Abstract
Objectives: The distinction of clinically uncertain, poorly differentiated liver masses into primary liver cancer (PLC) of cholangiocytic origin (intrahepatic cholangiocarcinoma; CCA) or hepatocellular origin (hepatocellular carcinoma; HCC) vs. metastasis is highly relevant to guiding patient treatment. Protocols differ in terms of resection, local ablation, liver transplantation, or systemic therapies with immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs). Methods: This retrospective case series exemplifies a multidisciplinary, practical guide to clinically uncertain liver masses using imaging, histomorphology, immune phenotyping, and mutational testing of telomerase promoter (TERT) combined with a literature review. Results: In 2/3 patients with uncertain liver masses and inconclusive immunohistochemistry profiles, TERT testing supported the diagnosis of poorly differentiated hepatocellular carcinoma. The third case with a history of sclerosing cholangitis and vague adenoid morphology yielded mutations in ARID1a and TP53 and was identified as primary liver cancer, consistent with poorly differentiated intrahepatic cholangiocarcinoma or mixed hepatocellular cholangiocarcinoma (cHCC/CCA). Conclusions: Finding HCC-typical TERT promoter mutations is a useful diagnostic tool in poorly differentiated primary liver cancer.
1. Introduction
Sorting clinically uncertain liver masses into hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (iCCA), or metastasis in the setting of CUP (cancer of unknown primary) can be difficult when neoplastic cells are poorly differentiated and immunohistochemistry markers show inconclusive phenotypes. Broad-panel sequencing is often not feasible upfront. Metastases are by far the most frequent reason for liver nodules. Primary liver cancer (PLC) comprises hepatocellular carcinoma (HCC; 75–85% of cases), intrahepatic cholangiocarcinoma (iCCA; 10–15% of cases), and combined hepatocellular-cholangiocarcinoma (cHCC/CCA; <5%) [1]. The distinction is clinically relevant, because treatment protocols for HCC patients include surgical resection, local ablation, liver transplantation, or systemic therapies with immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) [2]. In contrast, patients with intrahepatic CCA are rarely eligible for surgical resection (~25% of cases) and survival benefit after liver transplantation has been reported as unsatisfactory [3]. Patients with unresectable tumors are submitted to undergo palliative systemic chemotherapy with gemcitabine and cisplatin [4]. This retrospective case series exemplifies a practical guide to clinically uncertain liver masses using imaging, histomorphology, and immune phenotyping supplemented with the mutational testing of telomerase reverse transcriptase promoter (TERT).
In our center, patients with suspect liver masses receive contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI), as recommended in international guidelines [5] (Figure 1). If the patient is eligible for biopsy, tissue analysis consists of morphology, immunohistochemistry, and molecular testing if applicable. Broad-panel sequencing is only performed if there is a pre-test probability of detecting cholangiocarcinoma with predictive FGFR or IDH alterations. The main reason is the lack of therapeutic targets in hepatocellular carcinoma. All findings are discussed in the interdisciplinary liver tumor board to determine the treatment regimen.
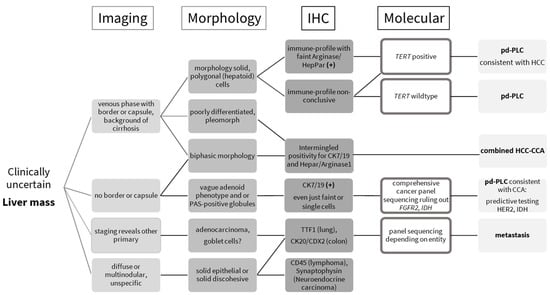
Figure 1.
Practical guide to clinically uncertain liver masses, essentially combining morphology, immunohistochemistry, and molecular testing. TERT—telomerase reverse transcriptase promoter; pd-PLC—poorly differentiated primary liver cancer; HCC—hepatocellular carcinoma; combined HCC-CCA—combined hepatocellular cholangiocarcinoma; CCA—cholangiocarcinoma.
