Knockdown of IER3 Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA–Seq Analysis of hMSCs
2.2. Cell Culture
2.3. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
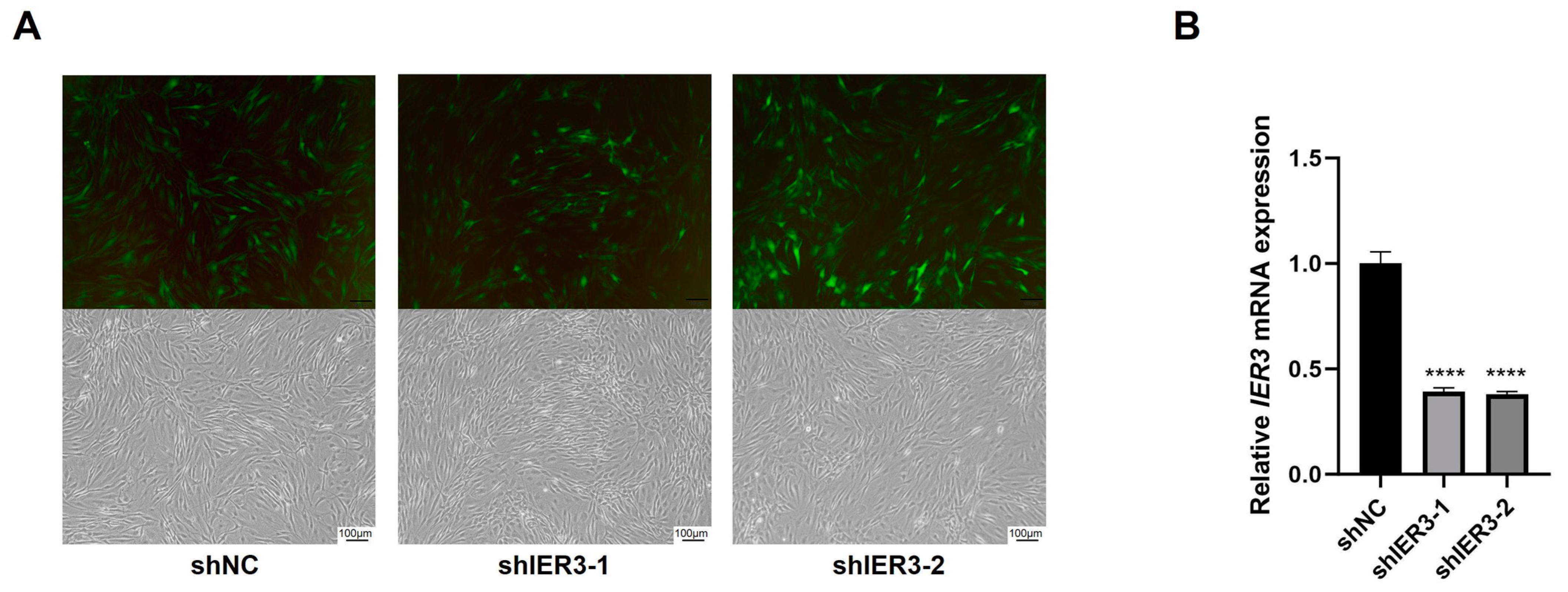
2.4. Lentiviral Transfection of hMSCs for IER3 Knockdown
2.5. ALP and Alizarin Red S (ARS) Staining
2.6. Oil Red O Staining
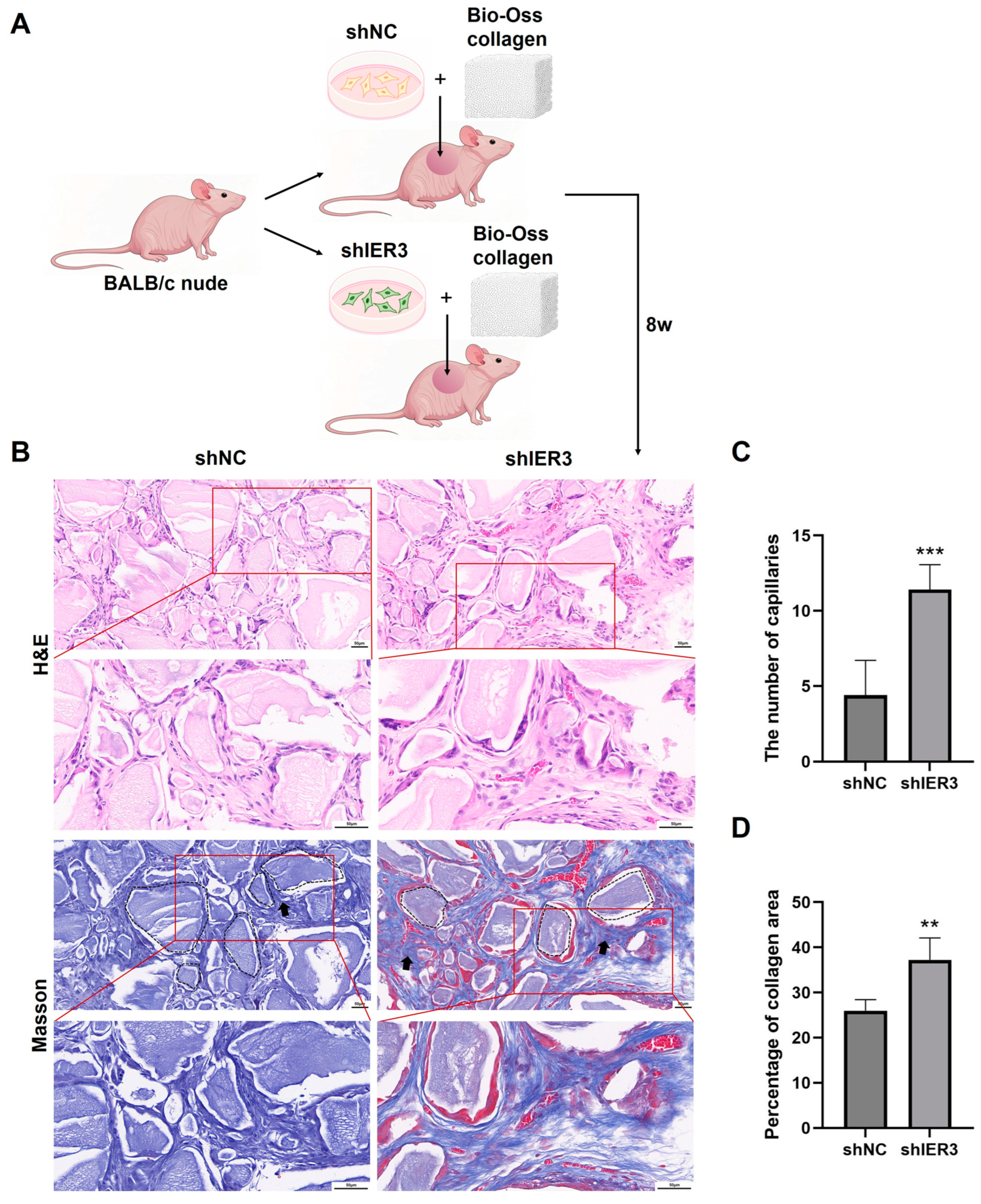
2.7. Ectopic Osteogenesis in Nude Mice
2.8. Histological Staining
2.9. RNA Sequencing and Analysis
2.10. Protein Extraction and Western Blot Analysis
2.11. Inhibition of the ERK Signaling Pathway
2.12. Statistical Analysis
3. Results
3.1. Expression of IER3 During Osteogenic Differentiation of hMSCs
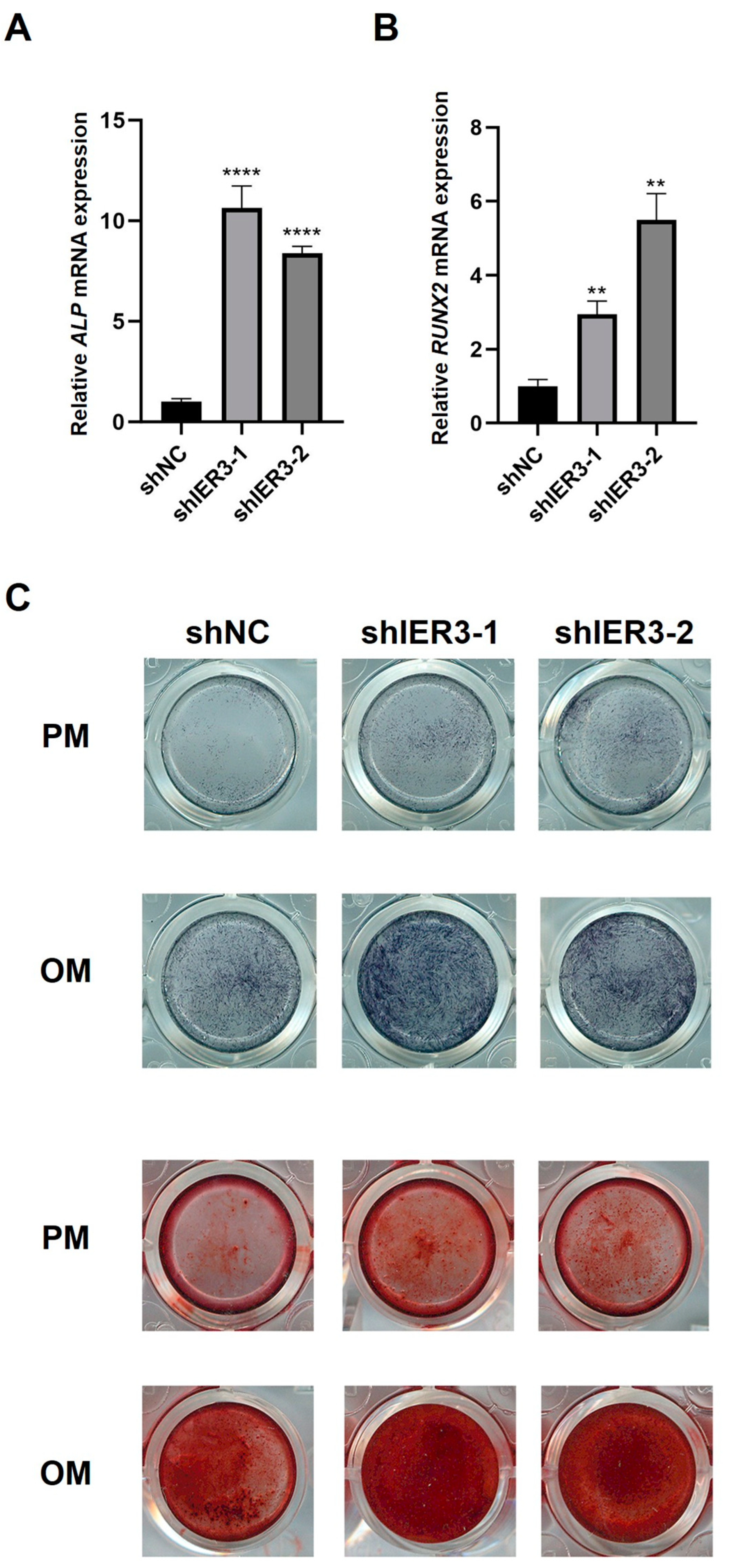
3.2. The Effect of IER3 on Osteogenic Differentiation of hMSCs In Vitro
3.3. The Effect of IER3 on Osteogenic Differentiation of hMSCs In Vivo
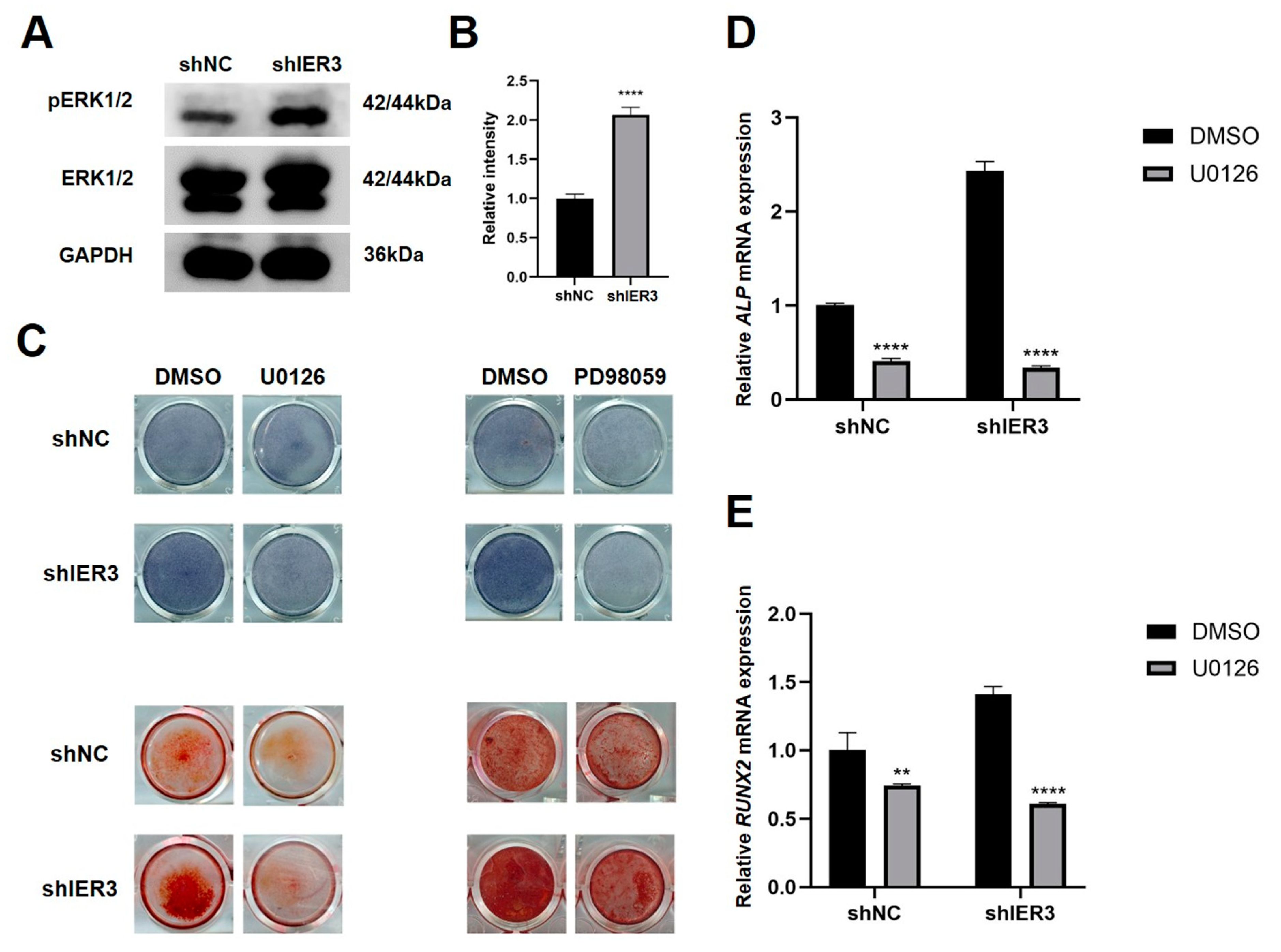
3.4. IER3 Influences Osteogenic Differentiation Through the MAPK/ERK Pathway
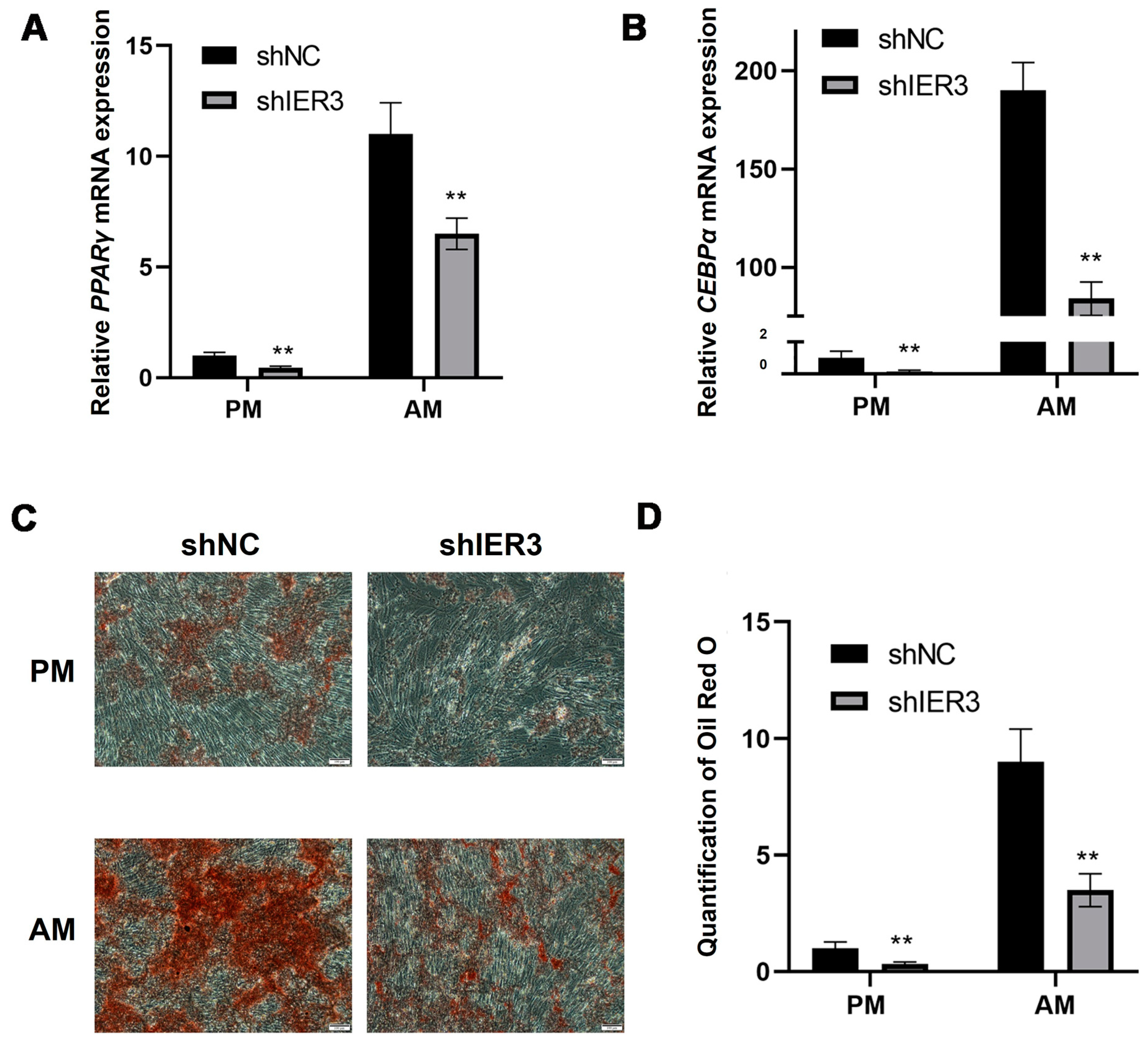
3.5. The Effect of IER3 on Adipogenic Differentiation of hMSCs In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| AM | Adipogenic medium |
| ARS | Alizarin Red S |
| CEBPα | CCAAT/enhancer-binding protein alpha |
| DEGs | Differentially expressed genes |
| DMSO | Dimethyl sulfoxide |
| ERK | Extracellular regulated protein kinases |
| H&E | Hematoxylin and eosin |
| hMSCs | Human mesenchymal stem cells |
| IER3 | Immediate Early Response 3 |
| MAPK | Mitogen-activated protein kinase |
| MSCs | Mesenchymal stem cells |
