Closed-Loop Spinal Cord Stimulation in Chronic Pain Management: Mechanisms, Clinical Evidence, and Emerging Perspectives
Abstract
1. Introduction
2. Methodology
3. Current State of Lower Back Pain
3.1. Epidemiology
3.2. Pathophysiology
3.3. Pharmacological and Non-Pharmacological Treatment Strategies
4. Principles of Closed-Loop Spinal Cord Stimulation
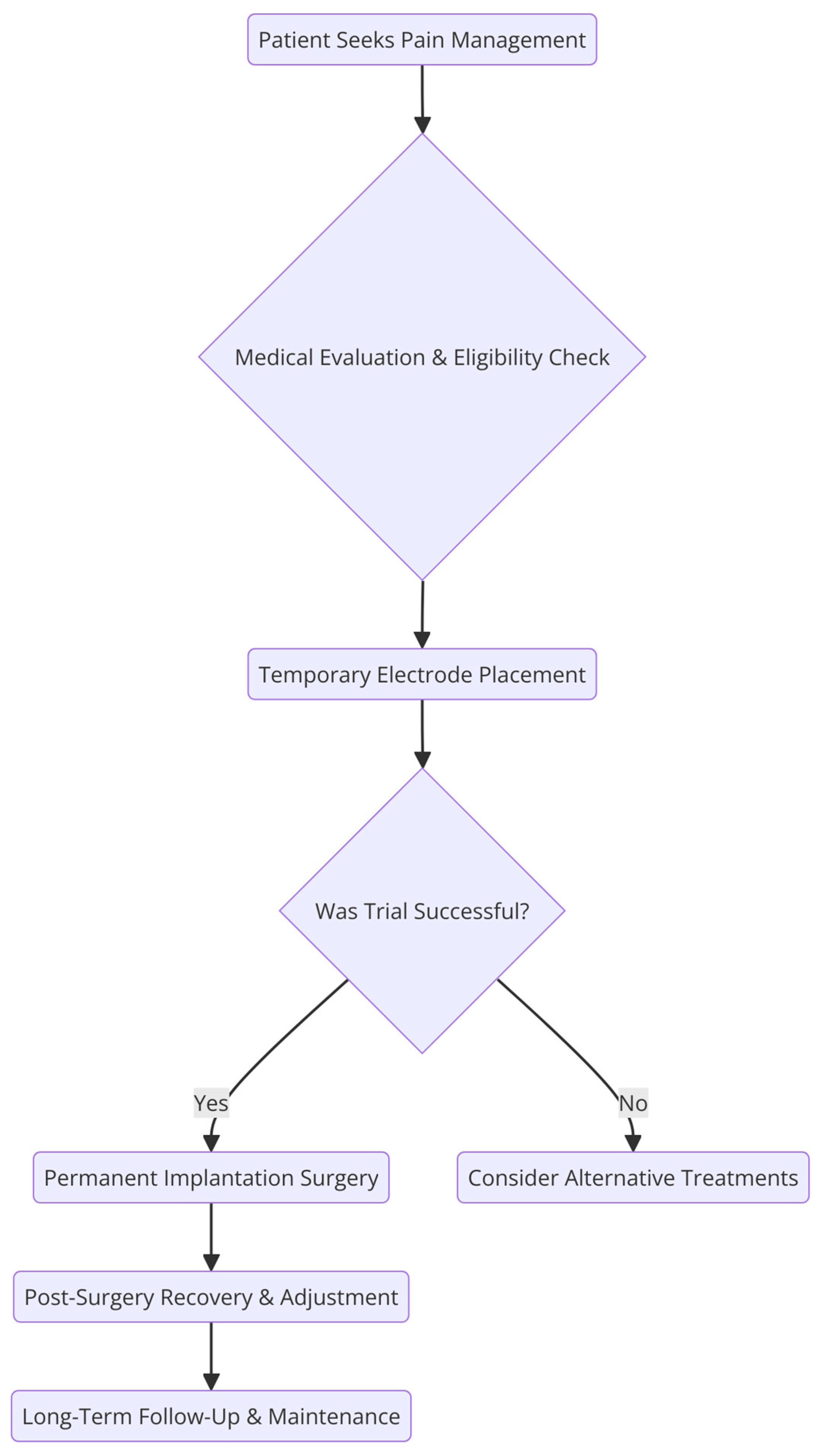
5. Periprocedural Considerations for SCS Therapy
5.1. Pre-Procedural Care
5.2. Perioperative Care
5.3. Postoperative Care
6. Clinical Data
6.1. Avalon Study
6.2. Evoke Trial
6.3. ECAP Study
6.4. ECHO-MAC Trial
6.5. Durability Study
6.6. Additional Studies
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCSs | Spinal cord stimulators |
| ECAP | Evoked compound action potential |
| CL-SCS | Closed-loop spinal cord stimulation |
| MeSHs | Medical Subject Headings |
| MRI | Magnetic resonance imaging |
| P1 | Small positive spike |
| N1 | Sharp negative spike |
| P2 | Large positive spike |
| GA | General anesthesia |
| IPG | Implantable pulse generator |
| IMMPACTs | Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials |
| MMEs | Morphine milligram equivalents |
| OL-SCS | Open-loop spinal cord stimulation |
| EOT | End of the trial |
| ADLs | Activities of daily living |
| RP | Raynaud’s phenomenon |
| DRG | Dorsal root ganglion |
| VAS | Visual Analog Scale |
| BPI | Brief pain inventory |
| EQ-5D-5L | EuroQOL instrument |
| ODI | Oswestry disability index |
| PSQI | Pittsburgh sleep quality index |
| SD | Standard deviation |
| GABA | Gamma-aminobutyric acid |
| RIII | Nociceptive sensorimotor reflex |
| SSEPs | Somatosensory excitation potentials |
References
- Moayedi, M.; Davis, K.D. Theories of pain: From specificity to gate control. J. Neurophysiol. 2013, 109, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.S.; Johnson, J.A.; Zernicke, K.A. Gate Control Theory of Pain. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 914–916. [Google Scholar] [CrossRef]
- Khelemsky, Y.; Malhotra, A.; Gritsenko, K. Academic Pain Medicine A Practical Guide to Rotations, Fellowship, and Beyond: A Practical Guide to Rotations, Fellowship, and Beyond. Anesth. Analg. 2019, 131, e1. [Google Scholar] [CrossRef]
- Brooker, C.; Russo, M.; Cousins, M.J.; Taylor, N.; Holford, L.; Martin, R.; Boesel, T.; Sullivan, R.; Hanson, E.; Gmel, G.E.; et al. ECAP-Controlled Closed-Loop Spinal Cord Stimulation Efficacy and Opioid Reduction over 24-Months: Final Results of the Prospective, Multicenter, Open-Label Avalon Study. Pain. Pract. 2021, 21, 680–691. [Google Scholar] [CrossRef]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [CrossRef]
- Urits, I.; Burshtein, A.; Sharma, M.; Testa, L.; Gold, P.A.; Orhurhu, V.; Viswanath, O.; Jones, M.R.; Sidransky, M.A.; Spektor, B.; et al. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr. Pain. Headache Rep. 2019, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef]
- Delitto, A.; George, S.Z.; Van Dillen, L.; Whitman, J.M.; Sowa, G.; Shekelle, P.; Denninger, T.R.; Godges, J.J. Low back pain. J. Orthop. Sports Phys. Ther. 2012, 42, A1-57. [Google Scholar] [CrossRef]
