Pirfenidone Alleviates Against Fine Particulate Matter-Induced Pulmonary Fibrosis Modulating via TGF-β1/TAK1/MKK3/p38 MAPK Signaling Pathway in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Groups
2.2. PM10 Injection and Pirfenidone Treatment
2.3. BALF Collection and Lung Tissue Preparation
2.4. BALF Cell Counting
2.5. Concentration of Pro-Inflammatory Cytokines and Fibrotic Factors
2.6. Hematoxylin and Eosin Staining for Lung Histopathological Evaluation
2.7. Picrosirius Red Staining for Pulmonary Fibrosis Analysis
2.8. Western Blotting
2.9. Immunofluorescence Staining for Collagen Type I and Fibronectin
2.10. Statistical Analysis
3. Results
3.1. BALF Cells, Lung Injury Score, and Pulmonary Fibrosis
3.2. Pro-Inflammatory Cytokines
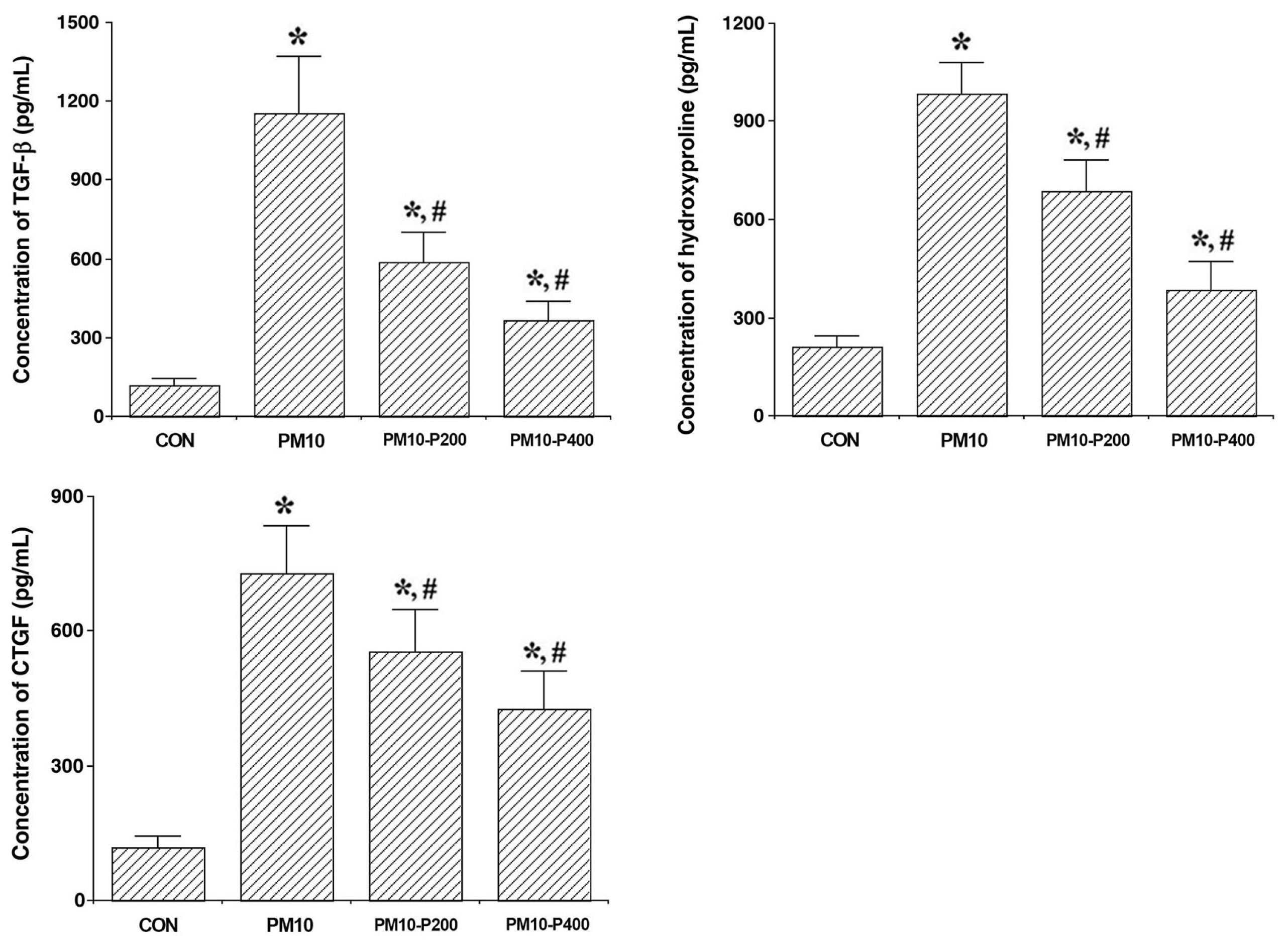
3.3. Fibrotic Factors
3.4. TAK1/MKK3/p38 Signaling Pathway
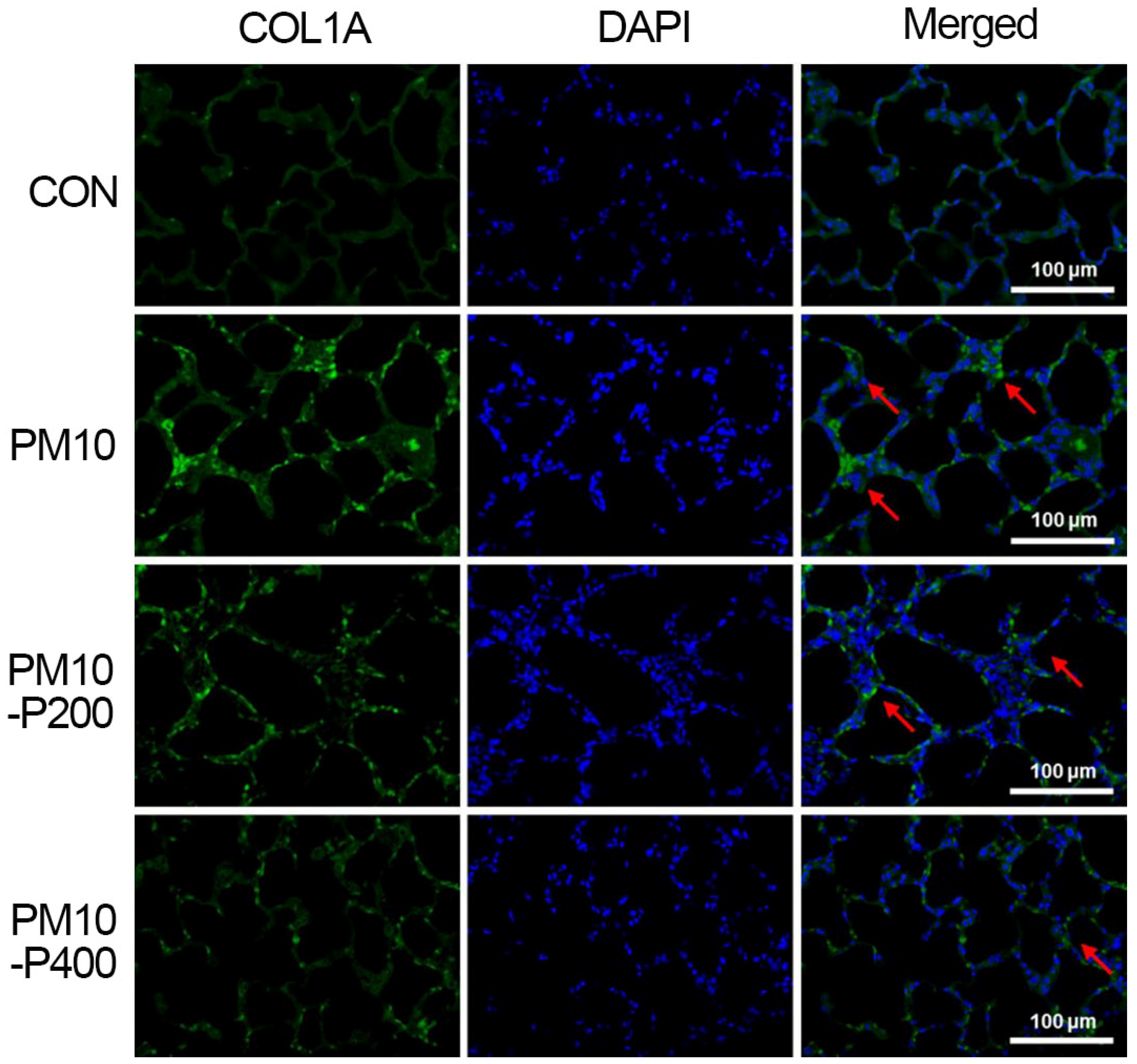
3.5. Collagen Type I
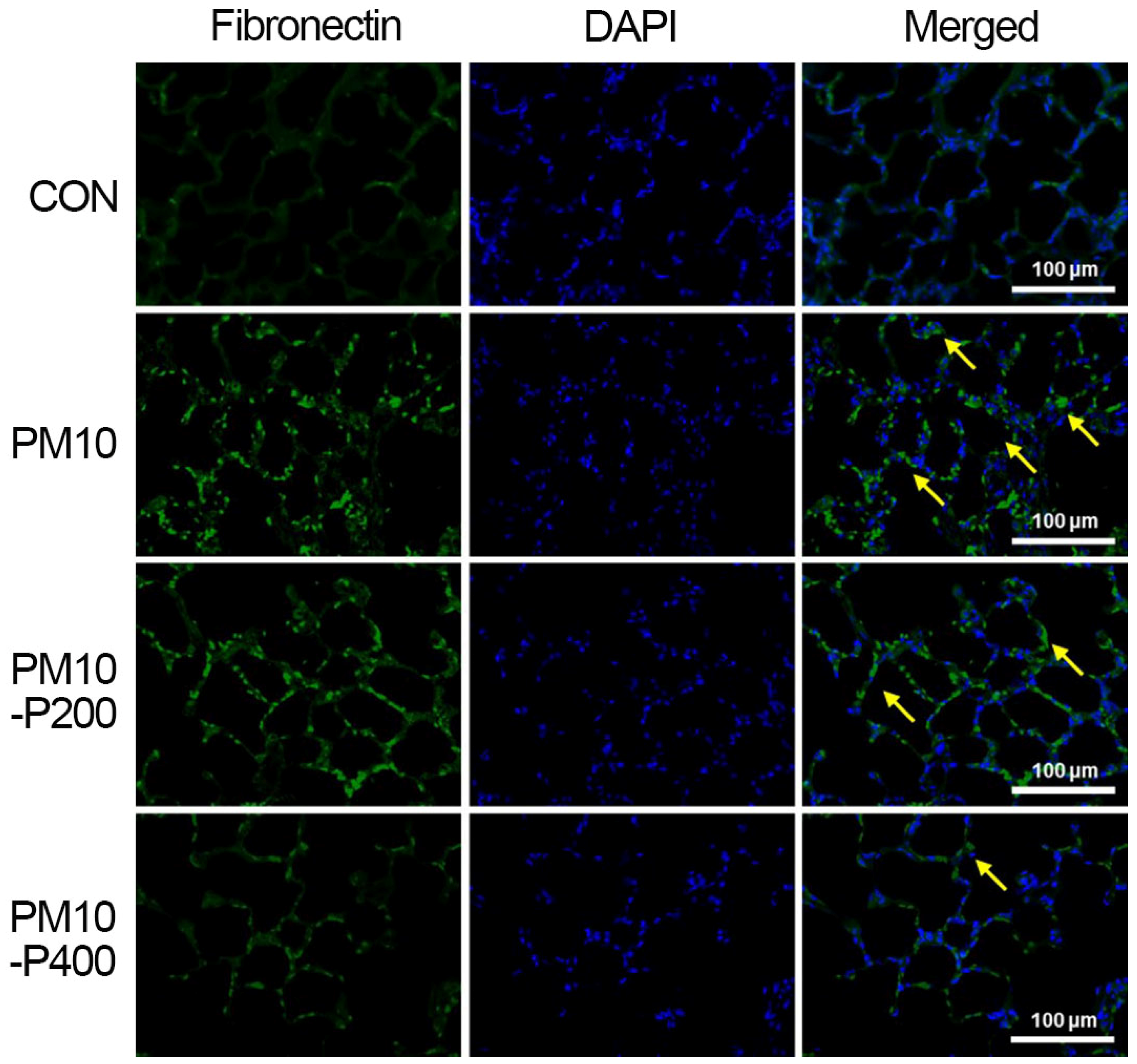
3.6. Fibronectin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM | Particulate matter |
| TGF | Transforming growth factor |
| TAK1 | Transforming growth factor-β-activated kinase 1 |
| MAPK | Mitogen-activated protein kinase |
| TNF | Tumor necrosis factor |
| IL | Interleukin |
| BALF | Bronchoalveolar lavage fluid |
| ELISA | Enzyme-linked immunosorbent assay |
| CTGF | Connective tissue growth factor |
| MKK | Mitogen-activated protein kinase kinase |
References
- Xia, J.; Huang, Y.H.; Li, J.; Liu, S.; Chen, Y.L.; Li, L.L.; Jiang, C.Z.; Chen, Z.J.; Wang, Y.; Liu, X.M.; et al. Maternal exposure to ambient particulate matter 10 μm or less in diameter before and after pregnancy, and anencephaly risk: A population-based case-control study in China. Environ. Res. 2020, 188, 109757. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Bassey, E.; Bos, B.; Makacha, L.; Varaden, D.; Arku, R.E.; Baumgartner, J.; Brauer, M.; Ezzati, M.; Kelly, F.J.; et al. Comparing human exposure to fine particulate matter in low and high-income countries: A systematic review of studies measuring personal PM2.5 exposure. Sci. Total Environ. 2022, 833, 155207. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent insights into particulate matter (PM2.5)-mediated toxicity in humans: An overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Kyung, S.Y.; Jeong, S.H. Particulate-matter related respiratory diseases. Tuberc. Respir. Dis. 2020, 83, 116–121. [Google Scholar] [CrossRef]
- Sun, B.; Shi, Y.; Li, Y.; Jiang, J.; Liang, S.; Duan, J.; Sun, Z. Short-term PM2.5 exposure induces sustained pulmonary fibrosis development during post-exposure period in rats. J. Hazard. Mater. 2020, 385, 121566. [Google Scholar] [CrossRef]
- Dong, J.; Yu, X.; Porter, D.W.; Battelli, L.A.; Kashon, M.L.; Ma, Q. Common and distinct mechanisms of induced pulmonary fibrosis by particulate and soluble chemical fibrogenic agents. Arch. Toxicol. 2016, 90, 385–402. [Google Scholar] [CrossRef]
- Choi, M.E.; Ding, Y.; Kim, S.I. TGF-β signaling via TAK1 pathway: Role in kidney fibrosis. Semin. Nephrol. 2012, 32, 244–252. [Google Scholar] [CrossRef]
- Wu, H.; Wang, D.; Shi, H.; Liu, N.; Wang, C.; Tian, J.; Wang, X.; Zhang, Z. PM2.5 and water-soluble components induce airway fibrosis through TGF-β1/Smad3 signaling pathway in asthmatic rats. Mol. Immunol. 2021, 137, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Gao, W.; Zhu, Q.; Alasbahi, A.; Seki, E.; Yang, L. TAK1: A Molecular link between liver inflammation, fibrosis, steatosis, and carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 734749. [Google Scholar] [CrossRef]
- Kaminska, B.; Wesolowska, A.; Danilkiewicz, M. TGF-β signalling and its role in tumour pathogenesis. Acta Biochim. Pol. 2005, 52, 329–337. [Google Scholar] [CrossRef]
