Enhancing Mitochondrial Function Through Pharmacological Modification: A Novel Approach to Mitochondrial Transplantation in a Sepsis Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Mitochondrial Isolation and Transplantation
2.4. Mitochondrial Respiration Measurement
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. RNA Extraction and Reverse Transcription–Quantitative Polymerase Chain Reaction (RT–qPCR)
2.7. Statistical Analysis
3. Results
3.1. The Drug Treatment Affected Mitochondrial Respiration
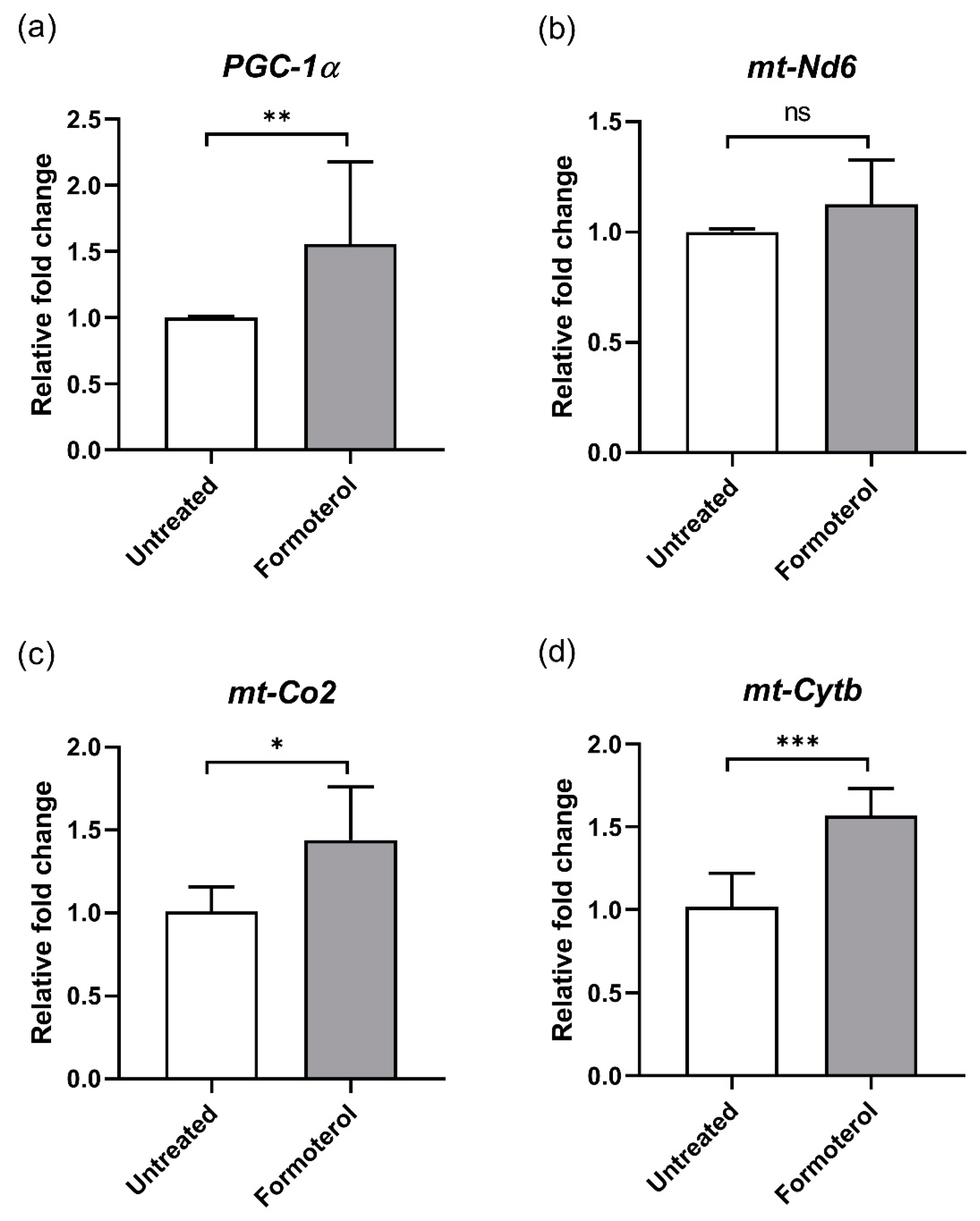
3.2. Formoterol Increases the Expression of Mitochondrial Genes
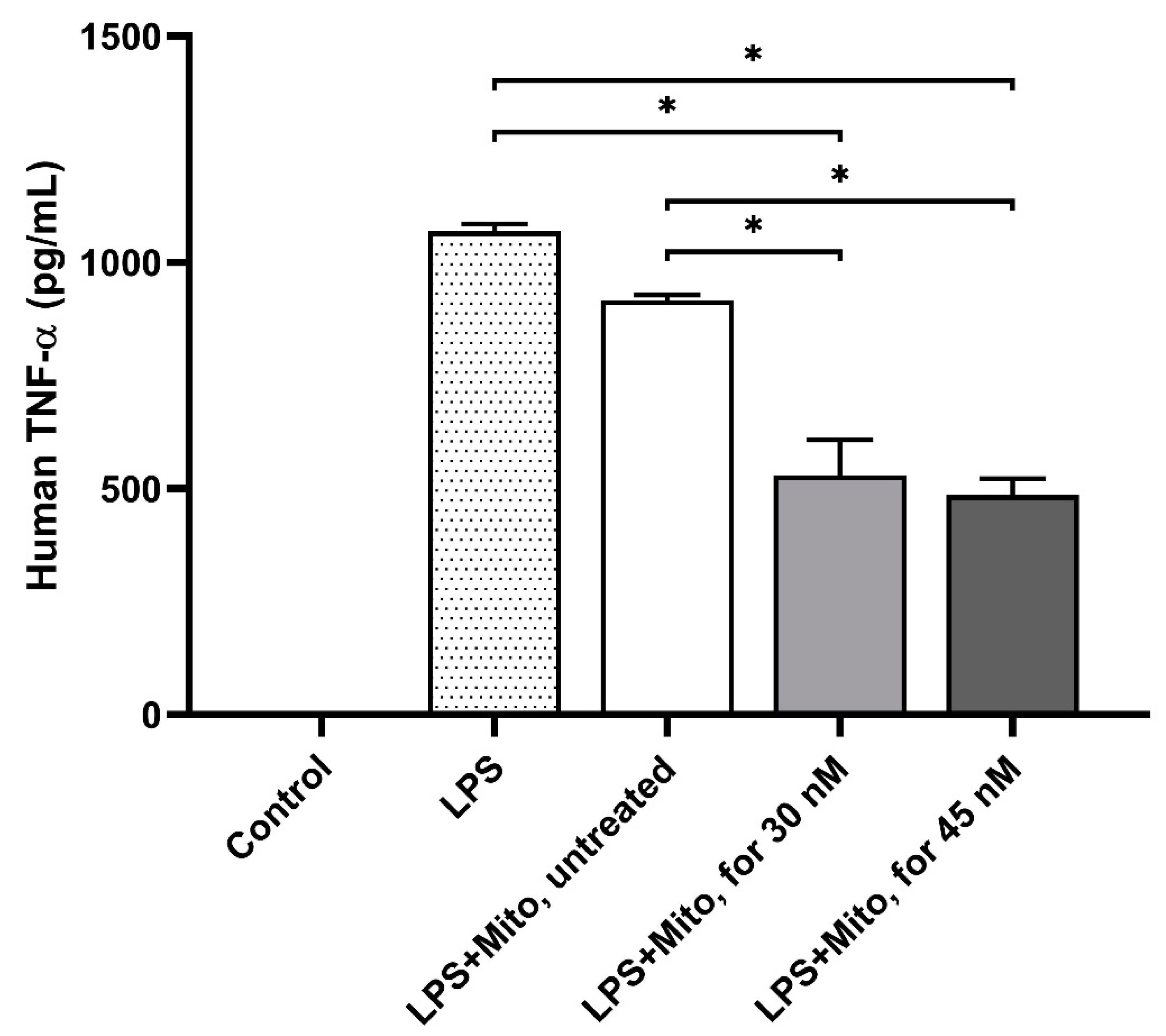
3.3. Formoterol-Enhanced Mitochondrial Transplantation Alleviated the Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Actb | actin, beta; |
| ETC | electron transport chain; |
| LPS | lipopolysaccharide; |
| mt-Cytb | cytochrome b, mitochondrial; |
| mt-Co2 | cytochrome c oxidase II, mitochondrial; |
| mt-Nd6 | NADH dehydrogenase 6, mitochondrial; |
| Ppargc1a | PPARG coactivator 1 alpha; |
| TNF-α | Tumor Necrosis Factor-alpha. |
References
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Zmijewski, J.W. Mitochondrial Dysfunction and Immune Cell Metabolism in Sepsis. Infect. Chemother. 2017, 49, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Ning, B.T. Signaling pathways and intervention therapies in sepsis. Signal Transduct. Target. Ther. 2021, 6, 407. [Google Scholar] [CrossRef]
- Rossmann, M.P.; Dubois, S.M.; Agarwal, S.; Zon, L.I. Mitochondrial function in development and disease. Dis. Model. Mech. 2021, 14, dmm048912. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. Redox Regulation of Mitochondrial Function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef]
- Yasukawa, K.; Oshiumi, H.; Takeda, M.; Ishihara, N.; Yanagi, Y.; Seya, T.; Kawabata, S.; Koshiba, T. Mitofusin 2 Inhibits Mitochondrial Antiviral Signaling. Sci. Signal. 2009, 2, ra47. [Google Scholar] [CrossRef]