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The Epidemiology of low back pain. Best. Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and Physiology. Clin. Plast. Surg. 2020, 47, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Atlas, S.J.; Deyo, R.A. Evaluating and managing acute low back pain in the primary care setting. J. Gen. Intern. Med. 2001, 16, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Chenot, J.F.; Greitemann, B.; Kladny, B.; Petzke, F.; Pfingsten, M.; Schorr, S.G. Non-Specific Low Back Pain. Dtsch. Arztebl. Int. 2017, 114, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Glattacker, M.; Heyduck, K.; Jakob, T. Yellow flags as predictors of rehabilitation outcome in chronic low back pain. Rehabil. Psychol. 2018, 63, 408–417. [Google Scholar] [CrossRef]
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M.; et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): A double-blind, randomised, controlled trial. Lancet Neurol. 2020, 19, 123–134. [Google Scholar] [CrossRef]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007, 132, 179–188. [Google Scholar] [CrossRef]
- Petersen, E.A.; Stauss, T.G.; Scowcroft, J.A.; Brooks, E.S.; White, J.L.; Sills, S.M.; Amirdelfan, K.; Guirguis, M.N.; Xu, J.; Yu, C.; et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients with Painful Diabetic Neuropathy: A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 687–698. [Google Scholar] [CrossRef]
- Duarte, R.V.; Nevitt, S.; Maden, M.; Meier, K.; Taylor, R.S.; Eldabe, S.; de Vos, C.C. Spinal cord stimulation for the management of painful diabetic neuropathy: A systematic review and meta-analysis of individual patient and aggregate data. Pain 2021, 162, 2635–2643. [Google Scholar] [CrossRef]
- Eldabe, S.; Thomson, S.; Duarte, R.; Brookes, M.; deBelder, M.; Raphael, J.; Davies, E.; Taylor, R. The Effectiveness and Cost-Effectiveness of Spinal Cord Stimulation for Refractory Angina (RASCAL Study): A Pilot Randomized Controlled Trial. Neuromodulation 2016, 19, 60–70. [Google Scholar] [CrossRef]
- Miękisiak, G. Failed Back Surgery Syndrome: No Longer a Surgeon’s Defeat-A Narrative Review. Medicina 2023, 59, 1255. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Wall, P.D. Presynaptic control of impulses at the first central synapse in the cutaneous pathway. Prog. Brain Res. 1964, 12, 92–118. [Google Scholar] [CrossRef] [PubMed]
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth. Analg. 1967, 46, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Shealy, C.N.; Taslitz, N.; Mortimer, J.T.; Becker, D.P. Electrical inhibition of pain: Experimental evaluation. Anesth. Analg. 1967, 46, 299–305. [Google Scholar] [CrossRef]
- Shealy, C.N.; Mortimer, J.T.; Hagfors, N.R. Dorsal column electroanalgesia. J. Neurosurg. 1970, 32, 560–564. [Google Scholar] [CrossRef]
- Heijmans, L.; Joosten, E.A. Mechanisms and mode of action of spinal cord stimulation in chronic neuropathic pain. Postgrad. Med. 2020, 132, 17–21. [Google Scholar] [CrossRef]
