Abstract
Background/Objectives: Quantitative sensory testing (QST) is one of the most reliable methods for assessing Fibromyalgia Syndrome (FMS). Despite its importance, there are still controversies regarding the correct interpretation of evoked responses, as they may vary depending on the protocol, individual characteristics, disease severity, and other factors. This study aims to examine how QST has been applied as an outcome measure in FMS. Methods: We considered three databases (Medline, Embase, and Web of Science) until June 2024. From a total of 2512 studies, 126 (39 RCTs and 87 non-RCTs) were selected for full reading after assessment for risk of bias and eligibility criteria. These criteria included at least one type of QST and a clear diagnosis of fibromyalgia (FMS). Results: The results highlighted a lack of standardization in QST, as no reported protocols were followed and there was no specific number of tender points tested for FMS. Additionally, there was inconsistency in the selection of sites and types of tests conducted. Conclusions: This heterogeneity in methodology may affect the comparability and interpretation of results, underscoring the urgent need for standardized guidelines for conducting QST in fibromyalgia studies. A clear understanding of how QST has been measured could prompt a reevaluation of current approaches to FMS assessment, leading to more accurate interpretations and, ultimately, improved management of this complex condition.
1. Introduction
Characterized as a widespread chronic pain syndrome, Fibromyalgia Syndrome (FMS) has a complex multifactorial etiopathogenesis that remains not fully understood [1,2] and affects 3 to 6% of global population [3]. FMS is often associated with impairments in mental health and quality of life [4,5,6,7,8,9].
Since 1980, various FMS diagnostic criteria have been developed to reduce subjective clinical judgment, most notably the American College of Rheumatology (ACR), which consider FMS diagnosis as a combined score of the Widespread Pain Index (WPI) and Symptom Severity Scale (SSS) [5]. In this context, Quantitative Sensory Testing (QST) has emerged to improve the precision of sensory deficit detection in FMS by assessing pain thresholds through a combination of static and dynamic protocols that allows the assessment of pain thresholds through isolated stimuli, measuring hyperalgesia or hypoalgesia in specific areas and the perception of pain [10,11].
QST is based on measurements of responses to calibrated, graded innocuous, or noxious stimuli (generally mechanical or thermal) [12,13,14]. Despite its potential, its implementation can be complex due to cost and protocol selection [15,16]. In FMS, QST protocol variability, combined with individual differences and comorbidities, can hinder the interpretation of evoked responses [17,18]. This lack of standardization impedes understanding, comparison of studies, and development of effective diagnostic and therapeutic strategies [14].
Equivalent difficulties have been observed in other chronic pain conditions, already postulated for previous reviews [18,19]. In brief, the use of QST for painful experiences demonstrated the need for a more standardized approach [18,19]. QST standardization issues, including test site variability and inconsistent definitions, have been reported in other chronic pain conditions like knee osteoarthritis [20] and pediatric populations [21], highlighting the need for consistent protocols to improve reproducibility and clinical applicability [21]. This heterogeneity compromises QST’s potential in chronic pain research, including fibromyalgia [22].
Therefore, despite favorable evidence for QST application, there are no previous studies debating the implications of QST protocols in FMS. In this sense, the current scoping review aims to clarify the complexities and variations inherent in QST methodologies in FMS. By examining QST protocols and identifying factors that influence their reliability as outcome measures, we believe it will be possible to develop more effective approaches for fibromyalgia syndrome (FMS).
2. Materials and Methods
This scoping review protocol was developed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews, PRISMA-ScR [23] (Supplementary Materials Data S1). The protocol was previously registered [24].
2.1. Eligibility Criteria
This study considered studies where participants were adults, both sexes, aged 18+ with a clear diagnosis of Fibromyalgia Syndrome (FMS) considering any of the ACR criteria. As we are looking for Quantitative Sensory Testing-QST measurement, studies with at least one measure of pain threshold or sensitivity (any study design), were selected. We excluded studies if they had less than 50% of the participants with FMS. Duplicates, reviews, and commentaries on findings from other studies or documents that were not the primary research (for example, conference abstracts) were also excluded.
2.2. Search
This review extracted studies from the following databases: Pubmed (n = 508), Embase (n = 817), and Web of Science (n = 1187). We replicated the primary database search terms (Pubmed) for the others (see Supplementary Materials Data S2). The search was not limited by language or year. The search was conducted up to 3 June 2024.
2.3. Selection of Sources of Evidence and Critical Appraisal
The quality of the included studies was assessed by two independent reviewers (AMC and VAS), and disagreements will be solved by a third reviewer (MGS). For the RCTs, the ROB 2 tool was used, and for the non-RCTs, the STROBE (see Supplementary Materials Data S3 and S4).
2.4. Data Charting Process
The Rayyan software (https://www.rayyan.ai/, accessed on 4 April 2025) [25] was used to select studies by title and abstract. It was made by two independent reviewers (AMC and VAS) based on our previously established inclusion/exclusion criteria. The third reviewer (MGS) remained on standby if needed. After a full reading, data were extracted from papers by AMC and VAS using an extraction table developed by the reviewers independently. In cases where it is not possible, we search for the data protocol or other similar studies made by the author/group in order to clarify the information. In our study, it was not necessary to consult the authors.
2.5. Data Items and Synthesis of Results
For extraction, we considered the study design, quality of the study, sample characteristics, age, sex, distribution, inclusion criteria, diagnosis, type of QST (static or dynamic), and methods applied by the studies. We also obtained information from other measurements and main conclusions. The data were tabulated and presented in a narrative way, answering the research objectives.
3. Results
The search (up to June 2024) yielded 2512 records: 1187 from Web of Science, 817 from Embase, and 508 from PubMed. No filters (e.g., article type, species, language, or age) were applied during the search process to avoid unintentionally excluding relevant records. The search query used for each database is detailed in Supplementary Material Data S2. The study selection process is summarized in Figure 1. The characteristics of the included studies are summarized in Table 1.
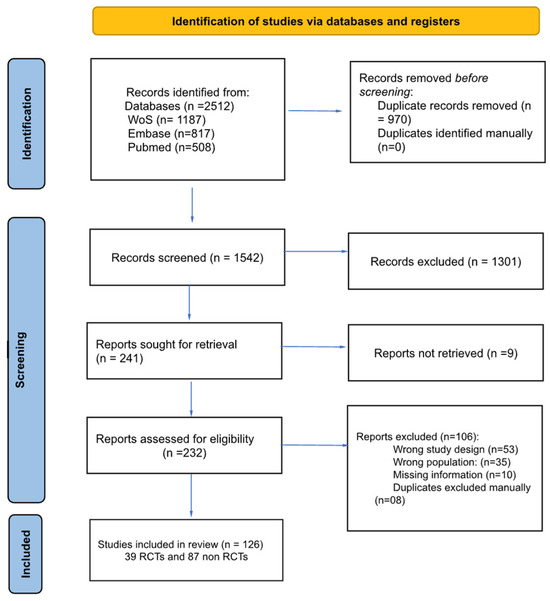
Figure 1.
Study flow chart.

Table 1.
Characterization of the studies using QST methods in patients with FMS from the retrieved studies (n = 126).
3.1. Quality Assessment
Only studies classified as having a low risk of bias or some concerns were considered, and 30 (76.9%) RCTs and 59 (67.81%) non RCT’s studies met this criterion. RCT limitations involved lack of information regarding the original protocol and data analysis plan (Supplementary Material Data S3). In terms of nonRCTs, limitations were related to sample (i.e., recruitment, inclusion, sample size calculation), generalizability and insufficient information regarding sample size and bias (Supplementary Material Data S4).
3.2. Narrative Synthesis of Quantitative Sensory Testing Methods
A total of 42.9% of studies included both static and dynamic QST assessments, offering a comprehensive approach to sensory evaluation. (Table 2). For these studies, we divided our results considering both assessments.

Table 2.
Summary of Static and Dynamic Quantitative Sensory Testing Across Body Locations.
