Trends and Disparities in Cardiovascular Disease in US Adults with Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Population
2.2. Assessment of MASLD
2.3. Outcome Ascertainment
2.4. Sociodemographic Variables
2.5. Metabolic Variables
2.6. Statistical Analysis
3. Results
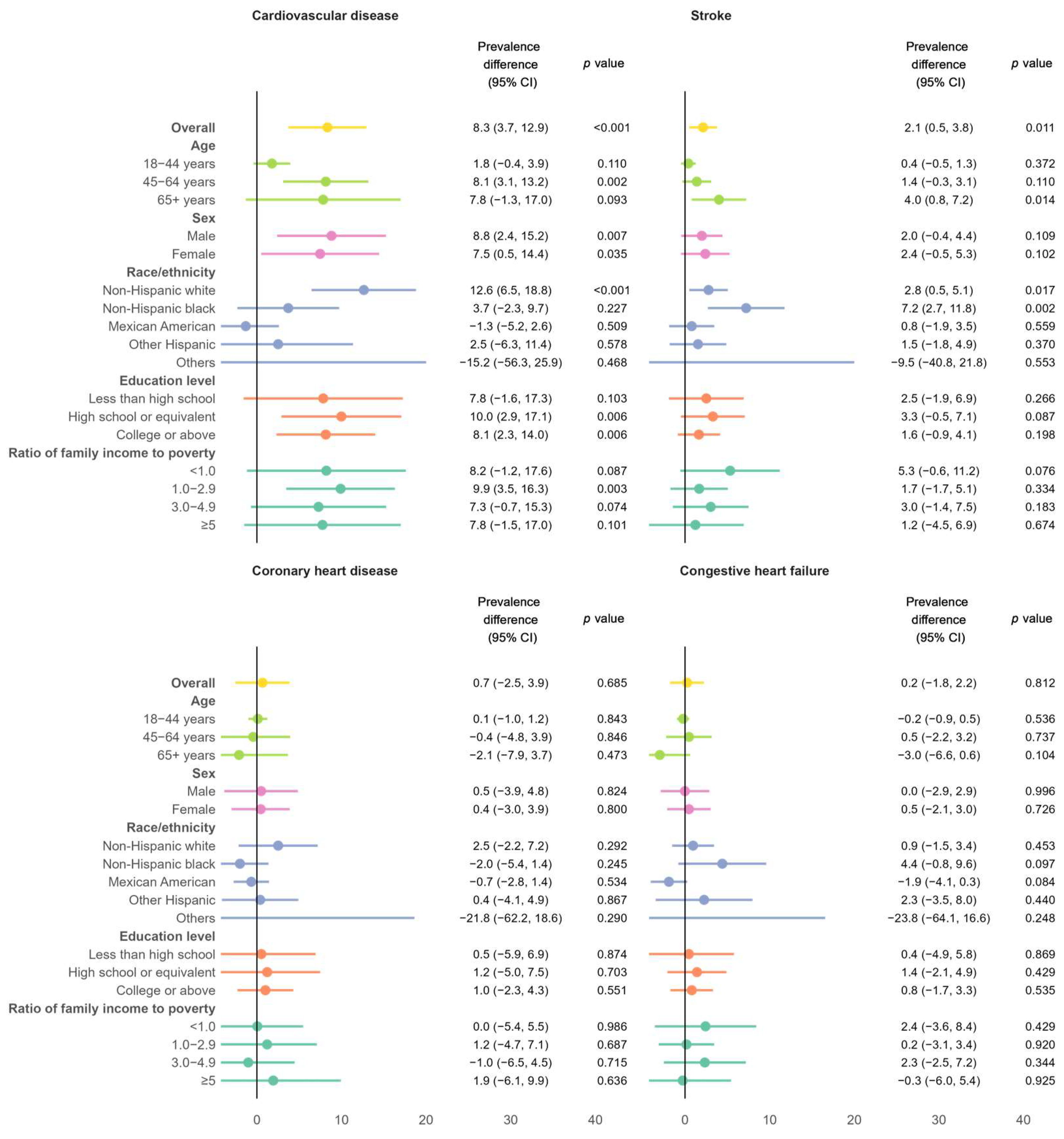
3.1. Absolute Burden of CVD Among Individuals with MASLD
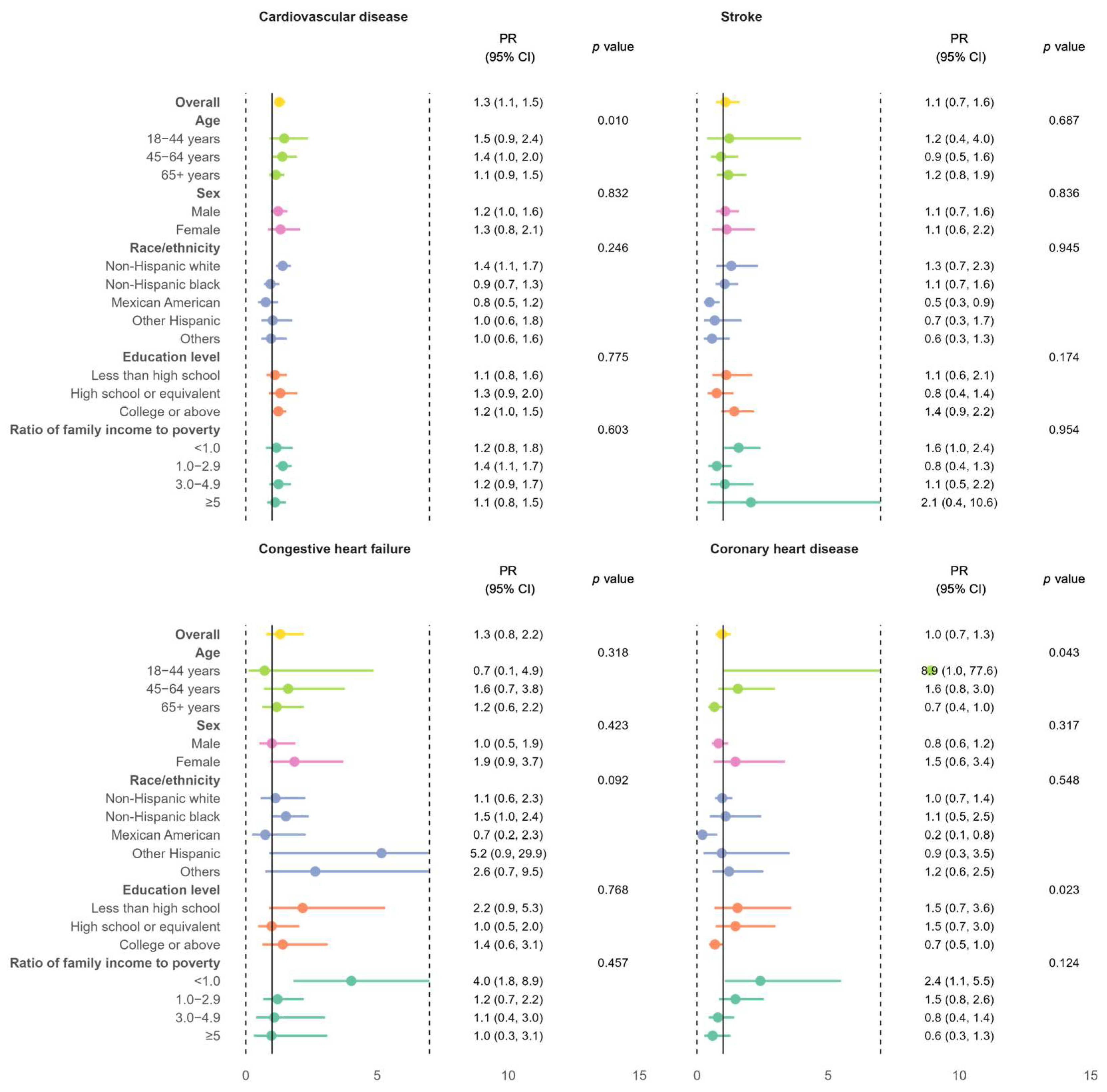
3.2. Relative Burden of CVD Among Individuals with MASLD Versus Those Without
4. Discussion
4.1. Principle Findings
4.2. Rising CVD Prevalence in Adults with MASLD
4.3. Elevated CVD Risk Among Individuals with MASLD Versus Those Without
4.4. Sociodemographic Disparities
4.5. Implications
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CVD | Cardiovascular disease |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NAFLD | Non-alcoholic fatty liver disease |
| NHANES | National Health and Nutrition Examination Survey |
| PR | Prevalence ratio |
References
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Yi, M.; Peng, W.; Feng, X.; Teng, F.; Tang, Y.; Kong, Q.; Chen, Z. Extrahepatic morbidities and mortality of NAFLD: An umbrella review of meta-analyses. Aliment. Pharmacol. Ther. 2022, 56, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Csermely, A.; Petracca, G.; Beatrice, G.; Corey, K.E.; Simon, T.G.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Drury, T.F.; Winn, D.M.; Snowden, C.B.; Kingman, A.; Kleinman, D.V.; Lewis, B. An overview of the oral health component of the 1988–1991 National Health and Nutrition Examination Survey (NHANES III-Phase 1). J. Dent. Res. 1996, 75, 620–630. [Google Scholar] [CrossRef]
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Stat. Ser. 2 Data Eval. Methods Res. 2014, 162, 1–33. [Google Scholar]
- CDC/National Center for Health Statistics. NHANES Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#sample-design (accessed on 14 November 2024).