Tissue marker expression analysis (Table 1) is performed to rule out the liver metastasis of other primary tumors (gastrointestinal tract, lung, breast, female and male genital tract). This always occurs in cooperation with radiology/staging. Poorly differentiated lesions might not exhibit specific marker profiles. Once a primary liver lesion is determined to be the most likely, cholangiocytic vs. hepatocellular origin can be further evaluated for vague morphological hepatoid or adenoid features, as suggested by Kikuchi et al. [6]. Single-target testing of telomerase reverse transcriptase promoter (TERT) is helpful for diagnosis if cost- and time-intensive genomic sequencing is restricted for technical or economic reasons. Approx. 70% of HCC bear TERT mutations. The rate of occurrence ranges from 31 to 80% [7,8,9,10]. TERT constitutes the catalytic unit of the telomerase that maintains the length of telomeres [11]. Most normal tissues have no telomerase activity, except germ cells of the ovary or testis, and display weak telomerase activity in proliferative cells of renewal tissues [12]. Malignant cells reactivate the telomerase to escape replicative telomere reduction during aging, thereby facilitating unlimited proliferation [13]. This reactivation is often brought about by TERT promoter mutations, which induce telomerase transcription [14]. In HCC specimens, mutations generally occur very early during tumor evolution at two main hotspots [15]. The more frequent one occurs at −124 bp from the TERT transcription start site, and is characterized by either a G to A, or G to T, substitution. The second hotspot at −146 bp is defined by G to A substitution [8]. In contrast, TERT mutations are rare in intrahepatic CCA; larger studies have reported mutations in 0–8% of cases [16]. TERT mutations might occur in gliomas and papillary thyroid carcinoma, two entities that rarely metastasize into the liver [17,18].

Table 1.
Literature review of useful biomarkers to distinguish uncertain liver masses found in imaging.
2. Materials and Methods
Three patients with clinically uncertain liver masses are reported. Imaging studies were performed as contrast-enhanced CT or MRI scans, but radiology left diagnosis open. Other primaries could not be detected. Biopsies were performed as recommended for non-cirrhotic patients or indeterminate nodules > 1 cm in size [5,33]. The tissue samples were formalin-fixed, paraffin-embedded, and cut into 3 µm slides (liver tumor protocol: hematoxylin–eosin, elastica–van-Gieson, periodic–acid shift). Case 1 was a liver segment resection specimen; Case 2 and 3 were transjugular liver biopsies (both < 1 cm in length) with limited amounts of tissue. The morphology revealed solid growth and poor differentiation (G3) according to the 5th edition of the WHO classification [34]. Based on the work by Kikuchi et al., we further categorized samples as polygonal/hepatoid, pleomorph, or vaguely adenoid [6]. Immunohistochemistry staining included a minimum panel of hepatocyte-specific Arginase1 (sensitivity > 90%, specificity 94%), HepPar1 (sensitivity > 90%, specificity 97%), and cholangiocyte CK7/CK19 (Table 1) [35]. For Arginase1, we used clone SP156 (dilution 1:100), a monoclonal rabbit antibody (CellMarque, Rocklin, CA, USA). For HepPar1, we used OCH1E5 at a dilution of 1:200 (Roche Diagnostics, Rotkreuz, Switzerland). CK7 had the following parameters: clone OV-TL12/30 and dilution 1:800. Searching for BAP1 (BSB-109, BioSB, Santa Barbara, CA, USA), Glypican (1G12, CellMarque, Rocklin, CA, USA), and AFP (polyclonal, Dako, Santa Clara, CA, USA) expression supplemented the panel with HCC- and CCA-specific alterations. Only the resection specimen could be further stained for other primary origins: TTF1 (SPT24, Novacastra, Biosystems, Muttenz, Switzerland), PAX8 (RM436, BioSB, Santa Barbara, CA, USA), PSA (polyclonal, Dako, Santa Clara, CA, USA), CK20 (Ks20.8, CellMarque, Rocklin, CA, USA), CDX2 (EPR2764Y, CellMarque), HMB45 (monoclonal, CellMarque), MelanA (A103, Dako), SOX11 (MRQ-58, CellMarque), CD45 (PD7/26, 2B11, Dako), CD10 (56C6, Novacastra). This was because of continuous uncertainty about other primaries.
