Chloroquine Enhances Chemosensitivity of Breast Cancer via mTOR Inhibition
Abstract
1. Introduction
2. Materials and Methods
- Materials
- 2.
- Cell culture
- 3.
- EDU proliferation assay
- 4.
- Measurement of ROS and ATP
- 5.
- Immunoblot analysis
- 6.
- Real-time fluorescent quantitative reverse transcription PCR (RT-qPCR)
- 7.
- Colocalization analysis of P2X4 and LAMP1 immunostaining
- 8.
- Lysosomal pH measurement
- 9.
- MTT assay
- 10.
- Intracellular calcium concentration assay
- 11.
- Treatment in vivo
- 12.
- Statistical analysis
3. Results
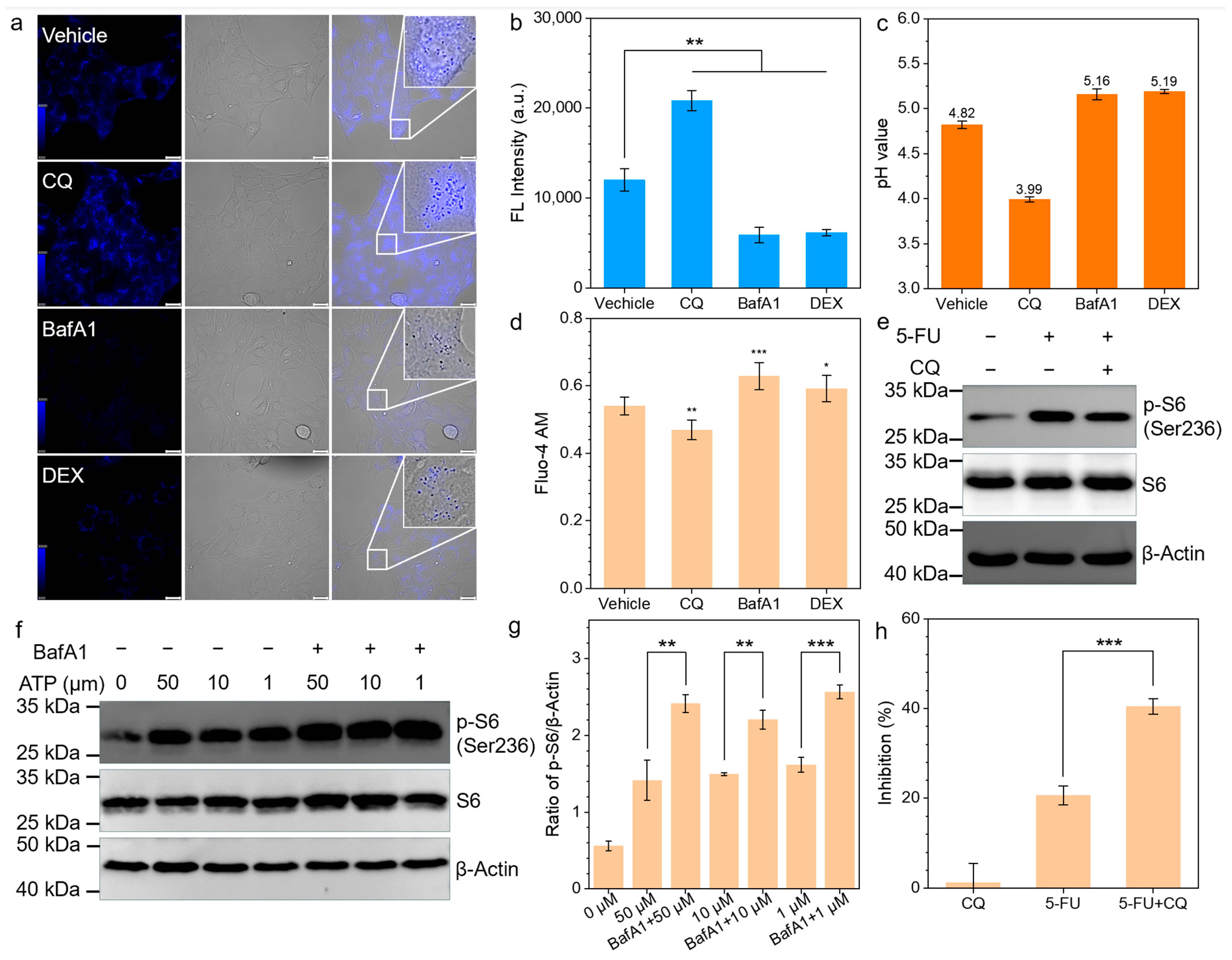
3.1. CQ-Mediated Chemosensitization Prevented by N-Acetylcysteine
3.2. ATP-Induced mTOR Activation in Reaction to 5-FU
3.3. CQ Inhibits ATP-P2X4-mTOR Pathway
3.4. CQ Promotes 5-FU Through P2X4-mTOR Inhibition
3.5. In Vivo Validation of CQ-Mediated Chemosensization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine triphosphate |
| BafA1 | Bafilomycin A1 |
| CHKA | choline kinase alpha |
| CQ | Chloroquine |
| DEX | Dexamethasone |
| LAMP1 | Lysosome-associated membrane protein 1 |
| mTOR | Mammalian target of rapamycin |
| NAC | N-Acetylcysteine |
| PFKM | Phosphofructokinase muscle |
| p-S6 | Phosphorylated ribosomal protein S6 |
| ROS | Reactive oxygen species |
| RAPA | Rapamycin |
| S6 | Ribosomal protein S6 |
| 5-FU | 5-Fluorouracil |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| DCFH | 2′,7′-Dichlorodihydrofluorescein |
References
- Li, J.; Chen, X.; Kang, R.; Zeh, H.; Klionsky, D.J.; Tang, D. Regulation and function of autophagy in pancreatic cancer. Autophagy 2021, 17, 3275–3296. [Google Scholar] [CrossRef] [PubMed]
- Pangilinan, C.; Klionsky, D.J.; Liang, C. Emerging dimensions of autophagy in melanoma. Autophagy 2024, 20, 1700–1711. [Google Scholar] [CrossRef]
- González-Pastor, R.; Lancelot, A.; Morcuende-Ventura, V.; San Anselmo, M.; Sierra, T.; Serrano, J.L.; Martin-Duque, P. Combination Chemotherapy with Cisplatin and Chloroquine: Effect of Encapsulation in Micelles Formed by Self-Assembling Hybrid Dendritic-Linear-Dendritic Block Copolymers. Int. J. Mol. Sci. 2021, 22, 5223. [Google Scholar] [CrossRef]
- Kang, C.; Ju, S.; Kim, J.; Jung, Y. Chloroquine prevents hypoxic accumulation of HIF-1α by inhibiting ATR kinase: Implication in chloroquine-mediated chemosensitization of colon carcinoma cells under hypoxia. Pharmacol. Rep. 2023, 75, 211–221. [Google Scholar] [CrossRef]
