Converted Macrophage Polarization and Expression of COL6α3—Early Predictors of Remodeling Processes in Adipose Tissue of Male Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric Measurements
2.2. Body Mass Index and Z-Score
2.3. Blood Sample and Serum Analysis
2.4. Subcutaneous and Visceral Adipose Tissue Samples
2.5. Adipose Tissue Morphology
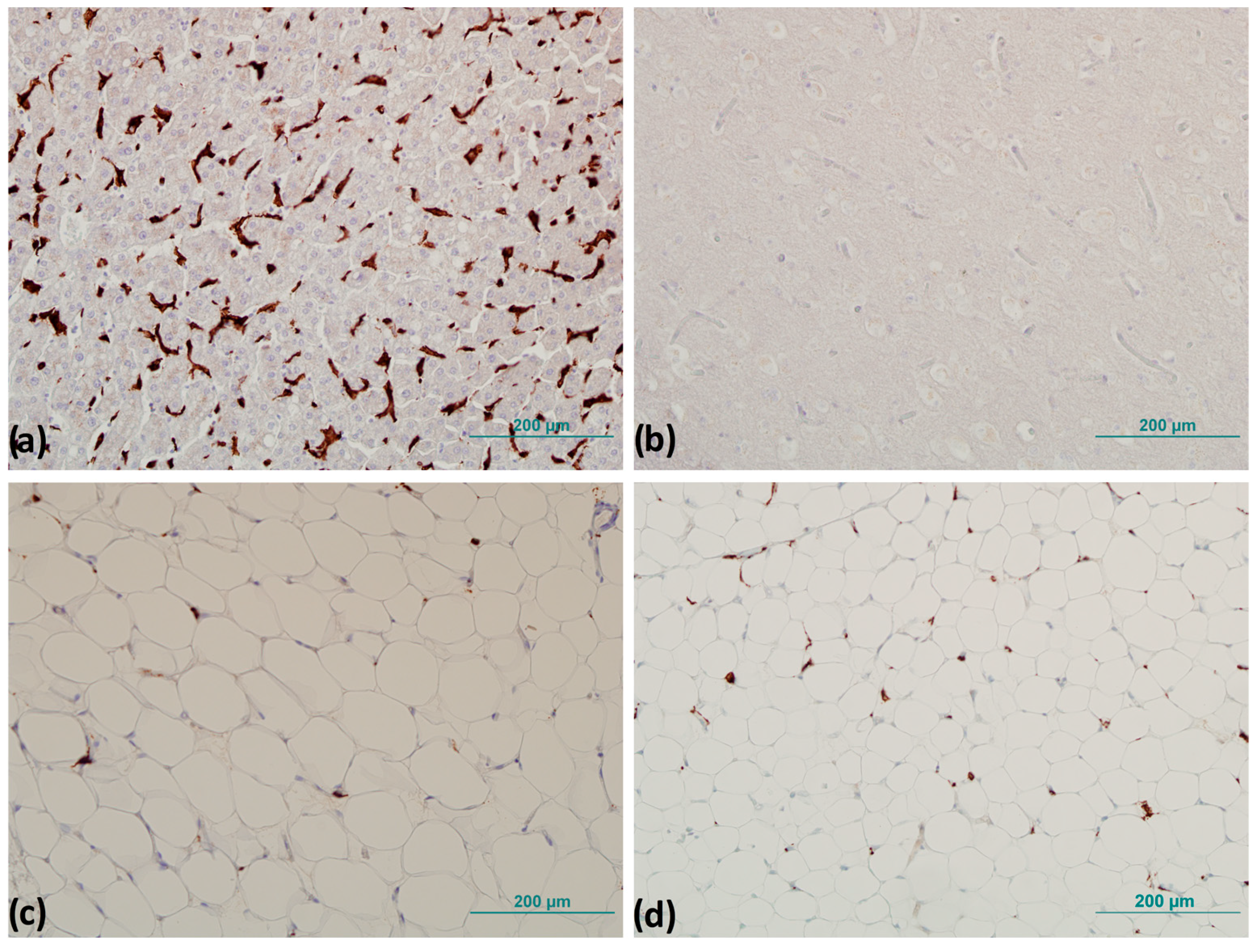
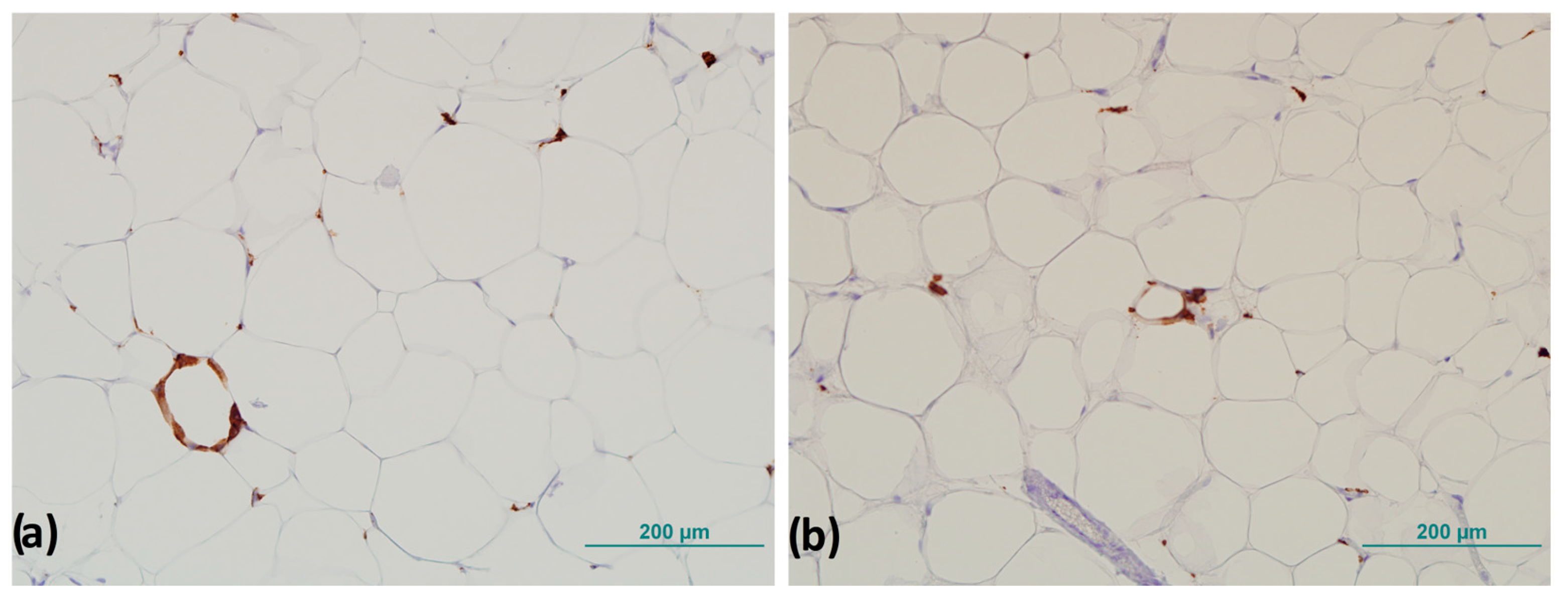
2.6. Immunohistochemistry
2.7. Determination of Relative Gene Expression
2.8. Statistical Analysis
3. Results