TERT promoter sequencing was conducted as simple targeted Sanger sequencing in 2 cases (Primer: Forward 5-CACCCGTCCTGCCCCTTCACCTT-3; Reverse 5-GGCTTCCCACGTGCGCAGCAGGA-3). Mutational analysis of the TERT promoter hot-spot regions, located 124 and 146 bp upstream of the ATG start site, was conducted by PCR followed by Sanger sequencing. The specimen of case 3 was directly submitted to broad-panel sequencing (tumor board consensus) because of the clinical presentation with a history of sclerosing cholangitis and imaging features. Sequencing analysis was performed using the TruSight Oncology 500 (TSO500) panel; https://www.illumina.com/, NovaSeq 6000 platform (Illumina, San Diego, CA 92122, USA), which targets exonic and splice site regions of 523 cancer-related genes. Paired-end sequencing was carried out at the Clinical Genomics Lab of the Inselspital, Bern. The threshold for VAF detection was set at 5%, with an exon coverage of >500×.
The local ethics committee (Kantonale Ethikkommission Bern) approved the report of this retrospective case series. All patients signed a general consent form. Informed consent was waived due to the observational nature of the study (Req-2021–01336).
3. Results
Case 1 was a 71-year-old female with a suspect lesion in liver segment II/III without evidence of chronic liver disease. There was neither detection of tumor marker alpha-fetoprotein (AFP), CA19–9, nor CEA. Contrast-enhanced magnetic resonance imaging showed a 6 cm large, suspicious liver lesion in segment II/III without capsular retraction and a central enhancement in the late venous phase. This was more distinct for cholangiocarcinoma (Figure 2; upper panel). The tumor board consensus was to perform laparoscopic surgical resection.
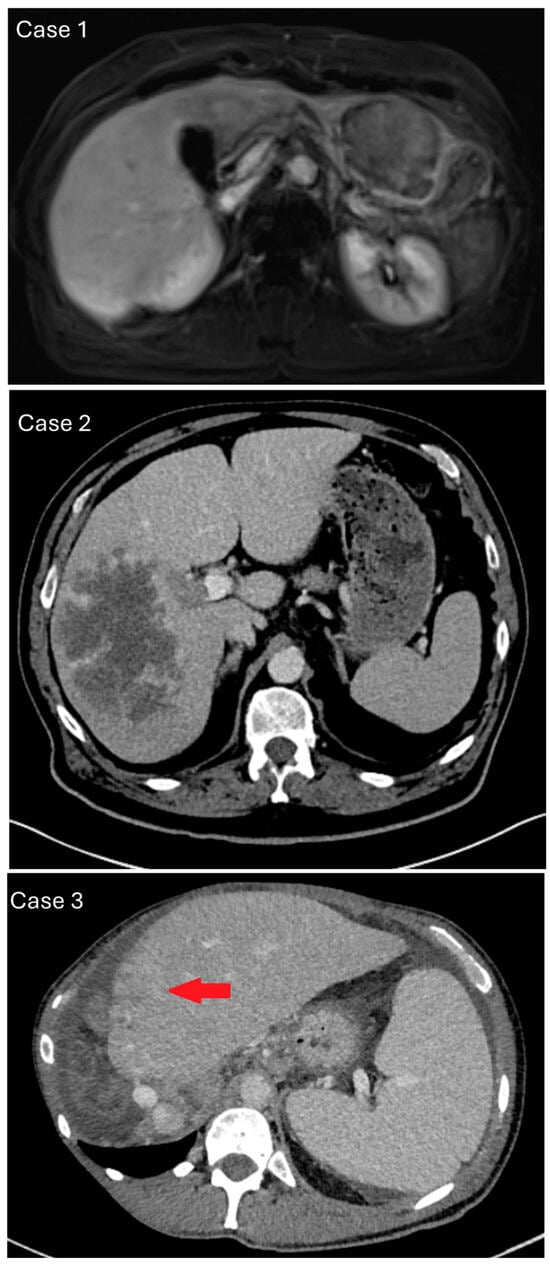
Figure 2.
