Mucosal Immunity: Lessons from the Lower Respiratory and Small Intestinal Epithelia
Abstract
1. Introduction
2. General Structure and Function
3. Cell Populations
3.1. Non-Secretory Cells
3.2. Secretory Cells
4. Mechanical and Physical Defense Systems
5. Immune Response by Mucosal Epithelial Cells
5.1. Pathogen Detection
5.2. Response to Pathogen Recognition Receptor Signaling
5.3. Anti-Microbial Peptides, Proteins, and Other Effectors
6. Impact of Innate Immune Activation on the Epithelium
7. Lessons from the Respiratory and Small Intestinal Epithelia
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Wu, Y.; Zhu, Z.; Lu, C.; Zhang, C.; Zeng, L.; Xie, F.; Zhang, L.; Zhou, F. Mucosal Immune Response in Biology, Disease Prevention and Treatment. Signal Transduct. Target. Ther. 2025, 10, 7. [Google Scholar] [PubMed]
- Lemke, S.B.; Nelson, C.M. Dynamic Changes in Epithelial Cell Packing during Tissue Morphogenesis. Curr. Biol. 2021, 31, R1098–R1110. [Google Scholar] [CrossRef]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight Junctions and Cell Polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Streuli, C.H. Integrins and Epithelial Cell Polarity. J. Cell Sci. 2014, 127, 3217–3225. [Google Scholar] [CrossRef]
- Riga, A.; Castiglioni, V.G.; Boxem, M. New Insights into Apical-Basal Polarization in Epithelia. Curr. Opin. Cell Biol. 2020, 62, 1–8. [Google Scholar] [CrossRef]
- Buckley, C.E.; St Johnston, D. Apical–Basal Polarity and the Control of Epithelial Form and Function. Nat. Rev. Mol. Cell Biol. 2022, 23, 559–577. [Google Scholar]
- Zuo, L.; Kuo, W.T.; Turner, J.R. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Tariq, H.; Bella, J.; Jowitt, T.A.; Holmes, D.F.; Rouhi, M.; Nie, Z.; Baldock, C.; Garrod, D.; Tabernero, L. Cadherin Flexibility Provides a Key Difference between Desmosomes and Adherens Junctions. Proc. Natl. Acad. Sci. USA 2015, 112, 5395–5400. [Google Scholar] [CrossRef]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell—Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2017, 10, a029181. [Google Scholar] [CrossRef]
- Ragupathy, S.; Eesmaeili, F.; Paschoud, S.; Sublet, E.; Citi, S.; Borchard, G. Toll-like Receptor 2 Regulates the Barrier Function of Human Bronchial Epithelial Monolayers through Atypical Protein Kinase C Zeta, and an Increase in Expression of Claudin-1. Tissue Barriers 2014, 2, e29166. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Umar, S. Intestinal Stem Cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, L.E.; Bennett, W.D. Cilia and Mucociliary Clearance. Encycl. Respir. Med. Four-Vol. Set. 2006, 9, 466–470. [Google Scholar] [CrossRef]
- Karam, S.M. Lineage Commitment and Maturation of Epithelial Cells in the Gut. Front. Biosci. 1999, 4, 286–298. [Google Scholar] [CrossRef]
- Dean, C.H.; Snelgrove, R.J. New Rules for Club Development: New Insights into Human Small Airway Epithelial Club Cell Ontogeny and Function. Am. J. Respir. Crit. Care Med. 2018, 198, 1355–1356. [Google Scholar] [CrossRef]
- Crystal, R.G. Airway Basal Cells: The “Smoking Gun” of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2014, 190, 1355–1362. [Google Scholar] [CrossRef]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L.M. Basal Cells as Stem Cells of the Mouse Trachea and Human Airway Epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.; Yoon, J.K.; Lee, D.; Kim, Y.; Han, Y.B.; Kim, R.; Moon, S.; Park, Y.J.; Park, K.; et al. A Single-Cell Atlas of in Vitro Multiculture Systems Uncovers the in Vivo Lineage Trajectory and Cell State in the Human Lung. Exp. Mol. Med. 2023, 55, 1831–1842. [Google Scholar] [CrossRef]
- Van Der Flier, L.G.; Clevers, H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Cheng, H.; Leblond, C.P. Origin, Differentiation and Renewal of the Four Main Epithelial Cell Types in the Mouse Small Intestine I. Columnar Cell. Am. J. Anat. 1974, 141, 461–479. [Google Scholar] [CrossRef]
- Rawlins, E.L.; Hogan, B.L.M. Ciliated Epithelial Cell Lifespan in the Mouse Trachea and Lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, 231–234. [Google Scholar] [CrossRef]
- Tam, A.; Wadsworth, S.; Dorscheid, D.; Man, S.f.P.; Sin, D.D. The Airway Epithelium: More than Just a Structural Barrier. Ther. Adv. Respir. Dis. 2011, 5, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, R.J.; Lloyd, C.M. Regulation of Immune Responses by the Airway Epithelial Cell Landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Khoury Damaa, M.; Serizay, J.; Balagué, R.; Boudjema, A.-R.; Faucourt, M.; Delgehyr, N.; Goh, K.J.; Lu, H.; Tan, E.K.; James, C.T.; et al. Cyclin O Controls Entry into the Cell-Cycle Variant Required for Multiciliated Cell Differentiation. Cell Rep. 2025, 44, 115117. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; Okubo, T.; Xue, Y.; Brass, D.M.; Auten, R.L.; Hasegawa, H.; Wang, F.; Hogan, B.L.M. The Role of Scgb1a1+ Clara Cells in the Long-Term Maintenance and Repair of Lung Airway, but Not Alveolar, Epithelium. Cell Stem Cell 2009, 4, 525–534. [Google Scholar] [CrossRef]
- McCauley, K.B.; Alysandratos, K.D.; Jacob, A.; Hawkins, F.; Caballero, I.S.; Vedaie, M.; Yang, W.; Slovik, K.J.; Morley, M.; Carraro, G.; et al. Single-Cell Transcriptomic Profiling of Pluripotent Stem Cell-Derived SCGB3A2+ Airway Epithelium. Stem Cell Rep. 2018, 10, 1579–1595. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A Revised Airway Epithelial Hierarchy Includes CFTR-Expressing Ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef]
- Lin, B.; Shah, V.S.; Chernoff, C.; Sun, J.; Shipkovenska, G.G.; Vinarsky, V.; Waghray, A.; Xu, J.; Leduc, A.D.; Hintschich, C.A.; et al. Airway Hillocks Are Injury-Resistant Reservoirs of Unique Plastic Stem Cells. Nature 2024, 629, 869–877. [Google Scholar] [CrossRef]
- Kersten, E.T.G.; Pett, J.P.; Malmström, K.; Chun, Y.; Jonker, M.R.; Wilbrey-Clark, A.; Worlock, K.B.; van den Berge, M.; Vermeulen, R.C.H.; Vonk, J.M.; et al. Childhood-Onset Asthma Is Characterized by Airway Epithelial Hillock-to-Squamous Differentiation in Early Life. bioRxiv 2023.