3.1. Anthropometric and Laboratory Measurements
3.2. Adipocyte Morphometry
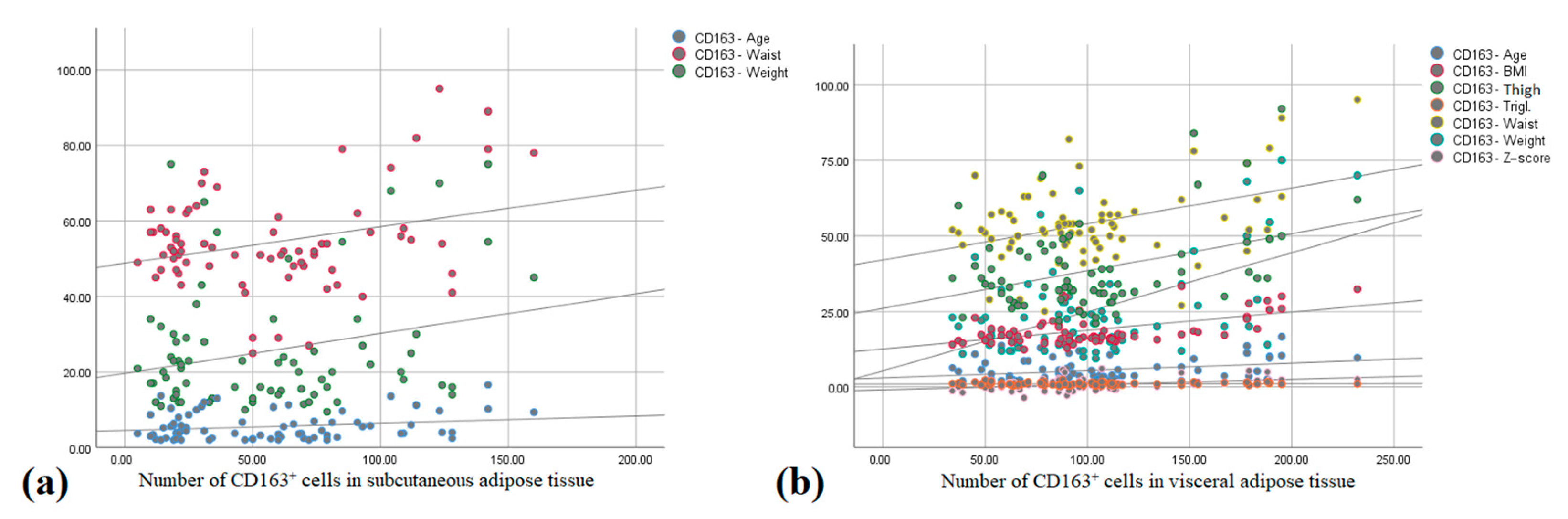
3.3. CD163+ Cells and Number of Crown-like Structures
3.4. Relative Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAFLD | Nonalcoholic fatty liver disease |
| AT | Adipose tissue |
| SAT | Subcutaneous adipose tissue |
| VAT | Visceral adipose tissue |
| WAT | White adipose tissue |
| FFAs | Free fatty acids |
| MetS | Metabolic syndrome |
| ECM | Extracellular matrix |
| COL6α3 | Collagen VI subtype alpha 3 |
| ATMs | Adipose tissue macrophages |
| CLS | Crown-like structure |
| BMI | Body mass index |
| HDL | High density lipoprotein |
| LDL | Low density lipoprotein |
| IHC | Immunohistochemistry |
| LRP10 | Low-density lipoprotein receptor related protein 10 |
| IPO8 | Importin 8 |
| IR | Insulin resistance |
References
- Noncommunicable Diseases: Childhood Overweight and Obesity [Internet]. Available online: https://www.who.int/news-room/questions-and-answers/item/noncommunicable-diseases-childhood-overweight-and-obesity (accessed on 3 February 2022).