- North, R.B.; Ewend, M.G.; Lawton, M.T.; Piantadosi, S. Spinal cord stimulation for chronic, intractable pain: Superiority of “multi-channel” devices. Pain 1991, 44, 119–130. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.H.; Zahurak, M.; James, C.S.; Long, D.M. Spinal cord stimulation for chronic, intractable pain: Experience over two decades. Neurosurgery 1993, 32, 384–394; discussion 394–395. [Google Scholar] [CrossRef]
- Racz, G.B.; McCarron, R.F.; Talboys, P. Percutaneous dorsal column stimulator for chronic pain control. Spine 1989, 14, 1–4. [Google Scholar] [CrossRef]
- Demirel, T.; Braun, W.; Reimers, C.D. Results of spinal cord stimulation in patients suffering from chronic pain after a two year observation period. Neurochirurgia 1984, 27, 47–50. [Google Scholar] [CrossRef]
- Holsheimer, J.; den Boer, J.A.; Struijk, J.J.; Rozeboom, A.R. MR assessment of the normal position of the spinal cord in the spinal canal. AJNR Am. J. Neuroradiol. 1994, 15, 951–959. [Google Scholar] [PubMed]
- Olin, J.C.; Kidd, D.H.; North, R.B. Postural changes in spinal cord stimulation perceptual thresholds. Neuromodulation 1998, 1, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Shimoji, K.; Shimizu, H.; Kuribayashi, H.; Fujioka, H. Human spinal cord potentials evoked by different sources of stimulation and conduction velocities along the cord. J. Neurophysiol. 1982, 48, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Anaya, C.J.; Zander, H.J.; Graham, R.D.; Sankarasubramanian, V.; Lempka, S.F. Evoked Potentials Recorded From the Spinal Cord During Neurostimulation for Pain: A Computational Modeling Study. Neuromodulation 2020, 23, 64–73. [Google Scholar] [CrossRef]
- Vallejo, R.; Chakravarthy, K.; Will, A.; Trutnau, K.; Dinsmoor, D. A New Direction for Closed-Loop Spinal Cord Stimulation: Combining Contemporary Therapy Paradigms with Evoked Compound Action Potential Sensing. J. Pain Res. 2021, 14, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.P.; Gerard, S.; Raijmakers, M.E.; Truin, M.; Van Kleef, M.; Joosten, E.A. Decreased intracellular GABA levels contribute to spinal cord stimulation-induced analgesia in rats suffering from painful peripheral neuropathy: The role of KCC2 and GABA(A) receptor-mediated inhibition. Neurochem. Int. 2012, 60, 21–30. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Sluka, K.A.; Lisi, T.L.; Westlund, K.N. Increased release of serotonin in the spinal cord during low, but not high, frequency transcutaneous electric nerve stimulation in rats with joint inflammation. Arch. Phys. Med. Rehabil. 2006, 87, 1137–1140. [Google Scholar] [CrossRef]
- Song, Z.; Ultenius, C.; Meyerson, B.A.; Linderoth, B. Pain relief by spinal cord stimulation involves serotonergic mechanisms: An experimental study in a rat model of mononeuropathy. Pain 2009, 147, 241–248. [Google Scholar] [CrossRef]
- Vallejo, R.; Kelley, C.A.; Gupta, A.; Smith, W.J.; Vallejo, A.; Cedeño, D.L. Modulation of neuroglial interactions using differential target multiplexed spinal cord stimulation in an animal model of neuropathic pain. Mol. Pain 2020, 16, 1744806920918057. [Google Scholar] [CrossRef]