3.3. Static QST
Static QST methods comprised 76% of all assessments. Pressure pain thresholds/tolerances (PPTh/PPT) were the most frequently measured (n = 84), predominantly at 18 tender points (n = 24), hands (n = 17), trapezius (n = 8), and forearm (n = 5), using primarily the Somac algometer (n = 54). Mechanical detection/pain thresholds/sensitivity (MDTh/MPTh/MPS, n = 10) were assessed mainly at the forearm (n = 3) and hands (n = 4), often with Von Frey monofilaments (n = 5). Thermal pain thresholds/tolerances (TPTh/TPT, n = 36) were typically measured at hands (n = 9) and forearm (n = 7), often with the TSA II Medoc.
3.4. Dynamic QST
Dynamic QST methods constituted 24% of assessments. Temporal Summation (TS, n = 15) was primarily assessed at hands (n = 7) and forearm (n = 4). Conditioned pain modulation (CPM, n = 26) was frequently tested at the forearm (n = 12) and hands (n = 4), using PPT as the test stimulus and cold water immersion as the conditioned stimulus.
4. Discussion
This scoping review examined how QST is used in FMS, a complex condition with widespread pain and variable symptom presentation, that per se makes diagnosis and measurement challenging [15,16]. While QST offers a potential surrogate measure to improve pain assessment reliability and validity, and understand neuropathic pain [4,149], this review revealed important methodological issues.
Although static QST is prevalent, variations in body location, stimulus duration, and intensity may affect results. Researchers demonstrated PPT and CPM variability across test points, reflecting altered pain modulation in FMS. Given the diffuse pain and altered sensation characteristic of FMS, QST at remote sites may reinforce information regarding the central nervous system [18,45,104]. The NeuPSIG consensus [14] reinforces the use of multiple test sites, or preferably standardizing test locations in order to improve the accuracy and interpretation by reducing variability and potentially revealing more consistent patterns of somatosensory dysfunction in FMS.
Given the scarcity of studies measuring QST both before and after interventions, QST stability in FMS remains poorly understood. No consistent information regarding the presence of other symptoms was controlled (e.g., sleep disturbances and other non-physical symptoms), neither the impact of psychological factors, the presence of psychopathology or neuropathic pain conditions [14,18,20]. There is an amount of literature available claiming for a more controlled information of those variables, once they are related to the severity of this disease [5,7]. Further research should explore how internal and external factors contribute to FMS progression [110]. Consequently, despite efforts to minimize bias, the generalizability of findings remains limited due to substantial methodological variation.
The diversity in test locations—from high muscle areas (e.g., trapezius, deltoid) to minimal muscle sites (e.g., wrist, thumbnail)—further adds to this heterogeneity [150]. This inconsistency could limit the synthesis of findings across studies and impact the reliability of QST as a biomarker in FMS. Standardization in test locations and stimuli parameters could facilitate future meta-analyses and enhance the clinical applicability of QST.
Furthermore, QST modality definitions might be implicit. For example, while TS and CPM are often used as measures of central sensitization, they also involve the peripheral nervous system–the parameters of the sensitization analysis must be defined to each study. Additional limitations include variability in test parameters (number of tests, duration, rest intervals, stimulus intensity/increment) and equipment. Despite recommended protocols, application remains uncommon, hindering full standardization in this review.
These factors include variations in body location of QST application, differences in stimulus parameters (e.g., duration, intensity, rest intervals), test modality definitions (e.g., TS and CPM interpretation), and equipment used. Each of these methodological differences can introduce heterogeneity between studies. Although standardized protocols have been recommended, our findings show that their consistent application remains uncommon. In our review, even when controlling this information, it was not possible to standardize fully across studies.
Finally, as a strength, this is one of the first studies to recruit the state of the art by considering QST measures in FMS. We hope that this scoping review might be able to summarize the need for a more standardized approach to measuring FMS, particularly considering the complex and unpredictable nature of endogenous pain inhibition mechanisms.
5. Conclusions
While promising for FMS assessment, QST’s potential is hampered by significant lack of information regarding its validity and reliability. Future research should stratify studies by treatment modality (e.g., pharmacological vs. neuromodulatory) to elucidate treatment-specific effects and optimize patient care. Addressing these gaps promises to significantly advance FMS understanding and improve patient outcomes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines13040988/s1. Data S1: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist; Data S2: Search strategies for systematic review. Data S3: Rob2 from the RCT studies included (n = 39). Data S4: Rob2—Individual quality of RCT studies included (n = 39). Data S5: STROBE from nonRCT studies included (n = 93).
Author Contributions
Conceptualization, A.M.C. and V.A.d.S.; methodology, A.M.C. and V.A.d.S.; validation, A.M.C., V.A.d.S. and M.d.G.S.; formal analysis, A.M.C. and V.A.d.S.; investigation, A.M.C. and V.A.d.S.; resources, A.M.C.; data curation, A.M.C. and V.A.d.S.; writing—original draft preparation, A.M.C. and V.A.d.S.; writing—review and editing, A.M.C., V.A.d.S., M.d.G.S. and C.S.D.; visualization, A.M.C. and V.A.d.S.; supervision, M.d.G.S. and C.S.D.; project administration, A.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FMS | Fibromyalgia Syndrome |
| QST | Quantitative Sensory Testing |
| ACR | American College of Rheumatology |
| VAS | Visual Analog Scale |
| PPTh/PPT | Pressure Pain Threshold/Tolerance |
| MDTh/MPTh/MPS | Mechanical Detection/Pain Thresholds/Sensitivity |
| TPTh/TPT | Thermal Pain Threshold/Tolerance |
| TS | Temporal Summation |
| CPM | Conditioned Pain Modulation |
| RCT | Randomized Controlled Trial |
| ROB 2 | Risk of Bias Tool 2 |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
References
- D’Agnelli, S.; Arendt-Nielsen, L.; Gerra, M.C.; Zatorri, K.; Boggiani, L.; Baciarello, M.; Bignami, E. Fibromyalgia: Genetics and Epigenetics Insights May Provide the Basis for the Development of Diagnostic Biomarkers. Mol. Pain 2019, 15, 1744806918819944. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Fang, Y.-H.D.; Jones, C.; McConathy, J.E.; Raman, F.; Lapi, S.E.; Younger, J.W. Evidence of Neuroinflammation in Fibromyalgia Syndrome: A [18F]DPA-714 Positron Emission Tomography Study. Pain 2023, 164, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- The National Fibromyalgia Association, the Best Place for Patients with Chronic Pain. Available online: https://www.fmaware.org/ (accessed on 4 April 2025).