- CDC/National Center for Health Statistics. NHANES 2017-March 2020 Pre-Pandemic. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2017-2020 (accessed on 14 November 2024).
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, Z.; Bundy, J.D.; Dorans, K.S.; Chen, J.; Hamm, L.L. Trends in Cardiovascular Risk Factors in US Adults by Race and Ethnicity and Socioeconomic Status, 1999–2018. JAMA 2021, 326, 1286–1298. [Google Scholar] [CrossRef]
- Hales, C.M.; Kit, B.K.; Gu, Q.; Ogden, C.L. Trends in Prescription Medication Use Among Children and Adolescents-United States, 1999–2014. JAMA 2018, 319, 2009–2020. [Google Scholar] [CrossRef]
- National Center for Health Statistics. Heart Disease Prevalence. Available online: https://www.cdc.gov/nchs/hus/topics/heart-disease-prevalence.htm#explore-data (accessed on 15 November 2024).
- Andres, W.; Rothstein, A.; Elser, H.; Sloane, K.L.; Gottesman, R.F.; Kasner, S.E.; Schneider, A.L.C. Trends in the Prevalence of Stroke Among Community-Dwelling Individuals in the US, 1999–2018. JAMA Neurol. 2023, 80, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Wang, Y.Y.; Chen, C.; Lu, Y.L.; Wang, N.J. Cardiovascular and renal burdens of metabolic associated fatty liver disease from serial US national surveys, 1999–2016. Chin. Med. J. 2021, 134, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.R.; Officer, A.; De Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.-P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.G.; Mahanani, W.R. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J.; IDF Diabetes Atlas 10th Edition Scientific Committee. IDF Diabetes Atlas [Internet], 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581934/ (accessed on 5 February 2025).
- Cao, Y.-T.; Xiang, L.-L.; Qi, F.; Zhang, Y.-J.; Chen, Y.; Zhou, X.-Q. Accuracy of controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) for assessing steatosis and fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. eClinicalMedicine 2022, 51, 101547. [Google Scholar] [CrossRef]
- Strain, T.; Flaxman, S.; Guthold, R.; Semenova, E.; Cowan, M.; Riley, L.M.; Bull, F.C.; Stevens, G.A.; Abdul Raheem, R.; Agoudavi, K.; et al. National, regional, and global trends in insufficient physical activity among adults from 2000 to 2022: A pooled analysis of 507 population-based surveys with 5 × 7 million participants. Lancet Glob. Health 2024, 12, e1232–e1243. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Webb, P.; Cudhea, F.; Shi, P.; Zhang, J.; Reedy, J.; Erndt-Marino, J.; Coates, J.; Mozaffarian, D.; Bas, M.; et al. Global dietary quality in 185 countries from 1990 to 2018 show wide differences by nation, age, education, and urbanicity. Nat. Food 2022, 3, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Hong, S.; Han, K.; Park, C.-Y. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: Nationwide population based study. BMJ 2024, 384, e076388. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 372–381. [Google Scholar] [CrossRef]