Representative chronological imaging of the case series. Upper panel/case 1: contrast-enhanced magnetic resonance imaging, arterial rim enhancement of approx. 6 cm large, suspicious liver lesion in segment II/III without CCA-typical capsular retraction. Middle panel/case 2: contrast-enhanced computed tomography, venous phase with cauliflower-like mass of right liver without border or capsule, suggesting intrahepatic cholangiocarcinoma (iCCA). Recognizable macrovascular infiltration, which is more common in hepatocellular carcinoma. Lower panel/case 3: contrast-enhanced computed tomography and a new, rim-enhancing mass in liver segment II with capsular retraction and signs of macrovascular infiltration (red arrow).
In a liver segment II/III resection specimen (278 g, 18 × 9 × 5 cm), we observed a whitish-yellowish tumor, max. 7 cm in diameter, with multiple adjacent satellite nodules, max. 1.9 cm in diameter. Histology revealed a poorly differentiated carcinoma with a predominantly solid growth pattern with a mesenchymal appearance, lymphocyte infiltration, and necrosis (Figure 3). Tumor cells showed focal intracellular iron storage. The surrounding non-tumorous liver parenchyma had no significant fibrosis and minimal steatosis. Specimen staining showed faint positivity for hepatocyte paraffin-1 (HepPar-1). Arginase 1 was negative and CK7 < 5%. Unspecific markers like CD10, CK18, and PanCK were positive. Negative staining involved GS-6, EBER, CD117, DOG1, ERG, CDX2, SF1, Inhibin, Glypican-3, MelanA, HMB45, SOX10, and TTF1. BAP1 nuclear staining was preserved, indicative of BAP1 retention. TERT mutation analysis through Sanger sequencing yielded a TERT mutation at the hotspot 228 C > T (−124 bp). In summary, the combination of TERT mutation and faint HepPar-1 staining made us identify the case as poorly differentiated primary liver cancer, consistent with poorly differentiated hepatocellular carcinoma. The patient received adjuvant chemotherapy with Capecitabin and later a cisplatin/gemcitabine regimen because of tumor recurrence. The overall survival was 43 months.
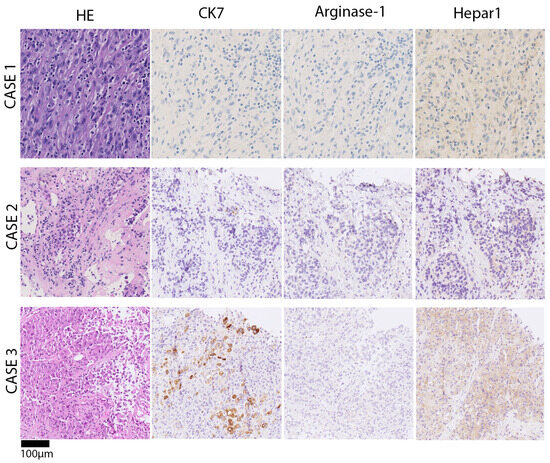
Figure 3.
Chronological presentation of histomorphology and representative immune phenotypes of case series. Upper panel/case 1: poorly differentiated carcinoma, solid growth pattern, lymphocyte-infiltrate, and necrosis. Very faint positivity for hepatocyte paraffin-1 (HepPar1), which was initially interpreted as unspecific. Middle panel/case 2: poorly differentiated carcinoma, solid growth pattern, and necrosis in the right liver. Immunohistochemistry showed focal very faint HepPar1 positivity in tumor cells, Arginase-1 and CK7 negativity. Lower panel/case 3: poorly differentiated carcinoma with a predominantly solid, partial trabecular growth pattern and necrosis. Vague adenoid features, i.e., intracytoplasmatic globules. Single-cell positivity for CK7 and faint HepPar1 positivity.
Case 2 was a 72-year-old male with a suspect lesion in the right liver and a smaller one in the left lateral (Figure 2; middle panel). There was a moderate rise in tumor marker alpha-fetoprotein, which went from 223 to 310 ng/mL within one month. CT imaging showed a cauliflower-like, large, malignant mass in the venous phase. It was localized in the right liver without a sharp border or capsule and recognizable macrovascular infiltration. The histology of the biopsy revealed poorly differentiated carcinoma with solid growth patterns and necrosis (Figure 3). Immunohistochemistry showed focal HepPar1 positivity in tumor cells; Arginase-1 and CK7 were negative. TERT mutation analysis through Sanger sequencing yielded a TERT mutation at 250 C > T (−146 bp). The case was signed out as poorly differentiated primary liver cancer, consistent with poorly differentiated hepatocellular carcinoma. The patient was treated with palliative chemotherapy (Atelzolizumab/Bevacizumab) but unfortunately deceased after 6 months.