- Döhla, J.; Kuuluvainen, E.; Gebert, N.; Amaral, A.; Englund, J.I.; Gopalakrishnan, S.; Konovalova, S.; Nieminen, A.I.; Salminen, E.S.; Muñumer, R.T.; et al. Metabolic determination of cell fate through selective inheritance of mitochondria. Nat. Cell Biol. 2022, 24, 148–154. [Google Scholar] [CrossRef]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic Reticulum–Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef]
- Garrabou, G.; Morén, C.; López, S.; Tobías, E.; Cardellach, F.; Miró, Ò.; Casademont, J. The Effects of Sepsis on Mitochondria. J. Infect. Dis. 2012, 205, 392–400. [Google Scholar] [CrossRef]
- Lira Chavez, F.M.; Gartzke, L.P.; van Beuningen, F.E.; Wink, S.E.; Henning, R.H.; Krenning, G.; Bouma, H.R. Restoring the infected powerhouse: Mitochondrial quality control in sepsis. Redox Biol. 2023, 68, 102968. [Google Scholar] [CrossRef]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Wu, G.; Huang, P.; Wang, H.; An, H.; Liu, S.; Zhang, W. The pathogenesis and potential therapeutic targets in sepsis. MedComm 2023, 4, e418. [Google Scholar] [CrossRef]
- Kim, K. Future of sepsis: Perspective on diagnosis. Clin. Exp. Emerg. Med. 2022, 9, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.H.; Greenwood, J.C.; Spyres, M.B.; Eckmann, D.M. Measurement of Mitochondrial Respiration and Motility in Acute Care: Sepsis, Trauma, and Poisoning. J. Intensive Care Med. 2017, 32, 86–94. [Google Scholar] [CrossRef]
- Wills, L.P.; Trager, R.E.; Beeson, G.C.; Lindsey, C.C.; Peterson, Y.K.; Beeson, C.C.; Schnellmann, R.G. The β2-Adrenoceptor Agonist Formoterol Stimulates Mitochondrial Biogenesis. J. Pharmacol. Exp. Ther. 2012, 342, 106–118. [Google Scholar] [CrossRef]
- Calkins, M.J.; Manczak, M.; Mao, P.; Shirendeb, U.; Reddy, P.H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2011, 20, 4515–4529. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, M.; Hao, S.; Jia, M.; Ji, M.; Qiu, L.; Sun, X.; Yang, J.; Li, K. Mitochondria-Targeted Peptide Reverses Mitochondrial Dysfunction and Cognitive Deficits in Sepsis-Associated Encephalopathy. Mol. Neurobiol. 2015, 52, 783–791. [Google Scholar] [CrossRef]
