Evaluating Magnetic Stimulation as an Innovative Approach for Treating Dry Eye Disease: An Initial Safety and Efficacy Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Study Aims
2.1.2. Study Endpoints
2.2. Participants
2.2.1. Inclusion and Exclusion Criteria
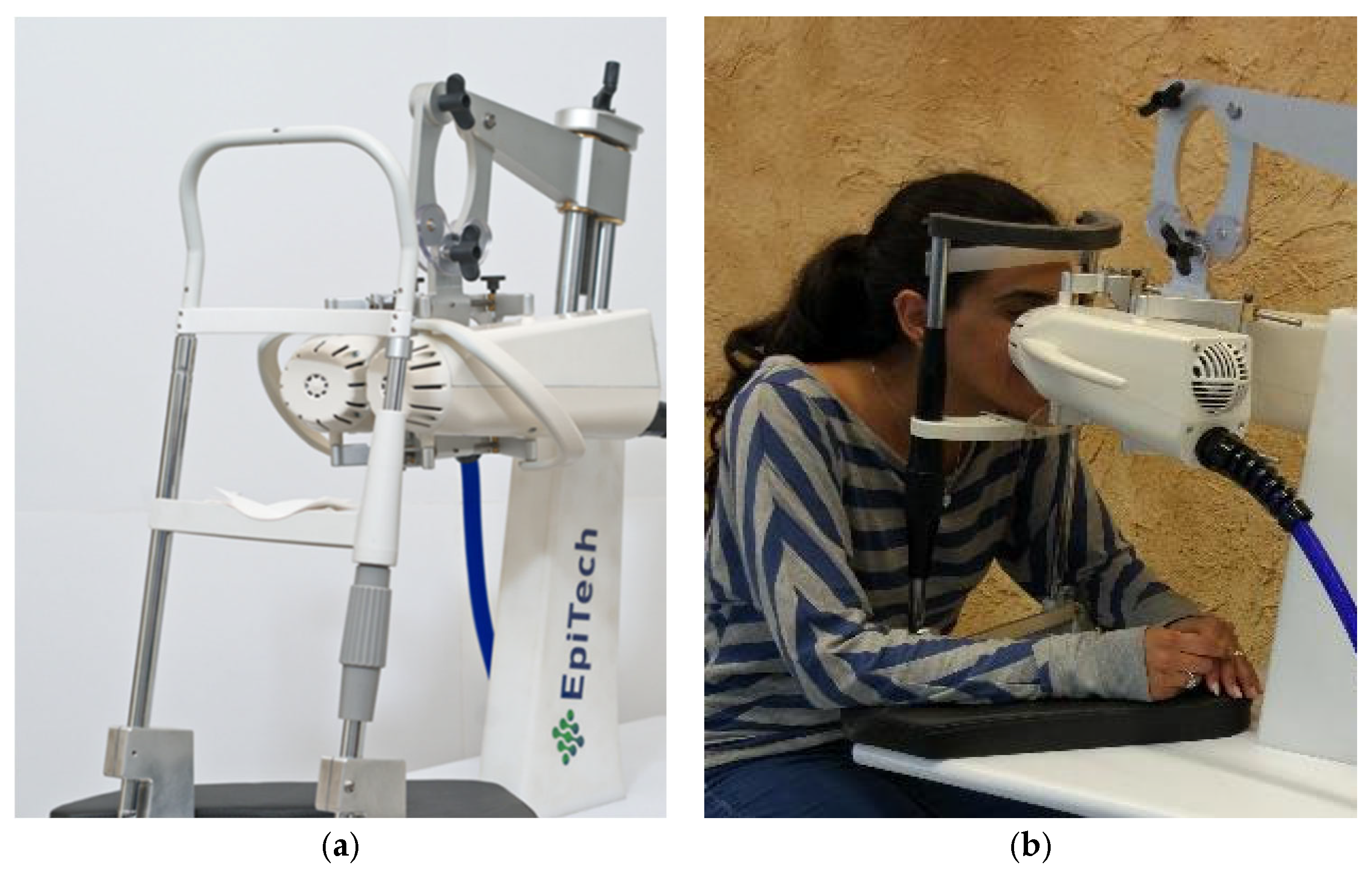
2.2.2. Instrument
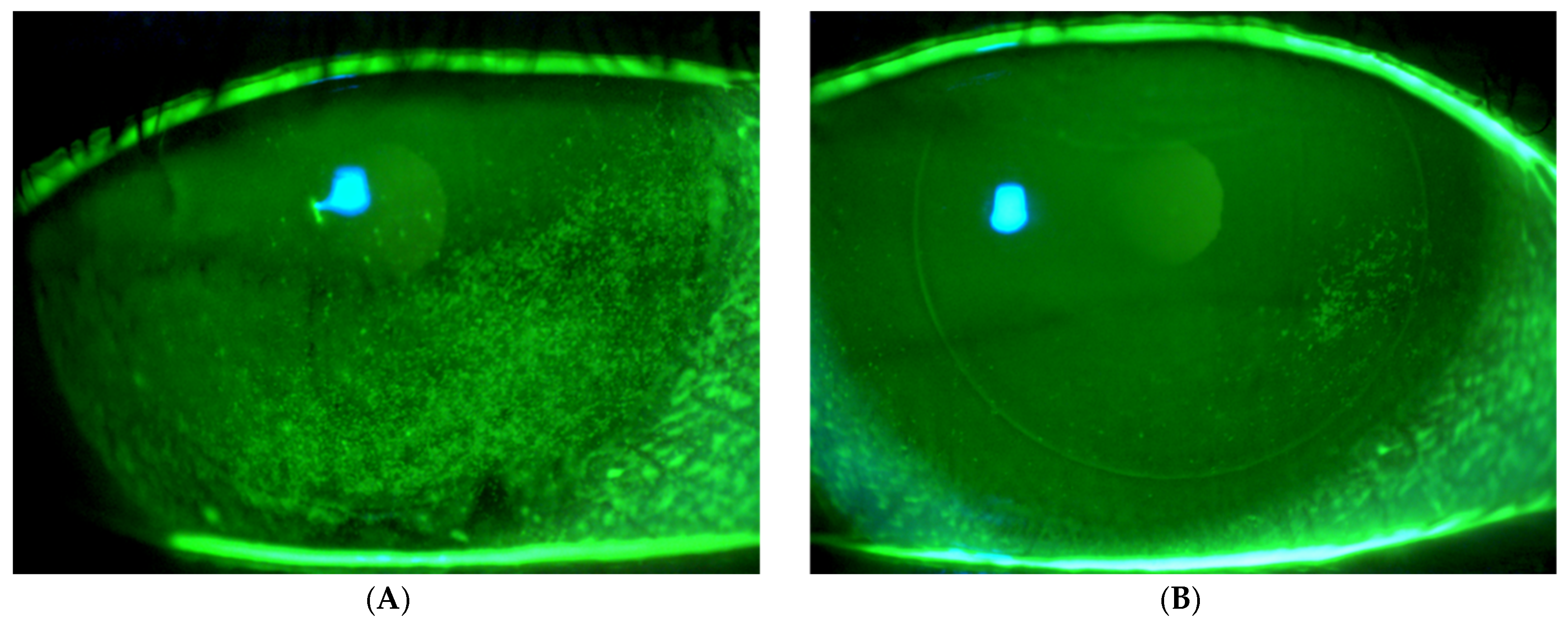
2.2.3. Safety and Efficacy Tests
2.2.4. The Course of the Experiment
2.2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Dry Eye Workshop (DEWS). The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. Ocul. Surface 2007, 5, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Gayton, J. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009, 3, 405–412. [Google Scholar] [CrossRef]
- Courtin, R.; Pereira, B.; Naughton, G.; Chamoux, A.; Chiambaretta, F.; Lanhers, C.; Dutheil, F. Prevalence of dry eye disease in visual display terminal workers: A systematic review and meta-analysis. BMJ Open 2016, 6, e009675. [Google Scholar] [CrossRef]
- Galor, A. Painful Dry Eye Symptoms: A Nerve Problem or a Tear Problem? Ophthalmology 2019, 126, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Sawale, G.; Ghuge, S.; Sathaye, S. Quintessence of currently approved and upcoming treatments for dry eye disease. Graefes. Arch. Clin. Exp. Ophthalmol. 2025, 263, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Coco, G.; Ambrosini, G.; Poletti, S.; Meliante, L.A.; Taloni, A.; Scorcia, V.; Giannaccare, G. Recent advances in drug treatments for dry eye disease. Expert Opin. Pharmacother. 2023, 24, 2059–2079. [Google Scholar] [CrossRef]