Case 3 was a 53-year-old male with liver mass, peritoneal carcinosis, and unclear primary. There was a history of sclerosing cholangitis and tumor marker CA19-9 was elevated to 1198 IU/mL. CT imaging showed a new rim-enhancing mass in segment II with capsular retraction in the venous phase (Figure 2; lower panel). There were signs of direct macrovascular infiltration and peritoneal carcinomatosis. Histology revealed a poorly differentiated carcinoma with a predominantly solid, partial trabecular growth pattern and necrosis. A closer review revealed vague adenoid features (sprinkled intracytoplasmatic globules). Non-tumor tissue showed incomplete cirrhotic remodeling. Specimen staining showed single-cell tumor cells positive for CK7 > CK20; there was very weak and patchy positivity for HepPar1, and negativity for Arginase-1 (Figure 3). Next-generation sequencing analysis was performed, because imaging and clinical presentation made CCA more likely. Mutations were found in ARID1A, TP53, PBRM1, LATS1 and other variants with unclear significance. The tumor mutational burden yielded 11 mutations/MB, microsattelites stable. TERT was reported to be wildtype (exon coverage, median > 150; fragment size, median > 70 bp). The case was signed out as poorly differentiated primary liver cancer, consistent with poorly differentiated intrahepatic cholangiocarcinoma or mixed hepatocellular cholangiocarcinoma (cHCC/CCA). The patient was lost to follow-up after 12 months.
4. Discussion
The practical approach to clinically uncertain, poorly differentiated liver masses has the goal of distinguishing metastasis from primary liver cancer of cholangiocytic (intrahepatic CCA) or hepatocellular (HCC) origin. If specific dynamics of contrast enhancement in imaging studies are lacking, tumor morphology must critically be reviewed for polygonal/hepatoid, pleomorph, or vaguely adenoid features, as suggested by a recent study by Kikuchi et al. [6].
The immunohistochemistry marker panel as a minimum should contain liver specific marker (HepPar1, Arginase-1) and CK7/19 [20,36]. CK20 might help to initially rule out adenocarcinoma metastasis from the gastrointestinal tract and is usually neither expressed in HCC nor CCA, although aberrant expression has been reported in both tumors [21]. HepPar1 stains normal and neoplastic hepatocytes, having a high sensitivity and specificity (>90%) [19,20,24]. Hence, there is a gradient from well-differentiated HCC (strong positivity) to poorly differentiated HCC (weak positivity or negative). Arginase-1 is the most specific and sensitive (>90%) marker for HCC, including poorly differentiated tumors and scirrhous HCC [24]. However, in our case series, Hepar1 was more helpful in diagnosis; even very faint partial staining <10% can still be considered as indicative of hepatocellular origin. CK7 (97%) and CK19 (77%) positivity generally points to a tumor origin of the pancreato-biliary tract, including intrahepatic CCA. HCC specimens can be strongly positive if they exhibit a progenitor phenotype with worse prognosis, as was first described in 2006 by Durnez et al. [22]. In this case, it is crucial to distinguish true glandular or tubular tumor formations in intrahepatic CCA or mixed HCC-CCA from pseudoglandular growth pattern in HCCs. The hallmarks of pseudoglandular growth in HCC are round, luminal structures lined with hepatocytes, but not ductal cells.
The genomic sequencing analysis of selected poorly differentiated primary liver cancer by Kikuchi et al. reported that TERT and TP53 mutations as supportive for hepatocellular carcinoma. IHD1, BAP1, and ERBB2 mutations, as well as predictive FGFR fusions are supportive of iCCA [6]. BRCA1-associated protein 1 (BAP1) is a tumor suppressor gene; its inactivation is observed in 25% of cholangiocarcinoma cases and (in the absence of mesothelioma) it is of potential value for differential diagnosis with very low sensitivity [25]. In the mentioned genomic analysis of poorly differentiated primary liver cancer by Kikuchi et al., 2/16 patients (each n = 1 with polygonal cell and indeterminate morphology) showed a BAP1 mutation. This finding illustrates that BAP1 mutations seem to be very rare in pd-PLC and not necessarily coherent with other findings diagnostic for CCA. In our case, we found no CCA-typical BAP1, FGFR2, IDH1, or ERBB2 alterations, but we are aware of the limitations in this study, i.e., a small number of cases and a difference in sequencing methods due to restrictions in predictive testing. Nevertheless, CCA-typical FGFR2 fusions were equally rare in Kikuchi’s pd-PLC cohort (1/16). It was recently reported that, among CCA patients, 14% are eligible for targeted FGFR inhibitors [37].