- Gerbe, F.; Van Es, J.H.; Makrini, L.; Brulin, B.; Mellitzer, G.; Robine, S.; Romagnolo, B.; Shroyer, N.F.; Bourgaux, J.F.; Pignodel, C.; et al. Distinct ATOH1 and Neurog3 Requirements Define Tuft Cells as a New Secretory Cell Type in the Intestinal Epithelium. J. Cell Biol. 2011, 192, 767–780. [Google Scholar] [CrossRef]
- Vieira Braga, F.A.; Kar, G.; Berg, M.; Carpaij, O.A.; Polanski, K.; Simon, L.M.; Brouwer, S.; Gomes, T.; Hesse, L.; Jiang, J.; et al. A Cellular Census of Human Lungs Identifies Novel Cell States in Health and in Asthma. Nat. Med. 2019, 25, 1153–1163. [Google Scholar] [CrossRef]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A Single-Cell Survey of the Small Intestinal Epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Kotas, M.E.; O’leary, C.E.; Locksley, R.M. Tuft Cells: Context-and Tissue-Specific Programming for a Conserved Cell Lineage. Annu. Rev. Pathol. 2024, 18, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal Epithelial Tuft Cells Initiate Type 2 Mucosal Immunity to Helminth Parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Ualiyeva, S.; Lemire, E.; Aviles, E.C.; Wong, C.; Boyd, A.A.; Lai, J.; Liu, T.; Matsumoto, I.; Barrett, N.A.; Boyce, J.A.; et al. Tuft Cell–Produced Cysteinyl Leukotrienes and IL-25 Synergistically Initiate Lung Type 2 Inflammation. Sci. Immunol. 2021, 6, eabj0474. [Google Scholar] [CrossRef] [PubMed]
- Bemark, M.; Angela Linterman, M.; Bimczok, D.; Kimura, S.; Mutoh, M.; Hisamoto, M.; Saito, H.; Takahashi, S.; Asakura, T.; Ishii, M.; et al. Airway M Cells Arise in the Lower Airway Due to RANKL Signaling and Reside in the Bronchiolar Epithelium Associated With IBALT in Murine Models of Respiratory Disease. Front. Immunol. 2019, 1, 1323. [Google Scholar] [CrossRef]
- Han, S.H. Unveiling an Important New Cell Type in the Lung: Microfold Cells. Am. J. Respir. Cell Mol. Biol. 2024, 70, 235–236. [Google Scholar] [CrossRef]
- Nair, V.R.; Franco, L.H.; Zacharia, V.M.; Khan, H.S.; Stamm, C.E.; You, W.; Marciano, D.K.; Yagita, H.; Levine, B.; Shiloh, M.U. Microfold Cells Actively Translocate Mycobacterium Tuberculosis to Initiate Infection. Cell Rep. 2016, 16, 1253–1258. [Google Scholar] [CrossRef]
- Rogers, A.P.; Mileto, S.J.; Lyras, D. Impact of Enteric Bacterial Infections at and beyond the Epithelial Barrier. Nat. Rev. Microbiol. 2023, 21, 260–274. [Google Scholar] [CrossRef]
- Beagley, K.; Husband, A.J. Intraepithelial Lymphocytes: Origins, Distribution, and Function. Crit. Rev. Immunol. 1998, 18, 237–254. [Google Scholar] [CrossRef]
- Davis, J.D.; Wypych, T.P. Cellular and Functional Heterogeneity of the Airway Epithelium. Mucosal Immunol. 2021, 14, 978–990. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, W.B.; James, A.L.; Tiddens, H.A.W.M. Structure and Function of Small Airways in Asthma Patients Revisited. Eur. Respir. Rev. 2021, 30, 200186. [Google Scholar] [CrossRef]
- Tokita, E.; Tanabe, T.; Asano, K.; Suzaki, H.; Rubin, B.K. Club Cell 10-KDa Protein Attenuates Airway Mucus Hypersecretion and Inflammation. Eur. Respir. J. 2014, 44, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.B.; Li, N.F.; Bartlett, N.W.; Richmond, B.W. An Update in Club Cell Biology and Its Potential Relevance to Chronic Obstructive Pulmonary Disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2023, 324, L652–L665. [Google Scholar] [CrossRef]
- Luan, X.; Henao Romero, N.; Campanucci, V.A.; Le, Y.; Mustofa, J.; Tam, J.S.; Ianowski, J.P. Pulmonary Ionocytes Regulate Airway Surface Liquid PH in Primary Human Bronchial Epithelial Cells. Am. J. Respir. Crit. Care Med. 2024, 210, 788–800. [Google Scholar] [CrossRef]
- Hawkins, F.J.; Kotton, D.N. Pulmonary Ionocytes Challenge the Paradigm in Cystic Fibrosis. Trends Pharmacol. Sci. 2018, 39, 852–854. [Google Scholar] [CrossRef]
- Lueschow, S.R.; McElroy, S.J. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front. Immunol. 2020, 11, 587. [Google Scholar] [CrossRef]
- Ramanan, D.; Cadwell, K. Intrinsic Defense Mechanisms of the Intestinal Epithelium. Cell Host Microbe 2016, 19, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Wallaeys, C.; Garcia-Gonzalez, N.; Pollaris, L.; Saeys, Y.; Libert, C. Identification and Characterization of Multiple Paneth Cell Types in the Mouse Small Intestine. Cells 2024, 13, 1435. [Google Scholar] [CrossRef]
- Quintero, M.; Samuelson, L.C. Paneth Cells: Dispensable yet Irreplaceable for the Intestinal Stem Cell Niche. Cell. Mol. Gastroenterol. Hepatol. 2025, 19, 101443. [Google Scholar] [CrossRef]
- Atanga, R.; Singh, V.; In, J.G. Intestinal Enteroendocrine Cells: Present and Future Druggable Targets. Int. J. Mol. Sci. 2023, 24, 8836. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J. Particle Size and Pathogenicity in the Respiratory Tract. Virulence 2013, 4, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and Function of the Polymeric Mucins in Airways Mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef]
- Ehre, C.; Worthington, E.N.; Liesman, R.M.; Grubb, B.R.; Barbier, D.; O’Neal, W.K.; Sallenave, J.-M.; Pickles, R.J.; Boucher, R.C. Overexpressing Mouse Model Demonstrates the Protective Role of MUC5AC in the Lungs. Proc. Natl. Acad. Sci. USA 2012, 109, 16528–16533. [Google Scholar] [CrossRef]
- Melton, L. Does Mucus Hypersecretion Matter in Airway Disease? Lancet 2002, 359, 1924. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of Intestinal Epithelial Cells in the Maintenance of Gut Homeostasis. Exp. Mol. Med. 2017, 49, e338-8. [Google Scholar] [CrossRef]