- Ruiz-Sauri, A.; Orduña-Valls, J.M.; Blasco-Serra, A.; Tornero-Tornero, C.; Cedeño, D.L.; Bejarano-Quisoboni, D.; Valverde-Navarro, A.A.; Benyamin, R.; Vallejo, R. Glia to neuron ratio in the posterior aspect of the human spinal cord at thoracic segments relevant to spinal cord stimulation. J. Anat. 2019, 235, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Roitbak, A.I.; Fanardjian, V.V. Depolarization of cortical glial cells in response to electrical stimulation of the cortical surface. Neuroscience 1981, 6, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Cedeño, D.L.; Smith, W.J.; Kelley, C.A.; Vallejo, R. Spinal cord stimulation using differential target multiplexed programming modulates neural cell-specific transcriptomes in an animal model of neuropathic pain. Mol. Pain 2020, 16, 1744806920964360. [Google Scholar] [CrossRef] [PubMed]
- Tilley, D.M.; Lietz, C.B.; Cedeno, D.L.; Kelley, C.A.; Li, L.; Vallejo, R. Proteomic Modulation in the Dorsal Spinal Cord Following Spinal Cord Stimulation Therapy in an In Vivo Neuropathic Pain Model. Neuromodulation 2021, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Ji, R.R. Activation of JNK pathway in persistent pain. Neurosci. Lett. 2008, 437, 180–183. [Google Scholar] [CrossRef]
- De Hert, S.; Staender, S.; Fritsch, G.; Hinkelbein, J.; Afshari, A.; Bettelli, G.; Bock, M.; Chew, M.S.; Coburn, M.; De Robertis, E.; et al. Pre-operative evaluation of adults undergoing elective noncardiac surgery: Updated guideline from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. 2018, 35, 407–465. [Google Scholar] [CrossRef]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 515–550; discussion 550. [Google Scholar] [CrossRef]
- Celestin, J.; Edwards, R.R.; Jamison, R.N. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: A systematic review and literature synthesis. Pain Med. 2009, 10, 639–653. [Google Scholar] [CrossRef]
- Paroli, M.; Bernini, O.; De Carolis, G.; Tollapi, L.; Bondi, F.; Martini, A.; Dario, A.; Paolicchi, A. Are Multidimensional Pain Inventory Coping Strategy Profiles Associated with Long-Term Spinal Cord Stimulation Effectiveness? Pain Med. 2018, 19, 1023–1032. [Google Scholar] [CrossRef]
- Deer, T.R.; Provenzano, D.A.; Hanes, M.; Pope, J.E.; Thomson, S.J.; Russo, M.A.; McJunkin, T.; Saulino, M.; Raso, L.J.; Lad, S.P.; et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Recommendations for Infection Prevention and Management. Neuromodulation 2017, 20, 31–50. [Google Scholar] [CrossRef]
- Law, J.A.; Broemling, N.; Cooper, R.M.; Drolet, P.; Duggan, L.V.; Griesdale, D.E.; Hung, O.R.; Jones, P.M.; Kovacs, G.; Massey, S.; et al. The difficult airway with recommendations for management--part 2--the anticipated difficult airway. Can. J. Anaesth. 2013, 60, 1119–1138. [Google Scholar] [CrossRef] [PubMed]