TERT mutations are rare in gastrointestinal and lung cancer but can occur in glioma and thyroid carcinoma [17,18,38]. In a Chinese cohort study of cholangiocarcinoma patients assessed by Tian et al., TERT alterations were observed in 21% of cases [39]. However, many of these patients had reportedly poorly or undifferentiated tumors that might as well have been poorly differentiated HCC. Further, it has been discussed that some tumors diagnosed as intrahepatic CCA in the cirrhotic liver may in fact be combined HCC/CCA due to sampling errors. The distinction between CCA and combined HCC/CCA is of relevance, because the latter is reported to reflect the mutational landscape of HCCs [40]. A technical aspect relevant to diagnosis, disease monitoring, and prognosis is the upcoming opportunities of circulating tumor (ct)−DNA in liquid biopsies [41]. Since liver cancer patients often have coagulation disorders and liver biopsies introduce many risks, considering ct DNA or microRNA-based serum biomarker might be pivotal in the future, enabling early diagnosis and prognosis [42].
In conclusion, a multidiscipline workup algorithm for poorly differentiated liver masses comprises imaging, clinical history, morphology, and immunohistochemistry to subtype poorly differentiated primary liver cancer into HCC or intrahepatic CCA. If broad-panel genomic sequencing is restricted, a closer look at polygonal morphology, faint, patchy Hepar1 staining, and single-target TERT sequencing can aid the diagnosis of poorly differentiated hepatocellular carcinoma.
Author Contributions
Conceptualization, J.F.; methodology, J.F., software, M.P.B. and E.V.; validation, M.M. and J.F.; formal analysis, G.S. (Greta Sökeland); investigation, G.S. (Greta Sökeland) and J.F.; resources, M.M.; data curation, G.S. (Greta Sökeland) and M.P.B.; writing—original draft preparation, G.S. (Greta Sökeland) and J.F.; writing—review and editing, E.V., M.P.B. and G.S. (Guido Stirnimann); visualization, J.F.; supervision, G.S. (Guido Stirnimann). All authors have read and agreed to the published version of the manuscript.
Funding
Intramural funding, University of Bern.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Kantonale Ethikkommission Bern (protocol code: Req 2021-01336 and date of approval: 8 November 2021).
Informed Consent Statement
All patients signed a general consent. Informed consent was waived due to the observational nature of the series with anonymized reporting (Req-2021–01336).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Krasnodebski, M.; Grat, M.; Jastrzebski, M.; Szczesniak, M.; Morawski, M.; Zajac, K.; Patkowski, W.; Zieniewicz, K. Unsatisfactory Long-term Results of Liver Transplant in Patients with Intrahepatic Cholangiocarcinoma. Transplant. Proc. 2020, 52, 2463–2467. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef]
- Kikuchi, A.T.; Umetsu, S.; Joseph, N.; Kakar, S. Genomic Analysis in the Categorization of Poorly Differentiated Primary Liver Carcinomas. Am. J. Surg. Pathol. 2023, 47, 1207–1218. [Google Scholar] [CrossRef]
- Guichard, C.; Amaddeo, G.; Imbeaud, S.; Ladeiro, Y.; Pelletier, L.; Maad, I.B.; Calderaro, J.; Bioulac-Sage, P.; Letexier, M.; Degos, F.; et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef]
- Quaas, A.; Oldopp, T.; Tharun, L.; Klingenfeld, C.; Krech, T.; Sauter, G.; Grob, T.J. Frequency of TERT promoter mutations in primary tumors of the liver. Virchows Arch. 2014, 465, 673–677. [Google Scholar] [CrossRef]