- Sotelo, J.; Briceño, E.; López-González, M.A. Adding chloroquine to conventional treatment for glioblastoma multiforme: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2006, 144, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Puentes, L.L.; Gonzalez-Pinedo, M.; Crismatt, A.; Ortega-Gomez, A.; Gamboa-Vignolle, C.; Nuñez-Gomez, R.; Dorantes-Gallareta, Y.; Arce-Salinas, C.; Arrieta, O. Phase II randomized, double-blind, placebo-controlled study of whole-brain irradiation with concomitant chloroquine for brain metastases. Radiat. Oncol. 2013, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Gu, L.; Li, K.; Ma, A.; Liu, L.; Meng, Y.; Zhang, J.; Shen, S.; Shi, Q.; et al. Chloroquine Suppresses Colorectal Cancer Progression via Targeting CHKA and PFKM to inhibit the PI3K/AKT Pathway and the Warburg Effect. Int. J. Biol. Sci. 2025, 21, 1619–1631. [Google Scholar] [CrossRef]
- Spears, L.D.; Tran, A.V.; Qin, C.Y.; Hobbs, S.B.; Burns, C.A.; Royer, N.K.; Zhang, Z.; Ralston, L.; Fisher, J.S. Chloroquine increases phosphorylation of AMPK and Akt in myotubes. Heliyon 2016, 2, e00083. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Chatterjee, A.; Kogan, D.; Patel, D.; Foster, D.A. 5-Aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) enhances the efficacy of rapamycin in human cancer cells. Cell Cycle 2015, 14, 3331–3339. [Google Scholar] [CrossRef]
- Borack, M.S.; Dickinson, J.M.; Fry, C.S.; Reidy, P.T.; Markofski, M.M.; Deer, R.R.; Jennings, K.; Volpi, E.; Rasmussen, B.B. Effect of the lysosomotropic agent chloroquine on mTORC1 activation and protein synthesis in human skeletal muscle. Nutr. Metab. 2021, 18, 61. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Vares, G.; Ahire, V.; Sunada, S.; Ho Kim, E.; Sai, S.; Chevalier, F.; Romeo, P.H.; Yamamoto, T.; Nakajima, T.; Saintigny, Y. A multimodal treatment of carbon ions irradiation, miRNA-34 and mTOR inhibitor specifically control high-grade chondrosarcoma cancer stem cells. Radiother. Oncol. 2020, 150, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Wang, C.; Vegna, S.; Jin, H.; Benedict, B.; Lieftink, C.; Ramirez, C.; de Oliveira, R.L.; Morris, B.; Gadiot, J.; Wang, W.; et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 2019, 574, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Taha-Mehlitz, S.; Bianco, G.; Coto-Llerena, M.; Kancherla, V.; Bantug, G.R.; Gallon, J.; Ercan, C.; Panebianco, F.; Eppenberger-Castori, S.; von Strauss, M.; et al. Adenylosuccinate lyase is oncogenic in colorectal cancer by causing mitochondrial dysfunction and independent activation of NRF2 and mTOR-MYC-axis. Theranostics 2021, 11, 4011–4029. [Google Scholar] [CrossRef]
- Liu, J.; Lyu, Q.; Wu, M.; Zhou, Y.; Wang, T.; Zhang, Y.; Fan, N.; Yang, C.; Wang, W. Integrating mTOR Inhibition and Photodynamic Therapy Based on Carrier-Free Nanodrugs for Breast Cancer Immunotherapy. Adv. Healthc. Mater. 2024, 13, e2402357. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Q.; Luo, X.; Lou, K.; Weiss, W.A.; Shokat, K.M. Brain-restricted mTOR inhibition with binary pharmacology. Nature 2022, 609, 822–828. [Google Scholar] [CrossRef]