| OCN | Osteocalcin |
| OM | Osteogenic medium |
| PFA | Paraformaldehyde |
| PM | Proliferation medium |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| RNA–seq | RNA sequencing |
| RUNX2 | Runx-related transcription factor 2 |
Appendix A

References
- Fuggle, N.; Laslop, A.; Rizzoli, R.; Al-Daghri, N.; Alokail, M.; Balkowiec-Iskra, E.; Beaudart, C.; Bruyère, O.; Bemden, A.B.; Burlet, N.; et al. Treatment of Osteoporosis and Osteoarthritis in the Oldest Old. Drugs 2025, 85, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.C.; Elias, A.; Reynolds, J.; Ball, J.R.; Lieberman, J.R. Regional Gene Therapy for Bone Tissue Engineering: A Current Concepts Review. Bioengineering 2025, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Lamy, O.; Everts-Graber, J.; Rodriguez, E.G. Denosumab for osteoporosis treatment: When, how, for whom, and for how long. A pragmatical approach. Aging Clin. Exp. Res. 2025, 37, 70. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Huang, M.; Wu, X.S.; Xiao, N.; Huang, X.; Lin, P.F. Genetically modified stem cells for osteoporosis: A systematic review and meta-analysis of preclinical studies. BMC Musculoskelet. Disord. 2025, 26, 259. [Google Scholar] [CrossRef]
- Tang, A.; Shu, Q.; Jia, S.; Lai, Z.; Tian, J. Adipose Mesenchymal Stem Cell-Derived Exosomes as Nanocarriers for Treating Musculoskeletal Disorders. Int. J. Nanomed. 2024, 19, 13547–13562. [Google Scholar] [CrossRef]