- Peng, H.; Wang, S.; Wang, M.; Ye, Y.; Xue, E.; Chen, X.; Wang, X.; Fan, M.; Gao, W.; Qin, X.; et al. Nonalcoholic fatty liver disease and cardiovascular diseases: A Mendelian randomization study. Metabolism 2022, 133, 155220. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Moore, K.J. Targeting inflammation in CVD: Advances and challenges. Nat. Rev. Cardiol. 2019, 16, 74–75. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Laslett, L.J.; Alagona, P., Jr.; Clark, B.A., III; Drozda, J.P., Jr.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef]
- Carnethon, M.R.; Pu, J.; Howard, G.; Albert, M.A.; Anderson, C.A.M.; Bertoni, A.G.; Mujahid, M.S.; Palaniappan, L.; Taylor, H.A., Jr.; Willis, M.; et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e393–e423. [Google Scholar] [CrossRef]
- Ostrominski, J.W.; Arnold, S.V.; Butler, J.; Fonarow, G.C.; Hirsch, J.S.; Palli, S.R.; Donato, B.M.K.; Parrinello, C.M.; O’Connell, T.; Collins, E.B.; et al. Prevalence and Overlap of Cardiac, Renal, and Metabolic Conditions in US Adults, 1999–2020. JAMA Cardiol. 2023, 8, 1050–1060. [Google Scholar] [CrossRef]
- Hamad, R.; Nguyen, T.T.; Bhattacharya, J.; Glymour, M.M.; Rehkopf, D.H. Educational attainment and cardiovascular disease in the United States: A quasi-experimental instrumental variables analysis. PLoS Med. 2019, 16, e1002834. [Google Scholar] [CrossRef]
- Abdalla, S.M.; Yu, S.; Galea, S. Trends in Cardiovascular Disease Prevalence by Income Level in the United States. JAMA Netw. Open 2020, 3, e2018150. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, H.; Fan, B.; Shi, M.; Lau, E.S.H.; Yang, A.; Chow, E.; Kong, A.P.S.; Chan, J.C.N.; Ma, R.C.W.; et al. The role of age on the risk relationship between prediabetes and major morbidities and mortality: Analysis of the Hong Kong diabetes surveillance database of 2 million Chinese adults. Lancet Reg. Health West. Pac. 2023, 30, 100599. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Farkouh, M.E.; Newman, J.D.; Garvey, W.T. Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 525–538. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef]