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Beiner, E.; Lucas, V.; Reichert, J.; Buhai, D.-V.; Jesinghaus, M.; Vock, S.; Drusko, A.; Baumeister, D.; Eich, W.; Friederich, H.-C.; et al. Stress Biomarkers in Individuals with Fibromyalgia Syndrome: A Systematic Review with Meta-Analysis. Pain 2023, 164, 1416–1427. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Rodrigues Lacerda, A.C.; Ribeiro, V.G.C.; Scheidt Figueiredo, P.H.; Fonseca, S.F.; da Silva Lage, V.K.; Costa, H.S.; Pereira Lima, V.; Sañudo, B.; Bernardo-Filho, M.; et al. Oxidative Stress Biomarkers and Quality of Life Are Contributing Factors of Muscle Pain and Lean Body Mass in Patients with Fibromyalgia. Biology 2022, 11, 935. [Google Scholar] [CrossRef]
- Telli, H.; Akkus, M. The Interplay of Pain, Emotional Regulation Difficulties, and Social Support in Individuals Diagnosed with Fibromyalgia: Impact on Depression, Anxiety, Functional Disability, and Quality of Life. Psychol. Health Med. 2025, 30, 1–20. [Google Scholar] [CrossRef]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, R.-D.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative Sensory Testing in the German Research Network on Neuropathic Pain (DFNS): Standardized Protocol and Reference Values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef]
- Häuser, W.; Ablin, J.; Fitzcharles, M.-A.; Littlejohn, G.; Luciano, J.V.; Usui, C.; Walitt, B. Fibromyalgia. Nat. Rev. Dis. Primers 2015, 1, 15022. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Graven-Nielsen, T. Translational Musculoskeletal Pain Research. Best. Pract. Res. Clin. Rheumatol. 2011, 25, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, V.; Akin-Akinyosoye, K.; Zhang, W.; McWilliams, D.F.; Hendrick, P.; Walsh, D.A. Quantitative Sensory Testing and Predicting Outcomes for Musculoskeletal Pain, Disability, and Negative Affect: A Systematic Review and Meta-Analysis. Pain 2019, 160, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Backonja, M.M.; Attal, N.; Baron, R.; Bouhassira, D.; Drangholt, M.; Dyck, P.J.; Edwards, R.R.; Freeman, R.; Gracely, R.; Haanpaa, M.H.; et al. Value of Quantitative Sensory Testing in Neurological and Pain Disorders: NeuPSIG Consensus. Pain 2013, 154, 1807–1819. [Google Scholar] [CrossRef]
- da Silva, L.A.; Kazyiama, H.H.S.; Teixeira, M.J.; de Siqueira, S.R.D.T. Quantitative Sensory Testing in Fibromyalgia and Hemisensory Syndrome: Comparison with Controls. Rheumatol. Int. 2013, 33, 2009–2017. [Google Scholar] [CrossRef]
- Hurtig, I.M.; Raak, R.I.; Kendall, S.A.; Gerdle, B.; Wahren, L.K. Quantitative Sensory Testing in Fibromyalgia Patients and in Healthy Subjects: Identification of Subgroups. Clin. J. Pain 2001, 17, 316–322. [Google Scholar] [CrossRef]
- Schmelz, M. What Can We Learn from the Failure of Quantitative Sensory Testing? Pain 2021, 162, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Yarnitsky, D. Experimental and Clinical Applications of Quantitative Sensory Testing Applied to Skin, Muscles and Viscera. J. Pain 2009, 10, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Backonja, M.-M.; Walk, D.; Edwards, R.R.; Sehgal, N.; Moeller-Bertram, T.; Wasan, A.; Irving, G.; Argoff, C.; Wallace, M. Quantitative Sensory Testing in Measurement of Neuropathic Pain Phenomena and Other Sensory Abnormalities. Clin. J. Pain 2009, 25, 641–647. [Google Scholar] [CrossRef]
- Rankin, J.; Rudy-Froese, B.; Hoyt, C.; Ramsahoi, K.; Gareau, L.; Howatt, W.; Carlesso, L. Quantitative Sensory Testing Protocols to Evaluate Central and Peripheral Sensitization in Knee OA: A Scoping Review. Pain Med. 2022, 23, 526–557. [Google Scholar] [CrossRef]
- Tutelman, P.R.; MacKenzie, N.E.; Chambers, C.T.; Coffman, S.; Cornelissen, L.; Cormier, B.; Higgins, K.S.; Phinney, J.; Blankenburg, M.; Walker, S. Quantitative Sensory Testing for Assessment of Somatosensory Function in Children and Adolescents: A Scoping Review. Pain Rep. 2024, 9, e1151. [Google Scholar] [CrossRef]
- Rolke, R.; Magerl, W.; Campbell, K.A.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R.-D. Quantitative Sensory Testing: A Comprehensive Protocol for Clinical Trials. Eur. J. Pain 2006, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.M.; Dalle, C.; Salvetti, M.G.; Silva, V.A. Quantitative Sensory Testing in Fibromyalgia Syndrome: A Scoping Review—Protocol. Open Sci. Framew. 2024. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, J.; Bengtsson, A.; Bäckman, E.; Henriksson, K.G.; Bengtsson, M. Pain Analysis in Patients with Fibromyalgia. Effects of Intravenous Morphine, Lidocaine, and Ketamine. Scand. J. Rheumatol. 1995, 24, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Ekholm, J.; Hansson, P. Sensory Dysfunction in Fibromyalgia Patients with Implications for Pathogenic Mechanisms. Pain 1996, 68, 375–383. [Google Scholar] [CrossRef]
- Ernberg, M.; Hedenberg-Magnusson, B.; Alstergren, P.; Kopp, S. Short-Term Effect of Glucocorticoid Injection into the Superficial Masseter Muscle of Patients with Chronic Myalgia: A Comparison between Fibromyalgia and Localized Myalgia. J. Orofac. Pain 1997, 11, 249–257. [Google Scholar]
- Price, D.D.; Staud, R.; Robinson, M.E.; Mauderli, A.P.; Cannon, R.; Vierck, C.J. Enhanced Temporal Summation of Second Pain and Its Central Modulation in Fibromyalgia Patients. Pain 2002, 99, 49–59. [Google Scholar] [CrossRef]
- Desmeules, J.A.; Cedraschi, C.; Rapiti, E.; Baumgartner, E.; Finckh, A.; Cohen, P.; Dayer, P.; Vischer, T.L. Neurophysiologic Evidence for a Central Sensitization in Patients with Fibromyalgia. Arthritis Rheum. 2003, 48, 1420–1429. [Google Scholar] [CrossRef]
- Staud, R.; Cannon, R.C.; Mauderli, A.P.; Robinson, M.E.; Price, D.D.; Vierck, C.J., Jr. Temporal Summation of Pain from Mechanical Stimulation of Muscle Tissue in Normal Controls and Subjects with Fibromyalgia Syndrome. Pain 2003, 102, 87–95. [Google Scholar] [CrossRef]
- Ernberg, M.; Lundeberg, T.; Kopp, S. Effects on Muscle Pain by Intramuscular Injection of Granisetron in Patients with Fibromyalgia. Pain 2003, 101, 275–282. [Google Scholar] [CrossRef]
- Kendall, S.A.; Henriksson, K.G.; Hurtig, I.; Raak, R.; Bengtsson, A.; Sören, B.; Wahren, L.-K.; Gerdle, B. Differences in Sensory Thresholds in the Skin of Women with Fibromyalgia Syndrome: A Comparison between Ketamine Responders and Ketamine Non-Responders. J. Musculoskelet. Pain 2003, 11, 3–9. [Google Scholar] [CrossRef]