- Ward, Z.J.; Long, M.W.; Resch, S.C.; Giles, C.M.; Cradock, A.L.; Gortmaker, S.L. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N. Engl. J. Med. 2017, 377, 2145–2153. [Google Scholar] [CrossRef]
- Kansra, A.R.; Lakkunarajah, S.; Jay, M.S. Childhood and adolescent obesity: A review. Front. Pediatr. 2021, 8, 581461. [Google Scholar] [CrossRef]
- Butte, N.F.; Hopkinson, J.M.; Wong, W.W.; Smith EO bria Ellis, K.J. Body Composition during the First 2 Years of Life: An Updated Reference. Pediatr. Res. 2000, 47, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Freedland, E.S. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef]
- Ebbert, J.O.; Jensen, M.D. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients 2013, 5, 498. [Google Scholar] [CrossRef]
- Choe, E.K.; Kim, D.; Kim, H.J.; Park, K.J. Association of visceral obesity and early colorectal neoplasia. World J. Gastroenterol. 2013, 19, 8349. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, C.; Lu, F.; Liao, Y.; Cai, J.; Gao, J. Challenges and opportunities in obesity: The role of adipocytes during tissue fibrosis. Front. Endocrinol. 2024, 15, 1365156. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef]
- Chun, T.-H. Peri-adipocyte ECM remodeling in obesity and adipose tissue fibrosis. Adipocyte 2012, 1, 89–95. [Google Scholar] [CrossRef]
- Mo, Y.-Y.; Han, Y.-X.; Xu, S.-N.; Jiang, H.-L.; Wu, H.-X.; Cai, J.-M.; Li, L.; Bu, Y.-H.; Xiao, F.; Liang, H.-D.; et al. Adipose Tissue Plasticity: A Comprehensive Definition and Multidimensional Insight. Biomolecules 2024, 14, 1223. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Santibañez, G.; Nien-Kai Lumeng, C. Macrophages and the Regulation of Adipose Tissue Remodeling. Annu. Rev. Nutr. 2014, 34, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced Adipose Tissue Oxygenation in Human Obesity Evidence for Rarefaction, Macrophage Chemotaxis, and Inflammation Without an Angiogenic Response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Dankel, S.N.; Grytten, E.; Bjune, J.-I.; Nielsen, H.J.; Dietrich, A.; Blüher, M.; Sagen, J.V.; Mellgren, G. COL6A3 expression in adipose tissue cells is associated with levels of the homeobox transcription factor PRRX1. Sci. Rep. 2020, 10, 20164. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef]
- Dahdah, N.; Tercero-Alcázar, C.; Malagón, M.M.; Garcia-Roves, P.M.; Guzmán-Ruiz, R. Interrelation of adipose tissue macrophages and fibrosis in obesity. Biochem. Pharmacol. 2024, 225, 116324. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, F.; Cheng, H.; Su, M.; Wang, Y. Macrophage polarization: An important role in inflammatory diseases. Front. Immunol. 2024, 15, 1352946. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Spencer, M.; Unal, R.; Zhu, B.; Rasouli, N.; McGehee, R.E.; Peterson, C.A.; Kern, P.A. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, E1990–E1998. [Google Scholar] [CrossRef]
- Obesity and Overweight [Internet]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 January 2022).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, M.; Campion, J.; Muñoz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-De-Solórzano, C. Adiposoft: Automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- van Steenberghe, M.; Schubert, T.; Guiot, Y.; Bouzin, C.; Bollen, X.; Gianello, P. Enhanced vascular biocompatibility of decellularized xeno-/allogeneic matrices in a rodent model. Cell Tissue Bank. 2017, 18, 249–262. [Google Scholar] [CrossRef]