- Farin, H.F.; Karthaus, W.R.; Kujala, P.; Rakhshandehroo, M.; Schwank, G.; Vries, R.G.J.; Kalkhoven, E.; Nieuwenhuis, E.E.S.; Clevers, H. Paneth Cell Extrusion and Release of Antimicrobial Products Is Directly Controlled by Immune Cell-Derived IFN-γ. J. Exp. Med. 2014, 211, 1393–1405. [Google Scholar] [CrossRef]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus Enhances Gut Homeostasis and Oral Tolerance by Delivering Immunoregulatory Signals. Science (1979) 2013, 342, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; Murphy, C.; O’Neill, L.A.J.; Creagh, E.M. The Role of TLRs, NLRs, and RLRs in Mucosal Innate Immunity and Homeostasis. Mucosal Immunol. 2010, 3, 17–28. [Google Scholar] [CrossRef]
- McClure, R.; Massari, P. TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Front. Immunol. 2014, 5, 386. [Google Scholar] [CrossRef]
- Price, A.E.; Shamardani, K.; Lugo, K.A.; Deguine, J.; Roberts, A.W.; Lee, B.L.; Barton, G.M. A Map of Toll-like Receptor Expression in the Intestinal Epithelium Reveals Distinct Spatial, Cell Type-Specific, and Temporal Patterns. Immunity 2018, 49, 560–575.e6. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Vora, P.; Faure, E.; Thomas, L.S.; Arnold, E.T.; Arditi, M. Decreased Expression of Toll-Like Receptor-4 and MD-2 Correlates with Intestinal Epithelial Cell Protection Against Dysregulated Proinflammatory Gene Expression in Response to Bacterial Lipopolysaccharide. J. Immunol. 2001, 167, 1609–1616. [Google Scholar] [CrossRef]
- Jia, H.P.; Kline, J.N.; Penisten, A.; Apicella, M.A.; Gioannini, T.L.; Weiss, J.; McCray, P.B. Endotoxin Responsiveness of Human Airway Epithelia Is Limited by Low Expression of MD-2. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Hornef, M.W.; Frisan, T.; Vandewalle, A.; Normark, S.; Richter-Dahlfors, A. Toll-like Receptor 4 Resides in the Golgi Apparatus and Colocalizes with Internalized Lipopolysaccharide in Intestinal Epithelial Cells. J. Exp. Med. 2002, 195, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, V.; Moranta, D.; Campos, M.A.; Margareto, J.; Garmendia, J.; Bengoechea, J.A. Klebsiella Pneumoniae Increases the Levels of Toll-like Receptors 2 and 4 in Human Airway Epithelial Cells. Infect. Immun. 2009, 77, 714–724. [Google Scholar] [CrossRef]
- Abreu, M.T.; Arnold, E.T.; Thomas, L.S.; Gonsky, R.; Zhou, Y.; Hu, B.; Arditi, M. TLR4 and MD-2 Expression Is Regulated by Immune-Mediated Signals in Human Intestinal Epithelial Cells. J. Biol. Chem. 2002, 277, 20431–20437. [Google Scholar] [CrossRef]
- Yu, S.; Gao, N. Compartmentalizing Intestinal Epithelial Cell Toll-like Receptors for Immune Surveillance. Cell. Mol. Life Sci. 2015, 72, 3343–3353. [Google Scholar] [CrossRef]
- Hörmann, N.; Brandão, I.; Jäckel, S.; Ens, N.; Lillich, M.; Walter, U.; Reinhardt, C. Gut Microbial Colonization Orchestrates TLR2 Expression, Signaling and Epithelial Proliferation in the Small Intestinal Mucosa. PLoS ONE 2014, 9, 2–12. [Google Scholar] [CrossRef]
- Melmed, G.; Thomas, L.S.; Lee, N.; Tesfay, S.Y.; Lukasek, K.; Michelsen, K.S.; Zhou, Y.; Hu, B.; Arditi, M.; Abreu, M.T. Human Intestinal Epithelial Cells Are Broadly Unresponsive to Toll-Like Receptor 2-Dependent Bacterial Ligands: Implications for Host-Microbial Interactions in the Gut. J. Immunol. 2003, 170, 1406–1415. [Google Scholar] [CrossRef]
- Soong, G.; Reddy, B.; Sokol, S.; Adamo, R.; Prince, A. TLR2 Is Mobilized into an Apical Lipid Raft Receptor Complex to Signal Infection in Airway Epithelial Cells. J. Clin. Investig. 2004, 113, 1482–1489. [Google Scholar] [CrossRef]
- Denney, L.; Ho, L.P. The Role of Respiratory Epithelium in Host Defence against Influenza Virus Infection. Biomed. J. 2018, 41, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Spalluto, C.M.; Singhania, A.; Cellura, D.; Woelk, C.H.; Sanchez-Elsner, T.; Staples, K.J.; Wilkinson, T.M.A. IFN-g Influences Epithelial Antiviral Responses via Histone Methylation of the RIG-I Promoter. Am. J. Respir. Cell Mol. Biol. 2017, 57, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Shikhagaie, M.M.; Andersson, C.K.; Mori, M.; Kortekaas Krohn, I.; Bergqvist, A.; Dahl, R.; Ekblad, E.; Hoffmann, H.J.; Bjermer, L.; Erjefält, J.S. Mapping of TLR5 and TLR7 in Central and Distal Human Airways and Identification of Reduced TLR Expression in Severe Asthma. Clin. Exp. Allergy 2014, 44, 184–196. [Google Scholar] [CrossRef]
- Adamo, R.; Sokol, S.; Soong, G.; Gomez, M.I.; Prince, A. Pseudomonas Aeruginosa Flagella Activate Airway Epithelial Cells through AsialoGM1 and Toll-like Receptor 2 as Well as Toll-like Receptor 5. Am. J. Respir. Cell Mol. Biol. 2004, 30, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, I.; Ye, F.; McNally, B.; Willette, M.; Flano, E. Toll-Like Receptor Expression and Induction of Type I and Type III Interferons in Primary Airway Epithelial Cells. J. Virol. 2013, 87, 3261–3270. [Google Scholar] [CrossRef]
- Hug, H.; Mohajeri, M.H.; La Fata, G. Toll-like Receptors: Regulators of the Immune Response in the Human Gut. Nutrients 2018, 10, 203. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-ΚB by Toll-like Receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Creagh, E.M.; O’Neill, L.A.J. TLRs, NLRs and RLRs: A Trinity of Pathogen Sensors That Co-Operate in Innate Immunity. Trends Immunol. 2006, 27, 352–357. [Google Scholar] [CrossRef]
- Liu, J.Z.; Van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association Analyses Identify 38 Susceptibility Loci for Inflammatory Bowel Disease and Highlight Shared Genetic Risk across Populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef]
- Biswas, A.; Liu, Y.J.; Hao, L.; Mizoguchi, A.; Salzman, N.H.; Bevins, C.L.; Kobayashi, K.S. Induction and Rescue of Nod2-Dependent Th1-Driven Granulomatous Inflammation of the Ileum. Proc. Natl. Acad. Sci. USA 2010, 107, 14739–14744. [Google Scholar] [CrossRef]