- Falowski, S.M.; Celii, A.; Sestokas, A.K.; Schwartz, D.M.; Matsumoto, C.; Sharan, A. Awake vs. asleep placement of spinal cord stimulators: A cohort analysis of complications associated with placement. Neuromodulation 2011, 14, 130–134; discussion 134–135. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Baranidharan, G. Spinal cord stimulators and implications for anaesthesia. BJA Educ. 2020, 20, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.M.; Veizi, E.; Hanes, M. Treatment-Limiting Complications of Percutaneous Spinal Cord Stimulator Implants: A Review of Eight Years of Experience from an Academic Center Database. Neuromodulation 2015, 18, 603–608; discussion 608–609. [Google Scholar] [CrossRef]
- Caylor, J.; Reddy, R.; Yin, S.; Cui, C.; Huang, M.; Huang, C.; Rao, R.; Baker, D.G.; Simmons, A.; Souza, D.; et al. Spinal cord stimulation in chronic pain: Evidence and theory for mechanisms of action. Bioelectron. Med. 2019, 5, 12. [Google Scholar] [CrossRef]
- Sato, K.L.; Johanek, L.M.; Sanada, L.S.; Sluka, K.A. Spinal cord stimulation reduces mechanical hyperalgesia and glial cell activation in animals with neuropathic pain. Anesth. Analg. 2014, 118, 464–472. [Google Scholar] [CrossRef]
- Ranger, M.R.; Irwin, G.J.; Bunbury, K.M.; Peutrell, J.M. Changing body position alters the location of the spinal cord within the vertebral canal: A magnetic resonance imaging study. Br. J. Anaesth. 2008, 101, 804–809. [Google Scholar] [CrossRef]
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M.; et al. Durability of Clinical and Quality-of-Life Outcomes of Closed-Loop Spinal Cord Stimulation for Chronic Back and Leg Pain: A Secondary Analysis of the Evoke Randomized Clinical Trial. JAMA Neurol. 2022, 79, 251–260. [Google Scholar] [CrossRef]
- Sdrulla, A.D.; Guan, Y.; Raja, S.N. Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms. Pain Pract. 2018, 18, 1048–1067. [Google Scholar] [CrossRef]
- Bentley, L.D.; Duarte, R.V.; Furlong, P.L.; Ashford, R.L.; Raphael, J.H. Brain activity modifications following spinal cord stimulation for chronic neuropathic pain: A systematic review. Eur. J. Pain. 2016, 20, 499–511. [Google Scholar] [CrossRef]
- Turk, D.C.; Dworkin, R.H.; Allen, R.R.; Bellamy, N.; Brandenburg, N.; Carr, D.B.; Cleeland, C.; Dionne, R.; Farrar, J.T.; Galer, B.S.; et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003, 106, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, N.A.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Pope, J.E.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; et al. ECAP-controlled closed-loop versus open-loop SCS for the treatment of chronic pain: 36-month results of the EVOKE blinded randomized clinical trial. Reg. Anesth. Pain Med. 2024, 49, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Will, A.; Fishman, M.; Schultz, D.; Danko, M.; Verill, D.; Davies, C.; Retterath, P.; Miller, N.; Tonder, L.; Johanek, L.; et al. Improvements in Therapy Experience with Evoked Compound Action Potential Controlled, Closed-Loop Spinal Cord Stimulation-Primary Outcome of the ECHO-MAC Randomized Clinical Trial. J. Pain 2024, 25, 104646. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Antony, A.; Petersen, E.A.; Rosen, S.M.; Sayed, D.; Hunter, C.W.; Goree, J.H.; Vu, C.M.; Bhandal, H.S.; Shumsky, P.M.; et al. Identifying SCS Trial Responders Immediately After Postoperative Programming with ECAP Dose-Controlled Closed-Loop Therapy. Pain Ther. 2024, 13, 1173–1185. [Google Scholar] [CrossRef]