- Yildiz, S.; Kiralp, M.Z.; Akin, A.; Keskin, I.; Ay, H.; Dursun, H.; Cimsit, M. A New Treatment Modality for Fibromyalgia Syndrome: Hyperbaric Oxygen Therapy. J. Int. Med. Res. 2004, 32, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Vierck, C.J.; Robinson, M.E.; Price, D.D. Spatial Summation of Heat Pain within and across Dermatomes in Fibromyalgia Patients and Pain-Free Subjects. Pain 2004, 111, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Price, D.D.; Robinson, M.E.; Vierck, C.J., Jr. Body Pain Area and Pain-Related Negative Affect Predict Clinical Pain Intensity in Patients with Fibromyalgia. J. Pain 2004, 5, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, T.; Gracely, R.H.; Williams, D.A.; Geisser, M.E.; Petzke, F.W.; Clauw, D.J. The Relationship between Depression, Clinical Pain, and Experimental Pain in a Chronic Pain Cohort. Arthritis Rheum. 2005, 52, 1577–1584. [Google Scholar] [CrossRef]
- Montoya, P.; Pauli, P.; Batra, A.; Wiedemann, G. Altered Processing of Pain-Related Information in Patients with Fibromyalgia. Eur. J. Pain 2005, 9, 293–303. [Google Scholar] [CrossRef]
- Staud, R.; Robinson, M.E.; Price, D.D. Isometric Exercise Has Opposite Effects on Central Pain Mechanisms in Fibromyalgia Patients Compared to Normal Controls. Pain 2005, 118, 176–184. [Google Scholar] [CrossRef]
- Geisser, M.E.; Gracely, R.H.; Giesecke, T.; Petzke, F.W.; Williams, D.A.; Clauw, D.J. The Association between Experimental and Clinical Pain Measures among Persons with Fibromyalgia and Chronic Fatigue Syndrome. Eur. J. Pain 2007, 11, 202–207. [Google Scholar] [CrossRef]
- Jespersen, A.; Dreyer, L.; Kendall, S.; Graven-Nielsen, T.; Arendt-Nielsen, L.; Bliddal, H.; Danneskiold-Samsoe, B. Computerized Cuff Pressure Algometry: A New Method to Assess Deep-Tissue Hypersensitivity in Fibromyalgia. Pain 2007, 131, 57–62. [Google Scholar] [CrossRef]
- Smith, B.W.; Tooley, E.M.; Montague, E.Q.; Robinson, A.E.; Cosper, C.J.; Mullins, P.G. Habituation and Sensitization to Heat and Cold Pain in Women with Fibromyalgia and Healthy Controls. Pain 2008, 140, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Targino, R.A.; Imamura, M.; Kaziyama, H.H.S.; Souza, L.P.M.; Hsing, W.T.; Furlan, A.D.; Imamura, S.T.; Azevedo Neto, R.S. A Randomized Controlled Trial of Acupuncture Added to Usual Treatment for Fibromyalgia. J. Rehabil. Med. 2008, 40, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Diers, M.; Koeppe, C.; Yilmaz, P.; Thieme, K.; Markela-Lerenc, J.; Schiltenwolf, M.; van Ackern, K.; Flor, H. Pain Ratings and Somatosensory Evoked Responses to Repetitive Intramuscular and Intracutaneous Stimulation in Fibromyalgia Syndrome. J. Clin. Neurophysiol. 2008, 25, 153–160. [Google Scholar] [CrossRef]
- Staud, R.; Craggs, J.G.; Perlstein, W.M.; Robinson, M.E.; Price, D.D. Brain Activity Associated with Slow Temporal Summation of C-Fiber Evoked Pain in Fibromyalgia Patients and Healthy Controls. Eur. J. Pain 2008, 12, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Suman, A.L.; Biagi, B.; Biasi, G.; Carli, G.; Gradi, M.; Prati, E.; Bonifazi, M. One-Year Efficacy of a 3-Week Intensive Multidisciplinary Non-Pharmacological Treatment Program for Fibromyalgia Patients. Clin. Exp. Rheumatol. 2009, 27, 7–14. [Google Scholar]
- Ge, H.-Y.; Nie, H.; Madeleine, P.; Danneskiold-Samsøe, B.; Graven-Nielsen, T.; Arendt-Nielsen, L. Contribution of the Local and Referred Pain from Active Myofascial Trigger Points in Fibromyalgia Syndrome. Pain 2009, 147, 233–240. [Google Scholar] [CrossRef]
- Stening, K.D.; Eriksson, O.; Henriksson, K.G.; Brynhildsen, J.; Lindh-Åstrand, L.; Berg, G.; Hammar, M.; Amandusson, A.; Blomqvist, A. Hormonal Replacement Therapy Does Not Affect Self-Estimated Pain or Experimental Pain Responses in Post-Menopausal Women Suffering from Fibromyalgia: A Double-Blind, Randomized, Placebo-Controlled Trial. Rheumatology 2011, 50, 544–551. [Google Scholar] [CrossRef]
- Nelson, D.V.; Bennett, R.M.; Barkhuizen, A.; Sexton, G.J.; Jones, K.D.; Esty, M.L.; Ochs, L.; Donaldson, C.C.S. Neurotherapy of Fibromyalgia? Pain Med. 2010, 11, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Tastekin, N.; Uzunca, K.; Sut, N.; Birtane, M.; Mercimek, O.B. Discriminative Value of Tender Points in Fibromyalgia Syndrome. Pain Med. 2010, 11, 466–471. [Google Scholar] [CrossRef]
- De Bruijn, S.T.; van Wijck, A.J.M.; Geenen, R.; Snijders, T.J.; van der Meulen, W.J.T.M.; Jacobs, J.W.G.; Veldhuijzen, D.S. Relevance of Physical Fitness Levels and Exercise-Related Beliefs for Self-Reported and Experimental Pain in Fibromyalgia: An Explorative Study. J. Clin. Rheumatol. 2011, 17, 295–301. [Google Scholar] [CrossRef]
- Hassett, A.L.; Epel, E.; Clauw, D.J.; Harris, R.E.; Harte, S.E.; Kairys, A.; Buyske, S.; Williams, D.A. Pain Is Associated with Short Leukocyte Telomere Length in Women with Fibromyalgia. J. Pain 2012, 13, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jauand, M.; Sitges, C.; Rodríguez, V.; Picornell, A.; Ramon, M.; Buskila, D.; Montoya, P. Pain Sensitivity in Fibromyalgia Is Associated with Catechol-O-Methyltransferase (COMT) Gene. Eur. J. Pain 2013, 17, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Paul-Savoie, E.; Marchand, S.; Morin, M.; Bourgault, P.; Brissette, N.; Rattanavong, V.; Cloutier, C.; Bissonnette, A.; Potvin, S. Is the Deficit in Pain Inhibition in Fibromyalgia Influenced by Sleep Impairments? Open Rheumatol. J. 2012, 6, 296–302. [Google Scholar] [CrossRef]
- Hargrove, J.B.; Bennett, R.M.; Simons, D.G.; Smith, S.J.; Nagpal, S.; Deering, D.E. A Randomized Placebo-Controlled Study of Noninvasive Cortical Electrostimulation in the Treatment of Fibromyalgia Patients. Pain Med. 2012, 13, 115–124. [Google Scholar] [CrossRef]
- Hooten, M.W.; Qu, W.; Townsend, C.O.; Judd, J.W. Effects of Strength vs. Aerobic Exercise on Pain Severity in Adults with Fibromyalgia: A Randomized Equivalence Trial. Pain 2012, 153, 915–923. [Google Scholar] [CrossRef]
- Hassett, A.L.; Whibley, D.; Kratz, A.; Williams, D.A. Measures for the Assessment of Pain in Adults. Arthritis Care Res. 2020, 72 (Suppl. 10), 342–357. [Google Scholar] [CrossRef]
- Castro-Sánchez, A.M.; Matarán-Peñarrocha, G.A.; López-Rodríguez, M.M.; Lara-Palomo, I.C.; Arendt-Nielsen, L.; Fernández-de-las-Peñas, C. Gender Differences in Pain Severity, Disability, Depression, and Widespread Pressure Pain Sensitivity in Patients with Fibromyalgia Syndrome without Comorbid Conditions. Pain Med. 2012, 13, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Burgmer, M.; Pfleiderer, B.; Maihöfner, C.; Gaubitz, M.; Wessolleck, E.; Heuft, G.; Pogatzki-Zahn, E. Cerebral Mechanisms of Experimental Hyperalgesia in Fibromyalgia. Eur. J. Pain 2012, 16, 636–647. [Google Scholar] [CrossRef]
- Van Oosterwijck, J.; Meeus, M.; Paul, L.; De Schryver, M.; Pascal, A.; Lambrecht, L.; Nijs, J. Pain Physiology Education Improves Health Status and Endogenous Pain Inhibition in Fibromyalgia: A Double-Blind Randomized Controlled Trial. Clin. J. Pain 2013, 29, 873–882. [Google Scholar] [CrossRef]