- Valdés-Arias, D.; Locatelli, E.V.T.; Sepulveda-Beltran, P.A.; Mangwani-Mordani, S.; Navia, J.C.; Galor, A. Recent United States Developments in the Pharmacological Treatment of Dry Eye Disease. Drugs 2024, 84, 549–563. [Google Scholar] [CrossRef]
- Dieckmann, G.; Fregni, F.; Hamrah, P. Neurostimulation in dry eye disease-past, present, and future. Ocul. Surf. 2019, 17, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.D.; Evans, D.G.; Ousler, G.W.; Wilson, J.; Baba, S.N.; Senchyna, M.; Holland, E.J. Characterization of tear production in subjects with dry eye disease during intranasal tear neurostimulation: Results from two pivotal clinical trials. Ocul. Surf. 2019, 17, 142–150. [Google Scholar] [CrossRef]
- Carmi, L.; Alyagon, U.; Barnea-ygael, N.; Zohar, J.; Dar, R.; Zangen, A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018, 11, 158–165. [Google Scholar] [CrossRef]
- Fiocchi, S.; Longhi, M.; Ravazzani, P.; Roth, Y.; Zangen, A.; Parazzini, M. Modelling of the Electric Field Distribution in Deep Transcranial Magnetic Stimulation in the Adolescence, in the Adulthood, and in the Old Age. Comput. Math. Methods Med. 2016, 2016, 9039613. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Otake, H. Novel drug delivery systems for the management of dry eye. Adv. Drug Deliv. Rev. 2022, 191, 114582. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Patel, S.; Galor, A. Alternative therapies for dry eye disease. Curr. Opin. Ophthalmol. 2021, 32, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Sher, I.; Tzameret, A.; Goldberg, Z.; Bubis, E.; Avni-Zauberman, N.; Kalter-Leibovici, O.; Marcovich, A.L.; Simon, G.B.; Rotenstreich, Y. Repetitive magnetic stimulation protects corneal epithelium in a rabbit model of short-term exposure keratopathy. Ocul. Surf. 2020, 18, 64–73. [Google Scholar] [CrossRef]
- Seewoo, B.J.; Feindel, K.W.; Etherington, S.J.; Rodger, J. Frequency-specific effects of low-intensity rTMS can persist for up to 2 weeks post-stimulation: A longitudinal rs-fMRI/MRS study in rats. Brain Stimul. 2019, 12, 1526–1536. [Google Scholar] [CrossRef]
- Savulescu, S.E.; Berteanu, M.; Filipescu, I.; Beiu, C.; Mihai, M.-M.; Popa, L.G.; Popescu, S.I.; Balescu, I.; Bacalbasa, N.; Popescu, M.-N. Repetitive Peripheral Magnetic Stimulation (rPMS) in Subjects with Lumbar Radiculopathy: An Electromyography-guided Prospective, Randomized Study. In Vivo 2021, 35, 623–627. [Google Scholar] [CrossRef]
- Behrens, A.; John, J.D.; Lee, S.; Roy, S.C.; Peter, J.M.; Dimitri, T.A.; Harminder, S.D.; Milton, H.; Paul, M.K.; Laibson, P.R.; et al. Dysfunctional tear syndrome: A Delphi approach to treatment recommendations. Cornea 2006, 25, 900–907. [Google Scholar] [CrossRef]
- NCT03012698@ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03012698 (accessed on 1 March 2025).