- Oleksak, P.; Nepovimova, E.; Chrienova, Z.; Musilek, K.; Patocka, J.; Kuca, K. Contemporary mTOR inhibitor scaffolds to diseases breakdown: A patent review (2015–2021). Eur. J. Med. Chem. 2022, 238, 114498. [Google Scholar] [CrossRef]
- Mao, B.; Zhang, Q.; Ma, L.; Zhao, D.S.; Zhao, P.; Yan, P. Overview of Research into mTOR Inhibitors. Molecules 2022, 27, 5295. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Buratto, D.; Han, P.; Yang, Z.; Zhou, R. A pH-responsive nanoparticle delivery system containing dihydralazine and doxorubicin-based prodrug for enhancing antitumor efficacy. Aggregate 2023, 5, e434. [Google Scholar] [CrossRef]
- Liang, X.; Tang, J.; Liang, Y.; Jin, R.; Cai, X. Suppression of autophagy by chloroquine sensitizes 5-fluorouracil-mediated cell death in gallbladder carcinoma cells. Cell Biosci. 2014, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponenko, V.; Hay, N.; Gartel, A.L. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Ceteci, F.; Gupta, J.; Pesic, M.; Bottger, T.W.; Nicolas, A.M.; Kennel, K.B.; Engel, E.; Schewe, M.; Callak Kirisozu, A.; et al. Colon tumour cell death causes mTOR dependence by paracrine P2X4 stimulation. Nature 2022, 612, 347–353. [Google Scholar] [CrossRef]
- Kanellopoulos, J.M.; Almeida-da-Silva, C.L.C.; Ruutel Boudinot, S.; Ojcius, D.M. Structural and Functional Features of the P2X4 Receptor: An Immunological Perspective. Front. Immunol. 2021, 12, 645834. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Wu, X.; Yin, D.; Jia, X.H.; Chen, X.; Gu, Z.Y.; Zhu, X.M. Autophagy inhibitors for cancer therapy: Small molecules and nanomedicines. Pharmacol. Ther. 2023, 249, 108485. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Cocco, S.; Leone, A.; Roca, M.S.; Lombardi, R.; Piezzo, M.; Caputo, R.; Ciardiello, C.; Costantini, S.; Bruzzese, F.; Sisalli, M.J.; et al. Inhibition of autophagy by chloroquine prevents resistance to PI3K/AKT inhibitors and potentiates their antitumor effect in combination with paclitaxel in triple negative breast cancer models. J. Transl. Med. 2022, 20, 290. [Google Scholar] [CrossRef]
- Chen, Z.; Ouyang, C.; Zhang, H.; Gu, Y.; Deng, Y.; Du, C.; Cui, C.; Li, S.; Wang, W.; Kong, W.; et al. Vascular smooth muscle cell-derived hydrogen sulfide promotes atherosclerotic plaque stability via TFEB (transcription factor EB)-mediated autophagy. Autophagy 2022, 18, 2270–2287. [Google Scholar] [CrossRef]