- LaBrecque, D.R.; Abbas, Z.; Anania, F.; Ferenci, P.; Khan, A.G.; Goh, K.L.; Hamid, S.S.; Isakov, V.; Lizarzabal, M.; Peñaranda, M.M.; et al. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2014, 48, 467–473. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Stefan, N.; Yki-Järvinen, H.; Neuschwander-Tetri, B.A. Metabolic dysfunction-associated steatotic liver disease: Heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025, 13, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Suo, C.; Zhao, R.; Yuan, H.; Jin, L.; Zhang, T.; Chen, X. Genetic predisposition, lifestyle risk, and obesity associate with the progression of nonalcoholic fatty liver disease. Dig. Liver Dis. 2021, 53, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.M.; Deshpande, R.; Golabi, P.; Younossi, I.; Henry, L.; Younossi, Z.M. The impact of modifiable risk factors on the long-term outcomes of non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2020, 51, 291–304. [Google Scholar] [CrossRef]
- Akinbami, L.J.; Chen, T.C.; Davy, O.; Ogden, C.L.; Fink, S.; Clark, J.; Riddles, M.K.; Mohadjer, L.K. National Health and Nutrition Examination Survey, 2017–March 2020 Prepandemic File: Sample Design, Estimation, and Analytic Guidelines. Vital Health Stat. Ser. 2 2022, 2, 1–36. [Google Scholar] [CrossRef]
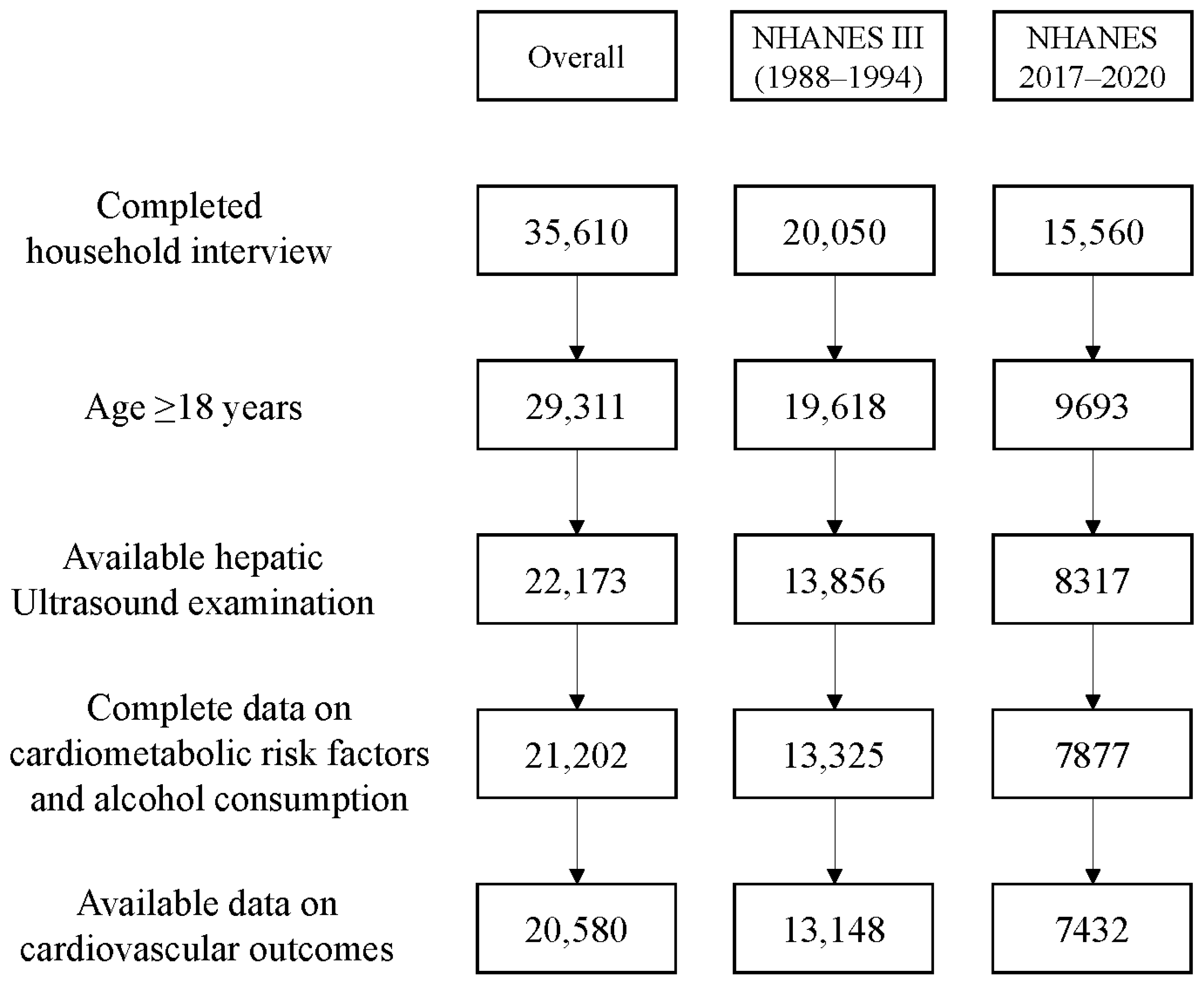
| With MASLD | Without MASLD | p-Value | |
|---|---|---|---|
| No. of adults | 2344 | 5088 | |
| Age, years [mean (SD)] | 53.1 (15.7) | 49.3 (17.3) | <0.001 |
| Age group, years (%) | <0.001 | ||
| 18–44 years | 2179 (48.6) | 721 (35.8) | |
| 45–64 years | 1729 (33.3) | 1006 (41.6) | |
| 65+ years | 1180 (18.2) | 617 (22.6) | |
| Sex (%) | |||
| Male | 1335 (60.0) | 2364 (45.5) | <0.001 |
| Female | 1009 (43.0) | 2724 (53.5) | |
| Race (%) | <0.001 | ||
| Mexican American | 392 (12.3) | 466 (6.6) | |
| Non-Hispanic black | 511 (8.7) | 1508 (12.7) | |
| Non-Hispanic white | 888 (63.5) | 1690 (62.9) | |
| Other Hispanic | 243 (7.0) | 529 (7.9) | |
| Others a | 310 (8.4) | 895 (9.9) | |
| Education (%) | 0.019 | ||
| Less than high school | 413 (9.8) | 910 (10.4) | |
| High school or equivalent | 590 (30.0) | 1219 (25.4) | |
| College or above | 1338 (60.2) | 2955 (64.2) | |
| Ratio of family income to poverty level (%) | 0.367 | ||
| <1.0 | 348 (10.9) | 842 (12.6) | |
| 1.0–2.9 | 903 (35.3) | 1825 (32.6) | |
| 3.0–4.9 | 427 (26.1) | 868 (24.6) | |
| ≥5.0 | 385 (27.6) | 846 (30.2) |
| Total CVD | p-Value | Stroke | p-Value | Congestive Heart Failure | p-Value | Coronary Heart Disease | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Overall | 17.0 (13.7, 20.3) | 4.1 (2.8, 5.4) | 3.9 (2.3, 5.5) | 5.3 (3.3, 7.3) | ||||
| Age | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| 18–44 years | 3.3 (1.3, 5.3) | 0.9 (0.2, 1.5) | 0.4 (−0.1, 0.8) | 0.7 (−0.3, 1.7) | ||||
| 45–64 years | 18.7 (14.3, 23.1) | 3.9 (2.6, 5.1) | 4.3 (2.1, 6.4) | 6.3 (2.4, 10.2) | ||||