- Fischer, I.P.; Irmler, M.; Meyer, C.W.; Sachs, S.J.; Neff, F.; de Angelis, M.H.; Beckers, J.; Tschöp, M.H.; Hofmann, S.M.; Ussar, S. A history of obesity leaves an inflammatory fingerprint in liver and adipose tissue. Int. J. Obes. 2018, 42, 507. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Wang, H.; Peng, D.Q. New insights into the mechanism of low high-density lipoprotein cholesterol in obesity. Lipids Health Dis. 2011, 10, 176. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 968–976. [Google Scholar] [CrossRef]
- De Grooth, G.J.; Klerkx, A.H.E.M.; Stroes, E.S.G.; Stalenhoef, A.F.H.; Kastelein, J.J.P.; Kuivenhoven, J.A. A review of CETP and its relation to atherosclerosis. J. Lipid Res. 2004, 45, 1967–1974. [Google Scholar] [CrossRef]
- Linsel-Nitschke, P.; Götz, A.; Erdmann, J.; Braenne, I.; Braund, P.; Hengstenberg, C.; Stark, K.; Fischer, M.; Schreiber, S.; El Mokhtari, N.E.; et al. Lifelong Reduction of LDL-Cholesterol Related to a Common Variant in the LDL-Receptor Gene Decreases the Risk of Coronary Artery Disease—A Mendelian Randomisation Study. PLoS ONE 2008, 3, e2986. [Google Scholar] [CrossRef]
- Alligier, M.; Gabert, L.; Meugnier, E.; Lambert-Porcheron, S.; Chanseaume, E.; Pilleul, F.; Debard, C.; Sauvinet, V.; Morio, B.; Vidal-Puig, A.; et al. Visceral Fat Accumulation During Lipid Overfeeding Is Related to Subcutaneous Adipose Tissue Characteristics in Healthy Men. J. Clin. Endocrinol. Metab. 2013, 98, 802–810. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Kuchibhotla, S.; Westfall, K.M.; Silverstein, R.L.; Morton, R.E.; Febbraio, M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc. Res. 2011, 89, 604. [Google Scholar] [CrossRef] [PubMed]
- Cali’, A.M.G.; Bonadonna, R.C.; Trombetta, M.; Weiss, R.; Caprio, S. Metabolic Abnormalities Underlying the Different Prediabetic Phenotypes in Obese Adolescents. J. Clin. Endocrinol. Metab. 2008, 93, 1767. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338. [Google Scholar] [CrossRef]
- Poissonnet, C.M.; Burdi, A.R.; Garn’, S.M. The chronology of adipose tissue appearance and distribution in the human fetus *. Early Hum. Dev. 1984, 10, 1–11. [Google Scholar] [CrossRef]
- Knittle, J.L.; Timmers, K.; Ginsberg-Fellner, F.; Brown, R.E.; Katz, D.P. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J. Clin. Investig. 1979, 63, 239. [Google Scholar] [CrossRef]
- Siegel, M.J.; Hildebolt, C.F.; Bae, K.T.; Hong, C.; White, N.H. Total and intraabdominal fat distribution in preadolescents and adolescents: Measurement with MR imaging. Radiology 2007, 242, 846–856. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- von Krüchten, R.; Lorbeer, R.; Müller-Peltzer, K.; Rospleszcz, S.; Storz, C.; Askani, E.; Kulka, C.; Schuppert, C.; Rathmann, W.; Peters, A.; et al. Association between Adipose Tissue Depots and Dyslipidemia: The KORA-MRI Population-Based Study. Nutrients 2022, 14, 797. [Google Scholar] [CrossRef]
- Muir, L.A.; Baker, N.A.; Washabaugh, A.R.; Neeley, C.K.; Flesher, C.G.; DelProposto, J.B.; Geletka, L.M.; Ghaferi, A.A.; Finks, J.F.; Singer, K.; et al. Adipocyte hypertrophy-hyperplasia balance contributes to weight loss after bariatric surgery. Adipocyte 2017, 6, 134. [Google Scholar] [CrossRef]
- Orsso, C.E.; Colin-Ramirez, E.; Field, C.J.; Madsen, K.L.; Prado, C.M.; Haqq, A.M. Adipose Tissue Development and Expansion from the Womb to Adolescence: An Overview. Nutrients 2020, 12, 2735. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Bernard, S.; Arner, P. Adipocyte Turnover: Relevance to Human Adipose Tissue Morphology. Diabetes 2010, 59, 105. [Google Scholar] [CrossRef]