- Chen, L.; Peng, J.; Wang, Y.; Jiang, H.; Wang, W.; Dai, J.; Tang, M.; Wei, Y.; Kuang, H.; Xu, G.; et al. Fenofibrate-induced mitochondrial dysfunction and metabolic reprogramming reversal: The anti-tumor effects in gastric carcinoma cells mediated by the PPAR pathway. Am. J. Transl. Res. 2020, 12, 428–446. [Google Scholar]
- de Marañón, A.M.; Díaz-Pozo, P.; Canet, F.; Díaz-Morales, N.; Abad-Jiménez, Z.; López-Domènech, S.; Vezza, T.; Apostolova, N.; Morillas, C.; Rocha, M.; et al. Metformin modulates mitochondrial function and mitophagy in peripheral blood mononuclear cells from type 2 diabetic patients. Redox Biol. 2022, 53, 102342. [Google Scholar] [CrossRef]
- Mollo, N.; Nitti, M.; Zerillo, L.; Faicchia, D.; Micillo, T.; Accarino, R.; Secondo, A.; Petrozziello, T.; Calì, G.; Cicatiello, R.; et al. Pioglitazone Improves Mitochondrial Organization and Bioenergetics in Down Syndrome Cells. Front. Genet. 2019, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef]
- Lenaz, G.; Fato, R.; Formiggini, G.; Genova, M.L. The role of Coenzyme Q in mitochondrial electron transport. Mitochondrion 2007, 7, S8–S33. [Google Scholar] [CrossRef]
- Borcherding, N.; Brestoff, J.R. The power and potential of mitochondria transfer. Nature 2023, 623, 283–291. [Google Scholar] [CrossRef]
- Hwang, J.W.; Lee, M.J.; Chung, T.N.; Lee, H.A.R.; Lee, J.H.; Choi, S.Y.; Park, Y.J.; Kim, C.H.; Jin, I.; Kim, S.H.; et al. The immune modulatory effects of mitochondrial transplantation on cecal slurry model in rat. Crit. Care 2021, 25, 20. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.A.R.; Lee, M.J.; Park, Y.J.; Mun, S.; Yune, C.J.; Chung, T.N.; Bae, J.; Kim, M.J.; Choi, Y.S.; et al. The Effects of Mitochondrial Transplantation on Sepsis Depend on the Type of Cell from Which They Are Isolated. Int. J. Mol. Sci. 2023, 24, 10113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Noh, J.H.; Lee, M.J.; Park, Y.J.; Kim, B.M.; Kim, Y.S.; Hwang, S.; Park, C.; Kim, K. Effects of Mitochondrial Transplantation on Transcriptomics in a Polymicrobial Sepsis Model. Int. J. Mol. Sci. 2023, 24, 15326. [Google Scholar] [CrossRef]