- Li, J.; Liang, C.; Zhang, Z.K.; Pan, X.; Peng, S.; Lee, W.S.; Lu, A.; Lin, Z.; Zhang, G.; Leung, W.N.; et al. TAK1 inhibition attenuates both inflammation and fibrosis in experimental pneumoconiosis. Cell Discov. 2017, 3, 17023. [Google Scholar] [CrossRef] [PubMed]
- Shi-wen, X.; Parapuram, S.K.; Pala, D.; Chen, Y.; Carter, D.E.; Eastwood, M.; Denton, C.P.; Abraham, D.J.; Leask, A. Requirement of transforming growth factor β-activated kinase 1 for transforming growth factor β-induced α-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009, 60, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Togo, S.; Kadoya, K.; Tulafu, M.; Namba, Y.; Iwai, M.; Watanabe, J.; Nagahama, K.; Okabe, T.; Hidayat, M.; et al. Pirfenidone attenuates lung fibrotic fibroblast responses to transforming growth factor-β1. Respir. Res. 2019, 20, 119. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, Y.; Qin, Y.; Yu, L.; Li, R.; Chen, Y.; Xu, Y. Quercetin intervention alleviates offspring’s oxidative stress, inflammation, and tight junction damage in the colon induced by maternal fine particulate matter (PM2.5) exposure through the reduction of Bacteroides. Nutrients 2020, 12, 3095. [Google Scholar] [CrossRef]
- Mao, M.; Li, J.; Bi, A.; Jia, H.; Li, Q.; Liu, Y.; Jiang, X.; Huang, D.; Xia, S. Thymoquinone ameliorates the PM2.5-induced lung injury in rats. Exp. Lung Res. 2020, 46, 297–307. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Tian, Y.; Hu, X. The Lung Microbiota Affects Pulmonary Inflammation and Oxidative Stress Induced by PM2.5 Exposure. Environ. Sci. Technol. 2022, 56, 12368–12379. [Google Scholar] [CrossRef]
- Ko, I.G.; Hwang, J.J.; Chang, B.S.; Kim, S.H.; Jin, J.J.; Hwang, L.; Kim, C.J.; Choi, C.W. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced acute lung injury via modulation of the MAPK/NF-κB signaling pathway in rats. Int. Immunopharmacol. 2020, 83, 106444. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Dong, L.; Li, L. Lats2-underexpressing bone marrow-derived mesenchymal stem cells ameliorate LPS-induced acute lung injury in mice. Mediat. Inflamm. 2019, 21, 4851431. [Google Scholar] [CrossRef]