- Chassaing, B.; Rolhion, N.; De Vallée, A.; Salim, S.Y.; Prorok-hamon, M.; Neut, C.; Campbell, B.J.; Söderholm, J.D.; Hugot, J. Crohn Disease–Associated Adherent-Invasive E. Coli Bacteria Target Mouse and Human Peyer’s Patches via Long Polar Fimbriae. J. Clin. Investig. 2018, 121, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shan, P.; Jiang, G.; Cohn, L.; Lee, P.J. Toll-like Receptor 4 Deficiency Causes Pulmonary Emphysema. J. Clin. Investig. 2006, 116, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Maglione, P.J.; Simchoni, N.; Cunningham-Rundles, C. Toll-like Receptor Signaling in Primary Immune Deficiencies. Ann. N. Y. Acad. Sci. 2015, 1356, 1–21. [Google Scholar] [CrossRef]
- Kunii, J.; Takahashi, K.; Kasakura, K.; Tsuda, M.; Nakano, K.; Hosono, A.; Kaminogawa, S. Commensal Bacteria Promote Migration of Mast Cells into the Intestine. Immunobiology 2011, 216, 692–697. [Google Scholar] [CrossRef]
- Umesaki, Y.; Setoyama, H.; Matsumoto, S.; Okada, Y. Expansion of Alpha Beta T-Cell Receptor-Bearing Intestinal Intraepithelial Lymphocytes after Microbial Colonization in Germ-Free Mice and Its Independence from Thymus. Immunology 1993, 79, 32–37. [Google Scholar]
- Nguyen, H.D.; Aljamaei, H.M.; Stadnyk, A.W. The Production and Function of Endogenous Interleukin-10 in Intestinal Epithelial Cells and Gut Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1343–1352. [Google Scholar] [CrossRef]
- Hyun, J.; Romero, L.; Riveron, R.; Flores, C.; Kanagavelu, S.; Chung, K.D.; Alonso, A.; Sotolongo, J.; Ruiz, J.; Manukyan, A.; et al. Human Intestinal Epithelial Cells Express Interleukin-10 through Toll-like Receptor 4-Mediated Epithelial-Macrophage Crosstalk. J. Innate Immun. 2015, 7, 87–101. [Google Scholar] [CrossRef]
- Yeruva, L.; Spencer, N.E.; Saraf, M.K.; Hennings, L.; Bowlin, A.K.; Cleves, M.A.; Mercer, K.; Chintapalli, S.V.; Shankar, K.; Rank, R.G.; et al. Formula Diet Alters Small Intestine Morphology, Microbial Abundance and Reduces VE-Cadherin and IL-10 Expression in Neonatal Porcine Model. BMC Gastroenterol. 2016, 16, 40. [Google Scholar] [CrossRef]
- Liu, L.; Gong, T.; Tao, W.; Lin, B.; Li, C.; Zheng, X.; Zhu, S.; Jiang, W.; Zhou, R. Commensal Viruses Maintain Intestinal Intraepithelial Lymphocytes via Noncanonical RIG-I Signaling. Nat. Immunol. 2019, 20, 1681–1691. [Google Scholar] [CrossRef]
- Özçam, M.; Lynch, S.V. The Gut–Airway Microbiome Axis in Health and Respiratory Diseases. Nat. Rev. Microbiol. 2024, 22, 492–506. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Zhou, X. Lung Microbiome: New Insights into the Pathogenesis of Respiratory Diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [PubMed]
- Kucharzik, T.; Hudson, J.T.; Lügering, A.; Abbas, J.A.; Bettini, M.; Lake, J.G.; Evans, M.E.; Ziegler, T.R.; Merlin, D.; Madara, J.L.; et al. Acute Induction of Human IL-8 Production by Intestinal Epithelium Triggers Neutrophil Infiltration without Mucosal Injury. Gut 2005, 54, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Lee, J.; Stamm, D.S.; Sanderson, I.R. MIP-2 Secreted by Epithelial Cells Increases Neutrophil and Lymphocyte Recruitment in the Mouse Intestine. Gut 2001, 49, 526–533. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ahyi, A.N.N.; Pepper-Cunningham, Z.A.; Ferrari, J.D.; Wilson, A.A.; Jones, M.R.; Quinton, L.J.; Mizgerd, J.P. Roles of Lung Epithelium in Neutrophil Recruitment during Pneumococcal Pneumonia. Am. J. Respir. Cell Mol. Biol. 2014, 50, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Rösler, B.; Herold, S. Lung Epithelial GM-CSF Improves Host Defense Function and Epithelial Repair in Influenza Virus Pneumonia—A New Therapeutic Strategy? Mol. Cell Pediatr. 2016, 3, 29. [Google Scholar] [CrossRef]
- Patel, J.A.; Jiang, Z.; Nakajima, N.; Kunimoto, M. Autocrine Regulation of Interleukin-8 by Interleukin-1α in Respiratory Syncytial Virus-Infected Pulmonary Epithelial Cells in Vitro. Immunology 1998, 95, 501–506. [Google Scholar] [CrossRef]
- Corren, J.; Ziegler, S.F. TSLP: From Allergy to Cancer. Nat. Immunol. 2019, 20, 1603–1609. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Fornasa, G.; Rescigno, M. Thymic Stromal Lymphopoietin: To Cut a Long Story Short. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 174–182. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Wechsler, M.E.; Brightling, C.E. Unmet Need in Severe, Uncontrolled Asthma: Can Anti-TSLP Therapy with Tezepelumab Provide a Valuable New Treatment Option? Respir. Res. 2020, 21, 268. [Google Scholar] [CrossRef]
- Leiva-Juárez, M.M.; Kolls, J.K.; Evans, S.E. Lung Epithelial Cells: Therapeutically Inducible Effectors of Antimicrobial Defense. Mucosal Immunol. 2018, 11, 21–34. [Google Scholar] [CrossRef]
- Frye, M.; Bargon, J.; Dauletbaev, N.; Weber, A.; Wagner, T.O.F.; Gropp, R. Expression of Human α-Defensin 5 (HD5) MRNA in Nasal and Bronchial Epithelial Cells. J. Clin. Pathol. 2000, 53, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth Cells Directly Sense Gut Commensals and Maintain Homeostasis at the Intestinal Host-Microbial Interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef]
- McCray, P.B.; Bentley, L. Human Airway Epithelia Express a β-Defensin. Am. J. Respir. Cell Mol. Biol. 1997, 16, 343–349. [Google Scholar] [CrossRef]
- Vora, P.; Youdim, A.; Thomas, L.S.; Fukata, M.; Tesfay, S.Y.; Lukasek, K.; Michelsen, K.S.; Wada, A.; Hirayama, T.; Arditi, M.; et al. β-Defensin-2 Expression Is Regulated by TLR Signaling in Intestinal Epithelial Cells. J. Immunol. 2004, 173, 5398–5405. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-J. Intestinal Mucosal Lesion in Low-Flow States. Arch. Surg. 1970, 101, 478. [Google Scholar] [CrossRef] [PubMed]
- Chairatana, P.; Chiang, I.L.; Nolan, E.M. Human α-Defensin 6 Self-Assembly Prevents Adhesion and Suppresses Virulence Traits of Candida Albicans. Biochemistry 2017, 56, 1033–1041. [Google Scholar] [CrossRef]
- Wu, H.; Kuzmenko, A.; Wan, S.; Schaffer, L.; Weiss, A.; Fisher, J.H.; Kim, K.S.; McCormack, F.X. Surfactant Proteins A and D Inhibit the Growth of Gram-Negative Bacteria by Increasing Membrane Permeability. J. Clin. Investig. 2003, 111, 1589–1602. [Google Scholar] [CrossRef]