- Versura, P.; Profazio, V.; Campos, E.C. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr. Eye Res. 2010, 35, 553–564. [Google Scholar] [CrossRef]
- Sher, I.; Tzameret, A.; Szalapak, A.M.; Carmeli, T.; Derazne, E.; Avni-Zauberman, N.; Marcovich, A.L.; Ben Simon, G.; Rotenstreich, Y. Multimodal Assessment of Corneal Erosions Using Optical Coherence Tomography and Automated Grading of Fluorescein Staining in a Rabbit Dry Eye Model. Transl. Vis. Sci. Technol. 2019, 8, 27. [Google Scholar] [CrossRef]
- Sweeney, D.F.; Millar, T.J.; Raju, S.R. Tear film stability: A review. Exp. Eye Res. 2013, 117, 28–38. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Chun, Y.S.; Yoon, W.B.; Kim, K.G.; Park, I.K. Objective assessment of corneal staining using digital image analysis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7896–7903. [Google Scholar] [CrossRef] [PubMed]
- Ngo, W.; Situ, P.; Keir, N.; Korb, D.; Blackie, C.; Simpson, T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness Questionnaire. Cornea 2013, 32, 1204–1210. [Google Scholar] [CrossRef]
- Hays, R.D.; Tarver, M.E.; Spritzer, K.L.; Reise, S.; Hilmantel, G.; Hofmeister, E.M.; Hammel, K.; May, J.; Ferris, F.; Eydelman, M. Assessment of the Psychometric Properties of a Questionnaire Assessing Patient-Reported Outcomes with Laser In Situ Keratomileusis (PROWL). JAMA Ophthalmol. 2017, 135, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Lader, M. The use of analogue scales in rating subjective feelings. Br. J. Med. Psychol. 1974, 47, 211–218. [Google Scholar] [CrossRef]
- Farhangi, M.; Cheng, A.M.; Baksh, B.; Sarantopoulos, C.D.; Felix, E.R.; Levitt, R.C.; Galor, A. Effect of non-invasive intranasal neurostimulation on tear volume, dryness and ocular pain. Br. J. Ophthalmol. 2020, 104, 1310–1316. [Google Scholar] [CrossRef]