- Williams, K.J.; Robinson, N.E.; Lim, A.; Brandenberger, C.; Maes, R.; Behan, A.; Bolin, S.R. Experimental induction of pulmonary fibrosis in horses with the gammaherpesvirus equine herpesvirus 5. PLoS ONE 2013, 8, e77754. [Google Scholar] [CrossRef]
- Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, S.H.; Kim, C.J.; Won, K.Y.; Na, Y.G.; Kim, K.H.; Kim, S.J. Adenosine A2A receptor agonist polydeoxyribonucleotide alleviates interstitial cystitis-induced voiding dysfunction by suppressing inflammation and apoptosis in rats. J. Inflamm. Res. 2021, 14, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, S.; Ma, X.; Li, R.; Zhang, H.; Li, J.; Yan, X. MiR-217-5p inhibits smog (PM2.5)-induced inflammation and oxidative stress response of mouse lung tissues and macrophages through targeting STAT1. Aging 2022, 14, 6796–6808. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Ansari, M.A.; Zoheir, K.M.; Bakheet, S.A.; Korashy, H.M.; Nadeem, A.; Ashour, A.E.; Attia, S.M. Regulation of TNF-α and NF-κB activation through the JAK/STAT signaling pathway downstream of histamine 4 receptor in a rat model of LPS-induced joint inflammation. Immunobiology 2015, 220, 889–898. [Google Scholar] [CrossRef]
- Valderrama, A.; Ortiz-Hernández, P.; Agraz-Cibrián, J.M.; Tabares-Guevara, J.H.; Gómez, D.M.; Zambrano-Zaragoza, J.F.; Taborda, N.A.; Hernandez, J.C. Particulate matter (PM10) induces in vitro activation of human neutrophils, and lung histopathological alterations in a mouse model. Sci. Rep. 2022, 9, 7581. [Google Scholar] [CrossRef]
- Park, S.Y.; An, K.S.; Lee, B.; Kang, J.H.; Jung, H.J.; Kim, M.W.; Ryu, H.Y.; Shim, K.S.; Nam, K.T.; Yoon, Y.S.; et al. Establishment of particulate matter-induced lung injury model in mouse. Lab. Anim. Res. 2021, 30, 20. [Google Scholar] [CrossRef]