- Lau, C.S.; Park, S.Y.; Ethiraj, L.P.; Singh, P.; Raj, G.; Quek, J.; Prasadh, S.; Choo, Y.; Goh, B.T. Role of Adipose-Derived Mesenchymal Stem Cells in Bone Regeneration. Int. J. Mol. Sci. 2024, 25, 6805. [Google Scholar] [CrossRef]
- Robert, A.W.; Marcon, B.H.; Dallagiovanna, B.; Shigunov, P. Adipogenesis, Osteogenesis, and Chondrogenesis of Human Mesenchymal Stem/Stromal Cells: A Comparative Transcriptome Approach. Front. Cell Dev. Biol. 2020, 8, 561. [Google Scholar] [CrossRef]
- Chen, Q.; Su, Y.; Yang, Z.; Lin, Q.; Ke, Y.; Xing, D.; Li, H. Bibliometric mapping of mesenchymal stem cell therapy for bone regeneration from 2013 to 2023. Front. Med. 2024, 11, 1484097. [Google Scholar] [CrossRef]
- Peng, Y.; Jiang, H.; Zuo, H.D. Factors affecting osteogenesis and chondrogenic differentiation of mesenchymal stem cells in osteoarthritis. World J. Stem Cells 2023, 15, 548–560. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Tahoori, M.; Tafreshi, A.P.; Naghshnejad, F.; Zeynali, B. Transforming Growth Factor-β Signaling Inhibits the Osteogenic Differentiation of Mesenchymal Stem Cells via Activation of Wnt/β-Catenin Pathway. J. Bone Metab. 2025, 32, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xue, Y.; Yang, K. LINC00899 promotes osteogenic differentiation by targeting miR-374a and RUNX2 expression. Exp. Ther. Med. 2021, 22, 1071. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.L.; Begun, D.L.; Westendorf, J.J.; McGee-Lawrence, M.E. Defining osteoblast and adipocyte lineages in the bone marrow. Bone 2019, 118, 2–7. [Google Scholar] [CrossRef]