- Grubor, B.; Meyerholz, D.K.; Ackermann, M.R. Collectins and Cationic Antimicrobial Peptides of the Respiratory Epithelia. Vet. Pathol. 2006, 43, 595–612. [Google Scholar] [CrossRef]
- Miki, T.; Holsts, O.; Hardt, W.D. The Bactericidal Activity of the C-Type Lectin RegIIIβ against Gram-Negative Bacteria Involves Binding to Lipid A. J. Biol. Chem. 2012, 287, 34844–34855. [Google Scholar] [CrossRef]
- Mukherjee, S.; Zheng, H.; Derebe, M.G.; Callenberg, K.M.; Partch, C.L.; Rollins, D.; Propheter, D.C.; Rizo, J.; Grabe, M.; Jiang, Q.X.; et al. Antibacterial Membrane Attack by a Pore-Forming Intestinal C-Type Lectin. Nature 2014, 505, 103–107. [Google Scholar] [CrossRef]
- Natividad, J.M.M.; Hayes, C.L.; Motta, J.P.; Jury, J.; Galipeau, H.J.; Philip, V.; Garcia-Rodenas, C.L.; Kiyama, H.; Bercik, P.; Verdú, E.F. Differential Induction of Antimicrobial REGIII by the Intestinal Microbiota and Bifidobacterium Breve NCC2950. Appl. Environ. Microbiol. 2013, 79, 7745–7754. [Google Scholar] [CrossRef] [PubMed]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L. V The Antibacterial Lectin RegIIIγ Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science (1979) 2011, 334, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science (1979) 2006, 313, 1126–1130. [Google Scholar] [CrossRef]
- Matsumoto, S.; Konishi, H.; Maeda, R.; Kiryu-Seo, S.; Kiyama, H. Expression Analysis of the Regenerating Gene (Reg) Family Members Reg-IIIβ and Reg-IIIγ in the Mouse during Development. J. Comp. Neurol. 2012, 520, 479–494. [Google Scholar] [CrossRef]
- Ito, T.; Hirose, K.; Saku, A.; Kono, K.; Takatori, H.; Tamachi, T.; Goto, Y.; Renauld, J.C.; Kiyono, H.; Nakajima, H. IL-22 Induces Reg3γ and Inhibits Allergic Inflammation in House Dust Mite-Induced Asthma Models. J. Exp. Med. 2017, 214, 3037–3050. [Google Scholar] [CrossRef]
- De Luna, X.; Hsieh, I.-N.; White, M.; Hartshorn, K.L. Exploring Anti-Viral and Immunomodulatory Activities of Regenerating Islet-Derived Proteins. J. Immunol. 2018, 200, 168.3. [Google Scholar] [CrossRef]
- Fischer, H. Mechanisms and Function of DUOX in Epithelia of the Lung. Antioxid. Redox Signal 2009, 11, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, A.; Pang, L.; Hanson, J.; Dlugolenski, D.; Streich, R.; Lafontaine, E.R.; Nagy, T.; Tripp, R.A.; Rada, B. Hypothiocyanite Produced by Human and Rat Respiratory Epithelial Cells Inactivates Extracellular H1N2 Influenza A Virus. Inflamm. Res. 2016, 65, 71–80. [Google Scholar] [CrossRef]
- Grasberger, H.; Gao, J.; Nagao-Kitamoto, H.; Kitamoto, S.; Zhang, M.; Kamada, N.; Eaton, K.A.; El-Zaatari, M.; Shreiner, A.B.; Merchant, J.L.; et al. Increased Expression of DUOX2 Is an Epithelial Response to Mucosal Dysbiosis Required for Immune Homeostasis in Mouse Intestine. Gastroenterology 2015, 149, 1849–1859. [Google Scholar] [CrossRef]
- Kumar, A.; Wu, H.; Collier-Hyams, L.S.; Hansen, J.M.; Li, T.; Yamoah, K.; Pan, Z.Q.; Jones, D.P.; Neish, A.S. Commensal Bacteria Modulate Cullin-Dependent Signaling via Generation of Reactive Oxygen Species. EMBO J. 2007, 26, 4457–4466. [Google Scholar] [CrossRef]
- Lin, P.W.; Myers, L.E.S.; Ray, L.; Song, S.C.; Nasr, T.R.; Berardinelli, A.J.; Kundu, K.; Murthy, N.; Hansen, J.M.; Neish, A.S. Lactobacillus Rhamnosus Blocks Inflammatory Signaling in Vivo via Reactive Oxygen Species Generation. Free Radic. Biol. Med. 2009, 47, 1205–1211. [Google Scholar] [CrossRef]
- Lipinski, S.; Till, A.; Sina, C.; Arlt, A.; Grasberger, H.; Schreiber, S.; Rosenstiel, P. DUOX2-Derived Reactive Oxygen Species Are Effectors of NOD2-Mediated Antibacterial Responses. J. Cell Sci. 2009, 122, 3522–3530. [Google Scholar] [CrossRef]
- Granberg, S.; Wikland, M. Implication of Oxidative Stress in Small Intestine Disorders, Constipation and Diarrhea: A Mini Review. Recent. Adv. Biol. Med. 2017, 3, 66–68. [Google Scholar] [CrossRef]
- Henriksen, P.A.; Hitt, M.; Xing, Z.; Wang, J.; Haslett, C.; Riemersma, R.A.; Webb, D.J.; Kotelevtsev, Y.V.; Sallenave, J.-M. Adenoviral Gene Delivery of Elafin and Secretory Leukocyte Protease Inhibitor Attenuates NF-ΚB-Dependent Inflammatory Responses of Human Endothelial Cells and Macrophages to Atherogenic Stimuli. J. Immunol. 2004, 172, 4535–4544. [Google Scholar] [CrossRef]
- Meyer, M.; Jaspers, I. Respiratory Protease/Antiprotease Balance Determines Susceptibility to Viral Infection and Can Be Modified by Nutritional Antioxidants. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Brockbank, S.; Lowry, P.; Fitzgerald, S.P.; Taggart, C.; Weldon, S. The Role of Serine Proteases and Antiproteases in the Cystic Fibrosis Lung. Mediat. Inflamm. 2015, 2015, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Si-Tahar, M.; Merlin, D.; Sitaraman, S.; Madara, J.L. Constitutive and Regulated Secretion of Secretory Leukocyte Proteinase Inhibitor by Human Intestinal Epithelial Cells. Gastroenterology 2000, 118, 1061–1071. [Google Scholar] [CrossRef]
- Galipeau, H.J.; Wiepjes, M.; Motta, J.; Schulz, J.D.; Jury, J.; Natividad, J.M.; Pinto-Sanchez, I.; Sinclair, D.; Rousset, P.; Martin-Rosique, R.; et al. Novel Role of the Serine Protease Inhibitor Elafin in Gluten-Related Disorders. Am. J. Gastroenterol. 2014, 109, 748–756. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Girolamo, S.D.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 903. [Google Scholar] [CrossRef]
- Ellison, R.T.; Giehl, T.J. Killing of Gram-Negative Bacteria by Lactoferrin and Lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef]
- Mason, D.Y.; Taylor, C.R. The Distribution of Muramidase (Lysozyme) in Human Tissues. J. Clin. Pathol. 1975, 28, 124–132. [Google Scholar] [CrossRef]