- Üçeyler, N.; Zeller, D.; Kahn, A.-K.; Kewenig, S.; Kittel-Schneider, S.; Schmid, A.; Casanova-Molla, J.; Reiners, K.; Sommer, C. Small Fibre Pathology in Patients with Fibromyalgia Syndrome. Brain 2013, 136, 1857–1867. [Google Scholar] [CrossRef]
- Crettaz, B.; Marziniak, M.; Willeke, P.; Young, P.; Hellhammer, D.; Stumpf, A.; Burgmer, M. Stress-Induced Allodynia--Evidence of Increased Pain Sensitivity in Healthy Humans and Patients with Chronic Pain after Experimentally Induced Psychosocial Stress. PLoS ONE 2013, 8, e69460. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, B.; Rivas, P.; Rodero, B.; Quintial, C.; Llorca, J.; González-Gay, M.A. Short-Term Improvement Following Dry Needle Stimulation of Tender Points in Fibromyalgia. Rheumatol. Int. 2014, 34, 861–866. [Google Scholar] [CrossRef]
- Belenguer-Prieto, R.; Morales-Espinoza, E.M.; Martín-González, R.M.; Brito-Zerón, P.; Pastor-Oliver, J.F.; Kostov, B.; Buss, D.; Gómez-Gálvez, C.; Salazar-Cifre, A.; Sisó-Almirall, A.; et al. Specificity and Sensitivity of Objective Tests to Detect Possible Malingering in Fibromyalgia: A Case-Control Study in 211 Spanish Patients. Clin. Exp. Rheumatol. 2013, 31, S86–S93. [Google Scholar]
- Staud, R.; Weyl, E.E.; Riley, J.L., 3rd; Fillingim, R.B. Slow Temporal Summation of Pain for Assessment of Central Pain Sensitivity and Clinical Pain of Fibromyalgia Patients. PLoS ONE 2014, 9, e89086. [Google Scholar] [CrossRef] [PubMed]
- Bokarewa, M.I.; Erlandsson, M.C.; Bjersing, J.; Dehlin, M.; Mannerkorpi, K. Smoking Is Associated with Reduced Leptin and Neuropeptide Y Levels and Higher Pain Experience in Patients with Fibromyalgia. Mediat. Inflamm. 2014, 2014, 627041. [Google Scholar] [CrossRef]
- Castro-Sánchez, A.M.; Aguilar-Ferrándiz, M.E.; Matarán-Peñarrocha, G.A.; Sánchez-Joya, M.D.M.; Arroyo-Morales, M.; Fernández-de-las-Peñas, C. Short-Term Effects of a Manual Therapy Protocol on Pain, Physical Function, Quality of Sleep, Depressive Symptoms, and Pressure Sensitivity in Women and Men with Fibromyalgia Syndrome: A Randomized Controlled Trial. Clin. J. Pain 2014, 30, 589–597. [Google Scholar] [CrossRef]
- Staud, R.; Weyl, E.E.; Bartley, E.; Price, D.D.; Robinson, M.E. Analgesic and Anti-Hyperalgesic Effects of Muscle Injections with Lidocaine or Saline in Patients with Fibromyalgia Syndrome. Eur. J. Pain 2014, 18, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, S.; Crombez, G.; Harrar, V.; Brusselmans, G.; Devulder, J.; Spence, C.; Goubert, L. Fibromyalgia Patients and Controls Are Equally Accurate in Detecting Tactile Stimuli While Observing Another in Pain: An Experimental Study. Atten. Percept. Psychophys. 2014, 76, 2548–2559. [Google Scholar] [CrossRef]
- Staud, R.; Lucas, Y.E.; Price, D.D.; Robinson, M.E. Effects of Milnacipran on Clinical Pain and Hyperalgesia of Patients with Fibromyalgia: Results of a 6-Week Randomized Controlled Trial. J. Pain 2015, 16, 750–759. [Google Scholar] [CrossRef]
- Qin, D.; De Wilde, V.-A.; Masquelier, E. Évaluation quantitative sensorielle thermique auprès de 251 patients atteints du syndrome de fibromyalgie. Douleur Analg. 2015, 28, 179–185. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Ortega, F.B.; Munguía-Izquierdo, D. Association of Cardiorespiratory Fitness with Pressure Pain Sensitivity and Clinical Pain in Women with Fibromyalgia. Rheumatol. Int. 2015, 35, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Loggia, M.L.; Cahalan, C.M.; Harris, R.E.; Beissner, F.; Garcia, R.G.; Kim, H.; Wasan, A.D.; Edwards, R.R.; Napadow, V. The Somatosensory Link in Fibromyalgia: Functional Connectivity of the Primary Somatosensory Cortex Is Altered by Sustained Pain and Is Associated with Clinical/autonomic Dysfunction. Arthritis Rheumatol. 2015, 67, 1395–1405. [Google Scholar] [CrossRef]
- Zamunér, A.R.; Andrade, C.P.; Forti, M.; Marchi, A.; Milan, J.; Avila, M.A.; Catai, A.M.; Porta, A.; Silva, E. Effects of a Hydrotherapy Programme on Symbolic and Complexity Dynamics of Heart Rate Variability and Aerobic Capacity in Fibromyalgia Patients. Clin. Exp. Rheumatol. 2015, 33, S73–S81. [Google Scholar] [PubMed]
- Efrati, S.; Golan, H.; Bechor, Y.; Faran, Y.; Daphna-Tekoah, S.; Sekler, G.; Fishlev, G.; Ablin, J.N.; Bergan, J.; Volkov, O.; et al. Hyperbaric Oxygen Therapy Can Diminish Fibromyalgia Syndrome--Prospective Clinical Trial. PLoS ONE 2015, 10, e0127012. [Google Scholar] [CrossRef]
- Oudejans, L.; He, X.; Niesters, M.; Dahan, A.; Brines, M.; van Velzen, M. Cornea Nerve Fiber Quantification and Construction of Phenotypes in Patients with Fibromyalgia. Sci. Rep. 2016, 6, 23573. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Marchand, S. Pain Facilitation and Pain Inhibition during Conditioned Pain Modulation in Fibromyalgia and in Healthy Controls. Pain 2016, 157, 1704–1710. [Google Scholar] [CrossRef]
- Schoen, C.J.; Ablin, J.N.; Ichesco, E.; Bhavsar, R.J.; Kochlefl, L.; Harris, R.E.; Clauw, D.J.; Gracely, R.H.; Harte, S.E. A Novel Paradigm to Evaluate Conditioned Pain Modulation in Fibromyalgia. J. Pain Res. 2016, 9, 711–719. [Google Scholar]
- Barbero, M.; Fernández-de-Las-Peñas, C.; Palacios-Ceña, M.; Cescon, C.; Falla, D. Pain Extent Is Associated with Pain Intensity but Not with Widespread Pressure or Thermal Pain Sensitivity in Women with Fibromyalgia Syndrome. Clin. Rheumatol. 2017, 36, 1427–1432. [Google Scholar] [CrossRef]
- Forti, M.; Zamunér, A.R.; Andrade, C.P.; Silva, E. Lung Function, Respiratory Muscle Strength, and Thoracoabdominal Mobility in Women With Fibromyalgia Syndrome. Respir. Care 2016, 61, 1384–1390. [Google Scholar] [CrossRef]
- Gómez-Perretta, C.; Triñanes, Y.; González-Villar, A.J.; Carrillo-de-la-Peña, M.T. Evaluation of the Accuracy of Several Symptoms and Domains in Distinguishing Patients Diagnosed with Fibromyalgia from Healthy Controls. Clin. Exp. Rheumatol. 2016, 34, S14–S25. [Google Scholar]
- Saral, I.; Sindel, D.; Esmaeilzadeh, S.; Sertel-Berk, H.O.; Oral, A. The Effects of Long- and Short-Term Interdisciplinary Treatment Approaches in Women with Fibromyalgia: A Randomized Controlled Trial. Rheumatol. Int. 2016, 36, 1379–1389. [Google Scholar] [CrossRef]