- Billet, B.; De Vos, R.; Hanssens, K. P111 holistic outcomes with ecap-controlled closed-loop scs: Interim az delta experience. Neuromodul. Technol. Neural Interface 2025, 28, S231. [Google Scholar] [CrossRef]
- Nijhuis, H.; Kallewaard, J.W.; van de Minkelis, J.; Hofsté, W.J.; Elzinga, L.; Armstrong, P.; Gültuna, I.; Almac, E.; Baranidharan, G.; Nikolic, S.; et al. Durability of Evoked Compound Action Potential (ECAP)-Controlled, Closed-Loop Spinal Cord Stimulation (SCS) in a Real-World European Chronic Pain Population. Pain Ther. 2024, 13, 1119–1136. [Google Scholar] [CrossRef]
- Levy, R.M.; Mekhail, N.A.; Kapural, L.; Gilmore, C.A.; Petersen, E.A.; Goree, J.H.; Pope, J.E.; Costandi, S.J.; Kallewaard, J.W.; Thomson, S.; et al. Maximal Analgesic Effect Attained by the Use of Objective Neurophysiological Measurements with Closed-Loop Spinal Cord Stimulation. Neuromodulation 2024, 27, 1393–1405. [Google Scholar] [CrossRef]
- Chung, M.; Abd-Elsayed, A. Comparative efficacy of closed-loop spinal cord stimulation and dorsal root ganglion stimulation through combination trialing for cancer pain—A retrospective case series. Pain Pract. 2025, 25, e70010. [Google Scholar] [CrossRef]
- Maciaczyk, J.; Bara, G.; Basilaia, B.; Abuassi, M.; Dietz, B.E.; Mugan, D.; Mayr, A.; Staerk, C.; Karakostas, P.; Schäfer, V.S. A Prospective Single-center Pilot Study on the Use of Closed-loop Spinal Cord Stimulation to Treat Chronic Pain Associated with Raynaud’s Phenomenon. Neuromodulation 2024, 27, 1457–1469. [Google Scholar] [CrossRef]
- Briggi, D.R.; Vangeison, C.T.; Vu, P.D.; Shah, Z.; Bruel, B.M. Closed-loop spinal cord stimulation as a novel treatment for chronic pelvic pain: A letter to the editor. Interv. Pain Med. 2024, 3, 100415. [Google Scholar] [CrossRef]
- Levy, R.; Deer, T.R.; Poree, L.; Rosen, S.M.; Kapural, L.; Amirdelfan, K.; Soliday, N.; Leitner, A.; Mekhail, N. Multicenter, Randomized, Double-Blind Study Protocol Using Human Spinal Cord Recording Comparing Safety, Efficacy, and Neurophysiological Responses Between Patients Being Treated with Evoked Compound Action Potential-Controlled Closed-Loop Spinal Cord Stimulation or Open-Loop Spinal Cord Stimulation (the Evoke Study). Neuromodulation 2019, 22, 317–326. [Google Scholar] [CrossRef] [PubMed]
- North, R.; Desai, M.J.; Vangeneugden, J.; Raftopoulos, C.; Van Havenbergh, T.; Deruytter, M.; Remacle, J.M.; Shipley, J.; Tan, Y.; Johnson, M.J.; et al. Postoperative Infections Associated with Prolonged Spinal Cord Stimulation Trial Duration (PROMISE RCT). Neuromodulation 2020, 23, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.L.; Stauss, T.; Li, S.; Rotte, A.; Subbaroyan, J. A Prospective, Multi-Center, Clinical Trial of a 10-kHz Spinal Cord Stimulation System in the Treatment of Chronic Pelvic Pain. Pain Pract. 2021, 21, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Versantvoort, E.M.; Dietz, B.E.; Mugan, D.; Vuong, Q.C.; Luli, S.; Obara, I. Evoked compound action potential (ECAP)-controlled closed-loop spinal cord stimulation in an experimental model of neuropathic pain in rats. Bioelectron. Med. 2024, 10, 2. [Google Scholar] [CrossRef]