- Friedl, A.; Stoesz, S.P.; Buckley, P.; Gould, M.N. Neutrophil Gelatinase-Associated Lipocalin in Normal and Neoplastic Human Tissues. Cell Type-Specific Pattern of Expression. Histochem. J. 1999, 31, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.; Gakhar, L.; Penterman, J.; Singh, P.; Mallampalli, R.K.; Porter, E.; McCray, P.B. PLUNC: A Multifunctional Surfactant of the Airways. Biochem. Soc. Trans. 2011, 39, 1012–1016. [Google Scholar] [CrossRef]
- Gakhar, L.; Bartlett, J.A.; Penterman, J.; Mizrachi, D.; Singh, P.K.; Mallampalli, R.K.; Ramaswamy, S.; McCray, P.B. PLUNC Is a Novel Airway Surfactant Protein with Anti-Biofilm Activity. PLoS ONE 2010, 5, e9098. [Google Scholar] [CrossRef] [PubMed]
- Britto, C.J.; Cohn, L. Bactericidal/Permeability-Increasing Protein Fold-Containing Family Member A1 in Airway Host Protection and Respiratory Disease. Am. J. Respir. Cell Mol. Biol. 2015, 52, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Bingle, C.D.; Craven, C.J. PLUNC: A Novel Family of Candidate Host Defence Proteins Expressed in the Upper Airways and Nasopharynx. Hum. Mol. Genet. 2002, 11, 937–943. [Google Scholar] [CrossRef]
- Stockinger, S.; Albers, T.; Duerr, C.U.; Ménard, S.; Pütsep, K.; Andersson, M.; Hornef, M.W. Interleukin-13-Mediated Paneth Cell Degranulation and Antimicrobial Peptide Release. J. Innate Immun. 2014, 6, 530–541. [Google Scholar] [CrossRef]
- Zuyderduyn, S.; Ninaber, D.K.; Schrumpf, J.A.; van Sterkenburg, M.A.J.A.; Verhoosel, R.M.; Prins, F.A.; van Wetering, S.; Rabe, K.F.; Hiemstra, P.S. IL-4 and IL-13 Exposure during Mucociliary Differentiation of Bronchial Epithelial Cells Increases Antimicrobial Activity and Expression of Antimicrobial Peptides. Respir. Res. 2011, 12, 59. [Google Scholar] [CrossRef]
- O’Hara, J.R.; Sharkey, K.A. Proliferative Capacity of Enterochromaffin Cells in Guinea-Pigs with Experimental Ileitis. Cell Tissue Res. 2007, 329, 433–441. [Google Scholar] [CrossRef]
- Lin, R.; Lu, H.; Zhou, G.; Wei, Q.; Liu, Z. Clinicopathological and Ileocolonoscopic Characteristics in Patients with Nodular Lymphoid Hyperplasia in the Terminal Ileum. Int. J. Med. Sci. 2017, 14, 750–757. [Google Scholar] [CrossRef][Green Version]
- Shaykhiev, R. Emerging Biology of Persistent Mucous Cell Hyperplasia in COPD. Thorax 2019, 74, 4–6. [Google Scholar] [CrossRef] [PubMed]
- McGraw, M.D.; Rioux, J.S.; Garlick, R.B.; Rancourt, R.C.; White, C.W.; Veress, L.A. Impaired Proliferation and Differentiation of the Conducting Airway Epithelium Associated with Bronchiolitis Obliterans after Sulfur Mustard Inhalation Injury in Rats. Toxicol. Sci. 2017, 157, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Tschurtschenthaler, M.; Wang, J.; Fricke, C.; Fritz, T.M.J.; Niederreiter, L.; Adolph, T.E.; Sarcevic, E.; Künzel, S.; Offner, F.A.; Kalinke, U.; et al. Type I Interferon Signalling in the Intestinal Epithelium Affects Paneth Cells, Microbial Ecology and Epithelial Regeneration. Gut 2014, 63, 1921–1931. [Google Scholar] [CrossRef]
- Katlinskaya, Y.V.; Katlinski, K.V.; Lasri, A.; Li, N.; Beiting, D.P.; Durham, A.C.; Yang, T.; Pikarsky, E.; Lengner, C.J.; Johnson, F.B.; et al. Type I Interferons Control Proliferation and Function of the Intestinal Epithelium. Mol. Cell. Biol. 2016, 36, 1124–1135. [Google Scholar] [CrossRef]
- Major, J.; Crotta, S.; Llorian, M.; McCabe, T.M.; Gad, H.H.; Priestnall, S.L.; Hartmann, R.; Wack, A. Type I and III Interferons Disrupt Lung Epithelial Repair during Recovery from Viral Infection. Science (1979) 2020, 369, 712–717. [Google Scholar] [CrossRef]
- Davidson, S.; McCabe, T.M.; Crotta, S.; Gad, H.H.; Hessel, E.M.; Beinke, S.; Hartmann, R.; Wack, A. IFN λ Is a Potent Anti-influenza Therapeutic without the Inflammatory Side Effects of IFN α Treatment. EMBO Mol. Med. 2016, 8, 1099–1112. [Google Scholar] [CrossRef]
- Chiriac, M.T.; Buchen, B.; Wandersee, A.; Hundorfean, G.; Günther, C.; Bourjau, Y.; Doyle, S.E.; Frey, B.; Ekici, A.B.; Büttner, C.; et al. Activation of Epithelial Signal Transducer and Activator of Transcription 1 by Interleukin 28 Controls Mucosal Healing in Mice With Colitis and Is Increased in Mucosa of Patients With Inflammatory Bowel Disease. Gastroenterology 2017, 153, 123–138.e8. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Beigel, F.; Olszak, T.; Zitzmann, K.; Eichhorst, S.T.; Otte, J.M.; Diebold, J.; Diepolder, H.; Adler, B.; Auernhammer, C.J.; et al. IL-28A and IL-29 Mediate Antiproliferative and Antiviral Signals in Intestinal Epithelial Cells and Murine CMV Infection Increases Colonic IL-28A Expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, 960–968. [Google Scholar] [CrossRef]
- Nava, P.; Koch, S.; Laukoetter, M.G.; Lee, W.Y.; Kolegraff, K.; Capaldo, C.T.; Beeman, N.; Addis, C.; Gerner-Smidt, K.; Neumaier, I.; et al. Interferon-γ Regulates Intestinal Epithelial Homeostasis through Converging β-Catenin Signaling Pathways. Immunity 2010, 32, 392–402. [Google Scholar] [CrossRef]
- Rodriguez, N.R.M.; Eloi, M.D.; Huynh, A.; Dominguez, T.; Lam, A.H.C.; Dayana, C.M.; Naser, Z.; Desharnais, R.; Salzman, N.H.; Porter, E. Expansion of Paneth Cell Population in Response to Enteric Salmonella Enterica Serovar Typhimurium Infection. Infect. Immun. 2012, 80, 266–275. [Google Scholar] [CrossRef]
- Walsh, R.; Seth, R.; Behnke, J.; Potten, C.S.; Mahida, Y.R. Epithelial Stem Cell-Related Alterations in Trichinella Spiralis-Infected Small Intestine. Cell Prolif. 2009, 42, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Guy-Grand, D.; DiSanto, J.P.; Henchoz, P.; Malassis-Séris, M.; Vassalli, P. Small Bowel Enteropathy: Role of Intraepithelial Lymphocytes and of Cytokines (IL-12, IFN-γ, TNF) in the Induction of Epithelial Cell Death and Renewal. Eur. J. Immunol. 1998, 28, 730–744. [Google Scholar] [CrossRef]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective Epithelial Barrier in Chronic Rhinosinusitis: The Regulation of Tight Junctions by IFN-γ and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096.e10. [Google Scholar] [CrossRef] [PubMed]