- Calixto, R.D.; Freitas, G.P.; Souza, P.G.; Ramos, J.I.R.; Santos, I.C.; de Oliveira, F.S.; Almeida, A.L.G.; Rosa, A.L.; Beloti, M.M. Effect of the secretome of mesenchymal stem cells overexpressing BMP-9 on osteoblast differentiation and bone repair. J. Cell. Physiol. 2023, 238, 2625–2637. [Google Scholar] [CrossRef]
- Shen, J.; Sun, Y.; Liu, X.; Zhu, Y.; Bao, B.; Gao, T.; Chai, Y.; Xu, J.; Zheng, X. EGFL6 regulates angiogenesis and osteogenesis in distraction osteogenesis via Wnt/β-catenin signaling. Stem Cell Res. Ther. 2021, 12, 415. [Google Scholar] [CrossRef]
- Thomas, S.; Jaganathan, B.G. Signaling network regulating osteogenesis in mesenchymal stem cells. J. Cell Commun. Signal. 2022, 16, 47–61. [Google Scholar] [CrossRef]
- Wang, N.; Li, Y.; Li, Z.; Ma, J.; Wu, X.; Pan, R.; Wang, Y.; Gao, L.; Bao, X.; Xue, P. IRS-1 targets TAZ to inhibit adipogenesis of rat bone marrow mesenchymal stem cells through PI3K-Akt and MEK-ERK pathways. Eur. J. Pharmacol. 2019, 849, 11–21. [Google Scholar] [CrossRef]
- Arlt, A.; Schäfer, H. Role of the immediate early response 3 (IER3) gene in cellular stress response, inflammation and tumorigenesis. Eur. J. Cell Biol. 2011, 90, 545–552. [Google Scholar] [CrossRef]
- Kanduri, M.; Subhash, S.; Putino, R.; Mahale, S.; Kanduri, C. IER3: Exploring its dual function as an oncogene and tumor suppressor. Cancer Gene Ther. 2025, 32, 450–463. [Google Scholar] [CrossRef]
- Jin, H.; Lee, K.; Kim, Y.H.; Oh, H.K.; Maeng, Y.I.; Kim, T.H.; Suh, D.S.; Bae, J. Scaffold protein FHL2 facilitates MDM2-mediated degradation of IER3 to regulate proliferation of cervical cancer cells. Oncogene 2016, 35, 5106–5118. [Google Scholar] [CrossRef] [PubMed]
