–819. [Google Scholar] [CrossRef]
- Kim, D.Y.; Abouljoud, M.; Parasuraman, R. The Role of Microscopic Hematuria in the Evaluation of Urologic Malignancy in Renal Transplant Recipients. Transpl. Proc. 2010, 42, 1641–1642. [Google Scholar] [CrossRef] [PubMed]
- Muruve, N.A.; Shoskes, D.A. Genitourinary Malignancies in Solid Organ Transplant Recipients. Transplantation 2005, 80, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Faba, O.; Palou, J.; Vila Reyes, H.; Guirado, L.; Palazzetti, A.; Gontero, P.; Vigués, F.; Garcia-Olaverri, J.; Fernández Gómez, J.M.; Olsburg, J.; et al. Opciones Terapéuticas y Factores Predictivos de Recurrencia y Mortalidad Cáncer-Específica En Pacientes Con Tumor Vesical Después de Trasplante Renal: Análisis Multiinstitucional. Actas Urol. Esp. 2017, 41, 639–645. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non–Muscle-Invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel [Formula Presented]. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef]
- Alkassis, M.; Abi Tayeh, G.; Khalil, N.; Mansour, R.; Lilly, E.; Sarkis, J.; Moukarzel, M. The Safety and Efficacy of Bacillus Calmette-Guerin Intravesical Therapy in Kidney Transplant Recipients with Superficial Bladder Cancer. Clin. Transplant 2021, 35, e14377. [Google Scholar] [CrossRef]
- Palou, J.; Angerri, O.; Segarra, J.; Caparrós, J.; Guirado, L.; Diaz, J.M.; Salvador-Bayarri, J.; Villavicencio-Mavrich, H. Intravesical Bacillus Calmette-Guèrin for the Treatment of Superficial Bladder Cancer in Renal Transplant Patients. Transplantation 2003, 76, 1514–1516. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fontán, F.; Cano Megías, M.; Sánchez-Conde, M.; Elías Triviño, S.; Fernández-Rodríguez, A.; Liaño, F. Subacute Interstitial Pneumonitis Due to Mycobacterium Bovis after Intravesical Bacillus Calmette-Guérin Instillation in a Renal Transplant Patient. Nefrologia 2016, 36, 711–713. [Google Scholar] [CrossRef]
- Ziegler, J.; Ho, J.; Gibson, I.W.; Nayak, J.G.; Stein, M.; Walkty, A.; Orr, P. Disseminated Mycobacterium Bovis Infection Post-Kidney Transplant Following Remote Intravesical Bcg Therapy for Bladder Cancer. J. Urol. 2019, 202, 457. [Google Scholar]
- Numakura, K.; Miyake, M.; Kobayashi, M.; Muto, Y.; Sekine, Y.; Nishimura, N.; Iida, K.; Shiga, M.; Morizane, S.; Yoneyama, T.; et al. Subsequent Upper Urinary Tract Carcinoma Related to Worse Survival in Patients Treated with BCG. Cancers 2023, 15, 2002. [Google Scholar] [CrossRef] [PubMed]
- Habuchi, T.; Yamada, H.; Kakehi, Y.; Yoshida, O.; Takahashi, R.; Suglyama, T. Metachronous Multifocal Development of Urothelial Cancers by Intraluminal Seeding. Lancet 1993, 342, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Penn, I. Cancers in Renal Transplant Recipients. Adv. Ren. Replace. Ther. 2000, 7, 147–156. [Google Scholar] [CrossRef]
- Kiselevskiy, M.V.; Gromova, E.G.; Kozlov, N.A.; Bezhanova, S.D.; Shubina, I.Z. Spontaneous Regression of a Metastatic Carcinoma Transmitted by a Kidney Graft. Explor. Target. Antitumor Ther. 2023, 4, 2011–2018. [Google Scholar] [CrossRef]
- Warren, H.; Olsburgh, J. Management of Renal Cell Carcinoma and Other Renal Masses in the Kidney Graft. Curr. Urol. Rep. 2020, 21, 8. [Google Scholar] [CrossRef]