- Cohn, G.S.; Corbett, D.; Tenen, A.; Coroneo, M.; McAlister, J.; Craig, J.P.; Gray, T.; Kent, D.; Murray, N.; Petsoglou, C.; et al. Randomized, Controlled, Double-Masked, Multicenter, Pilot Study Evaluating Safety and Efficacy of Intranasal Neurostimulation for Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2019, 60, 147–153. [Google Scholar] [CrossRef]
- Sabel, B.A.; Thut, G.; Haueisen, J.; Henrich-Noack, P.; Herrmann, C.S.; Hunold, A.; Kammer, T.; Matteo, B.; Sergeeva, E.G.; Waleszczyk, W.; et al. Vision modulation, plasticity and restoration using non-invasive brain stimulation-An IFCN-sponsored review. Clin. Neurophysiol. 2020, 131, 887–911. [Google Scholar] [CrossRef]
- Oswald, D.J.; Lee, A.; Trinidad, M.; Chi, C.; Ren, R.; Rich, C.B.; Trinkaus-Randall, V. Communication between corneal epithelial cells and trigeminal neurons is facilitated by purinergic (P2) and glutamatergic receptors. PLoS ONE 2012, 7, e44574. [Google Scholar] [CrossRef]
- Beuerman, R.W.; Schimmelpfennig, B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp. Neurol. 1980, 69, 196–201. [Google Scholar] [CrossRef]
- Toshida, H.; Nguyen, D.H.; Beuerman, R.W.; Murakami, A. Evaluation of novel dry eye model: Preganglionic parasympathetic denervation in rabbit. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4468–4475. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.; Jüttner, R.; Roth, C.; Bajbouj, M.; Kirste, I.; Heuser, I.; Gertz, K.; Endres, M.; Kronenberg, G. Repetitive magnetic stimulation of human-derived neuron-like cells activates cAMP-CREB pathway. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 87–91. [Google Scholar] [CrossRef]
- Kudo, K.; Yamada, M.; Takahashi, K.; Nishioka, G.; Tanaka, S.; Hashiguchi, T.; Fukuzako, H.; Takigawa, M.; Higuchi, T.; Momose, K.; et al. Repetitive transcranial magnetic stimulation Induces KF-1 expression in the rat brain. Life Sci. 2005, 76, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Crupi, D.; Liu, J.; Stucky, A.; Cruciata, G.; Di Rocco, A.; Friedman, E.; Quartarone, A.; Ghilardi, M.F. Repetitive transcranial magnetic stimulation enhances BDNF-TrkB signaling in both brain and lymphocyte. J. Neurosci. 2011, 31, 11044–11054. [Google Scholar] [CrossRef]