- Mendonca, M.E.; Simis, M.; Grecco, L.C.; Battistella, L.R.; Baptista, A.F.; Fregni, F. Transcranial Direct Current Stimulation Combined with Aerobic Exercise to Optimize Analgesic Responses in Fibromyalgia: A Randomized Placebo-Controlled Clinical Trial. Front. Hum. Neurosci. 2016, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Luciano, J.V.; Forero, C.G.; Cerdà-Lafont, M.; Peñarrubia-María, M.T.; Fernández-Vergel, R.; Cuesta-Vargas, A.I.; Ruíz, J.M.; Rozadilla-Sacanell, A.; Sirvent-Alierta, E.; Santo-Panero, P.; et al. Functional Status, Quality of Life, and Costs Associated With Fibromyalgia Subgroups: A Latent Profile Analysis. Clin. J. Pain 2016, 32, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, A.; Eich, W.; Treede, R.-D.; Tesarz, J. Conditioned Pain Modulation in Patients with Nonspecific Chronic Back Pain with Chronic Local Pain, Chronic Widespread Pain, and Fibromyalgia. Pain 2017, 158, 430. [Google Scholar] [CrossRef] [PubMed]
- de la Coba, P.; Bruehl, S.; Moreno-Padilla, M.; Reyes Del Paso, G.A. Responses to Slowly Repeated Evoked Pain Stimuli in Fibromyalgia Patients: Evidence of Enhanced Pain Sensitization. Pain Med. 2017, 18, 1778–1786. [Google Scholar] [CrossRef]
- de Abreu Freitas, R.P.; de Andrade, S.C.; Spyrides, M.H.C.; Micussi, M.T.A.B.C.; de Sousa, M.B.C. Impacts of Social Support on Symptoms in Brazilian Women with Fibromyalgia. Rev. Bras. Reumatol. 2016, 57, 197–203. [Google Scholar] [CrossRef]
- Baumueller, E.; Winkelmann, A.; Irnich, D.; Weigl, M. Electromyogram Biofeedback in Patients with Fibromyalgia: A Randomized Controlled Trial. Complement. Med. Res. 2017, 24, 33–39. [Google Scholar] [CrossRef]
- Harper, D.E.; Ichesco, E.; Schrepf, A.; Hampson, J.P.; Clauw, D.J.; Schmidt-Wilcke, T.; Harris, R.E.; Harte, S.E. Resting Functional Connectivity of the Periaqueductal Gray Is Associated With Normal Inhibition and Pathological Facilitation in Conditioned Pain Modulation. J. Pain 2018, 19, 635.e1–635.e15. [Google Scholar] [CrossRef]
- Pickering, G.; Macian, N.; Delage, N.; Picard, P.; Cardot, J.-M.; Sickout-Arondo, S.; Giron, F.; Dualé, C.; Pereira, B.; Marcaillou, F. Milnacipran Poorly Modulates Pain in Patients Suffering from Fibromyalgia: A Randomized Double-Blind Controlled Study. Drug Des. Dev. Ther. 2018, 12, 2485–2496. [Google Scholar] [CrossRef]
- Merriwether, E.N.; Frey-Law, L.A.; Rakel, B.A.; Zimmerman, M.B.; Dailey, D.L.; Vance, C.G.T.; Golchha, M.; Geasland, K.M.; Chimenti, R.; Crofford, L.J.; et al. Physical Activity Is Related to Function and Fatigue but Not Pain in Women with Fibromyalgia: Baseline Analyses from the Fibromyalgia Activity Study with TENS (FAST). Arthritis Res. Ther. 2018, 20, 199. [Google Scholar] [CrossRef]
- Wodehouse, T.; Poply, K.; Ramaswamy, S.; Snidvongs, S.; Bourke, J.; Tahir, H.; Ullrich, K.; Mehta, V. A Pilot Study Investigating Whether Quantitative Sensory Testing Alters after Treatment in Patients with Fibromyalgia. Br. J. Pain 2018, 12, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.; Jäkel, A.; Wellmann, K.; von Hehn, U.; Schmidt, T. Effectiveness of 2 Osteopathic Treatment Approaches on Pain, Pressure-Pain Threshold, and Disease Severity in Patients with Fibromyalgia: A Randomized Controlled Trial. Complement. Med. Res. 2018, 25, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Sánchez, C.M.; Muñoz Ladrón de Guevara, C.; Montoro, C.I.; Fernández-Serrano, M.J.; Duschek, S.; Reyes Del Paso, G.A. Cognitive Deficits in Fibromyalgia Syndrome Are Associated with Pain Responses to Low Intensity Pressure Stimulation. PLoS ONE 2018, 13, e0201488. [Google Scholar] [CrossRef] [PubMed]
- Eken, A.; Gökçay, D.; Yılmaz, C.; Baskak, B.; Baltacı, A.; Kara, M. Association of Fine Motor Loss and Allodynia in Fibromyalgia: An fNIRS Study. J. Mot. Behav. 2018, 50, 664–676. [Google Scholar] [CrossRef]
- de la Coba, P.; Bruehl, S.; Galvez-Sánchez, C.M.; Reyes Del Paso, G.A. Slowly Repeated Evoked Pain as a Marker of Central Sensitization in Fibromyalgia: Diagnostic Accuracy and Reliability in Comparison with Temporal Summation of Pain. Psychosom. Med. 2018, 80, 573–580. [Google Scholar] [CrossRef]
- Evdokimov, D.; Frank, J.; Klitsch, A.; Unterecker, S.; Warrings, B.; Serra, J.; Papagianni, A.; Saffer, N.; Meyer Zu Altenschildesche, C.; Kampik, D.; et al. Reduction of Skin Innervation Is Associated with a Severe Fibromyalgia Phenotype. Ann. Neurol. 2019, 86, 504–516. [Google Scholar] [CrossRef]
- Brietzke, A.P.; Antunes, L.C.; Carvalho, F.; Elkifury, J.; Gasparin, A.; Sanches, P.R.S.; da Silva Junior, D.P.; Dussán-Sarria, J.A.; Souza, A.; da Silva Torres, I.L.; et al. Potency of Descending Pain Modulatory System Is Linked with Peripheral Sensory Dysfunction in Fibromyalgia: An Exploratory Study. Medicine 2019, 98, e13477. [Google Scholar] [CrossRef]
- Amer-Cuenca, J.J.; Pecos-Martín, D.; Martínez-Merinero, P.; Lluch Girbés, E.; Nijs, J.; Meeus, M.; Ferrer Peña, R.; Fernández-Carnero, J. How Much Is Needed? Comparison of the Effectiveness of Different Pain Education Dosages in Patients with Fibromyalgia. Pain Med. 2020, 21, 782–793. [Google Scholar] [CrossRef]
- van de Donk, T.; van Velzen, M.; Dahan, A.; Niesters, M. Cornea Nerve Fibre State Determines Analgesic Response to Tapentadol in Fibromyalgia Patients without Effective Endogenous Pain Modulation. Eur. J. Pain 2019, 23, 1586–1595. [Google Scholar] [CrossRef]
- Andrade, C.P.; Zamunér, A.R.; Forti, M.; Tamburús, N.Y.; Silva, E. Effects of Aquatic Training and Detraining on Women with Fibromyalgia: Controlled Randomized Clinical Trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 79–88. [Google Scholar] [CrossRef]
- Udina-Cortés, C.; Fernández-Carnero, J.; Romano, A.A.; Cuenca-Zaldívar, J.N.; Villafañe, J.H.; Castro-Marrero, J.; Alguacil-Diego, I.M. Effects of Neuro-Adaptive Electrostimulation Therapy on Pain and Disability in Fibromyalgia: A Prospective, Randomized, Double-Blind Study. Medicine 2020, 99, e23785. [Google Scholar] [CrossRef]
- Uygur-Kucukseymen, E.; Castelo-Branco, L.; Pacheco-Barrios, K.; Luna-Cuadros, M.A.; Cardenas-Rojas, A.; Giannoni-Luza, S.; Zeng, H.; Gianlorenco, A.C.; Gnoatto-Medeiros, M.; Shaikh, E.S.; et al. Decreased Neural Inhibitory State in Fibromyalgia Pain: A Cross-Sectional Study. Neurophysiol. Clin. 2020, 50, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Kaziyama, H.; Barbour, J.; Galhardoni, R.; Aparecida da Silva, V.; R D Tesseroli de Siqueira, S.; Listik, C.; Dos Santos, G.J.; Yeng, L.T.; Marcolin, M.A.; Raicher, I.; et al. Sifting the Wheat from the Chaff? Evidence for the Existence of an Asymmetric Fibromyalgia Phenotype. Eur. J. Pain 2020, 24, 1635–1647. [Google Scholar] [CrossRef]
- Pickering, G.; Achard, A.; Corriger, A.; Sickout-Arondo, S.; Macian, N.; Leray, V.; Lucchini, C.; Cardot, J.-M.; Pereira, B. Electrochemical Skin Conductance and Quantitative Sensory Testing on Fibromyalgia. Pain Pract. 2020, 20, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, C.V.M.; Moon, S.; Pfeifer, T.; Smirnova, I.V.; Colgrove, Y.; Lai, S.M.; Liu, W. The Therapeutic Efficacy of Qigong Exercise on the Main Symptoms of Fibromyalgia: A Pilot Randomized Clinical Trial. Integr. Med. Res. 2020, 9, 100416. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.L.K.; Berssaneti, A.A.; Marques, A.P. Effects of Shiatsu in the Management of Fibromyalgia Symptoms: A Controlled Pilot Study. J. Manip. Physiol. Ther. 2013, 36, 436–443. [Google Scholar] [CrossRef]