- Mokhtari, B.; Hamidi, M.; Badalzadeh, R.; Mahmoodpoor, A. Mitochondrial transplantation protects against sepsis-induced myocardial dysfunction by modulating mitochondrial biogenesis and fission/fusion and inflammatory response. Mol. Biol. Rep. 2023, 50, 2147–2158. [Google Scholar] [CrossRef]
- Yan, C.; Ma, Z.; Ma, H.; Li, Q.; Zhai, Q.; Jiang, T.; Zhang, Z.; Wang, Q. Mitochondrial Transplantation Attenuates Brain Dysfunction in Sepsis by Driving Microglial M2 Polarization. Mol. Neurobiol. 2020, 57, 3875–3890. [Google Scholar] [CrossRef]
- Cameron, R.B.; Peterson, Y.K.; Beeson, C.C.; Schnellmann, R.G. Structural and pharmacological basis for the induction of mitochondrial biogenesis by formoterol but not clenbuterol. Sci. Rep. 2017, 7, 10578. [Google Scholar] [CrossRef]
- Jesinkey, S.R.; Funk, J.A.; Stallons, L.J.; Wills, L.P.; Megyesi, J.K.; Beeson, C.C.; Schnellmann, R.G. Formoterol Restores Mitochondrial and Renal Function after Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2014, 25, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Yamaoka, Y. Simple and inexpensive technique for measuring oxygen consumption rate in adherent cultured cells. J. Physiol. Sci. 2017, 67, 731–737. [Google Scholar] [CrossRef]
- Hao, S.; Huang, M.; Xu, X.; Wang, X.; Song, Y.; Jiang, W.; Huo, L.; Gu, J. Identification and validation of a novel mitochondrion-related gene signature for diagnosis and immune infiltration in sepsis. Front. Immunol. 2023, 14, 1196306. [Google Scholar] [CrossRef] [PubMed]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Stoccoro, A.; Coppedè, F. Mitochondrial DNA Methylation and Human Diseases. Int. J. Mol. Sci. 2021, 22, 4594. [Google Scholar] [CrossRef]
- Wallace, L.; Mehrabi, S.; Bacanamwo, M.; Yao, X.; Aikhionbare, F.O. Expression of mitochondrial genes MT-ND1, MT-ND6, MT-CYB, MT-COI, MT-ATP6, and 12S/MT-RNR1 in colorectal adenopolyps. Tumor Biol. 2016, 37, 12465–12475. [Google Scholar] [CrossRef]