| 65+ years | 35.5 (29.0, 42.0) | 9.6 (7.1, 12.0) | 5.6 (3.6, 7.7) | 10.6 (7.2, 13.9) | ||||
| Sex | 0.462 | 0.464 | 0.720 | 0.065 | ||||
| Male | 18.3 (13.3, 23.3) | 3.7 (2.0, 5.4) | 4.2 (2.2, 6.2) | 6.7 (4.0, 9.4) | ||||
| Female | 15.3 (9.8, 20.8) | 5.5 (3.0, 7.9) | 3.5 (1.4, 5.7) | 3.3 (0.8, 5.8) | ||||
| Race/ethnicity | <0.001 | 0.001 | 0.025 | <0.001 | ||||
| Non-Hispanic white | 21.6 (16.8, 26.3) | 4.8 (2.9, 6.7) | 4.3 (2.0, 6.7) | 7.3 (4.4, 10.3) | ||||
| Non-Hispanic black | 13.6 (8.7, 18.4) | 8.0 (4.4, 11.6) | 5.9 (2.5, 9.3) | 2.3 (0.1, 4.5) | ||||
| Mexican American | 6.8 (2.5, 11.1) | 1.3 (−0.7, 3.3) | 2.2 (−0.4, 4.7) | 3.3 (0.4, 6.2) | ||||
| Other Hispanic | 12.1 (4.9, 19.2) | 1.9 (−0.4, 4.1) | 3.2 (−0.4, 6.8) | 2.6 (0.8, 4.4) | ||||
| Others | 14.3 (5.4, 23.3) | 4.1 (−0.3, 8.5) | 2.7 (−0.8, 6.2) | 3.0 (0.3, 5.6) | ||||
| Education level | 0.294 | 0.130 | 0.056 | 0.357 | ||||
| Less than high school | 21.1 (13.5, 28.7) | 5.8 (2.3, 9.3) | 6.7 (2.9, 10.6) | 7.4 (2.6, 12.1) | ||||
| High school or equivalent | 22.7 (14.6, 30.8) | 6.5 (2.2, 10.8) | 3.3 (1.3, 5.2) | 7.4 (1.7, 13.1) | ||||
| College or above | 15.7 (11.9, 19.5) | 3.3 (1.9, 4.8) | 3.7 (1.5, 5.9) | 4.6 (2.8, 6.3) | ||||
| Ratio of family income to poverty | 0.149 | 0.259 | 0.176 | 0.430 | ||||
| <1.0 | 17.0 (9.3, 24.6) | 9.8 (3.8, 15.8) | 8.8 (3.0, 14.5) | 5.2 (1.1, 9.2) | ||||
| 1.0–2.9 | 20.4 (15.1, 25.6) | 5.5 (2.1, 9.0) | 4.6 (2.3, 6.9) | 6.6 (2.4, 10.8) | ||||
| 3.0–4.9 | 16.8 (10.0, 23.5) | 3.6 (1.0, 6.3) | 3.2 (−0.3, 6.6) | 4.5 (1.7, 7.3) | ||||
| ≥5 | 17.4 (10.8, 23.9) | 6.6 (2.2, 10.9) | 2.8 (0.4, 5.1) | 10.1 (4.1, 16.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, X.; Huang, C.; Zhu, L. Trends and Disparities in Cardiovascular Disease in US Adults with Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines 2025, 13, 956. https://doi.org/10.3390/biomedicines13040956
Zhang Y, Zhang X, Huang C, Zhu L. Trends and Disparities in Cardiovascular Disease in US Adults with Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines. 2025; 13(4):956. https://doi.org/10.3390/biomedicines13040956
Chicago/Turabian StyleZhang, Yanbing, Xinge Zhang, Chuiguo Huang, and Lei Zhu. 2025. "Trends and Disparities in Cardiovascular Disease in US Adults with Metabolic Dysfunction-Associated Steatotic Liver Disease" Biomedicines 13, no. 4: 956. https://doi.org/10.3390/biomedicines13040956
APA StyleZhang, Y., Zhang, X., Huang, C., & Zhu, L. (2025). Trends and Disparities in Cardiovascular Disease in US Adults with Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines, 13(4), 956. https://doi.org/10.3390/biomedicines13040956