- Feng, S.F.; Shi, T.Y.; Wang, W.N.; Chen, Y.C.; Tan, Q.R. Long-lasting effects of chronic rTMS to treat chronic rodent model of depression. Behav. Brain Res. 2012, 232, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.W.; Murphy, C.J.; Iwahashi, C.K.; Foster, B.A.; Mannis, M.J. Stimulation of epithelial cell growth by the neuropeptide substance P. J. Cell. Biochem. 1993, 52, 476–485. [Google Scholar] [CrossRef]
- Araki-Sasaki, K.; Aizawa, S.; Hiramoto, M.; Nakamura, M.; Iwase, O.; Nakata, K.; Sasaki, Y.; Mano, T.; Handa, H.; Tano, Y. Substance P-induced cadherin expression and its signal transduction in a cloned human corneal epithelial cell line. J. Cell. Physiol. 2000, 182, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hirschfeld, J.; Lopez-Briones, L.G.; Belmonte, C. Neurotrophic Influences on Corneal Epithelial Cells. Exp. Eye Res. 1994, 59, 597–605. [Google Scholar] [CrossRef]
- Cai, M.-M.; Zhang, J. Effectiveness of transcutaneous electrical stimulation combined with artificial tears for the treatment of dry eye: A randomized controlled trial. Exp. Ther. Med. 2020, 20, 175. [Google Scholar] [CrossRef]
- Park, J.K.; Cremers, S.; Kossler, A.L. Neurostimulation for tear production. Curr. Opin. Ophthalmol. 2019, 30, 386–394. [Google Scholar] [CrossRef]
- Brinton, M.; Chung, J.L.; Kossler, A.; Kook, K.H.; Loudin, J.; Franke, M.; Palanker, D. Electronic enhancement of tear secretion. J. Neural Eng. 2015, 13, 016006. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Treated Eye (N = 21) Mean ± SD | Non-Treated Eye (N = 19) Mean ± SD | p Value |
|---|---|---|---|
| Mean age (years) | 51.4 ± 18.6 | 47.7 ± 17.9 | 0.53 c |
| Age range (years) | 22–79 | 22–71 | |
| Female gender, N (%) | 18 (85%) | 16 (84%) | 0.72 b |