- Takaoka, E.I.; Miyazaki, J.; Kimura, T.; Kojima, T.; Kawai, K.; Murata, Y.; Itoguchi, N.; Minami, Y.; Nakamura, T.; Honda, K.; et al. Concurrent Urothelial Carcinoma in the Renal Pelvis of an Allograft Kidney and Native Recipient Bladder: Evidence of Donor Origin. Jpn. J. Clin. Oncol. 2014, 44, 366–369. [Google Scholar] [CrossRef]
- Kojima, Y.; Takahi, Y.; Ichimaru, N.; Okumi, M.; Takahara, S.; Nonomura, N. Successful Treatment of Metastatic Urothelial Carcinoma Arising in a Transplanted Renal Allograft with Paclitaxel, Cisplatin, and Gemcitabine Combination Therapy: A Case Report. BMC Res. Notes 2015, 8, 25. [Google Scholar] [CrossRef]
- Du, C.; Zheng, M.; Wang, Z.; Zhang, J.; Lin, J.; Zhang, L.; Tian, Y.; Zhu, Y. Clinical Characteristics and Treatment Outcomes of Kidney Transplant Recipients with de Novo Urothelial Carcinoma: Thirty Years of Experience from a Single Center. BMC Urol. 2023, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Okada, Y.; Okada, K.; Yoshida, O.; Amitanih, R.; Kuboh, Y.; Ushidac, S. Spontaneous Regression of Pulmonary Metastasis from Renal Pelvic Cancer. Urol. Int. 1987, 42, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Hejlesen, M.B.; Youssef, M.H.; Bech, J.N.; Mose, F.H. Urothelial Carcinoma in an Allograft Kidney. Case Rep. Nephrol. Dial. 2022, 2, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Kamei, J.; Yokoyama, H.; Niki, T.; Suda, R.; Sugihara, T.; Fujisaki, A.; Ando, S.; Iwami, D.; Fujimura, T. Complete Response to Pembrolizumab for Metastatic Urothelial Carcinoma in the Renal Pelvis of Allograft Kidney. IJU Case Rep. 2022, 3, 199–202. [Google Scholar] [CrossRef]
- Cuenca, A.G.; Rosales, I.; Lee, R.J.; Wu, C.L.; Colvin, R.; Feldman, A.S.; Efstathiou, J.A.; Tolkoff-Rubin, N.; Elias, N. Resolution of a High Grade and Metastatic BK Polyomavirus-Associated Urothelial Cell Carcinoma Following Radical Allograft Nephroureterectomy and Immune Checkpoint Treatment: A Case Report. Transpl. Proc. 2020, 52, 2720–2725. [Google Scholar] [CrossRef]
- Al-Adra, D.P.; Hammel, L.; Roberts, J.; Woodle, E.S.; Levine, D.; Mandelbrot, D.; Verna, E.; Locke, J.; D’Cunha, J.; Farr, M.; et al. Pretransplant Solid Organ Malignancy and Organ Transplant Candidacy: A Consensus Expert Opinion Statement. Am. J. Transplant. 2021, 21, 460–474. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldoveanu, O.; Baston, C.; Sorohan, B.; Discălicău, L.; Mirvald, C.; Baston, O.M.; Sinescu, I. Urothelial Carcinoma Arising on a Functional Kidney Graft. Biomedicines 2025, 13, 1118. https://doi.org/10.3390/biomedicines13051118
Moldoveanu O, Baston C, Sorohan B, Discălicău L, Mirvald C, Baston OM, Sinescu I. Urothelial Carcinoma Arising on a Functional Kidney Graft. Biomedicines. 2025; 13(5):1118. https://doi.org/10.3390/biomedicines13051118
Chicago/Turabian StyleMoldoveanu, Oana, Cătălin Baston, Bogdan Sorohan, Lucas Discălicău, Cristian Mirvald, Oana Mădălina Baston, and Ioanel Sinescu. 2025. "Urothelial Carcinoma Arising on a Functional Kidney Graft" Biomedicines 13, no. 5: 1118. https://doi.org/10.3390/biomedicines13051118
APA StyleMoldoveanu, O., Baston, C., Sorohan, B., Discălicău, L., Mirvald, C., Baston, O. M., & Sinescu, I. (2025). Urothelial Carcinoma Arising on a Functional Kidney Graft. Biomedicines, 13(5), 1118. https://doi.org/10.3390/biomedicines13051118