- He, F.Y.; Chen, G.; He, R.Q.; Huang, Z.G.; Li, J.D.; Wu, W.Z.; Chen, J.T.; Tang, Y.L.; Li, D.M.; Pan, S.L.; et al. Expression of IER3 in hepatocellular carcinoma: Clinicopathology, prognosis, and potential regulatory pathways. PeerJ 2022, 10, e12944. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, N.; Huang, G.; Dong, Y.; Zhu, P. Correlation study of NF-κB, IER3, and Recurrence of Ovarian Endometrioid Cysts. Reprod. Sci. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, S.L.; Careddu, G.; Stirparo, G.G.; Castagna, L.; Santoro, A.; Carlo-Stella, C. Dual PI3K/ERK inhibition induces necroptotic cell death of Hodgkin Lymphoma cells through IER3 downregulation. Sci. Rep. 2016, 6, 35745. [Google Scholar] [CrossRef]
- Xi, X.; Zhao, Y.; Liu, H.; Li, Z.; Chen, S.; Liu, D. Nrf2 activation is involved in osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. Exp. Cell Res. 2021, 403, 112598. [Google Scholar] [CrossRef]
- Yu, L.; Shen, Y.; Yang, J.; Feng, X.; Zhou, C.; Lin, J. Enhancing cranial defect repair in rats: Investigating the effect of combining Total Flavonoids from Rhizoma Drynariae with calcium phosphate/collagen scaffolds. J. Orthop. Surg. Res. 2023, 18, 903. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, W.J.; Ryoo, H.M. Post-Translational Regulations of Transcriptional Activity of RUNX2. Mol. Cells 2020, 43, 160–167. [Google Scholar]