- Han, C.-L.; Sheng, Y.-C.; Wang, S.-Y.; Chen, Y.-H.; Kang, J.-H. Serum Proteome Profiles Revealed Dysregulated Proteins and Mechanisms Associated with Fibromyalgia Syndrome in Women. Sci. Rep. 2020, 10, 12347. [Google Scholar] [CrossRef]
- Izquierdo-Alventosa, R.; Inglés, M.; Cortés-Amador, S.; Gimeno-Mallench, L.; Chirivella-Garrido, J.; Kropotov, J.; Serra-Añó, P. Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3634. [Google Scholar] [CrossRef]
- Rehm, S.; Sachau, J.; Hellriegel, J.; Forstenpointner, J.; Børsting Jacobsen, H.; Harten, P.; Gierthmühlen, J.; Baron, R. Pain Matters for Central Sensitization: Sensory and Psychological Parameters in Patients with Fibromyalgia Syndrome. Pain Rep. 2021, 6, e901. [Google Scholar] [CrossRef]
- Falaguera-Vera, F.J.; Garcia-Escudero, M.; Bonastre-Férez, J.; Zacarés, M.; Oltra, E. Pressure Point Thresholds and ME/CFS Comorbidity as Indicators of Patient’s Response to Manual Physiotherapy in Fibromyalgia. Int. J. Environ. Res. Public Health 2020, 17, 8044. [Google Scholar] [CrossRef]
- Staud, R.; Godfrey, M.M.; Robinson, M.E. Fibromyalgia Patients Are Not Only Hypersensitive to Painful Stimuli But Also to Acoustic Stimuli. J. Pain 2021, 22, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Jamison, R.N.; Curran, S.; Wan, L.; Ross, E.L.; Gilligan, C.J.; Edwards, R.R. Higher Pain Sensitivity Predicts Efficacy of a Wearable Transcutaneous Electrical Nerve Stimulation Device for Persons with Fibromyalgia: A Randomized Double-Blind Sham-Controlled Trial. Neuromodulation 2022, 25, 1410–1420. [Google Scholar] [CrossRef]
- Jamison, R.N.; Edwards, R.R.; Curran, S.; Wan, L.; Ross, E.L.; Gilligan, C.J.; Gozani, S.N. Effects of Wearable Transcutaneous Electrical Nerve Stimulation on Fibromyalgia: A Randomized Controlled Trial. J. Pain Res. 2021, 14, 2265–2282. [Google Scholar] [CrossRef] [PubMed]
- Soldatelli, M.D.; Siepmann, T.; Illigens, B.M.-W.; Souza Dos Santos, V.; Lucena da S Torres, I.; Fregni, F.; Caumo, W. Mapping of Predictors of the Disengagement of the Descending Inhibitory Pain Modulation System in Fibromyalgia: An Exploratory Study. Br. J. Pain 2021, 15, 221–233. [Google Scholar] [CrossRef]
- Karamanlioglu, D.S.; Geler Kulcu, D.; Ozturk, G.; Akpinar, P.; Unlu Ozkan, F.; Aktas, I. Effectiveness of Pregabalin Treatment for Trigger Points in Patients with Comorbid Myofascial Pain Syndrome and Fibromyalgia Syndrome: A Randomized Controlled Trial. Somatosens. Mot. Res. 2021, 38, 327–332. [Google Scholar] [CrossRef]
- Izquierdo-Alventosa, R.; Inglés, M.; Cortés-Amador, S.; Gimeno-Mallench, L.; Sempere-Rubio, N.; Serra-Añó, P. Effectiveness of High-Frequency Transcranial Magnetic Stimulation and Physical Exercise in Women with Fibromyalgia: A Randomized Controlled Trial. Phys. Ther. 2021, 101, pzab159. [Google Scholar] [CrossRef]
- van Campen, C.L.; Rowe, P.; Verheugt, F.; Visser, F. Orthostatic Stress Testing in Myalgic Encephalomyelitis/chronic Fatigue Syndrome (ME/CFS) Patients with or without Concomitant Fibromyalgia: Effects on Pressure Pain Thresholds and Temporal Summation. Authorea 2020. [Google Scholar]
- Weber, T.; Tatzl, E.; Kashofer, K.; Holter, M.; Trajanoski, S.; Berghold, A.; Heinemann, A.; Holzer, P.; Herbert, M.K. Fibromyalgia-Associated Hyperalgesia Is Related to Psychopathological Alterations but Not to Gut Microbiome Changes. PLoS ONE 2022, 17, e0274026. [Google Scholar] [CrossRef]
- Pacheco-Barrios, K.; Lima, D.; Pimenta, D.; Slawka, E.; Navarro-Flores, A.; Parente, J.; Rebello-Sanchez, I.; Cardenas-Rojas, A.; Gonzalez-Mego, P.; Castelo-Branco, L.; et al. Motor Cortex Inhibition as a Fibromyalgia Biomarker: A Meta-Analysis of Transcranial Magnetic Stimulation Studies. Brain Netw. Modul. 2022, 1, 88–101. [Google Scholar] [CrossRef]
- de Paula, T.M.H.; Castro, M.S.; Medeiros, L.F.; Paludo, R.H.; Couto, F.F.; da Costa, T.R.; Fortes, J.P.; de Oliveira Salbego, M.; Behnck, G.S.; de Moura, T.A.M.; et al. Association of Low-Dose Naltrexone and Transcranial Direct Current Stimulation in Fibromyalgia: A Randomized, Double-Blinded, Parallel Clinical Trial. Braz. J. Anesthesiol. 2023, 73, 409–417. [Google Scholar] [CrossRef]
- Tour, J.; Sandström, A.; Kadetoff, D.; Schalling, M.; Kosek, E. The OPRM1 Gene and Interactions with the 5-HT1a Gene Regulate Conditioned Pain Modulation in Fibromyalgia Patients and Healthy Controls. PLoS ONE 2022, 17, e0277427. [Google Scholar] [CrossRef] [PubMed]
- Serrano, P.V.; Zortea, M.; Alves, R.L.; Beltran, G.; Deliberali, C.B.; Maule, A.; Torres, I.L.S.; Fregni, F.; Caumo, W. Association between Descending Pain Modulatory System and Cognitive Impairment in Fibromyalgia: A Cross-Sectional Exploratory Study. Front. Behav. Neurosci. 2022, 16, 917554. [Google Scholar] [CrossRef] [PubMed]
- Alsouhibani, A.; Hoeger Bement, M. Impaired Conditioned Pain Modulation Was Restored after a Single Exercise Session in Individuals with and without Fibromyalgia. Pain. Rep. 2022, 7, e996. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Franco, Á.; da Silveira Alves, C.F.; Vicuña, P.; Bandeira, J.; de Aratanha, M.A.; Torres, I.L.S.; Fregni, F.; Caumo, W. Hyper-Connectivity between the Left Motor Cortex and Prefrontal Cortex Is Associated with the Severity of Dysfunction of the Descending Pain Modulatory System in Fibromyalgia. PLoS ONE 2022, 17, e0247629. [Google Scholar] [CrossRef]
- Samartin-Veiga, N.; Pidal-Miranda, M.; González-Villar, A.J.; Bradley, C.; Garcia-Larrea, L.; O’Brien, A.T.; Carrillo-de-la-Peña, M.T. Transcranial Direct Current Stimulation of 3 Cortical Targets Is No More Effective than Placebo as Treatment for Fibromyalgia: A Double-Blind Sham-Controlled Clinical Trial. Pain 2022, 163, e850–e861. [Google Scholar] [CrossRef]
- Lin, A.P.; Chiu, C.-C.; Chen, S.-C.; Huang, Y.-J.; Lai, C.-H.; Kang, J.-H. Using High-Definition Transcranial Alternating Current Stimulation to Treat Patients with Fibromyalgia: A Randomized Double-Blinded Controlled Study. Life 2022, 12, 1364. [Google Scholar] [CrossRef]
- Castelo-Branco, L.; Cardenas-Rojas, A.; Rebello-Sanchez, I.; Pacheco-Barrios, K.; de Melo, P.S.; Gonzalez-Mego, P.; Marduy, A.; Vasquez-Avila, K.; Costa Cortez, P.; Parente, J.; et al. Temporal Summation in Fibromyalgia Patients: Comparing Phasic and Tonic Paradigms. Front. Pain Res. 2022, 3, 881543. [Google Scholar] [CrossRef]