- Wu, N.; Wu, Z.; Zhang, C.; Wu, C.; Huo, X.; Bai, J.; Zhang, G. Retrograde evoked compound action potentials as an alternative for close-loop spinal cord stimulation. Sci. Rep. 2024, 14, 30141. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.E.; Titus, N.; Zhang, T.; Esteller, R.; Grill, W.M. Surround Inhibition Mediates Pain Relief by Low Amplitude Spinal Cord Stimulation: Modeling and Measurement. eNeuro 2022, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sokal, P.; Malukiewicz, A.; Kierońska, S.; Murawska, J.; Guzowski, C.; Rudaś, M.; Paczkowski, D.; Rusinek, M.; Krakowiak, M. Sub-Perception and Supra-Perception Spinal Cord Stimulation in Chronic Pain Syndrome: A Randomized, Semi-Double-Blind, Crossover, Placebo-Controlled Trial. J. Clin. Med. 2020, 9, 2810. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.V.; Bentley, A.; Soliday, N.; Leitner, A.; Gulve, A.; Staats, P.S.; Sayed, D.; Falowski, S.M.; Hunter, C.W.; Taylor, R.S. Cost-utility Analysis of Evoke Closed-loop Spinal Cord Stimulation for Chronic Back and Leg Pain. Clin. J. Pain 2023, 39, 551–559. [Google Scholar] [CrossRef]
- Eldabe, S.; Nevitt, S.; Bentley, A.; Mekhail, N.A.; Gilligan, C.; Billet, B.; Staats, P.S.; Maden, M.; Soliday, N.; Leitner, A.; et al. Network Meta-analysis and Economic Evaluation of Neurostimulation Interventions for Chronic Nonsurgical Refractory Back Pain. Clin. J. Pain 2024, 40, 507–517. [Google Scholar] [CrossRef]
| Study | Publication Year | Study Type | Patients (n) | Endpoints | Findings |
|---|---|---|---|---|---|
| Evoke [63] | 2024 | Multicenter, Double-blinded, randomized controlled trial | 134 | Reduction of ≥ 50% in overall back and leg pain, objective neural activation amplitude | Significantly greater number of patients endorsing a ≥50% and a ≥80% improvement in back and limb pain with CL versus OL Significantly more time in the therapeutic window with CL versus OL Fewer supratherapeutic stimulation amplitudes in CL versus OL significant increases in the No difference in the rate of adverse events between groups |
| ECHO-MAC [64] | 2024 | Multicenter, single-blinded, crossover, randomized controlled trial | 42 | Overstimulation and understimulation during ADLs with CL versus OL, patient preference, ECAP amplitudes and dose consistency | 97.6% reduction in overstimulation symptoms during CL-mode compared to OL-mode Mean ECAP amplitudes of 24.5 μV during OL and 9.3 μV during CL Median stimulation amplitudes of 6.4 mA during OL and 3.96 mA during CL 88.1% of patients preferred CL compared to OL |
| Study | Publication Year | Study Type | Patients (n) | Endpoints | Findings |
|---|---|---|---|---|---|
| Avalon [4] | 2021 | Prospective, multi-center, single-arm | 50 | Pain relief, opioid reduction | 85% of patients exhibited a response to treatment 77.3% reduction in pain scores 82.8% of patients had either reduction or discontinuation of opioid use Decrease in average MME from 62.9 to 29.1 per day |