- Zhang, J.; Zhang, W.; Dai, J.; Wang, X.; Shen, S.G. Overexpression of Dlx2 enhances osteogenic differentiation of BMSCs and MC3T3-E1 cells via direct upregulation of Osteocalcin and Alp. Int. J. Oral Sci. 2019, 11, 12. [Google Scholar] [CrossRef]
- Keil, C.; Gollmer, B.; Zeidler-Rentzsch, I.; Gredes, T.; Heinemann, F. Histological evaluation of extraction sites grafted with Bio-Oss Collagen: Randomized controlled trial. Ann. Anat.-Anat. Anz. 2021, 237, 151722. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, X.; Wang, C.; Dai, G.; Zhu, Z.; Gao, S.; He, B.; Liao, J.; Huang, W. BMP9 exhibits dual and coupled roles in inducing osteogenic and angiogenic differentiation of mesenchymal stem cells. Biosci. Rep. 2020, 40, BSR20201262. [Google Scholar] [CrossRef]
- Ribatti, D.; d’Amati, A. Bone angiocrine factors. Front. Cell Dev. Biol. 2023, 11, 1244372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, X.; Zheng, B.; Liu, Y. Regulation and mechanistic insights into tensile strain in mesenchymal stem cell osteogenic differentiation. Bone 2024, 187, 117197. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Xiao, G.; Jiang, D.; Franceschi, R.T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 2007, 176, 709–718. [Google Scholar] [CrossRef]
- Kim, J.M.; Yang, Y.S.; Hong, J.; Chaugule, S.; Chun, H.; van der Meulen, M.C.H.; Xu, R.; Greenblatt, M.B.; Shim, J.H. Biphasic regulation of osteoblast development via the ERK MAPK-mTOR pathway. eLife 2022, 11, e78069. [Google Scholar] [CrossRef]