- Theron, M.; Huang, K.J.; Chen, Y.W.; Liu, C.C.; Lei, H.Y. A Probable Role for IFN-γ in the Development of a Lung Immunopathology in SARS. Cytokine 2005, 32, 30–38. [Google Scholar] [CrossRef]
- Martini, E.; Schneider, E.; Neufert, C.; Neurath, M.F.; Becker, C. Survivin Is a Guardian of the Intestinal Stem Cell Niche and Its Expression Is Regulated by TGF-β. Cell Cycle 2016, 15, 2875–2881. [Google Scholar] [CrossRef]
- Ramesh, S.; Wildey, G.M.; Howe, P.H. Transforming Growth Factor β (TGFβ)-Induced Apoptosis: The Rise & Fall of Bim. Cell Cycle 2009, 8, 11–17. [Google Scholar] [CrossRef]
- Oshima, H.; Nakayama, M.; Han, T.S.; Naoi, K.; Ju, X.; Maeda, Y.; Robine, S.; Tsuchiya, K.; Sato, T.; Sato, H.; et al. Suppressing TGFβ Signaling in Regenerating Epithelia in an Inflammatory Microenvironment Is Sufficient to Cause Invasive Intestinal Cancer. Cancer Res. 2015, 75, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Cao, S.; Jiao, L.; Song, Z.; Lu, J.; Hu, C. TGF-Β1 Protects Intestinal Integrity and Influences Smads and MAPK Signal Pathways in IPEC-J2 after TNF-α Challenge. Innate Immun. 2017, 23, 276–284. [Google Scholar] [CrossRef]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef]
- Pittet, J.F.; Griffiths, M.J.D.; Geiser, T.; Kaminski, N.; Dalton, S.L.; Huang, X.; Brown, L.A.S.; Gotwals, P.J.; Koteliansky, V.E.; Matthay, M.A.; et al. TGF-β Is a Critical Mediator of Acute Lung Injury. J. Clin. Investig. 2001, 107, 1537–1544. [Google Scholar] [CrossRef]
- Li, M.; Krishnaveni, M.S.; Li, C.; Zhou, B.; Xing, Y.; Banfalvi, A.; Li, A.; Lombardi, V.; Akbari, O.; Borok, Z.; et al. Epithelium-Specific Deletion of TGF-β Receptor Type II Protects Mice from Bleomycin-Induced Pulmonary Fibrosis. J. Clin. Investig. 2011, 121, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Onnebrink, J.; Werner, C.; Loges, N.T.; Hormann, K.; Blum, A.; Schmidt, R.; Olbrich, H.; Omran, H. Effect of TH2 Cytokines and Interferon Gamma on Beat Frequency of Human Respiratory Cilia. Pediatr. Res. 2016, 79, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.F.; Oliveira, H.B.M.; das Neves Selis, N.; e Souza, C.L.S.; Júnior, M.N.S.; de Souza, E.P.; da Silva, L.S.C.; de Souza Nascimento, F.; Amorim, A.T.; Campos, G.B.; et al. Anti-Inflammatory Activity of β-Caryophyllene Combined with Docosahexaenoic Acid in a Model of Sepsis Induced by Staphylococcus Aureus in Mice. J. Sci. Food Agric. 2019, 99, 5870–5880. [Google Scholar] [CrossRef]
- Mcgee, D.W.; Vitkus, S.J.D. IL-4 Enhances IEC-6 Intestinal Epithelial Cell Proliferation yet Has No Effect on IL-6 Secretion. Clin. Exp. Immunol. 1996, 105, 274–277. [Google Scholar] [CrossRef]
- Mahapatro, M.; Foersch, S.; Hefele, M.; He, G.W.; Giner-Ventura, E.; Mchedlidze, T.; Kindermann, M.; Vetrano, S.; Danese, S.; Günther, C.; et al. Programming of Intestinal Epithelial Differentiation by IL-33 Derived from Pericryptal Fibroblasts in Response to Systemic Infection. Cell Rep. 2016, 15, 1743–1756. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Curley, G.F.; Rose-John, S.; McElvaney, N.G. Interleukin-6: Obstacles to Targeting a Complex Cytokine in Critical Illness. Lancet Respir. Med. 2021, 9, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.C.; Stappenbeck, T.S. IL-6 Stimulates Intestinal Epithelial Proliferation and Repair after Injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef]
- Jeffery, V.; Goldson, A.J.; Dainty, J.R.; Chieppa, M. Interleukin-6 Signaling Regulates Small Intestinal Crypt Homeostasis. J (Basel) 2019, 199, 304–311. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 Signalling Axis in Cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Le, T.-T.T.; Karmouty-Quintana, H.; Melicoff, E.; Le, T.-T.T.; Weng, T.; Chen, N.-Y.; Pedroza, M.; Zhou, Y.; Davies, J.; Philip, K.; et al. Blockade of IL-6 Trans Signaling Attenuates Pulmonary Fibrosis. J. Immunol. 2014, 193, 3755–3768. [Google Scholar] [CrossRef]
- Schumacher, N.; Rose-John, S. ADAM17 Activity and IL-6 Trans-Signaling in Inflammation and Cancer. Cancers 2019, 11, 1736. [Google Scholar] [CrossRef]
- Andrews, C.; McLean, M.H.; Durum, S.K. Cytokine Tuning of Intestinal Epithelial Function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Branchett, W.J.; Lloyd, C.M. Regulatory Cytokine Function in the Respiratory Tract. Mucosal Immunol. 2019, 12, 589–600. [Google Scholar] [CrossRef]
- Tadesse, S.; Corner, G.; Dhima, E.; Houston, M.; Guha, C.; Augenlicht, L.; Velcich, A. MUC2 Mucin Deficiency Alters Inflammatory and Metabolic Pathways in the Mouse Intestinal Mucosa. Oncotarget 2017, 8, 71456–71470. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Walsh, M.C.; Kim, K.S.; Hong, S.W.; Lee, J.; Yi, J.; Rivas, G.; Choi, Y.; Surh, C.D. Dendritic Cell Expression of the Signaling Molecule TRAF6 Is Required for Immune Tolerance in the Lung. Int. Immunol. 2017, 29, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Udomsopagit, T.; Miwa, A.; Seki, M.; Shimbori, E.; Kadota, Y.; Tochio, T.; Sonoyama, K. Intestinal Microbiota Transplantation Reveals the Role of Microbiota in Dietary Regulation of RegIIIβ and RegIIIγ Expression in Mouse Intestine. Biochem. Biophys. Res. Commun. 2020, 529, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Hu, Y.; Zhao, L.; Xia, J.; Li, C.; Shi, L.; Xu, F. Protective Role of Adipose-Derived Stem Cells in Staphylococcus Aureus -Induced Lung Injury Is Mediated by RegIIIγ Secretion. Stem Cells 2016, 34, 1947–1956. [Google Scholar] [CrossRef]
- Liu, J.; Gao, L.; Zhou, N.; Jiang, Z.; Che, S.; Deng, Y.; Zang, N.; Ren, L.; Xie, X.; Xie, J.; et al. P53 Suppresses the Inflammatory Response Following Respiratory Syncytial Virus Infection by Inhibiting TLR2. Virology 2024, 593, 110018. [Google Scholar] [CrossRef]