- Lendvai, G.; Szekerczés, T.; Illyés, I.; Csengeri, M.; Schlachter, K.; Szabó, E.; Lotz, G.; Kiss, A.; Borka, K.; Schaff, Z. Autophagy activity in cholangiocarcinoma is associated with anatomical localization of the tumor. PLoS ONE 2021, 16, e0253065. [Google Scholar] [CrossRef]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.; Liang, X.; Lv, J.; Jin, X.; Liu, J.; Mo, S.; Zhang, T.; et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 2018, 9, 873. [Google Scholar] [CrossRef]
- Lenk, G.M.; Meisler, M.H. Chloroquine corrects enlarged lysosomes in FIG4 null cells and reduces neurodegeneration in Fig4 null mice. Mol. Genet. Metab. 2022, 137, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Florey, O.; Gammoh, N.; Kim, S.E.; Jiang, X.; Overholtzer, M. V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation. Autophagy 2015, 11, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef]
- Chadet, S.; Allard, J.; Brisson, L.; Lopez-Charcas, O.; Lemoine, R.; Heraud, A.; Lerondel, S.; Guibon, R.; Fromont, G.; Le Pape, A.; et al. P2x4 receptor promotes mammary cancer progression by sustaining autophagy and associated mesenchymal transition. Oncogene 2022, 41, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chai, H.; Ehinger, K.; Egan, T.M.; Srinivasan, R.; Frick, M.; Khakh, B.S. Imaging P2X4 receptor subcellular distribution, trafficking, and regulation using P2X4-pHluorin. J. Gen. Physiol. 2014, 144, 81–104. [Google Scholar] [CrossRef]
- Iyer, D.P.; Khoei, H.H.; van der Weijden, V.A.; Kagawa, H.; Pradhan, S.J.; Novatchkova, M.; McCarthy, A.; Rayon, T.; Simon, C.S.; Dunkel, I.; et al. mTOR activity paces human blastocyst stage developmental progression. Cell 2024, 187, 6566–6583.e22. [Google Scholar] [CrossRef]
- Wu, Y.; Li, B.; Li, L.; Mitchell, S.E.; Green, C.L.; D’Agostino, G.; Wang, G.; Wang, L.; Li, M.; Li, J.; et al. Very-low-protein diets lead to reduced food intake and weight loss, linked to inhibition of hypothalamic mTOR signaling, in mice. Cell Metab. 2021, 33, 888–904.e6. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Xu, Y.; Li, M.; Liu, Y.; Yu, J.; Zhang, L. Chloroquine Enhances Chemosensitivity of Breast Cancer via mTOR Inhibition. Biomedicines 2025, 13, 948. https://doi.org/10.3390/biomedicines13040948
Lin Z, Xu Y, Li M, Liu Y, Yu J, Zhang L. Chloroquine Enhances Chemosensitivity of Breast Cancer via mTOR Inhibition. Biomedicines. 2025; 13(4):948. https://doi.org/10.3390/biomedicines13040948
Chicago/Turabian StyleLin, Zhihao, Yuting Xu, Mifang Li, Yibiao Liu, Jianbo Yu, and Lingyan Zhang. 2025. "Chloroquine Enhances Chemosensitivity of Breast Cancer via mTOR Inhibition" Biomedicines 13, no. 4: 948. https://doi.org/10.3390/biomedicines13040948
APA StyleLin, Z., Xu, Y., Li, M., Liu, Y., Yu, J., & Zhang, L. (2025). Chloroquine Enhances Chemosensitivity of Breast Cancer via mTOR Inhibition. Biomedicines, 13(4), 948. https://doi.org/10.3390/biomedicines13040948