- You, Y.; Niu, Y.; Zhang, J.; Huang, S.; Ding, P.; Sun, F.; Wang, X. U0126: Not only a MAPK kinase inhibitor. Front. Pharmacol. 2022, 13, 927083. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, Y.; Zhang, X.; Chu, Z.; Liu, Y.; Tang, C. MMP-1 promotes osteogenic differentiation of human bone marrow mesenchymal stem cells via the JNK and ERK pathway. Int. J. Biochem. Cell Biol. 2020, 129, 105880. [Google Scholar] [CrossRef]
- Jiang, Y.; Xin, N.; Xiong, Y.; Guo, Y.; Yuan, Y.; Zhang, Q.; Gong, P. αCGRP Regulates Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells Through ERK1/2 and p38 MAPK Signaling Pathways. Cell Transplant. 2022, 31, 9636897221107636. [Google Scholar] [CrossRef] [PubMed]
- Froemming, M.N.; Khosla, S.; Farr, J.N. Marrow Adipocyte Senescence in the Pathogenesis of Bone Loss. Curr. Osteoporos. Rep. 2024, 22, 378–386. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zhao, G.; Yang, K.; Tao, L. Obesity and lipid metabolism in the development of osteoporosis (Review). Int. J. Mol. Med. 2024, 54, 61. [Google Scholar] [CrossRef]
- Li, Y.; Jin, D.; Xie, W.; Wen, L.; Chen, W.; Xu, J.; Ding, J.; Ren, D. PPAR-γ and Wnt Regulate the Differentiation of MSCs into Adipocytes and Osteoblasts Respectively. Curr. Stem Cell Res. Ther. 2018, 13, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Schmidt, H.; Lai, B.; Ge, K. Transcriptional and Epigenomic Regulation of Adipogenesis. Mol. Cell. Biol. 2019, 39, e00601-18. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zheng, X.; Li, H.; Deng, Z.; Feng, S.; Tan, H.; Zhao, L. Carboxypeptidase M modulates BMSCs osteogenesis-adipogenesis via the MAPK/ERK pathway: An integrated single-cell and bulk transcriptomic study. FASEB J. 2024, 38, e23657. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer (5′–3′) | |