- Tarabra, E.; Nouws, J.; Vash-Margita, A.; Nadzam, G.S.; Goldberg, R.; Van Name, M.; Pierpont, B.; Knight, J.R.; Shulman, G.I.; Caprio, S. The omentum of obese girls harbors small adipocytes and browning transcripts. JCI Insight 2020, 5, e135448. [Google Scholar] [CrossRef]
- Landgraf, K.; Rockstroh, D.; Wagner, I.V.; Weise, S.; Tauscher, R.; Schwartze, J.T.; Löffler, D.; Bühligen, U.; Wojan, M.; Till, H.; et al. Evidence of Early Alterations in Adipose Tissue Biology and Function and Its Association With Obesity-Related Inflammation and Insulin Resistance in Children. Diabetes 2015, 64, 1249–1261. [Google Scholar] [CrossRef]
- Kazankov, K.; Barrera, F.; Møller, H.J.; Bibby, B.M.; Vilstrup, H.; George, J.; Grønbæk, H. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology 2014, 60, 521–530. [Google Scholar] [CrossRef]
- Kazankov, K.; Møller, H.J.; Lange, A.; Birkebaek, N.H.; Holland-Fischer, P.; Solvig, J.; Hørlyck, A.; Kristensen, K.; Rittig, S.; Handberg, A.; et al. The macrophage activation marker sCD163 is associated with changes in NAFLD and metabolic profile during lifestyle intervention in obese children. Pediatr. Obes. 2015, 10, 226–233. [Google Scholar] [CrossRef]
- Kotronen, A.; Juurinen, L.; Hakkarainen, A.; Westerbacka, J.; Corneér, A.; Bergholm, R.; Yki-Järvinen, H. Liver Fat Is Increased in Type 2 Diabetic Patients and Underestimated by Serum Alanine Aminotransferase Compared with Equally Obese Nondiabetic Subjects. Diabetes Care 2008, 31, 165–169. [Google Scholar] [CrossRef]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, E.S.; Koo, J.S. Tumor-associated macrophages and crown-like structures in adipose tissue in breast cancer. Breast Cancer Res. Treat. 2018, 170, 15–25. [Google Scholar] [CrossRef]
- Murano, I.; Barbatelli, G.; Parisani, V.; Latini, C.; Muzzonigro, G.; Castellucci, M.; Cinti, S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008, 49, 1562–1568. [Google Scholar] [CrossRef]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell. Biol. 2009, 29, 1575. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Yao-Borengasser, A.; Unal, R.; Rasouli, N.; Gurley, C.M.; Zhu, B.; Peterson, C.A.; Kern, P.A. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am. J. Physiol.—Endocrinol. Metab. 2010, 299, E1016. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Gowronska-Kozak, B.; Burk, D.; Remedios, I.; Hymel, D.; Gimble, J.; Ravussin, E.; Bray, G.A.; Smith, S.R. Adipose tissue collagen VI in obesity. J. Clin. Endocrinol. Metab. 2009, 94, 5155–5162. [Google Scholar] [CrossRef]
- Tam, C.S.; Heilbronn, L.K.; Henegar, C.; Wong, M.; Cowell, C.T.; Cowley, M.J.; Kaplan, W.; Clément, K.; Baur, L.A. An early inflammatory gene profile in visceral adipose tissue in children. Int. J. Pediatr. Obes. 2011, 6, e360–e363. [Google Scholar] [CrossRef]
- Henegar, C.; Tordjman, J.; Achard, V.; Lacasa, D.; Cremer, I.; Guerre-Millo, M.; Poitou, C.; Basdevant, A.; Stich, V.; Viguerie, N.; et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008, 9, R14. [Google Scholar] [CrossRef]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose Tissue Hypoxia in Obesity and Its Impact on Adipocytokine Dysregulation. Diabetes 2007, 56, 901–911. [Google Scholar] [CrossRef]
- Wang, B.; Wood, I.S.; Trayhurn, P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007, 455, 479. [Google Scholar] [CrossRef]