- Han, H.; Oh, E.Y.; Lee, J.H.; Park, J.W.; Park, H.J. Effects of Particulate Matter 10 Inhalation on Lung Tissue RNA expression in a Murine Model. Tuberc. Respir. Dis. 2021, 84, 55–66. [Google Scholar] [CrossRef]
- Hirano, A.; Kanehiro, A.; Ono, K.; Ito, W.; Yoshida, A.; Okada, C.; Nakashima, H.; Tanimoto, Y.; Kataoka, M.; Gelfand, E.W.; et al. Pirfenidone modulates airway responsiveness, inflammation, and remodeling after repeated challenge. Am. J. Respir. Cell Mol. Biol. 2006, 35, 366–377. [Google Scholar] [CrossRef]
- Oku, H.; Nakazato, H.; Horikawa, T.; Tsuruta, Y.; Suzuki, R. Pirfenidone suppresses tumor necrosis factor-α, enhances interleukin-10 and protects mice from endotoxic shock. Eur. J. Pharmacol. 2002, 446, 167–176. [Google Scholar] [CrossRef]
- Visner, G.A.; Liu, F.; Bizargity, P.; Liu, H.; Liu, K.; Yang, J.; Wang, L.; Hancock, W.W. Pirfenidone inhibits T-cell activation, proliferation, cytokine and chemokine production, and host alloresponses. Transplantation 2009, 88, 330–338. [Google Scholar] [CrossRef]
- Jia, Q.; Li, Q.; Wang, Y.; Zhao, J.; Jiang, Q.; Wang, H.; Xue, W.; Zhu, Z.; Tian, L. Lung microbiome and transcriptome reveal mechanisms underlying PM2.5 induced pulmonary fibrosis. Sci. Total Environ. 2022, 831, 154974. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Liao, Z.; Gao, S.; Hua, L.; Ye, X.; Wang, Y.; Jiang, S.; Wang, N.; Zhou, D.; et al. PM2.5 induced pulmonary fibrosis in vivo and in vitro. Ecotoxicol. Environ. Saf. 2019, 171, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.P.; Merryman, M.S.; Harris-Arnold, A.; McKinney, S.A.; Seidel, C.W.; Loethen, S.; Proctor, K.N.; Guo, L.; Sánchez Alvarado, A. Pathogenic shifts in endogenous microbiota impede tissue regeneration via distinct activation of TAK1/MKK/p38. Elife 2016, 5, e16793. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Deng, T.; Huo, T.; Dong, F.; Deng, J. MiR-140-5p/TLR4/NF-κB signaling pathway: Crucial role in inflammatory response in 16HBE cells induced by dust fall PM2.5. Ecotoxicol. Environ. Saf. 2021, 208, 111414. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, X.; Wang, Y.; Wang, J.; Zhou, F.; Wang, H.; Xie, W.; Kong, H. NLRP3 inflammasome inhibition attenuates silica-induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells. Exp. Cell Res. 2018, 362, 489–497. [Google Scholar] [CrossRef]
- Ono, K.; Ohtomo, T.; Ninomiya-Tsuji, J.; Tsuchiya, M. A dominant negative TAK1 inhibits cellular fibrotic responses induced by TGF-β. Biochem. Biophys. Res. Commun. 2003, 307, 332–337. [Google Scholar] [CrossRef]
- Kim, S.I.; Kwak, J.H.; Zachariah, M.; He, Y.; Wang, L.; Choi, M.E. TGF-β-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-β1-induced MKK3-p38 MAPK activation and stimulation of type I collagen. Am. J. Physiol. Renal. Physiol. 2007, 292, 1471–1478. [Google Scholar] [CrossRef]
- Hwang, J.J.; Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, S.H.; Jeon, J.W.; Paik, S.S.; Chang, B.S.; Choi, C.W. Combination therapy with polydeoxyribonucleotide and pirfenidone alleviates symptoms of acute respiratory distress syndrome in human lung epithelial A549 cells. Int. Neurourol. J. 2020, 24, S56–S64. [Google Scholar] [CrossRef]
- Okano, T.; Kobayashi, T.; Yasuma, T.; D’Alessandro-Gabazza, C.N.; Toda, M.; Fujimoto, H.; Nakahara, H.; Okano, Y.; Takeshita, A.; Nishihama, K.; et al. Low-dose of intrapulmonary pirfenidone improves human transforming growth factor-β1-driven lung fibrosis. Front. Pharmacol. 2020, 11, 593620. [Google Scholar] [CrossRef]
- Wu, S.B.; Hou, T.Y.; Kau, H.C.; Tsai, C.C. Effect of pirfenidone on TGF-β1-induced myofibroblast differentiation and extracellular matrix homeostasis of human orbital fibroblasts in Graves’ ophthalmopathy. Biomolecules 2021, 11, 1424. [Google Scholar] [CrossRef]