- Schulze, K.; Imbeaud, S.; Letouze, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef]
- Zvereva, M.I.; Shcherbakova, D.M.; Dontsova, O.A. Telomerase: Structure, functions, and activity regulation. Biochemistry 2010, 75, 1563–1583. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K.; Yokoyama, T.; Shay, J.W. Immunohistochemical detection of telomerase (hTERT) protein in human cancer tissues and a subset of cells in normal tissues. Neoplasia 2001, 3, 17–26. [Google Scholar] [CrossRef]
- Hafezi, F.; Perez Bercoff, D. The Solo Play of TERT Promoter Mutations. Cells 2020, 9, 749. [Google Scholar] [CrossRef]
- Hafezi, F.; Jaxel, L.; Lemaire, M.; Turner, J.D.; Perez-Bercoff, D. TERT Promoter Mutations Increase Sense and Antisense Transcription from the TERT Promoter. Biomedicines 2021, 9, 1773. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczanska, B.; Lacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT-Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A., Jr.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef]
- Tan, G.; Jin, B.; Qian, X.; Wang, Y.; Zhang, G.; Agyekum, E.A.; Wang, F.; Shi, L.; Zhang, Y.; Mao, Z.; et al. TERT promoter mutations contribute to adverse clinical outcomes and poor prognosis in radioiodine refractory differentiated thyroid cancer. Sci. Rep. 2024, 14, 23719. [Google Scholar] [CrossRef]
- Kakar, S.; Muir, T.; Murphy, L.M.; Lloyd, R.V.; Burgart, L.J. Immunoreactivity of Hep Par 1 in hepatic and extrahepatic tumors and its correlation with albumin in situ hybridization in hepatocellular carcinoma. Am. J. Clin. Pathol. 2003, 119, 361–366. [Google Scholar] [CrossRef]
- Choi, W.T.; Kakar, S. Immunohistochemistry in the Diagnosis of Hepatocellular Carcinoma. Gastroenterol. Clin. N. Am. 2017, 46, 311–325. [Google Scholar] [CrossRef]
- Stroescu, C.; Herlea, V.; Dragnea, A.; Popescu, I. The diagnostic value of cytokeratins and carcinoembryonic antigen immunostaining in differentiating hepatocellular carcinomas from intrahepatic cholangiocarcinomas. J. Gastrointestin Liver Dis. 2006, 15, 9–14. [Google Scholar]
- Durnez, A.; Verslype, C.; Nevens, F.; Fevery, J.; Aerts, R.; Pirenne, J.; Lesaffre, E.; Libbrecht, L.; Desmet, V.; Roskams, T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006, 49, 138–151. [Google Scholar] [CrossRef]
- Akita, M.; Ajiki, T.; Fukumoto, T.; Itoh, T.; Zen, Y. Keratin 19-expressing hepatocellular carcinoma and small-duct type intrahepatic cholangiocarcinoma show a similar postoperative clinical course but have distinct genetic features. Histopathology 2019, 75, 385–393. [Google Scholar] [CrossRef]
- Takahashi, Y.; Dungubat, E.; Kusano, H.; Ganbat, D.; Tomita, Y.; Odgerel, S.; Fukusato, T. Application of Immunohistochemistry in the Pathological Diagnosis of Liver Tumors. Int. J. Mol. Sci. 2021, 22, 5780. [Google Scholar] [CrossRef]
- Andrici, J.; Goeppert, B.; Sioson, L.; Clarkson, A.; Renner, M.; Stenzinger, A.; Tayao, M.; Watson, N.; Farzin, M.; Toon, C.W.; et al. Loss of BAP1 Expression Occurs Frequently in Intrahepatic Cholangiocarcinoma. Medicine 2016, 95, e2491. [Google Scholar] [CrossRef]
- Qu, Y.; Shi, L.; Wang, D.; Zhang, B.; Yang, Q.; Ji, M.; Shi, B.; Hou, P. Low frequency of TERT promoter mutations in a large cohort of gallbladder and gastric cancers. Int. J. Cancer 2014, 134, 2993–2994. [Google Scholar] [CrossRef]