| ECAP [65] | 2024 | Prospective | 132 | PPV and FPR of a successful Day 0 trial evaluation predicting a successful EOT evaluation | 98.4% PPV of a successful Day 0 evaluation predicting a successful EOT evaluation 5.6% FPR of successful Day 0 evaluations “converting” to non-successful EOT evaluations |
| Durability [66] | 2025 (projected to finish in 2027) | Prospective, multi-center | 70 | Percent change in pain severity after implant | Interim 6 months analysis with confirmed consistent device usage by patients, and an average improvement of 2.5 MCID points across various holistic measures of pain severity |
| Nijhuis et al. [67] | 2024 | Prospective, multi-center | 22 | Difference in overall back/leg pain from baseline | Similar rates of satisfaction and average pain relief at both 3 and 12 months after implantation |
| Levy et al. [68] | 2024 | Post-hoc analysis | 180 | Changes in MAE, dose accuracy, and ratio during ECAP and Durability studies | No significant difference in MAE or neurophysiologic ECAP amplitudes between these trials (ECAP and Durability) |
| Chung et al. [69] | 2025 | Retrospective case series | 4 | Pain relief and functional improvement in DRG and CL-SCS | All patients expressed pain relief and endorsed functional improvement after both the DRG stimulation or CL-SCS, with 3 preferring the CL-SCS |
| Maciaczyk et al. [70] | 2024 | Prospective, single center | 10 | Severity and frequency of RP exacerbations after CL-SCS | CL-SCS significantly improved the severity of exacerbations. However, the study was unable to demonstrate significant improvement in frequency of exacerbations |
| Briggi et al. [71] | 2024 | Case report | 1 | N/A | Complete alleviation of pain from CL-SCS implanted at T10-T11 in a patient with otherwise refractory pelvic pain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangano, N.; Torpey, A.; Devitt, C.; Wen, G.A.; Doh, C.; Gupta, A. Closed-Loop Spinal Cord Stimulation in Chronic Pain Management: Mechanisms, Clinical Evidence, and Emerging Perspectives. Biomedicines 2025, 13, 1091. https://doi.org/10.3390/biomedicines13051091
Mangano N, Torpey A, Devitt C, Wen GA, Doh C, Gupta A. Closed-Loop Spinal Cord Stimulation in Chronic Pain Management: Mechanisms, Clinical Evidence, and Emerging Perspectives. Biomedicines. 2025; 13(5):1091. https://doi.org/10.3390/biomedicines13051091
Chicago/Turabian StyleMangano, Nicholas, Andrew Torpey, Catherine Devitt, George A. Wen, Christopher Doh, and Abhishek Gupta. 2025. "Closed-Loop Spinal Cord Stimulation in Chronic Pain Management: Mechanisms, Clinical Evidence, and Emerging Perspectives" Biomedicines 13, no. 5: 1091. https://doi.org/10.3390/biomedicines13051091
APA StyleMangano, N., Torpey, A., Devitt, C., Wen, G. A., Doh, C., & Gupta, A. (2025). Closed-Loop Spinal Cord Stimulation in Chronic Pain Management: Mechanisms, Clinical Evidence, and Emerging Perspectives. Biomedicines, 13(5), 1091. https://doi.org/10.3390/biomedicines13051091