- Paveljšek, D.; Ivičak-Kocjan, K.; Treven, P.; Benčina, M.; Jerala, R.; Rogelj, I. Distinctive Probiotic Features Share Common TLR2-Dependent Signalling in Intestinal Epithelial Cells. Cell Microbiol. 2021, 23, e13264. [Google Scholar] [CrossRef]
- Girkin, J.; Loo, S.L.; Esneau, C.; Maltby, S.; Mercuri, F.; Chua, B.; Reid, A.T.; Veerati, P.C.; Grainge, C.L.; Wark, P.A.B.; et al. TLR2-Mediated Innate Immune Priming Boosts Lung Anti-Viral Immunity. Eur. Respir. J. 2021, 58, 2001584. [Google Scholar] [CrossRef]
- Lu, Y.Z.; He, X.L.; Liu, F.; Cheng, P.P.; Liang, L.M.; Wang, M.; Chen, S.J.; Huang, Y.; Yu, F.; Xin, J.B.; et al. Bleomycin Induced Apical-Basal Polarity Loss in Alveolar Epithelial Cell Contributes to Experimental Pulmonary Fibrosis. Exp. Cell Res. 2020, 396, 112295. [Google Scholar] [CrossRef]
- Schneeberger, K.; Roth, S.; Nieuwenhuis, E.E.S.; Middendorp, S. Intestinal Epithelial Cell Polarity Defects in Disease: Lessons from Microvillus Inclusion Disease. DMM Dis. Models Mech. 2018, 11, dmm031088. [Google Scholar] [CrossRef]
- Tran, C.S.; Eran, Y.; Ruch, T.R.; Bryant, D.M.; Datta, A.; Brakeman, P.; Kierbel, A.; Wittmann, T.; Metzger, R.J.; Mostov, K.E.; et al. Host Cell Polarity Proteins Participate in Innate Immunity to Pseudomonas Aeruginosa Infection. Cell Host Microbe 2014, 15, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, J.L.; Azin, M.; Dempsey, K.E.; Demehri, S. TSLP/Dendritic Cell Axis Promotes CD4+ T Cell Tolerance to the Gut Microbiome. JCI Insight 2023, 8, e160690. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Vanichsarn, C.; Nadeau, K.C. TSLP Directly Impairs Pulmonary Treg Function: Association with Aberrant Tolerogenic Immunity in Asthmatic Airway. Allergy Asthma Clin. Immunol. 2010, 6, 4. [Google Scholar] [CrossRef]
- Lin, J.D.; Feng, N.; Sen, A.; Balan, M.; Tseng, H.C.; McElrath, C.; Smirnov, S.V.; Peng, J.; Yasukawa, L.L.; Durbin, R.K.; et al. Distinct Roles of Type I and Type III Interferons in Intestinal Immunity to Homologous and Heterologous Rotavirus Infections. PLoS Pathog. 2016, 12, e1005600. [Google Scholar] [CrossRef]
- Lee, S.; Baldridge, M.T. Interferon-Lambda: A Potent Regulator of Intestinal Viral Infections. Front. Immunol. 2017, 8, 749. [Google Scholar] [CrossRef]
- Feld, J.J.; Kandel, C.; Biondi, M.J.; Kozak, R.A.; Zahoor, M.A.; Lemieux, C.; Borgia, S.M.; Boggild, A.K.; Powis, J.; McCready, J.; et al. Peginterferon Lambda for the Treatment of Outpatients with COVID-19: A Phase 2, Placebo-Controlled Randomised Trial. Lancet Respir. Med. 2021, 9, 498–510. [Google Scholar] [CrossRef]
- Sojati, J.; Parks, O.B.; Zhang, Y.; Walters, S.; Lan, J.; Eddens, T.; Lou, D.; Fan, L.; Chen, K.; Oury, T.D.; et al. IFN-λ Drives Distinct Lung Immune Landscape Changes and Antiviral Responses in Human Metapneumovirus Infection. mBio 2024, 15, e0055024. [Google Scholar] [CrossRef]

| Epithelium | ||
|---|---|---|
| Cell Function | Respiratory | Small Intestinal |
| Primary cell type | Ciliated | Enterocytes |
| Regeneration | Basal, Club Deuterosomal, KRT13+ (Hillock) | Intestinal stem |
| Chemosensory | Tuft | Tuft (1/2) |
| Mucus secretion | Goblet | Goblet |
| Secretory cells | Ionocytes, Club | Paneth |
| Antigen sampling | M | M |
| Endocrine cells | Pulmonary neuroendocrine | Enteroendocrine |
| Receptor | PAMP | Epithelium | ||||||
|---|---|---|---|---|---|---|---|---|
| Respiratory | Small Intestinal | |||||||
| Apical | Baso | Cyto | Apical | Baso | Cyto | |||
| TLRs | TLR4 | LPS | + | ++ | + | ++ | ||
| TLR2 | Lipoproteins, LTA | ++ * | + | ++ * | ||||
| TLR5 | Flagellin | + * | ++ | ++ | ||||
| TLR7 | ssRNA | + | + | - | ||||
| TLR3 | dsRNA | + | + | + | ||||
| TLR9 | ssDNA | + | + | + | ||||
| NLRs | NOD1 | γ-D-meso-DAP | + | + | ||||
| NOD2 | Muramyl Dipeptide | + | + | |||||
| RLRs | RIG-I | Viral RNA | + * | + | ||||
| MDA5 | Viral RNA | + * | + | |||||
| Epithelium | ||
|---|---|---|
| Mediator | Lower Respiratory | Small Intestinal |
| Type I IFNs | ↓ proliferation | ↓ proliferation |
| Type II IFNs (IFN-γ) | ↑ DUOX and RIG-I ↓ proliferation ↑ apoptosis ↓ barrier function | ↑ proliferation (acute exposure) ↓ proliferation and loss of Paneth cells (chronic exposure) ↑ Paneth/goblet cell secretion |
| Type III IFNs (IFN-λ) | ↓ proliferation ↓ epithelial repair (chronic exposure) | ↓ proliferation ↑ mucosal healing |
| TGF-β | ↓ proliferation ↑ apoptosis Maintenance of barrier permeability | ↓ proliferation ↑ apoptosis Maintenance of barrier permeability |
| IL-4/IL-13 | ↑ mucin/AMP secretion ↑ goblet cell proliferation (chronic exposure) ↓ decreased lung function (long-term) | ↑ mucin/AMP secretion ↑ tuft/goblet cell differentiation |
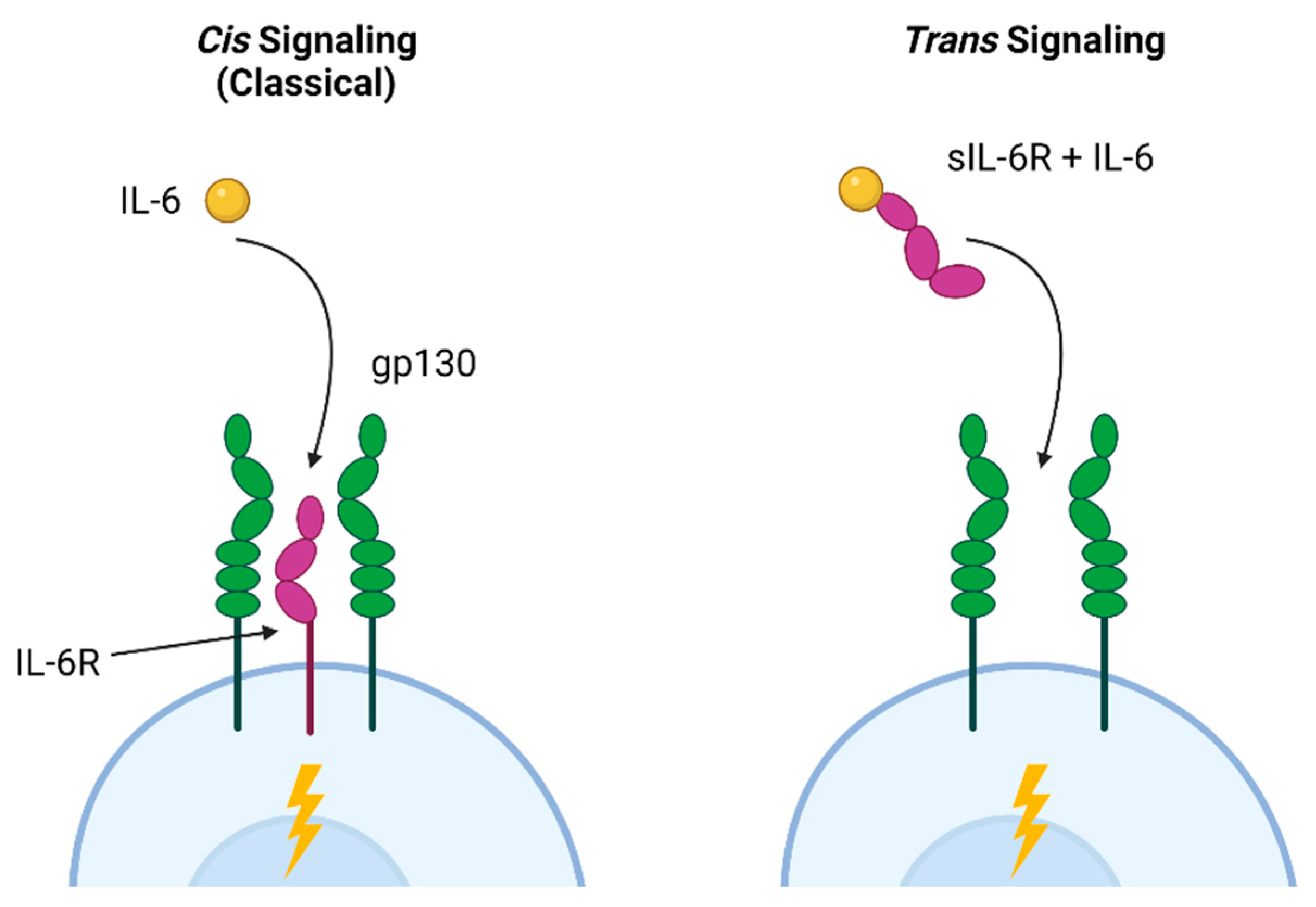
| IL-6 | ↑ proliferation (trans signaling) ↑ fibrosis (trans signaling) | Homeostasis and wound healing (classical signaling) ↑ proliferation (trans signaling) |
| Type I IFNs | ↓ proliferation | ↓ proliferation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickson, K.B.; Stadnyk, A.W.; Zhou, J.; Lehmann, C. Mucosal Immunity: Lessons from the Lower Respiratory and Small Intestinal Epithelia. Biomedicines 2025, 13, 1052. https://doi.org/10.3390/biomedicines13051052
Dickson KB, Stadnyk AW, Zhou J, Lehmann C. Mucosal Immunity: Lessons from the Lower Respiratory and Small Intestinal Epithelia. Biomedicines. 2025; 13(5):1052. https://doi.org/10.3390/biomedicines13051052
Chicago/Turabian StyleDickson, Kayle B., Andrew W. Stadnyk, Juan Zhou, and Christian Lehmann. 2025. "Mucosal Immunity: Lessons from the Lower Respiratory and Small Intestinal Epithelia" Biomedicines 13, no. 5: 1052. https://doi.org/10.3390/biomedicines13051052
APA StyleDickson, K. B., Stadnyk, A. W., Zhou, J., & Lehmann, C. (2025). Mucosal Immunity: Lessons from the Lower Respiratory and Small Intestinal Epithelia. Biomedicines, 13(5), 1052. https://doi.org/10.3390/biomedicines13051052