- Dow, M.; Pyke, R.M.; Tsui, B.Y.; Alexandrov, L.B.; Nakagawa, H.; Taniguchi, K.; Seki, E.; Harismendy, O.; Shalapour, S.; Karin, M.; et al. Integrative genomic analysis of mouse and human hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2018, 115, E9879–E9888. [Google Scholar] [CrossRef]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouze, E.; Blanc, J.F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239.e4. [Google Scholar] [CrossRef]
- Friemel, J.; Frick, L.; Unger, K.; Egger, M.; Parrotta, R.; Boge, Y.T.; Adili, A.; Karin, M.; Luedde, T.; Heikenwalder, M.; et al. Characterization of HCC Mouse Models: Towards an Etiology-Oriented Subtyping Approach. Mol. Cancer Res. 2019, 17, 1493–1502. [Google Scholar] [CrossRef]
- Cancer Genome Atlas, N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Shajani-Yi, Z.; de Abreu, F.B.; Peterson, J.D.; Tsongalis, G.J. Frequency of Somatic TP53 Mutations in Combination with Known Pathogenic Mutations in Colon Adenocarcinoma, Non-Small Cell Lung Carcinoma, and Gliomas as Identified by Next-Generation Sequencing. Neoplasia 2018, 20, 256–262. [Google Scholar] [CrossRef]
- Foerster, F.; Galle, P.R. Comparison of the current international guidelines on the management of HCC. JHEP Rep. 2019, 1, 114–119. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; Board, W.H.O.C.o.T.E. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Lagana, S.M.; Salomao, M.; Remotti, H.E.; Knisely, A.S.; Moreira, R.K. Bile salt export pump: A sensitive and specific immunohistochemical marker of hepatocellular carcinoma. Histopathology 2015, 66, 598–602. [Google Scholar] [CrossRef]
- Choi, W.T.; Ramachandran, R.; Kakar, S. Immunohistochemical approach for the diagnosis of a liver mass on small biopsy specimens. Hum. Pathol. 2017, 63, 1–13. [Google Scholar] [CrossRef]
- Chmiel, P.; Geca, K.; Rawicz-Pruszynski, K.; Polkowski, W.P.; Skorzewska, M. FGFR Inhibitors in Cholangiocarcinoma-A Novel Yet Primary Approach: Where Do We Stand Now and Where to Head Next in Targeting This Axis? Cells 2022, 11, 3929. [Google Scholar] [CrossRef]
- Cruvinel-Carloni, A.; Yamane, L.; Scapulatempo-Neto, C.; Guimaraes, D.; Reis, R.M. Absence of TERT promoter mutations in colorectal precursor lesions and cancer. Genet. Mol. Biol. 2018, 41, 82–84. [Google Scholar] [CrossRef]
- Tian, W.; Hu, W.; Shi, X.; Liu, P.; Ma, X.; Zhao, W.; Qu, L.; Zhang, S.; Shi, W.; Liu, A.; et al. Comprehensive genomic profile of cholangiocarcinomas in China. Oncol. Lett. 2020, 19, 3101–3110. [Google Scholar] [CrossRef]
- Joseph, N.M.; Tsokos, C.G.; Umetsu, S.E.; Shain, A.H.; Kelley, R.K.; Onodera, C.; Bowman, S.; Talevich, E.; Ferrell, L.D.; Kakar, S.; et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J. Pathol. 2019, 248, 164–178. [Google Scholar] [CrossRef]
- Kopystecka, A.; Patryn, R.; Lesniewska, M.; Budzynska, J.; Koziol, I. The Use of ctDNA in the Diagnosis and Monitoring of Hepatocellular Carcinoma-Literature Review. Int. J. Mol. Sci. 2023, 24, 9342. [Google Scholar] [CrossRef]
- Molina-Pelayo, F.A.; Zarate-Lopez, D.; Garcia-Carrillo, R.; Rodriguez-Beas, C.; Iniguez-Palomares, R.; Rodriguez-Mejia, J.L.; Soto-Guzman, A.; Velasco-Loyden, G.; Sierra-Martinez, M.; Virgen-Ortiz, A.; et al. miRNAs-Set of Plasmatic Extracellular Vesicles as Novel Biomarkers for Hepatocellular Carcinoma Diagnosis Across Tumor Stage and Etiologies. Int. J. Mol. Sci. 2025, 26, 2563. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).