- Hammarstedt, A.; Graham, T.E.; Kahn, B.B. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol. Metab. Syndr. 2012, 4, 42. [Google Scholar] [CrossRef]
- Isakson, P.; Hammarstedt, A.; Gustafson, B.; Smith, U. Impaired preadipocyte differentiation in human abdominal obesity: Role of Wnt, tumor necrosis factor-α, and inflammation. Diabetes 2009, 58, 1550–1557. [Google Scholar] [CrossRef]
- Mujkić, R.; Šnajder Mujkić, D.; Ilić, I.; Rođak, E.; Šumanovac, A.; Grgić, A.; Divković, D.; Selthofer-Relatić, K. Early Childhood Fat Tissue Changes—Adipocyte Morphometry, Collagen Deposition, and Expression of CD163+ Cells in Subcutaneous and Visceral Adipose Tissue of Male Children. Int. J. Environ. Res. Public Health 2021, 18, 3627. [Google Scholar] [CrossRef]
- Mariman, E.C.M.; Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol. Life Sci. 2010, 67, 1277. [Google Scholar] [CrossRef]
- Buechler, C.; Krautbauer, S.; Eisinger, K. Adipose tissue fibrosis. World J. Diabetes 2015, 6, 548–553. [Google Scholar] [CrossRef] [PubMed]

| Normal Body Weight | Overweight/Obese | p * | |
|---|---|---|---|
| n | 42 | 33 | |
| Age (years) | 5.08 ± 3.17 | 6.24 ± 3.85 | 0.157 |
| Body weight (kg) | 20.31 ± 9.61 | 32.35 ± 20.07 | 0.001 |
| Height (cm) | 110.76 ± 21.71 | 114.73 ± 30.16 | 0.510 |
| BMI (kg/m2) | 19.75 ± 1.43 | 26.72 ± 5.18 | <0.001 |
| Z-score | 0.40 ± 1.00 | 2.36 ± 1.49 | <0.001 |
| Waist circumference (cm) | 50.65 ± 10.97 | 58.61 ± 13.83 | 0.008 |
| Thigh circumference (cm) | 34.74 ± 11.63 | 43.71 ± 14.96 | 0.005 |
| Normal Body Weight | Overweight/Obese | p * | |
|---|---|---|---|
| n | 42 | 33 | |
| CD163+ cells | |||
| SAT | 73.36 ± 34.26 | 84.29 ± 34.60 | 0.403 |
| VAT | 98.57 ± 20.74 | 168.18 ± 33.13 | <0.001 |
| Crown-like structures | |||
| SAT | 0.50 ± 0.70 | 1.12 ± 1.08 | 0.004 |
| VAT | 2.93 ± 1.73 | 4.36 ± 2.32 | 0.003 |
| Normal Body Weight | Overweight/Obese | p * | |
|---|---|---|---|
| Collagen 6 α3 in SAT | 0.83 ± 0.15 | 1.14 ± 0.18 | 0.001 |
| Collagen 6 α3 in VAT | 0.68 ± 0.04 | 0.84 ± 0.08 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujkić, R.; Šnajder Mujkić, D.; Rožac, K.; Matić, A.; Kovač Lukić, T.; Divković, D.; Selthofer-Relatić, K. Converted Macrophage Polarization and Expression of COL6α3—Early Predictors of Remodeling Processes in Adipose Tissue of Male Children. Biomedicines 2025, 13, 935. https://doi.org/10.3390/biomedicines13040935
Mujkić R, Šnajder Mujkić D, Rožac K, Matić A, Kovač Lukić T, Divković D, Selthofer-Relatić K. Converted Macrophage Polarization and Expression of COL6α3—Early Predictors of Remodeling Processes in Adipose Tissue of Male Children. Biomedicines. 2025; 13(4):935. https://doi.org/10.3390/biomedicines13040935
Chicago/Turabian StyleMujkić, Robert, Darija Šnajder Mujkić, Karla Rožac, Anita Matić, Tanja Kovač Lukić, Dalibor Divković, and Kristina Selthofer-Relatić. 2025. "Converted Macrophage Polarization and Expression of COL6α3—Early Predictors of Remodeling Processes in Adipose Tissue of Male Children" Biomedicines 13, no. 4: 935. https://doi.org/10.3390/biomedicines13040935
APA StyleMujkić, R., Šnajder Mujkić, D., Rožac, K., Matić, A., Kovač Lukić, T., Divković, D., & Selthofer-Relatić, K. (2025). Converted Macrophage Polarization and Expression of COL6α3—Early Predictors of Remodeling Processes in Adipose Tissue of Male Children. Biomedicines, 13(4), 935. https://doi.org/10.3390/biomedicines13040935