|---|---|---|
| ALP | Forward | AGCCCTTCACTGCCATCCTGTAT |
| Reverse | CGCCTGGTAGTTGTTGTGAGCAT | |
| RUNX2 | Forward | CCGCCTCAGTGATTTAGGGC |
| Reverse | GGGTCTGTAATCTGACTCTGTCC | |
| OCN | Forward | CACTCCTCGCCCTATTGGC |
| Reverse | CCCTCCTGCTTGGACACAAG | |
| GAPDH | Forward | GGTCACCAGGGCTGCTTTTA |
| Reverse | GGATCTCGCTCCTGGAAGATG | |
| IER3 | Forward | CAGTCGAGGAACCGAACCC |
| Reverse | GATCTGGCAGAAGACGATGGT | |
| CEBPα | Forward | TAGGATAACCTTGTGCCTTGGAAAT |
| Reverse | GTCTGCTGTAGCCTCGGGAA | |
| PPARγ | Forward | GCCTGCATTTCTGCATTCTG |
| Reverse | CACGGAGCTGATCCCAAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Ma, H.; Tang, Z.; Jin, C. Knockdown of IER3 Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. Biomedicines 2025, 13, 947. https://doi.org/10.3390/biomedicines13040947
Han Y, Ma H, Tang Z, Jin C. Knockdown of IER3 Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. Biomedicines. 2025; 13(4):947. https://doi.org/10.3390/biomedicines13040947
Chicago/Turabian StyleHan, Yuqing, Hongyang Ma, Zhihui Tang, and Chanyuan Jin. 2025. "Knockdown of IER3 Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells" Biomedicines 13, no. 4: 947. https://doi.org/10.3390/biomedicines13040947
APA StyleHan, Y., Ma, H., Tang, Z., & Jin, C. (2025). Knockdown of IER3 Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. Biomedicines, 13(4), 947. https://doi.org/10.3390/biomedicines13040947