- Arif, E.; Solanki, A.K.; Srivastava, P.; Rahman, B.; Fitzgibbon, W.R.; Deng, P.; Budisavljevic, M.N.; Baicu, C.F.; Zile, M.R.; Megyesi, J.; et al. Mitochondrial biogenesis induced by the β2-adrenergic receptor agonist formoterol accelerates podocyte recovery from glomerular injury. Kidney Int. 2019, 96, 656–673. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Eo, H.; Yu, S.H.; Choi, Y.; Kim, Y.; Kang, Y.C.; Lee, H.; Kim, J.H.; Han, K.; Lee, H.K.; Chang, M.Y.; et al. Mitochondrial transplantation exhibits neuroprotective effects and improves behavioral deficits in an animal model of Parkinson’s disease. Neurotherapeutics 2024, 21, e00355. [Google Scholar] [CrossRef]


| Symbol | Primer Sequences (5’→3’) | |
|---|---|---|
| Actb | Forward | TGTGGATTGGTGGCTCTATC |
| Reverse | AGAAAGGGTGTAAAACGCAG | |
| PGC-1α (Ppargc1a) | Forward | AGGAAATCCGAGCTGAGCTGAACA |
| Reverse | GCAAGAAGGCGACACATCGAACAA | |
| mt-Cytb | Forward | CCCACAGGATTAAACTCCGA |
| Reverse | GTTGGGAATGGAGCGTAGAA | |
| mt-Co2 | Forward | CAAGACGCTACATCACCTATC |
| Reverse | CTAATAGACGAAGTTCACCTGG | |
| mt-Nd6 | Forward | TAGACCCTCAAGTCTCCGGG |
| Reverse | TGGTGGGCTTGGATTGATTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Kim, Y.-S.; Kim, K. Enhancing Mitochondrial Function Through Pharmacological Modification: A Novel Approach to Mitochondrial Transplantation in a Sepsis Model. Biomedicines 2025, 13, 934. https://doi.org/10.3390/biomedicines13040934
Kim B, Kim Y-S, Kim K. Enhancing Mitochondrial Function Through Pharmacological Modification: A Novel Approach to Mitochondrial Transplantation in a Sepsis Model. Biomedicines. 2025; 13(4):934. https://doi.org/10.3390/biomedicines13040934
Chicago/Turabian StyleKim, Bomi, Yun-Seok Kim, and Kyuseok Kim. 2025. "Enhancing Mitochondrial Function Through Pharmacological Modification: A Novel Approach to Mitochondrial Transplantation in a Sepsis Model" Biomedicines 13, no. 4: 934. https://doi.org/10.3390/biomedicines13040934
APA StyleKim, B., Kim, Y.-S., & Kim, K. (2025). Enhancing Mitochondrial Function Through Pharmacological Modification: A Novel Approach to Mitochondrial Transplantation in a Sepsis Model. Biomedicines, 13(4), 934. https://doi.org/10.3390/biomedicines13040934