| Classification | Items | Source | Titer | Company |
|---|---|---|---|---|
| Primary antibody | TAK1, p-MKK3, MKK3, p38, p-p38 | Anti-rabbit | 1:1000 | Cell Signaling Technology, Danvers, MA, USA |
| β-actin | Anti-mouse | 1:1000 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | |
| Secondary antibody | HRP-conjugated IgG | Mouse | 1:2000 | Vector Laboratories, Burlingame, CA, USA |
| Rabbit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.-S.; Ko, I.-G.; Hwang, L.; Kim, S.-H.; Han, J.H.; Jeon, J.W.; Kim, S.R.; Lee, J.M.; Choi, C.W. Pirfenidone Alleviates Against Fine Particulate Matter-Induced Pulmonary Fibrosis Modulating via TGF-β1/TAK1/MKK3/p38 MAPK Signaling Pathway in Rats. Biomedicines 2025, 13, 989. https://doi.org/10.3390/biomedicines13040989
Sung J-S, Ko I-G, Hwang L, Kim S-H, Han JH, Jeon JW, Kim SR, Lee JM, Choi CW. Pirfenidone Alleviates Against Fine Particulate Matter-Induced Pulmonary Fibrosis Modulating via TGF-β1/TAK1/MKK3/p38 MAPK Signaling Pathway in Rats. Biomedicines. 2025; 13(4):989. https://doi.org/10.3390/biomedicines13040989
Chicago/Turabian StyleSung, Jun-Seok, Il-Gyu Ko, Lakkyong Hwang, Sang-Hoon Kim, Jin Hee Han, Jung Won Jeon, Sae Rom Kim, Jeong Mi Lee, and Cheon Woong Choi. 2025. "Pirfenidone Alleviates Against Fine Particulate Matter-Induced Pulmonary Fibrosis Modulating via TGF-β1/TAK1/MKK3/p38 MAPK Signaling Pathway in Rats" Biomedicines 13, no. 4: 989. https://doi.org/10.3390/biomedicines13040989
APA StyleSung, J.-S., Ko, I.-G., Hwang, L., Kim, S.-H., Han, J. H., Jeon, J. W., Kim, S. R., Lee, J. M., & Choi, C. W. (2025). Pirfenidone Alleviates Against Fine Particulate Matter-Induced Pulmonary Fibrosis Modulating via TGF-β1/TAK1/MKK3/p38 MAPK Signaling Pathway in Rats. Biomedicines, 13(4), 989. https://doi.org/10.3390/biomedicines13040989