| Dry eye classification, N (%) | 0.26 d | ||
| Sjögren’s syndrome | 15 (57.9%) | 11 (57.9%) | |
| Aqueous tear deficiency | 3 (12%) | 5 (26%) | |
| MGD | 3 (12%) | 3 (15.8%) | |
| Treated eye RE, N (%) | 57.9% | 68% | 0.76 b |
| IOP at baseline (mmHg) | 14.5 ± 1.4 | 13.76 ± 0.9 | 0.31 a |
| BCVA at baseline (LogMAR) | 0.7 ± 0.1 | 0.7 ± 0.10 | 0.66 a |
| Schirmer’s test at baseline (mm) | 4.5 ± 1.5 | 5.2 ± 1.6 | 0.69 a |
| Treatment | Measurement | Time | Mean ± SD | p Value (Change over Time) |
|---|---|---|---|---|
| Treated N = 21 | BCVA | Enrollment | 0.22 ± 0.28 | 0.13 a |
| 1 week | 0.17 ± 0.26 | |||
| 1 month | 0.15 ± 0.26 | |||
| 2 months | 0.15 ± 0.26 | |||
| 3 months | 0.19 ± 0.26 | |||
| IOP | Enrollment | 13.76 ± 2.57 | 0.16 a | |
| 1 week | 12.40 ± 2.68 | |||
| 1 month | 11.96 ± 3.43 | |||
| 2 months | 11.96 ± 2.65 | |||
| 3 months | 12.90 ± 3.13 | |||
| Schirmer | Enrollment | 5.24 ± 4.32 | 0.42 b | |
| 1 week | 4.44 ± 3.65 | |||
| 1 month | 4.64 ± 4.00 | |||
| 2 months | 4.13 ± 3.70 | |||
| 3 months | 3.62 ± 2.71 | |||
| Not Treated N = 19 | BCVA | Enrollment | 0.14 ± 0.27 | 0.39 a |
| 1 week | 0.15 ± 0.28 | |||
| 1 month | 0.14 ± 0.26 | |||
| 2 months | 0.14 ± 0.26 | |||
| 3 months | 0.16 ± 0.28 | |||
| IOP | Enrollment | 14.53 ± 3.24 | 0.74 a | |
| 1 week | 13.05 ± 3.31 | |||
| 1 month | 13.84 ± 3.43 | |||
| 2 months | 13.42 ± 3.31 | |||
| 3 months | 13.89 ± 3.13 | |||
| Schirmer | Enrollment | 4.53 ± 3.65 | 0.51 b | |
| 1 week | 3.89 ± 2.76 | |||
| 1 month | 3.47 ± 2.95 | |||
| 2 months | 4.5 ± 4.1 | |||
| 3 months | 4.47 ± 3.60 |
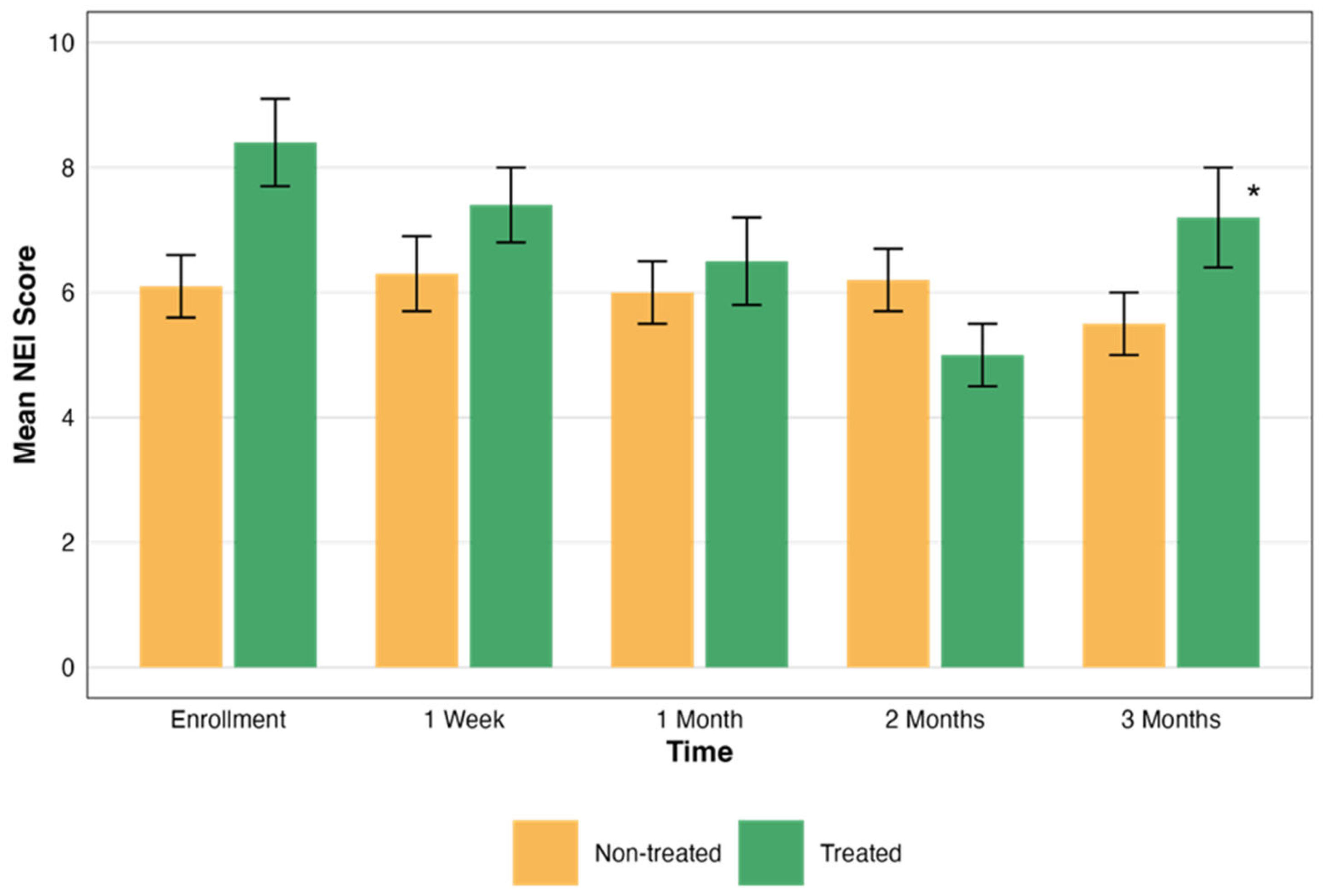
| Time Point | Group | NEI Mean ± SD | TBUT Mean Rank ± SD | NEI p Value | TBUT p Value |
|---|---|---|---|---|---|
| Enrollment | Non-treated N = 19 | 6.68 ± 3.65 | 3.24 ± 2.72 | 0.52 a | 0.19 b |
| Week 1 | 6.32 ± 3.71 | 3.64 ± 2.03 | |||
| Month 1 | 6.05 ± 3.29 | 2.7 ± 1.71 | |||
| Month 2 | 6.16 ± 3.16 | 4.22 ± 2.28 | |||