- Berwick, R.J.; Andersson, D.A.; Goebel, A.; Marshall, A. Aftersensations and Lingering Pain After Examination in Patients with Fibromyalgia Syndrome. Pain Med. 2022, 23, 1928–1938. [Google Scholar] [CrossRef]
- Fanton, S.; Sandström, A.; Tour, J.; Kadetoff, D.; Schalling, M.; Jensen, K.B.; Sitnikov, R.; Ellerbrock, I.; Kosek, E. The Translocator Protein Gene Is Associated with Endogenous Pain Modulation and the Balance between Glutamate and γ-Aminobutyric Acid in Fibromyalgia and Healthy Subjects: A Multimodal Neuroimaging Study. Pain 2022, 163, 274–286. [Google Scholar] [CrossRef]
- Ablin, J.N.; Lang, E.; Catalogna, M.; Aloush, V.; Hadanny, A.; Doenyas-Barak, K.; Finci, S.; Polak, N.; Fishlev, G.; Korin, C.; et al. Hyperbaric Oxygen Therapy Compared to Pharmacological Intervention in Fibromyalgia Patients Following Traumatic Brain Injury: A Randomized, Controlled Trial. PLoS ONE 2023, 18, e0282406. [Google Scholar] [CrossRef]
- Berardi, G.; Senefeld, J.W.; Hunter, S.K.; Bement, M.K.H. Impact of Isometric and Concentric Resistance Exercise on Pain and Fatigue in Fibromyalgia. Eur. J. Appl. Physiol. 2021, 121, 1389–1404. [Google Scholar] [CrossRef] [PubMed]
- Cigarán-Méndez, M.; Úbeda-D’Ocasar, E.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C.; Barbero, M.; Gallego-Sendarrubias, G.M.; Valera-Calero, J.A. Pain Extent Is Associated with Central Sensitization Inventory but Not Widespread Pressure Pain Sensitivity or Psychological Variables in Women with Fibromyalgia. Scand. J. Rheumatol. 2023, 52, 268–275. [Google Scholar] [CrossRef]
- Leone, C.; Galosi, E.; Esposito, N.; Falco, P.; Fasolino, A.; Di Pietro, G.; Di Stefano, G.; Camerota, F.; Vollert, J.; Truini, A. Small-Fibre Damage Is Associated with Distinct Sensory Phenotypes in Patients with Fibromyalgia and Small-Fibre Neuropathy. Eur. J. Pain 2023, 27, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.D.; Rosser, M.A.; Park, S.H.; Baker, A.K.; Martucci, K.T. Interplay between Noxious Heat Sensitivity and Temporal Summation Magnitude in Patients with Fibromyalgia and Long-Term Opioid Use. Front. Neurosci. 2023, 17, 1275921. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yadav, R.K.; Venkataraman, S.; Kumar, U.; Singh, A.; Deepak, K.K.; Bhatia, R. Exploring Pain Status and Flexibility in Fibromyalgia Patients: Effect of 20 Sessions of Yoga Therapy. Indian J. Physiol. Pharmacol. 2023, 67, 262–269. [Google Scholar] [CrossRef]
- Tapia-Haro, R.M.; Molina, F.; Rus, A.; Casas-Barragán, A.; Correa-Rodríguez, M.; Aguilar-Ferrándiz, M.E. Serum VEGF and CGRP Biomarkers: Relationships with Pain Intensity, Electric Pain, Pressure Pain Threshold, and Clinical Symptoms in Fibromyalgia-An Observational Study. Int. J. Mol. Sci. 2023, 24, 5533. [Google Scholar] [CrossRef]
- Sanzo, P.; Agostino, M.; Fidler, W.; Lawrence-Dewar, J.; Pearson, E.; Zerpa, C.; Niccoli, S.; Lees, S.J. Shockwave Therapy and Fibromyalgia and Its Effect on Pain, Blood Markers, Imaging, and Participant Experience—A Multidisciplinary Randomized Controlled Trial. Physiother. Theory Pract. 2025, 41, 99–114. [Google Scholar] [CrossRef]
- Bäumler, P.; Brenske, A.; Winkelmann, A.; Irnich, D.; Averbeck, B. Strong and Aversive Cold Processing and Pain Facilitation in Fibromyalgia Patients Relates to Augmented Thermal Grill Illusion. Sci. Rep. 2023, 13, 15982. [Google Scholar] [CrossRef]
- Rivas Neira, S.; Pasqual Marques, A.; Fernández Cervantes, R.; Seoane Pillado, M.T.; Vivas Costa, J. Efficacy of Aquatic vs. Land-Based Therapy for Pain Management in Women with Fibromyalgia: A Randomised Controlled Trial. Physiotherapy 2024, 123, 91–101. [Google Scholar] [CrossRef]
- Marshall, A.; Rapteas, L.; Burgess, J.; Riley, D.; Anson, M.; Matsumoto, K.; Bennett, A.; Kaye, S.; Marshall, A.; Dunham, J.; et al. Small Fibre Pathology, Small Fibre Symptoms and Pain in Fibromyalgia Syndrome. Sci. Rep. 2024, 14, 3947. [Google Scholar] [CrossRef]
- Boussi-Gross, R.; Catalogna, M.; Lang, E.; Shamai, Z.; Ablin, J.N.; Aloush, V.; Doenyas-Barak, K.; Lorberboym, M.; Lev-Wiesel, R.; Efrati, S. Hyperbaric Oxygen Therapy vs. Pharmacological Intervention in Adults with Fibromyalgia Related to Childhood Sexual Abuse: Prospective, Randomized Clinical Trial. Sci. Rep. 2024, 14, 11599. [Google Scholar] [CrossRef] [PubMed]
- Couëpel, B.; Daneau, C.; Tremblay, M.; Javelot, T.; Abboud, J.; Pagé, I.; Descarreaux, M. Effect of Physical Activity Education on Shoulder Girdle Pain and Muscle Strength in Participants with Fibromyalgia: A Pilot Experimental Study. Front. Pain Res. 2024, 5, 1328796. [Google Scholar] [CrossRef]
- Berardi, G.; Dailey, D.L.; Chimenti, R.; Merriwether, E.; Vance, C.G.T.; Rakel, B.A.; Crofford, L.J.; Sluka, K.A. Influence of Transcutaneous Electrical Nerve Stimulation (TENS) on Pressure Pain Thresholds and Conditioned Pain Modulation in a Randomized Controlled Trial in Women with Fibromyalgia. J. Pain 2024, 25, 104452. [Google Scholar] [CrossRef] [PubMed]
- Aoe, T.; Kawanaka, R.; Ohsone, F.; Hara, A.; Yokokawa, T. Functional Connectivity Associated with Attention Networks Differs among Subgroups of Fibromyalgia Patients: An Observational Case-Control Study. Sci. Rep. 2024, 14, 10197. [Google Scholar] [CrossRef]
- Castelo-Branco, L.; Pacheco-Barrios, K.; Cardenas-Rojas, A.; de Melo, P.S.; Gianlorenco, A.C.; Gonzalez-Mego, P.; Marduy, A.; Shaik, E.S.; Teixeira, P.; Caumo, W.; et al. Cross-Sectional and Longitudinal Analysis of Conditioned Pain Modulation and Pain in Fibromyalgia: CPM as an Effect Modifier of Pain Changes over Time. Physiother. Res. Int. 2024, 29, e2072. [Google Scholar] [CrossRef]
- Gil-Ugidos, A.; Vázquez-Millán, A.; Samartin-Veiga, N.; Carrillo-de-la-Peña, M.T. Conditioned Pain Modulation (CPM) Paradigm Type Affects Its Sensitivity as a Biomarker of Fibromyalgia. Sci. Rep. 2024, 14, 7798. [Google Scholar] [CrossRef] [PubMed]
- Gungormus, D.B.; Fernández-Martín, M.; Ortigosa-Luque, M.E.; Pérez-Mármol, J.M. Effects of Nature-Based Multisensory Stimulation on Pain Mechanisms in Women with Fibromyalgia Syndrome: A Randomized Double-Blind Placebo-Controlled Trial. Pain. Manag. Nurs. 2024, 25, 46–55. [Google Scholar] [CrossRef]
- Mücke, M.; Cuhls, H.; Radbruch, L.; Baron, R.; Maier, C.; Tölle, T.; Treede, R.-D.; Rolke, R. Quantitative Sensory Testing (QST). English Version. Schmerz 2021, 35, 153–160. [Google Scholar] [CrossRef]
- Maier, C.; Baron, R.; Tölle, T.R.; Binder, A.; Birbaumer, N.; Birklein, F.; Gierthmühlen, J.; Flor, H.; Geber, C.; Huge, V.; et al. Quantitative Sensory Testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory Abnormalities in 1236 Patients with Different Neuropathic Pain Syndromes. Pain 2010, 150, 439–450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).