| Month 3 | 5.47 ± 3.85 | 3.99 ± 2.06 | |||
| Enrollment | Treated N = 21 | 9.76 ± 3.80 | 2.94 ± 1.56 | 0.004 a | 0.04 b |
| Week 1 | 7.62 ± 4.43 | 3.26 ± 1.19 | |||
| Month 1 | 6.67 ± 4.35 | 3.19 ± 1.29 | |||
| Month 2 | 5.19 ± 3.60 | 3.77 ± 0.99 | |||
| Month 3 | 7.24 ± 5.02 | 3.99 ± 1.24 |
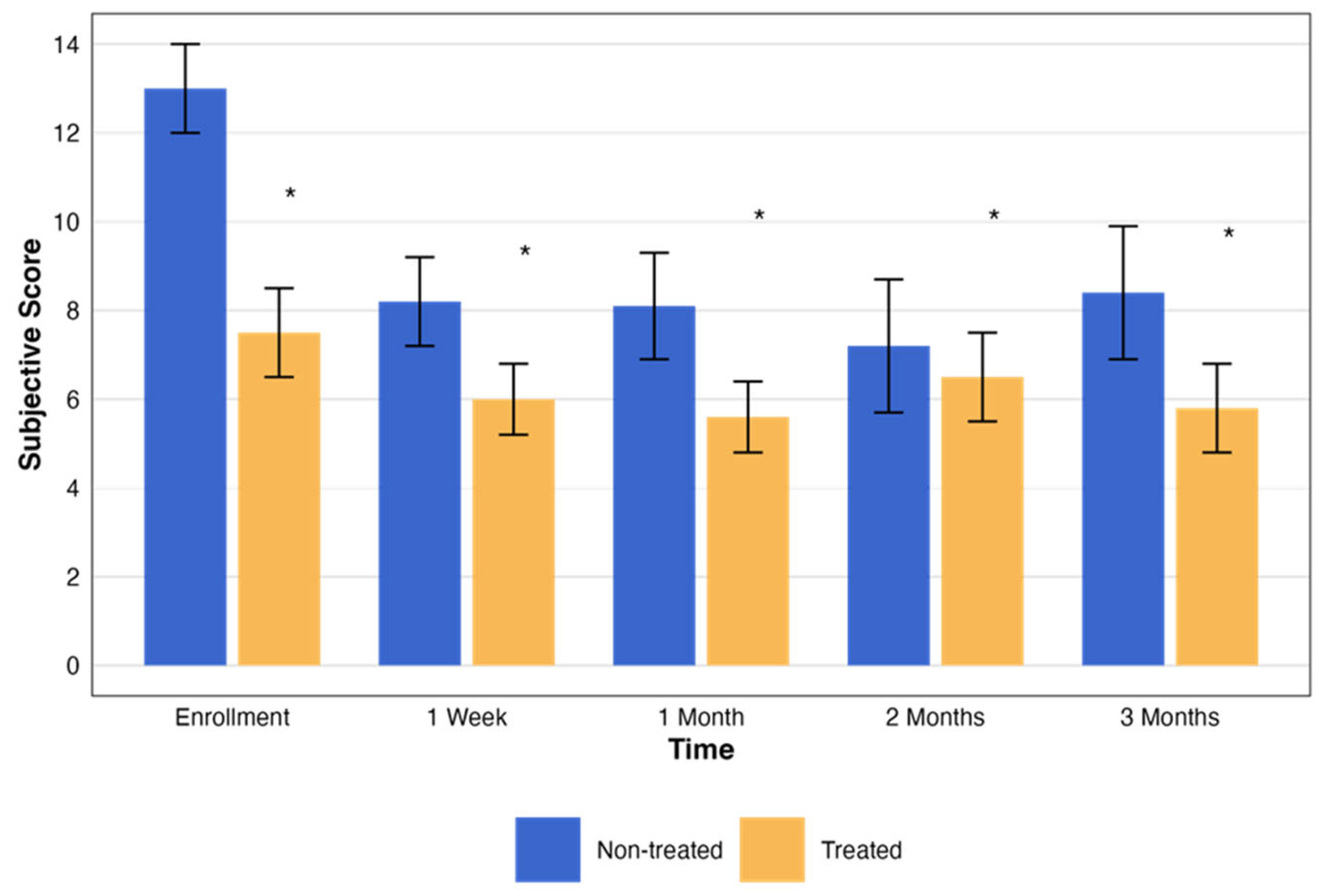
| Treatment | Score Mean ± SD | p Value a | |
|---|---|---|---|
| Group | Time | ||
| Non-treated | Enrollment score | 12.50 ± 1.55 | 0.008 |
| 1-week score | 8.27 ± 1.36 | ||
| 1-month score | 8.09 ± 1.62 | ||
| 2-month score | 7.27 ± 1.61 | ||
| 3-month score | 8.45 ± 1.45 | ||
| OCD | Enrollment score | 7.42 ± 1.26 | 0.007 |
| 1-week score | 5.95 ± 1.07 | ||
| 1-month score | 5.65 ± 1.23 | ||
| 2-month score | 6.65 ± 1.38 | ||
| 3-month score | 5.81 ± 1.45 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Eli, H.; Perelman, S.; Wajnsztajn, D.; Solomon, A. Evaluating Magnetic Stimulation as an Innovative Approach for Treating Dry Eye Disease: An Initial Safety and Efficacy Study. Biomedicines 2025, 13, 1064. https://doi.org/10.3390/biomedicines13051064
Ben-Eli H, Perelman S, Wajnsztajn D, Solomon A. Evaluating Magnetic Stimulation as an Innovative Approach for Treating Dry Eye Disease: An Initial Safety and Efficacy Study. Biomedicines. 2025; 13(5):1064. https://doi.org/10.3390/biomedicines13051064
Chicago/Turabian StyleBen-Eli, Hadas, Shimon Perelman, Denise Wajnsztajn, and Abraham Solomon. 2025. "Evaluating Magnetic Stimulation as an Innovative Approach for Treating Dry Eye Disease: An Initial Safety and Efficacy Study" Biomedicines 13, no. 5: 1064. https://doi.org/10.3390/biomedicines13051064
APA StyleBen-Eli, H., Perelman, S., Wajnsztajn, D., & Solomon, A. (2025). Evaluating Magnetic Stimulation as an Innovative Approach for Treating Dry Eye Disease: An Initial Safety and Efficacy Study. Biomedicines, 13(5), 1064. https://doi.org/10.3390/biomedicines13051064





